Abstract
Objective:
To determine whether high-sensitivity C-reactive protein (hsCRP) and serum amyloid A (SAA) predict stroke, vascular events, and mortality in a prospective cohort study.
Background:
Markers of inflammation have been associated with risk of myocardial infarction (MI). Their association with stroke is controversial.
Methods:
The Northern Manhattan Study includes a stroke-free community-based cohort study in participants aged ≥40 years (median follow-up 7.9 years). hsCRP and SAA were measured using nephelometry. Cox proportional hazards models were used to calculate hazard ratios (HR) and 95% confidence intervals (CI) for the association of markers with risk of ischemic stroke and other outcomes after adjusting for demographics and risk factors.
Results:
hsCRP measurements were available in 2,240 participants (mean age 68.9 ± 10.1 years; 64.2% women; 18.8% white, 23.5% black, and 55.1% Hispanic). The median hsCRP was 2.5 mg/L. Compared with those with hsCRP <1 mg/L, those with hsCRP >3 mg/L were at increased risk of ischemic stroke in a model adjusted for demographics (HR = 1.60, 95% CI 1.06–2.41), but the effect was attenuated after adjusting for other risk factors (adjusted HR = 1.20, 95% CI 0.78–1.86). hsCRP >3 mg/L was associated with risk of MI (adjusted HR = 1.70, 95% CI 1.04–2.77) and death (adjusted HR = 1.55, 95% CI 1.23–1.96). SAA was not associated with stroke risk.
Conclusion:
In this multiethnic cohort, high-sensitivity C-reactive protein (hsCRP) was not associated with ischemic stroke, but was modestly associated with myocardial infarction and mortality. The value of hsCRP and serum amyloid A may depend on population characteristics such as age and other risk factors.
GLOSSARY
- AHA
= American Heart Association;
- BP
= blood pressure;
- CDC
= Centers for Disease Control and Prevention;
- CI
= confidence interval;
- CRP
= C-reactive protein;
- CUMC
= Columbia University Medical Center;
- HR
= hazard ratio;
- hsCRP
= high-sensitivity C-reactive protein;
- IQR
= interquartile range;
- JUPITER
= Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin;
- MI
= myocardial infarction;
- NOMAS
= Northern Manhattan Study;
- SAA
= serum amyloid A.
Acute-phase proteins, such as C-reactive protein (CRP) and serum amyloid A (SAA), are nonspecific biomarkers of inflammation and have been proposed to be indicators of atherosclerosis and predictors of both cardiovascular and cerebrovascular endpoints.1 The Centers for Disease Control and Prevention (CDC) and the American Heart Association (AHA) jointly issued a consensus statement on inflammatory markers that identified hsCRP as the optimal inflammatory biomarker to estimate risk in primary prevention, but recommended limiting its use to persons in whom traditional risk factors place them at “intermediate” risk.2 Several studies using relatively young and disease-free populations confirm the value of hsCRP in predicting risk of incident myocardial infarction (MI) and stroke.3–5 Most studies, however, suggest that hsCRP is a more powerful risk marker for MI than stroke.6 The relationship of hsCRP to vascular outcomes may depend on the population studied, including ethnic and age differences, and medical and behavioral risk factors.7–10 The most consistent predictive value of hsCRP has been its association with all-cause mortality, established across prospective cohort studies of healthy men and women.11
We sought to determine whether hsCRP levels are associated with risk of incident ischemic stroke and other vascular events in the stroke-free, multiethnic urban Northern Manhattan Study (NOMAS) cohort after adjusting for demographic and traditional risk factors.
METHODS
NOMAS is a population-based prospective cohort study designed to evaluate stroke incidence and risk factors in an urban, multiethnic cohort, as previously described.12 The race/ethnic distribution of this cohort consists of 63% Hispanic, 20% non-Hispanic black, and 15% non-Hispanic white residents. In brief, participants were identified using random digit dialing using dual-frame sampling to allow for inclusion of both published and unpublished telephone numbers. Eligible participants 1) had no prior diagnosis of stroke, 2) were aged ≥39 years, and 3) resided in Northern Manhattan for >3 months in a household with a telephone. In-person evaluations were conducted for all participants either at the medical center or at home (6%). This study received approval from the institutional review board at Columbia University Medical Center (CUMC), and all participants provided informed consent.
Participant history and risk factor information were collected through interviews conducted by trained bilingual research assistants; study physicians conducted physical and neurologic examinations. Study participants self-reported race/ethnicity. Blood pressure, height, weight, fasting glucose, and lipid panels were collected as previously described.12 Standardized questions derived from the CDC Behavioral Risk Factor Surveillance System were used to establish risk factor status for hypertension, diabetes, peripheral vascular disease, TIA, cigarette smoking, and cardiac conditions.13 Hypertension was defined as systolic blood pressure (BP) ≥140 mm Hg or diastolic BP ≥90 mm Hg determined by the average of 2 BP measurements, prior history of diagnosis of hypertension, or patient self-reporting of hypertension or regimen of antihypertensive drug use. Diabetes mellitus was defined as fasting glucose ≥126 mg/dL or a participant’s self-report of history of disease or insulin or hypoglycemic drug use.
hsCRP and SAA biomarker measurements.
