Abstract
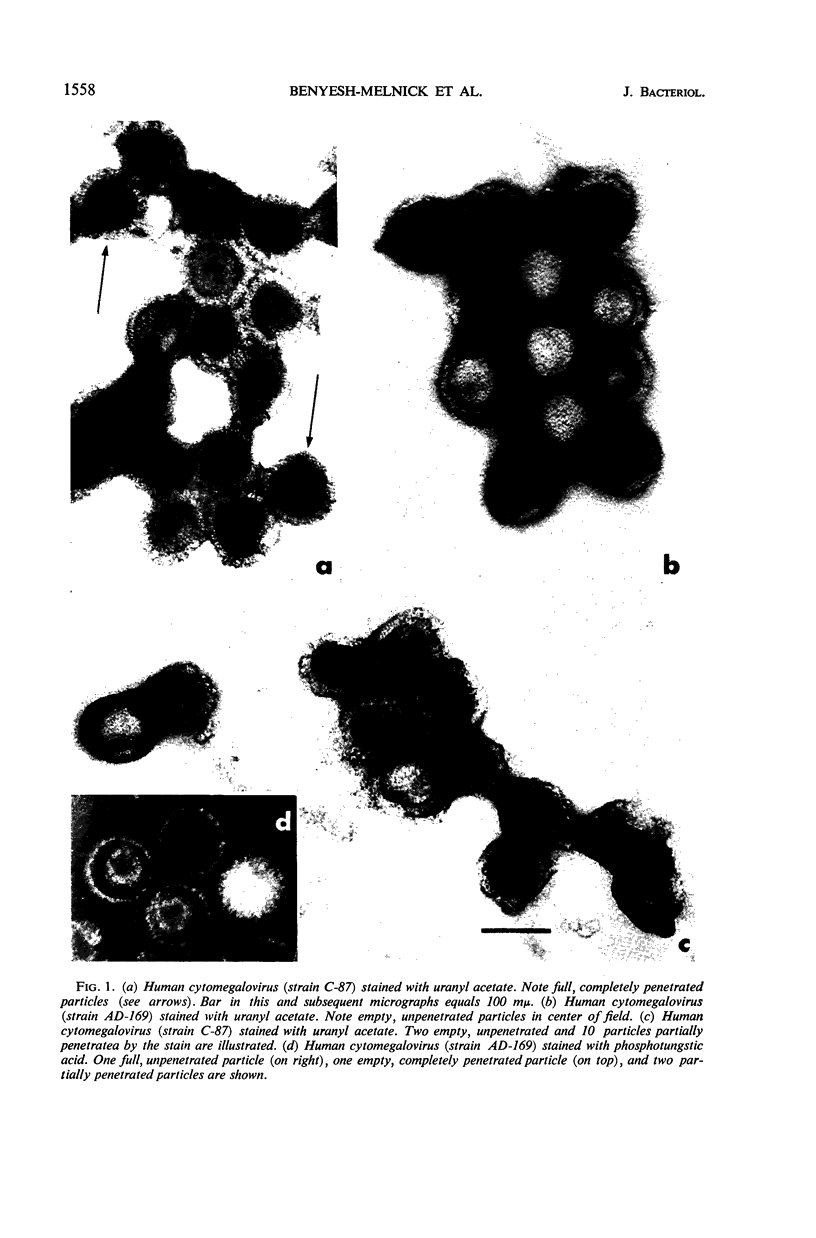
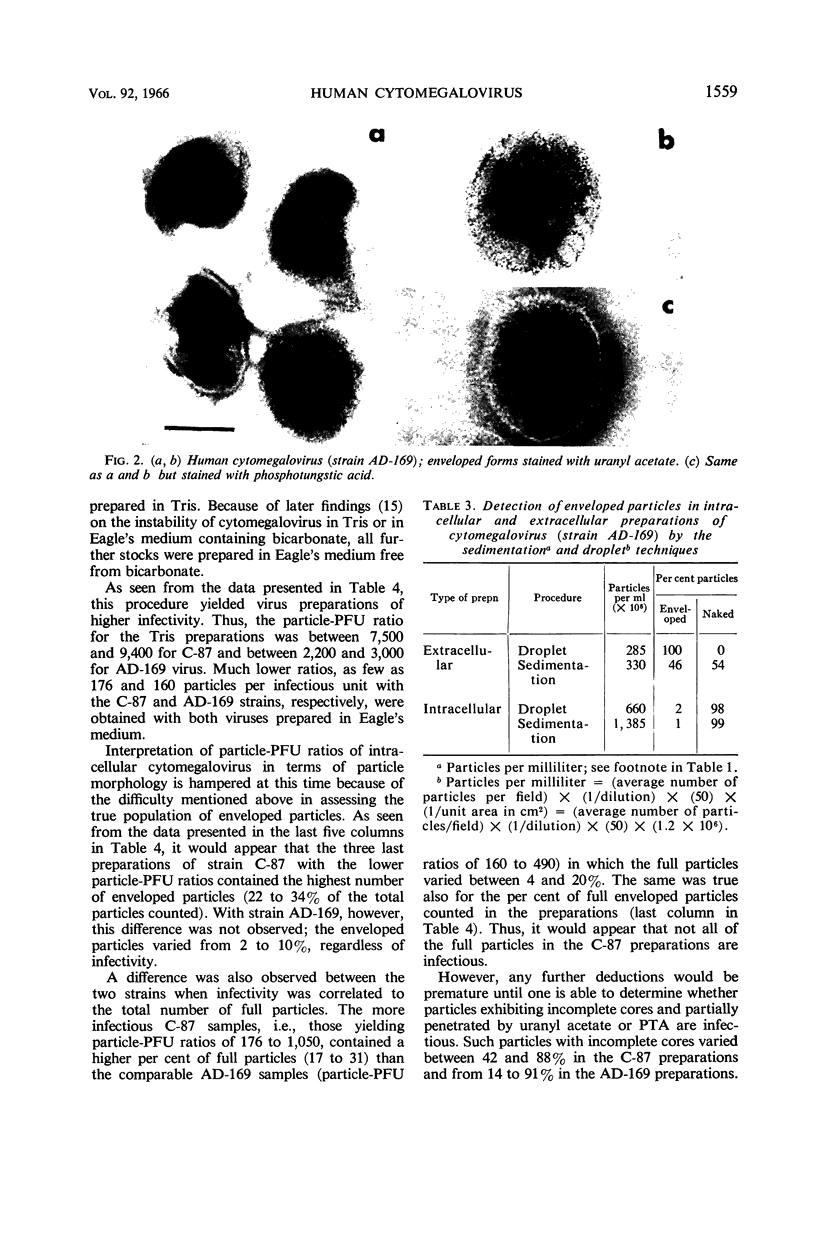
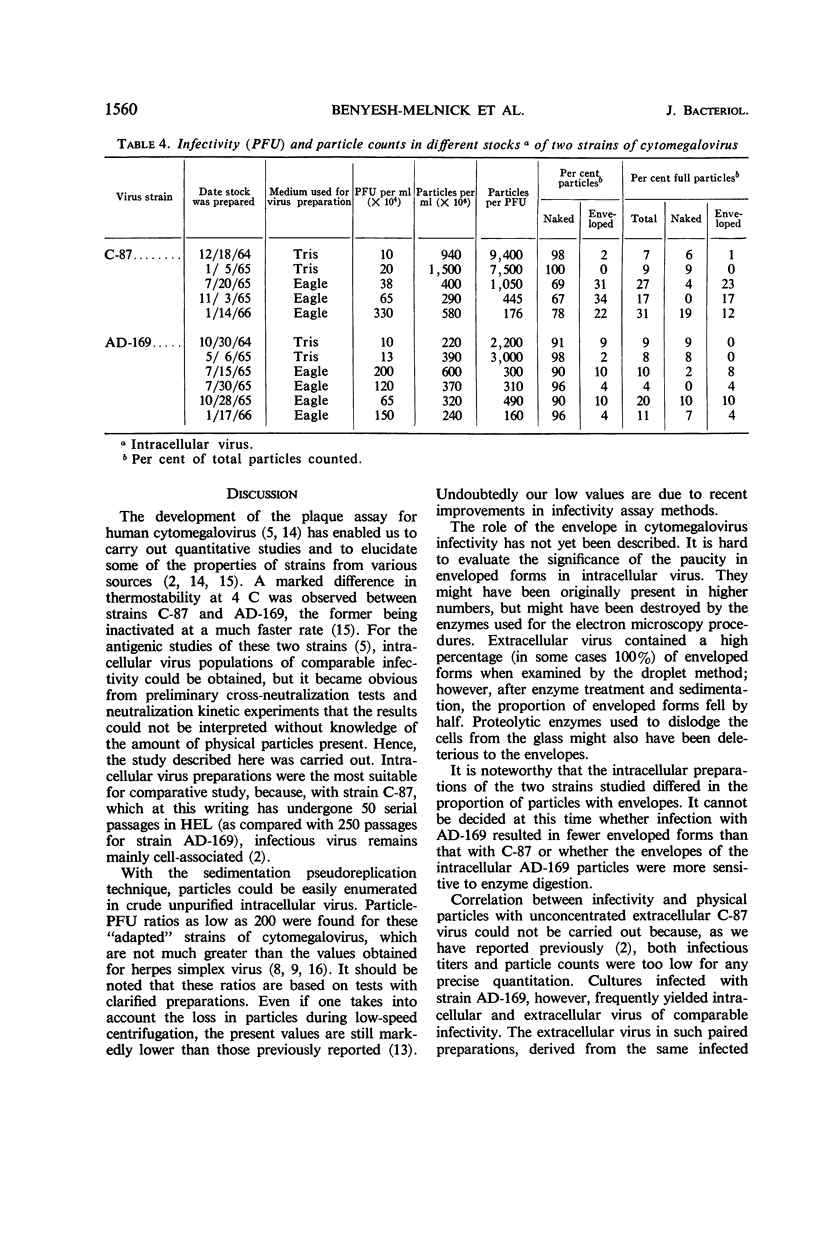
Benyesh-Melnick, Matilda (Baylor University College of Medicine, Houston, Tex.), Fern Probstmeyer, Robert McCombs, Jean P. Brunschwig, and Vladimir Vonka. Correlation between infectivity and physical virus particles in human cyto-megalovirus. J. Bacteriol. 92:1555–1561. 1966.—Infectivity titers [measured as plaque-forming units (PFU)] and particle counts by the sedimentation pseudo-replication technique were determined for crude, unpurified, intracellular preparations of two different strains of human cytomegalovirus. Unlike the high particle-infectivity ratio of 106 to 108 previously reported for these viruses, the number of total particles per PFU ranged from 160 to 490 with strain AD-169 and from 176 to 1,050 for strain C-87. Interpretation of particle-PFU ratios of intracellular cytomegalovirus in terms of particle morphology is not conclusive at this time. The number of enveloped forms found varied between 0 and 34% of the total particles counted. However, the true proportion is probably greater, because envelopes were found to be destroyed by the enzyme treatment used in preparing the specimens for