Abstract
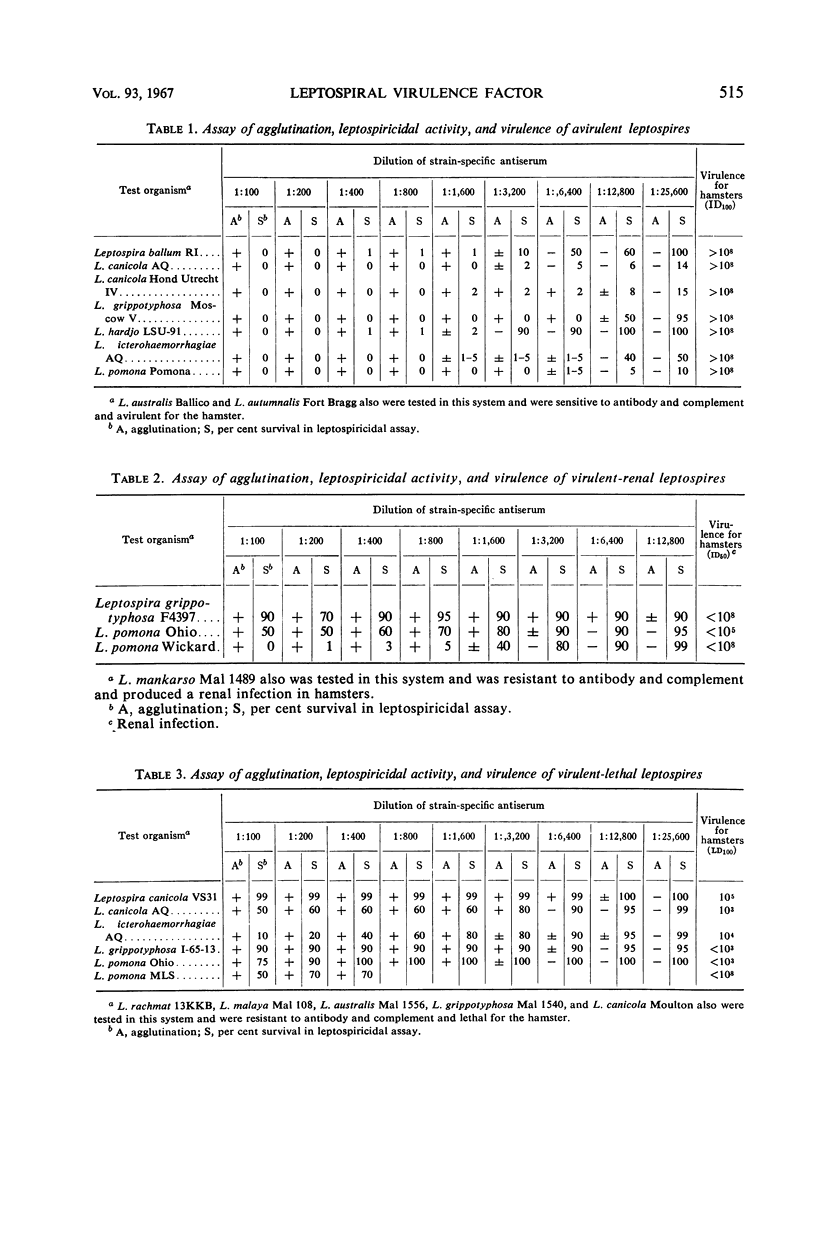
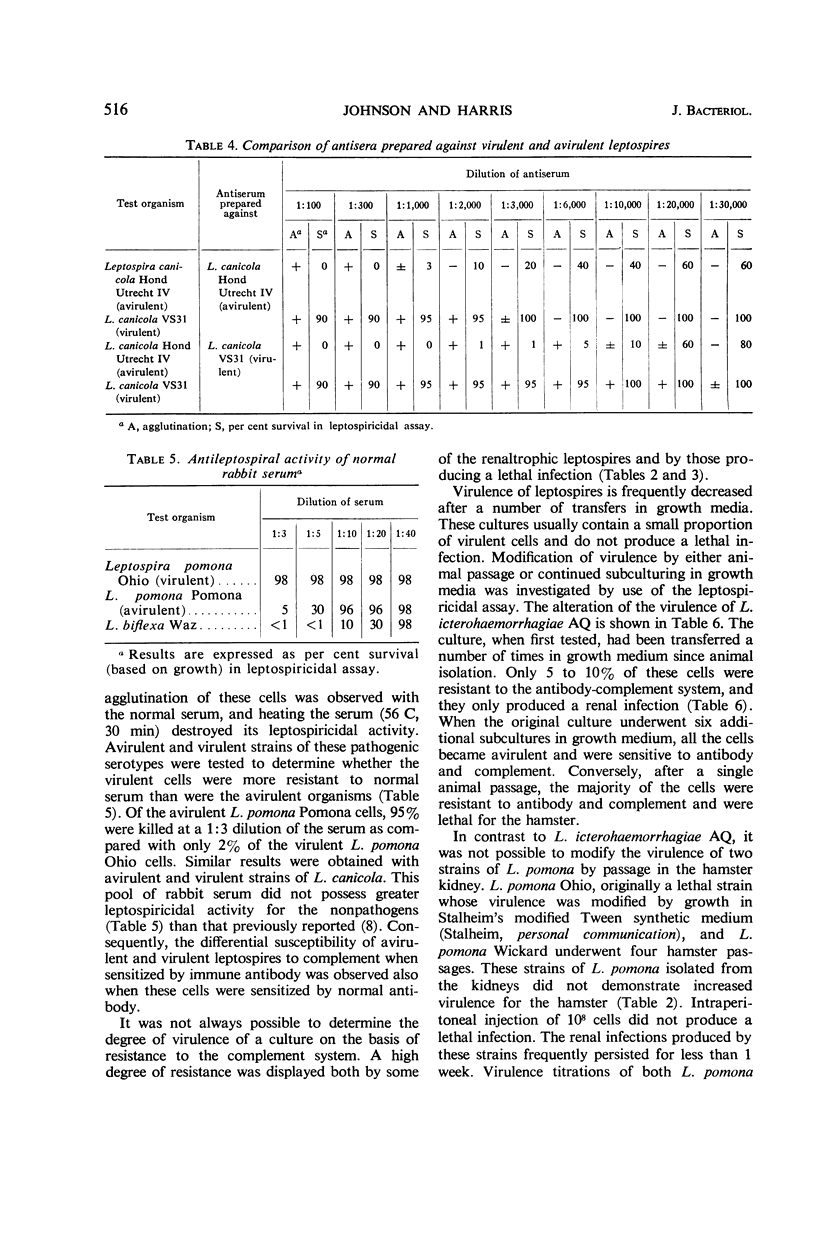
A definite relationship exists between the resistance of leptospires to the antibody-complement system and virulence. Leptospires capable of producing either lethal or renal infections in hamsters or guinea pigs were resistant to the leptospiricidal action of antibody and complement. Avirulent leptospires, in contrast to the virulent organisms, were rapidly immobilized and killed by these serum substances. The change of a virulent culture to the avirulent state as a result of growth in culture media was accompanied by the loss of resistance to antibody and complement. Virulent leptospires were phenotypically altered when grown in the presence of the purine analogue, 8-azaguanine. The cells became sensitive to antibody and complement without a corresponding decrease in virulence. The basis for a leptospiral virulence factor, the ability to multiply in vivo, appears to reside in their capacity to resist the leptospiricidal activity of the host antibody-complement system. The immune leptospiricidal assay provides a simple and rapid method of determining the virulence of a culture.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- FAINE S. RETICULOENDOTHELIAL PHAGOCYTOSIS OF VIRULENT LEPTOSPIRES. Am J Vet Res. 1964 May;25:830–835. [PubMed] [Google Scholar]
- FAINE S., SHAHAR A., ARONSON M. PHAGOCYTOSIS AND ITS SIGNIFICANCE IN LEPTOSPIRAL INFECTION. Aust J Exp Biol Med Sci. 1964 Oct;42:579–588. doi: 10.1038/icb.1964.54. [DOI] [PubMed] [Google Scholar]