Whole blood was collected in 5 mL ethylenediaminetetra-acetic acid anticoagulated tubes by a trained phlebotomist. Blood samples were centrifuged at 3,000 rpm for 10 minutes, with serum samples immediately separated, divided into aliquots, and stored in 1.2-mL cryovials at −70°C until ready for batch testing. hsCRP and SAA levels were determined using a BNII nephelometer (Dade Behring, Deerfield, IL) in the Center for Advanced Laboratory Medicine at CUMC by technicians blind to clinical status.
Cohort follow-up and endpoint adjudication.
Annual telephone follow-up assessed vital and functional status, and interval hospitalization or illness, including symptoms indicative of stroke, TIA, or myocardial infarction, as previously described.12 Stroke was defined by the first symptomatic occurrence of stroke according to World Health Organization criteria.12 Stroke subtype (ischemic or hemorrhagic) was decided by a consensus of 2 study neurologists, with a third neurologist adjudicating as needed. Myocardial infarction outcomes were defined according to criteria developed from the Cardiac Arrhythmia Suppression Trial14 and Lipid Research Clinics Coronary Primary Prevention Trial and required at least 2 of the following criteria: 1) typical angina ischemic cardiac pain, 2) abnormal creatine phosphokinase- MB fraction or troponin levels, or 3) EKG abnormalities.14,15 Myocardial infarction was diagnosed by a study cardiologist. Cause of death was reviewed by a study physician for all participants and classified as vascular or nonvascular. Stroke and MI outcomes were confirmed using hospital records.
Statistical analysis.
Distribution of hsCRP and other risk factors was calculated, both overall and by participant characteristics. The primary independent variable was hsCRP, categorized according to CDC/AHA risk levels: hsCRP <1 mg/L, hsCRP 1 to 3 mg/L, and hsCRP >3 mg/L.2 The primary outcome was ischemic stroke, and secondary outcomes included all strokes, MI, mortality, and a composite outcome (combined stroke, MI, and mortality). Cox proportional hazards modeling was used to estimate hazard ratios (HR) and 95% confidence intervals (CI) for hsCRP levels, unadjusted and after adjusting for demographics (age, sex, race/ethnicity, and education) and potential confounding medical and behavioral risk factors (hypertension, history of coronary artery disease, blood