examination in the electron microscope. The number of full particles found ranged between 4 and 31% of the total particles counted. The particle per PFU ratio of extracellular virus was found to be three- to fivefold lower than that of intracellular virus.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENYESH-MELNICK M., ROSENBERG H. S., WATSON B. VIRUSES IN CELL CULTURES OF KIDNEYS OF CHILDREN WITH CONGENITAL HEART MALFORMATIONS AND OTHER DISEASES. Proc Soc Exp Biol Med. 1964 Nov;117:452–459. doi: 10.3181/00379727-117-29607. [DOI] [PubMed] [Google Scholar]
- Benyesh-Melnick M., Vonka V., Probstmeyer F., Wimberly I. Human cytomegalovirus: properties of the complement-fixing antigen. J Immunol. 1966 Feb;96(2):261–267. [PubMed] [Google Scholar]
- GOODHEART C. R., JAROSS L. B. Human cytomegalovirus. Assay by counting infected cells. Virology. 1963 Apr;19:532–535. doi: 10.1016/0042-6822(63)90047-3. [DOI] [PubMed] [Google Scholar]
- McCombs R. M., Melnick M. B., Brunschwig J. P. Biophysical studies of vesicular stomatitis virus. J Bacteriol. 1966 Feb;91(2):803–812. doi: 10.1128/jb.91.2.803-812.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PLUMMER G., BENYESH-MELNICK M. A PLAQUE REDUCTION NEUTRALIZATION TEST FOR HUMAN CYTOMEGALOVIRUS. Proc Soc Exp Biol Med. 1964 Oct;117:145–150. doi: 10.3181/00379727-117-29520. [DOI] [PubMed] [Google Scholar]
