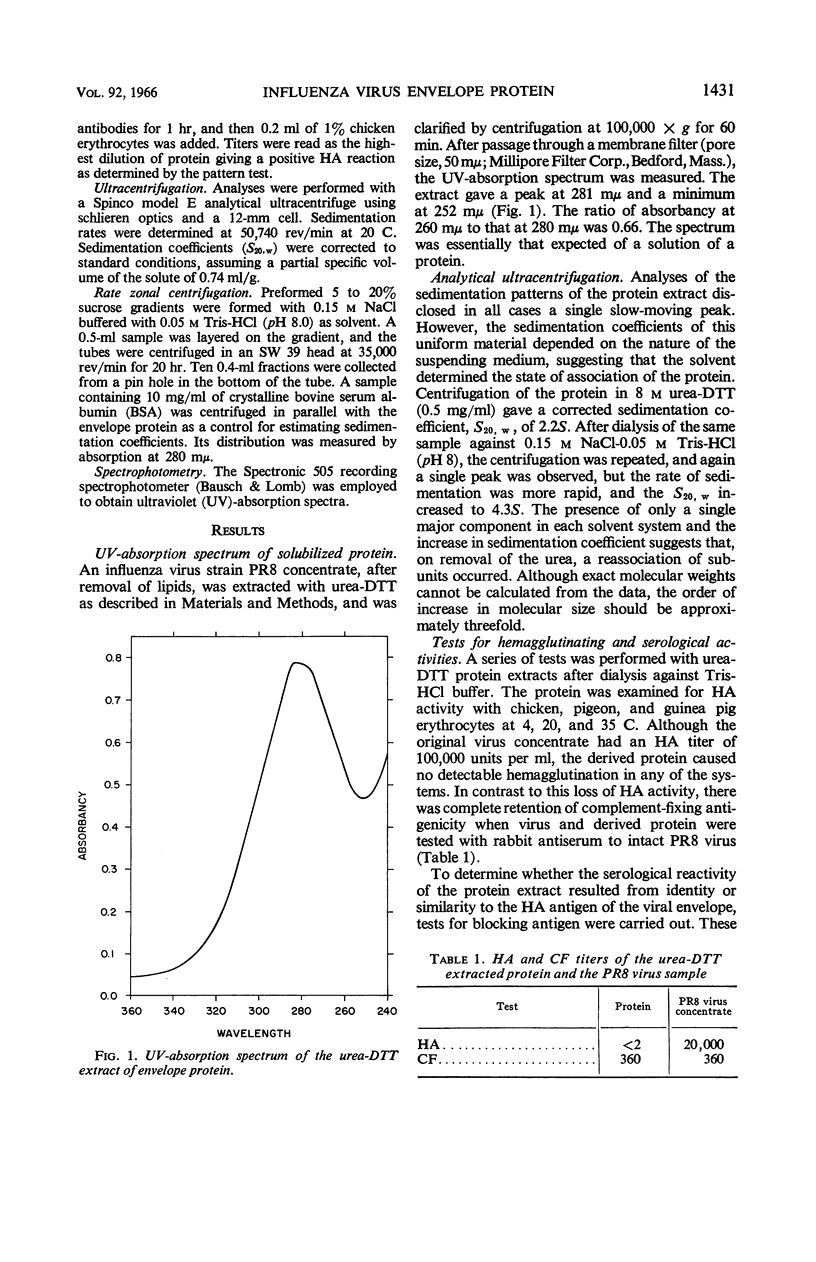
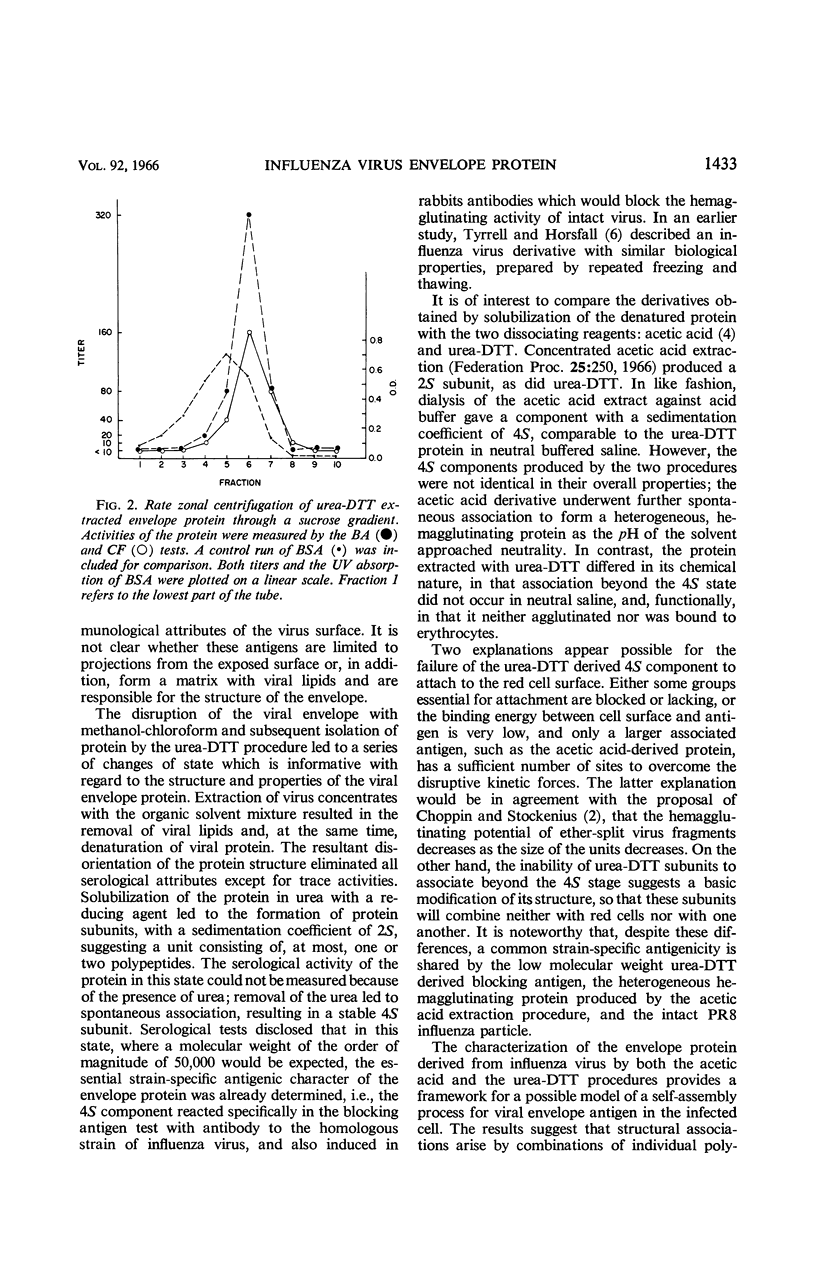
Abstract
Eckert, Edward A. (The University of Michigan, Ann Arbor). Characterization of a low molecular weight antigenic protein from the envelope of influenza virus. J. Bacteriol. 92:1430–1434. 1966.—An antigenic protein from the lipid-extracted residue of influenza virus strain PR8 was solubilized with urea-dithiothreitol (DTT). The protein subunits had a sedimentation coefficient of 2S in urea-DTT and reassociated to a 4S state on dialysis. This form of the envelope protein did not agglutinate erythrocytes, but reacted with strain-specific antisera in the complement-fixation and blocking-antigen tests.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHOPPIN P. W., STOECKENIUS W. INTERACTIONS OF ETHER-DISRUPTED INFLUENZA A2 VIRUS WITH ERYTHROCYTES, INHIBITORS, AND ANTIBODIES. Virology. 1964 Apr;22:482–492. doi: 10.1016/0042-6822(64)90069-8. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. DITHIOTHREITOL, A NEW PROTECTIVE REAGENT FOR SH GROUPS. Biochemistry. 1964 Apr;3:480–482. doi: 10.1021/bi00892a002. [DOI] [PubMed] [Google Scholar]
- Eckert E. A. Envelope protein(s) derived from influenza virus. J Bacteriol. 1966 May;91(5):1907–1910. doi: 10.1128/jb.91.5.1907-1910.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TYRRELL D. A., HORSFALL F. L., Jr Disruption of influenza virus; properties of degradation products of the virus particle. J Exp Med. 1954 Apr 1;99(4):321–342. doi: 10.1084/jem.99.4.321. [DOI] [PMC free article] [PubMed] [Google Scholar]