sugar, systolic BP, waist circumference, high-density lipoprotein, low-density lipoprotein, cigarette smoking, alcohol consumption, and physical activity). Analyses corrected for potential selection bias using the probability weighting method16 were performed, and assumptions for proportionality were met for the primary outcome. Interactions of hsCRP with age, diabetes, hypertension, and history of MI were examined. Secondary analyses used quartiles of hsCRP and, based on recent clinical trial results,17 hsCRP ≥2 mg/L thresholds. SAA quartiles were also analyzed for an association with outcomes, using the lowest quartile as the referent group.
RESULTS
Description of the cohort and association of risk factors with hsCRP.
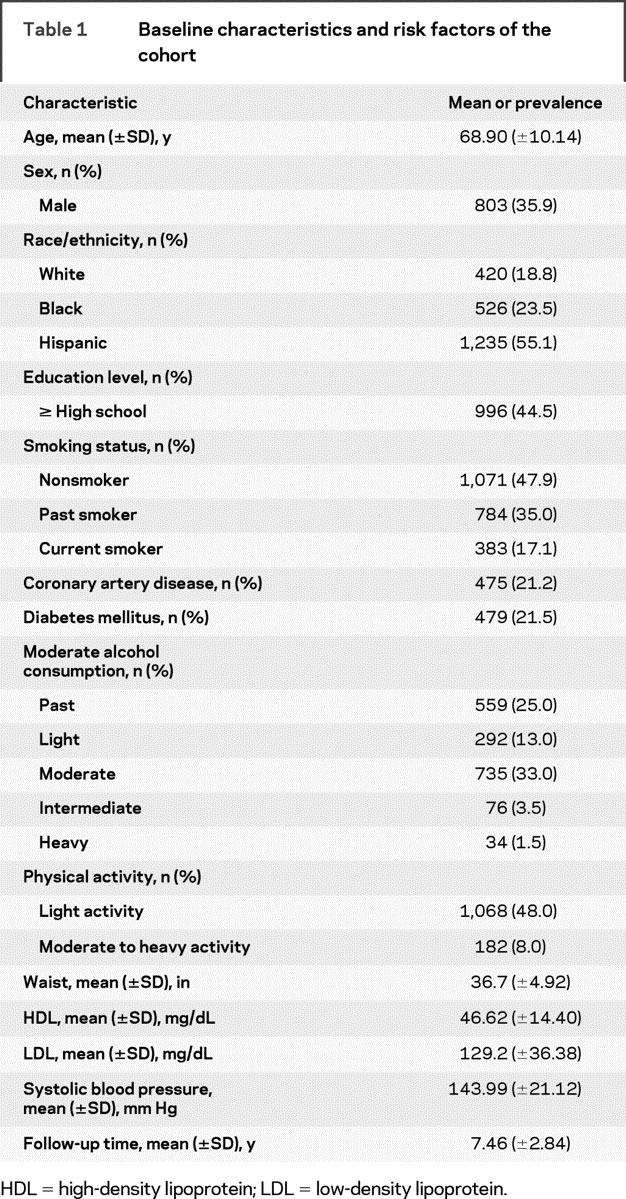
Of the total NOMAS cohort (n = 3,298), 2,240 (67.9%) had baseline hsCRP measured. Descriptive statistics and other baseline risk factors of this population are shown in table 1. The subgroup with hsCRP measured differed minimally from the cohort without hsCRP: those with hsCRP were more likely to be Hispanic (p < 0.0001) and to have a stroke during follow-up (p < 0.0001). The median follow-up was 7.9 years (interquartile range [IQR] 6.1–9.0 years). There were 198 strokes of all types, 171 (86.4%) ischemic strokes, 156 MIs, and 586 deaths, including 246 vascular deaths, 303 nonvascular deaths, and 37 deaths of unknown etiology. The median hsCRP was 2.5 mg/L (IQR 1.1–5.9 mg/L). There were 524 participants (23.4%) with hsCRP <1 mg/L, 710 (31.7%) with hsCRP 1 to 3 mg/L, and 1,006 (44.9%) with hsCRP ≥3 mg/L.
Table 1 Baseline characteristics and risk factors of the cohort
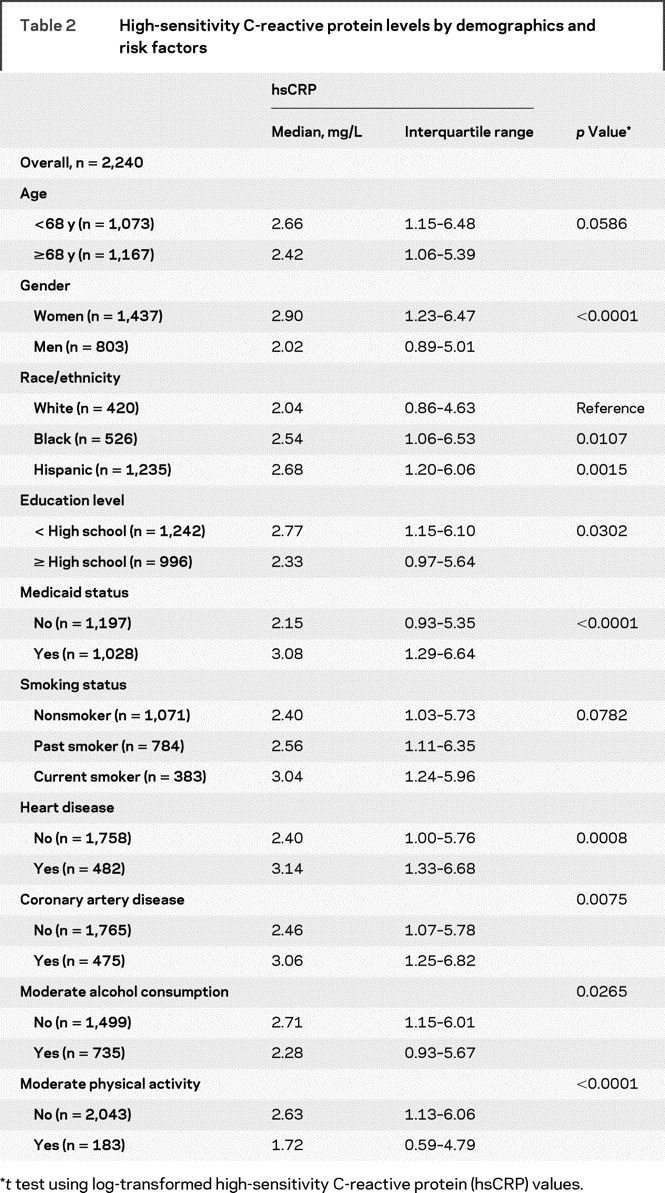
hsCRP levels stratified by demographic and risk factors are shown in table 2. hsCRP levels were higher in those aged <68 years compared with those aged ≥68 years (p = 0.0586), in women (p < 0.0001), and in both non-Hispanic blacks (p = 0.01) and Hispanics (p = 0.0015) than in non-Hispanic whites. hsCRP levels were also associated with educational level (p = 0.011), Medicaid status (p = 0.0001), smoking status (p = 0.0513), alcohol consumption (p = 0.0162), and physical activity (p < 0.0001). The median SAA value was 5.2 mg/L (IQR 3.0–8.8).
Table 2 High-sensitivity C-reactive protein levels by demographics and risk factors
Association of hsCRP with ischemic stroke and other outcomes.