- RAPP F., RASMUSSEN L. E., BENYESH-MELNICK M. THE IMMUNOFLUORESCENT FOCUS TECHNIQUE IN STUDYING THE REPLICATION OF CYTOMEGALOVIRUS. J Immunol. 1963 Nov;91:709–719. [PubMed] [Google Scholar]
- ROWE W. P., HARTLEY J. W., WATERMAN S., TURNER H. C., HUEBNER R. J. Cytopathogenic agent resembling human salivary gland virus recovered from tissue cultures of human adenoids. Proc Soc Exp Biol Med. 1956 Jun;92(2):418–424. [PubMed] [Google Scholar]
- SMITH K. O., MELNICK J. L. A method for staining virus particles and identifying their nucleic acid type in the electron microscope. Virology. 1962 Jul;17:480–490. doi: 10.1016/0042-6822(62)90143-5. [DOI] [PubMed] [Google Scholar]
- SMITH K. O., MELNICK J. L. Electron microscopic counting of virus particles by sedimentation on aluminized grids. J Immunol. 1962 Aug;89:279–284. [PubMed] [Google Scholar]
- SMITH K. O. PHYSICAL AND BIOLOGICAL OBSERVATIONS ON HERPESVIRUS. J Bacteriol. 1963 Nov;86:999–1009. doi: 10.1128/jb.86.5.999-1009.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
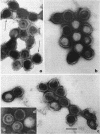
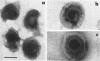