- FAINE S., VANDERHOEDEN J. VIRULENCE-LINKED COLONIAL AND MORPHOLOGICAL VARIATION IN LEPTOSPIRA. J Bacteriol. 1964 Nov;88:1493–1496. doi: 10.1128/jb.88.5.1493-1496.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocker N. D., Bauer D. C. The nature of antibodies synthesized during the immune response to Leptospira biflexa. J Immunol. 1965 Nov;95(5):887–894. [PubMed] [Google Scholar]
- JOHNSON R. C., GARY N. D. NUTRITION OF LEPTOSPIRA POMONA. II. FATTY ACID REQUIREMENTS. J Bacteriol. 1963 May;85:976–982. doi: 10.1128/jb.85.5.976-982.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON R. C., ROGERS P. 5-FLUOROURACIL AS A SELECTIVE AGENT FOR GROWTH OF LEPTOSPIRAE. J Bacteriol. 1964 Feb;87:422–426. doi: 10.1128/jb.87.2.422-426.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON R. C., ROGERS P. DIFFERENTIATION OF PATHOGENIC AND SAPROPHYTIC LEPTOSPIRES WITH 8-AZAGUANINE. J Bacteriol. 1964 Dec;88:1618–1623. doi: 10.1128/jb.88.6.1618-1623.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON R. C., WILSON J. B. Nutrition of Leptospira pomona. J Bacteriol. 1960 Sep;80:406–411. doi: 10.1128/jb.80.3.406-411.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Muschel L. H. Antileptospiral activity of serum. I. Normal and immune serum. J Bacteriol. 1966 Apr;91(4):1403–1409. doi: 10.1128/jb.91.4.1403-1409.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINSCOTT W. D. Immune-adherence with Leptospira antigens. II. Studies on hyperimmune, convalescent and normal sera. J Immunol. 1961 May;86:480–488. [PubMed] [Google Scholar]
- MUSCHEL L. H. Serum bactericidal actions. Ann N Y Acad Sci. 1960 Nov 21;88:1265–1272. doi: 10.1111/j.1749-6632.1960.tb20117.x. [DOI] [PubMed] [Google Scholar]