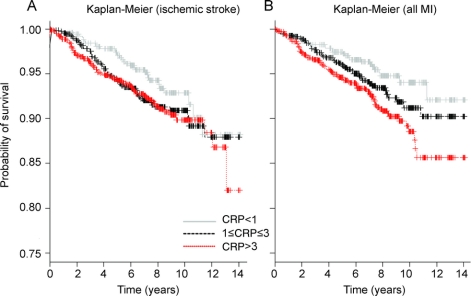
Compared with those with hsCRP <1 mg/L, participants with hsCRP >3 mg/L had increased ischemic stroke risk after adjusting for demographics (adjusted HR = 1.60, 95% CI 1.06–2.41), but not in an unadjusted model (HR = 1.46, 95% CI, 0.97–2.19). Kaplan-Meier curves are shown in the figure. The risk was attenuated, moreover, in a model fully adjusted for both demographics and other risk factors (adjusted HR = 1.20, 95% CI 0.78–1.86; table 3). Similar findings were observed for the outcome of all strokes (adjusted HR for hsCRP >3 mg/L = 1.17, 95% CI 0.79–1.74). Those with hsCRP 1 to 3 mg/L had no increased risk of ischemic stroke or all stroke. There were no interactions of hsCRP >3 mg/L with age, diabetes, hypertension, or history of MI. Results adjusted for possible selection bias were substantially the same.
Figure Kaplan-Meier survival analyses plot for stroke and myocardial infarction
Kaplan-Meier survival analyses plot for stroke (A) and myocardial infarction (MI) (B) using high-sensitivity C-reactive protein (CRP) clinical cutoffs: <1 mg/L, 1 to 3 mg/L, and >3 mg/L.
Table 3 HsCRP CDC/AHA risk categories as predictors of outcomes, unadjusted, adjusted for demographics, and adjusted for demographic and risk factors, as compared with reference hsCRP <1 mg/L
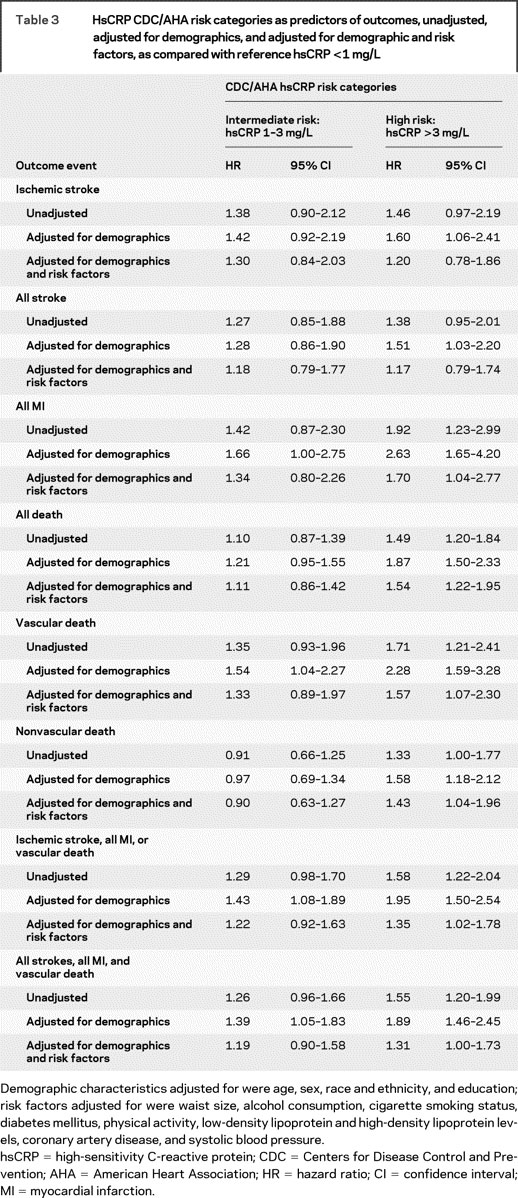
hsCRP >3 mg/L was associated with increased risk of MI in unadjusted models, and remained associated with MI risk after adjusting for demographics and risk factors (adjusted HR = 1.70, 95% CI 1.04–2.77). hsCRP 1 to 3 mg/L was not associated with MI (table 3).
hsCRP >3 mg/L was associated with all-cause mortality in unadjusted and fully adjusted models (adjusted HR = 1.54, 95% CI 1.22–1.95; table 3). The magnitude of association of hsCRP >3 mg/L was greater for vascular (adjusted HR = 1.57, 95% CI 1.07–2.30) than for nonvascular causes of death (adjusted HR = 1.43, 95% CI 1.04–1.96). hsCRP 1 to 3 mg/L was not associated with all-cause mortality.
Compared with the reference group, hsCRP >3 mg/L was associated with the combined vascular outcome endpoint of ischemic stroke, MI, or vascular death (adjusted HR = 1.35, 95% CI 1.02–1.78) and with the composite of all stroke, all MI, and all death (adjusted HR = 1.31, 95% CI 1.00–1.73). Intermediate hsCRP levels were not associated with these combined outcomes in fully adjusted models.