- SMITH K. O., RASMUSSEN L. MORPHOLOGY OF CYTOMEGALOVIRUS (SALIVARY GLAND VIRUS). J Bacteriol. 1963 Jun;85:1319–1325. doi: 10.1128/jb.85.6.1319-1325.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH K. O. RELATIONSHIP BETWEEN THE ENVELOPE AND THE INFECTIVITY OF HERPES SIMPLEX VIRUS. Proc Soc Exp Biol Med. 1964 Mar;115:814–816. doi: 10.3181/00379727-115-29045. [DOI] [PubMed] [Google Scholar]
- Vonka V., Benyesh-Melnick M. Interactions of human cytomegalovirus with human fibroblasts. J Bacteriol. 1966 Jan;91(1):213–220. doi: 10.1128/jb.91.1.213-220.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonka V., Benyeshmelnick M. Thermoinactivation of human cytomegalovirus. J Bacteriol. 1966 Jan;91(1):221–226. doi: 10.1128/jb.91.1.221-226.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON D. H., WILDY P., RUSSELL W. C. QUANTITATIVE ELECTRON MICROSCOPY STUDIES ON THE GROWTH OF HERPES VIRUS USING THE TECHNIQUES OF NEGATIVE STAINING AND ULTRAMICROTOMY. Virology. 1964 Dec;24:523–538. doi: 10.1016/0042-6822(64)90204-1. [DOI] [PubMed] [Google Scholar]
- WRIGHT H. T., Jr, GOODHEART C. R., LIELAUSIS A. HUMAN CYTOMEGALOVIRUS. MORPHOLOGY BY NEGATIVE STAINING. Virology. 1964 Jul;23:419–424. doi: 10.1016/0042-6822(64)90265-x. [DOI] [PubMed] [Google Scholar]