In analyses limited to those participants without prior history of MI, hsCRP >3 mg/L remained associated with all-cause mortality (adjusted HR = 1.59, 95% CI 1.23–2.05), nonvascular death, and the combined outcome of all stroke, MI, and all-cause mortality. There was no association with risk of the composite of stroke, MI, or vascular death (table e-1 on the Neurology® Web site at www.neurology.org). In additional models adjusting for Chlamydia pneumoniae serologies, homocysteine, left ventricular mass, peripheral artery disease, and renal function, results were essentially unchanged.
hsCRP was not associated with ischemic or total stroke risk in analyses using quartiles. Results for MI and other endpoints remained broadly similar to those using the other cutoffs. The third (2.5–5.9 mg/L) and fourth (>5.9 mg/L) quartiles of hsCRP were associated with mortality (adjusted HR for quartile 3 = 1.34, 95% CI 1.03–1.76 and for quartile 4 = 1.97, 95% CI 1.50–2.59) compared with the reference group (table e-2).
In a subgroup analysis designed to mimic the enrolment criteria and outcomes of a recently published trial of rosuvastatin therapy for those at low risk,17 among those (n = 686) without history of cardiovascular disease, diabetes, or use of cholesterol-lowering medication, and with baseline low-density lipoprotein level <130 mg/dL and creatinine <2.0 mg/dL, those with hsCRP ≥2 mg/L were not at increased risk compared with those with hsCRP <2 mg/L for the combined outcome of stroke, MI, or vascular death. Though patients on cholesterol-lowering medications during follow-up had a reduced risk for vascular outcomes (adjusted HR = 0.32, 95% CI 0.15–0.70), there was no interaction with hsCRP >2 mg/L (p = 0.1646).
Association of SAA with stroke and other outcomes.
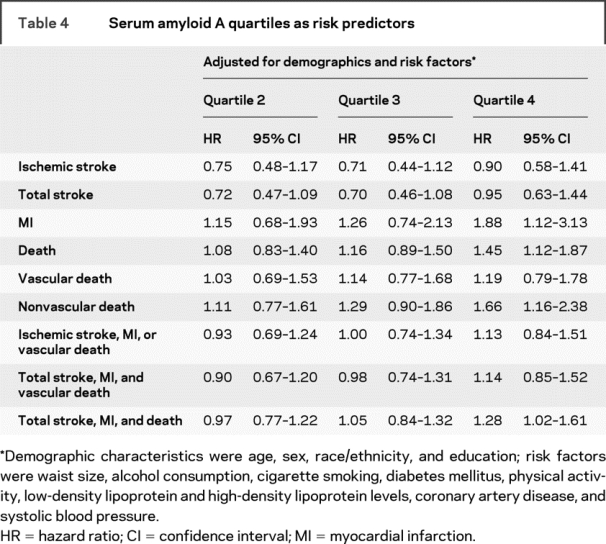
SAA quartiles were not associated with ischemic or total strokes (table 4). SAA demonstrated roughly similar abilities as hsCRP to predict MI and composite outcomes (all stroke, MI, death). Although the highest quartile of SAA was associated with all-cause mortality and nonvascular mortality, it was not associated with vascular causes of death.
Table 4 Serum amyloid A quartiles as risk predictors
DISCUSSION
hsCRP may serve as a sensitive but nonspecific marker of systemic inflammation, and it seems to predict future ischemic events, particularly MI, in many populations.1,2 The precise use of this marker for risk prediction over traditional risk factors for ischemic stroke, however, remains uncertain, and the effect of hsCRP and other acute-phase proteins varies across different populations. In NOMAS, hsCRP >3 mg/L was associated with ischemic stroke risk in the model adjusted for demographics, but these effects did not persist after controlling for other potential risk factors. Our findings corroborate previous studies showing that hsCRP is independently predictive of all-cause mortality,18,19 but we found no clear effect in stroke risk prediction. Differences between our findings and those of others may reflect differences in the burden of risk factors or underlying risk of the study population.
Many,20–22 but not all,23 studies that evaluated associations between hsCRP and incident stroke have provided evidence of increased risk for those in the topmost quantiles of hsCRP. Those studies in which predictive associations are found, however, tend to include relatively young, healthy cohorts.4 The absence of an association with stroke in our population may reflect the older age and heavy risk factor burden of our cohort. In our cohort, prevalence of diabetes mellitus is 21.5%, and that of hypertension is 74.6%. Other studies similarly found a difference in effect based on the presence or absence of other risk factors. In the Honolulu Heart Study, for example, hsCRP was not predictive of stroke among men with hypertension or diabetes, and among those older than 55 years.20 In the Cardiovascular Health Study, among persons aged ≥65 years, hsCRP in the upper quartile was modestly associated with stroke risk (adjusted HR = 1.60), but risk factor burden of the cohort was lower than in NOMAS, with diabetes affecting 15.7% and hypertension affecting 47.4% of the study population.22 A Japanese community-based study identified a relationship between hsCRP in the upper tertile and ischemic stroke risk (multivariate adjusted HR = 1.77), but diabetes was present in only 7.7% and hypertension was present in 45.8% of the population, and the population was younger (mean 63.9 years) than in NOMAS.24
Race/ethnic differences between NOMAS and other populations may also explain some of the differences. Hispanics, who constitute approximately 52% of NOMAS, had higher hsCRP levels than whites in NOMAS, and higher levels than those seen in predominantly white race/ethnic groups in several other studies.5,6,9 Some CRP gene polymorphisms are associated with hsCRP levels, and these may provide an explanation for different CRP levels across race/ethnic groups.25 We did not assess genetic polymorphisms in this study.
hsCRP predicted all-cause mortality, vascular, and nonvascular death in our cohort, even after adjusting for cardiovascular risk factors, including coronary artery disease and excluding those with a history of MI. The ability of hsCRP to predict nonvascular as well as vascular mortality suggests that hsCRP is nonspecific, and may be a marker of chronic illness more generally. Studies have found that hsCRP predicts in-hospital mortality in critical illnesses such as septic shock and traumatic subdural hematoma.26 Increased levels of CRP have also been linked to many chronic conditions not related to cardiovascular disease.18 In an analysis of a large hospital-based cohort (n = 274,515) admissions, low-sensitivity CRP predicted mortality for both cardiac and cancer deaths, though the study could not assess risk at CRP levels <5 mg/L.18 The Whitehall Study (n = 5,360) provided evidence of an association of hsCRP with vascular death (adjusted HR = 1.86), and the relationship remained consistent after excluding men with prior history of disease (adjusted HR = 1.82).19 The observed strength of association in NOMAS between hsCRP and vascular (adjusted HR = 1.83) and nonvascular (adjusted HR = 1.89) deaths is consistent with these findings.19 Additional studies found that joint elevation in hsCRP and other inflammatory markers was associated with all-cause mortality risk.27
hsCRP also predicted MI in our cohort, consistent with findings in other cohorts.28–30 However, after excluding those with prior history of MI, the effect of hsCRP >3 mg/L was greatly attenuated. In other studies that have simultaneously assessed the effect of hsCRP on MI and stroke, the effect on MI was greater than the effect on stroke.28,29
SAA, like CRP, is a major acute-phase reactant, but SAA levels reach higher plasma concentrations and may have different responses to stimuli than CRP.31 In our study, there was no association of SAA with stroke outcomes, though there was an effect on MI and mortality. SAA predicted nonvascular death, with no effect observed for prediction of vascular mortality outcomes. These results are broadly consistent with findings in other populations. In the Women’s Health Study, the upper quartile of SAA was associated with a 3-fold elevated risk of cardiovascular events.29 In another study, SAA had a marginal effect on future cardiovascular outcomes (adjusted HR = 1.03, 95% CI 1.02–1.05).32 SAA has also been linked to increased risk of early mortality in unstable coronary syndrome patients.33 Additionally, independent values of both hsCRP and SAA among persons with peripheral arterial disease are predictors of all-cause mortality (adjusted HR = 1.13, 95% CI 1.03–1.23).34
The role of hsCRP in predicting risk has been controversial. Recently, a large randomized trial, Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER), demonstrated a protective effect of rosuvastatin against vascular outcomes in otherwise healthy persons using a new clinical threshold of hsCRP ≥2.0 mg/L.17 We thus attempted to evaluate this threshold in secondary analyses in our cohort. Among those NOMAS participants who would have qualified for JUPITER, there was no difference in vascular outcomes for those with hsCRP ≥2 mg/L compared with those with hsCRP <2 mg/L, though there was evidence of an effect of use of cholesterol-lowering medications during follow-up on lowering risk of vascular events. Our data thus do not confirm that hsCRP ≥2 mg/L should be used generally to determine use of statin therapy, because the benefits may extend to those with levels <2 as well. Our observational study was not designed specifically to address the use of statin therapy, however.
Our study has several strengths. We studied a racially and ethnically diverse population, including a large number of Hispanics, a traditionally understudied population. Our follow-up was excellent, and there were a large number of strokes. Our study also had some potential limitations associated with characterizing underlying inflammatory disorders that may influence hsCRP levels. First, we had only a single hsCRP measurement rather than several measures over time. Most other studies have used a similar approach, however. Second, NOMAS was designed before inflammation was recognized to be a potential contributor to atherogenesis, and therefore a limitation of our design is that we did not have data on infections or inflammatory diseases at baseline or follow-up that may influence hsCRP levels. Other analyses in the NOMAS population have found that repeated hsCRP measures are stable over time.35
DISCLOSURE
Dr. Elkind serves as Resident and Fellow Section Editor for Neurology®; serves as a consultant to Bristol-Myers Squibb/Sanofi Pharmaceuticals Partnership, GlaxoSmithKline, Jarvik Heart, Tethys Bioscience, Inc., and Daiichi-Sankyo; serves on speakers’ bureaus for Boehringer-Ingelheim, Inc. and Bristol-Myers Squibb/Sanofi Pharmaceuticals Partnership; and receives research support from diaDexus, Inc., Bristol-Myers Squibb/Sanofi Pharmaceuticals Partnership, and the NIH/NINDS [K23 NS42912 (PI), R01 NS050724 (PI), NS048134 (PI), P50 NS049060 (Project PI), R37 NS029993 (Co-PI), and R01 NS55809 (Co-I)]; and has given expert testimony on behalf of Merck Serono (Vioxx® litigation), Pfizer Inc. (Shiley valve and Celebrex®/Bextra® litigation), and Novartis (Zelnorm® and stroke litigation). Mr. Luna, Ms. Moon, Ms. Liu, and Dr. Paik report no disclosures. Dr. Spitalnik has received speaker honoraria from the British Blood Transfusion Society; has served on NIH Study Sections; and receives research support from the NIH [R21 HL087906 (PI)]. Dr. Sacco has received speaker honoraria from Boehringer Ingelheim and Sanofi-aventis; serves as a consultant to Boehringer Ingelheim, Sanofi-aventis, and GlaxoSmithKline; and receives research support from the NIH [NINDS R37 NS29993 (Co-PI)].
Supplementary Material
Address correspondence and reprint requests to Dr. Mitchell S.V. Elkind, Neurological Institute, Box 182, 710 W. 168 St., New York, NY 10032
mse13@columbia.edu
Supplemental data at www.neurology.org
Supported by the NIH/National Institute of Neurological Disorders and Stroke (R01 NS48134 and R37 29993).
Disclosure: Author disclosures are provided at the end of the article.
Presented at the American Academy of Neurology Annual Meeting, April 2008, Chicago, IL.
Received December 16, 2008. Accepted in final form July 17, 2009.
REFERENCES
- 1.Di Napoli M, Schwaninger M, Cappelli R, et al. Evaluation of C-reactive protein measurement for assessing risk and prognosis in ischemic stroke: a statement for healthcare professional from the CRP Pooling Project member. Stroke 2005;36:1316–1329. [DOI] [PubMed] [Google Scholar]
- 2.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003;107:499–511. [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently health women. Circulation 1998;98:731–733. [DOI] [PubMed] [Google Scholar]
- 4.Rost NS, Wolf PA, Kase CS, et al. Plasma concentration of C-reactive protein and risk of ischemic stroke and transient ischemic attack: the Framingham study. Stroke 2001;32:2575–2579. [DOI] [PubMed] [Google Scholar]
- 5.Everett BM, Kurth T, Buring JE, Ridker PM, et al. The relative strength of C-reactive protein and lipid levels as determinants of ischemic stroke compared with coronary heart disease in women. J Am Coll Cardiol 2006;48:2235–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med 1997;336:973–979. [DOI] [PubMed] [Google Scholar]
- 7.Anand SS, Razak F, Yi Q, et al. C-reactive protein as a screening test for cardiovascular risk in a multiethnic population. Arterioscler Thromb Vasc Biol 2004;24:1509–1515. [DOI] [PubMed] [Google Scholar]
- 8.Yamada S, Gotoh T, Nakashima Y, et al. Distribution of serum C-reactive protein and its association with atherosclerotic risk factors in a Japanese population: Jichi Medical School cohort study. Am J Epidemiol 2001;153:1183–1190. [DOI] [PubMed] [Google Scholar]
- 9.Kelley-Hedgepeth A, Lloyd-Jones DM, Colvin A, et al. Ethnic differences in C-reactive protein concentrations. Clin Chem 2008;54:1027–1037. [DOI] [PubMed] [Google Scholar]
- 10.Tracy RP, Lemaitre RN, Psaty BM, et al. Relationship of C-reactive protein to risk of cardiovascular risk: results from the Cardiovascular Health Study and the Rural Health Promotion Project. Arterioscler Thromb Vasc 1997;17:1121–1127. [DOI] [PubMed] [Google Scholar]
- 11.Ridker PM. High-sensitivity C-reactive protein as a predictor of all-cause mortality: implication for research and patient care. Clin Chem 2008;54:234–237. [DOI] [PubMed] [Google Scholar]
- 12.Sacco RL, Anand K, Lee HS, et al. Homocysteine and the risk of ischemic stroke in a tri-ethnic cohort: the Northern Manhattan Study. Stroke 2004;35:2263–2269. [DOI] [PubMed] [Google Scholar]
- 13.Gentry EM, Kalsbeek WD, Hogelin GC, et al. The Behavioral Risk Factor Surveys, II: design, methods, and estimate from combined state data. Am J Prev Med 1985;1:9–14. [PubMed] [Google Scholar]
- 14.Greene HL, Richardson DW, Barker AH, et al; CAPS Investigators. Classification of deaths after myocardial infarction as arrhythmic and nonarrhythmic (the Cardiac Arrhythmia Pilot Study). Am J Cardiol 1989;63:1–6. [DOI] [PubMed] [Google Scholar]
- 15.Morris DL, Kritchevsky SB, Davis CE. Serum carotenoid and coronary heart disease: the Lipid Research Clinics Coronary Primary Prevention Trial and Follow-up Study. JAMA 1994;272:1439–1441. [DOI] [PubMed] [Google Scholar]
- 16.Wang CY, Chen HY. Augmented inverse probability weighted estimator for Cox regression missing covariate regression. Biometrics 2001;57:414–419. [DOI] [PubMed] [Google Scholar]
- 17.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008;359:2195–2207. [DOI] [PubMed] [Google Scholar]
- 18.Marsik C, Kazemi-Shirazi L, Schickbauer T, et al. C-reactive protein and all-cause mortality in a large-hospital based cohort. Clin Chem 2008;54:343–349. [DOI] [PubMed] [Google Scholar]
- 19.Clarke R, Emberson JR, Breeze E, et al. Biomarkers of inflammation predict both vascular and non-vascular mortality in older men. Eur Heart J 2008;29:800–809. [DOI] [PubMed] [Google Scholar]
- 20.Curb JD, Abbott RD, Rodriguez BL, et al. C-reactive protein and the future of thromboembolic stroke in health men. Circulation 2003;107:2016–2020. [DOI] [PubMed] [Google Scholar]
- 21.Wakugawa Y, Kiyohara Y, Tanizaki Y, et al. C-reactive protein and the risk of first ever ischemic and hemorrhagic stroke in a general Japanese population: the Hismaya Study. Stroke 2005;37:27–32. [DOI] [PubMed] [Google Scholar]
- 22.Cao JJ, Thach C, Manolio TA, et al. C-reactive protein, carotid intima-media thickness, and incidence of ischemic stroke in the elderly: the Cardiovascular Health Study. Circulation 2003;108:166–170. [DOI] [PubMed] [Google Scholar]
- 23.Cesari M, Penninx BW, Newman AB, et al. Inflammatory markers and onset of cardiovascular events results from the Health ABC Study. Circulation 2003;108:2317–2322. [DOI] [PubMed] [Google Scholar]
- 24.Makita S, Nakamura M, Satoh K, et al. Serum C-reactive protein levels can be used to predict future ischemic stroke and mortality in Japanese men from the general population. Atherosclerosis Epub 2008 Aug 12. [DOI] [PubMed]
- 25.Shen J, Arnett DK, Parnell LD, et al. Association of common C-reactive protein (CRP) gene polymorphisms with baseline plasma CRP levels and fenofibrate response: the GOLDN Study. Diabetes Care 2008;31:910–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Litton E, Ho KM, Chamberlain J, Dobb GJ, Webb SA. C-reactive protein concentration as a predictor of in-hospital mortality after ICU discharge: a nested-case control study. Critical Care Resusc 2007;9:19–25. [PubMed] [Google Scholar]
- 27.Harris T. Association of elevated interleukim-6 and C-reactive protein levels with mortality in the elderly. Am J Med 1999;106:506–512. [DOI] [PubMed] [Google Scholar]
- 28.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med 2002;55:445–451. [DOI] [PubMed] [Google Scholar]
- 29.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 2000;342:836–843. [DOI] [PubMed] [Google Scholar]
- 30.Yeh ET, Willerson JT. Coming of age of C-reactive protein: using inflammation markers in cardiology. Circulation 2005;107:370–371. [DOI] [PubMed] [Google Scholar]
- 31.Poole S, Walker D, Gaines Das RE, Gallimore JR, Pepys MB. The first international standard for serum amyloid A protein (SAA): evaluation in an international collaborative study. J Immunological Methods 1998;214:1–10. [DOI] [PubMed] [Google Scholar]
- 32.Johnson BD, Kip KE, Marroquin OC, et al. Serum amyloid A as a predictor of coronary artery disease and cardiovascular outcome in women: the National Heart, Lung, and Blood Institute–Sponsored Women’s Ischemia Syndrome Evaluation (WISE). Circulation 2004;109:726–732. [DOI] [PubMed] [Google Scholar]
- 33.Morrow DA, Rifai N, Antman EM, et al. Serum amyloid A predicts early mortality in acute coronary syndromes: a TIMI 11A substudy. J Am Coll Cardiol 1999;35:358–362. [DOI] [PubMed] [Google Scholar]
- 34.Vidula H, Tian L, Liu K, et al. Biomarkers of inflammation and thrombosis as predictors of near-term mortality in patients with peripheral arterial disease: a cohort study. Ann Intern Med 2008;148:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elkind MS, Leon V, Moon YP, Paik MC, Sacco RL. High-sensitivity C-reactive protein and lipoprotein-associated phospholipase A2 stability before and after stroke and myocardial infarction. Stroke (in press 2009). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.