SECTION 1
A 28-year-old woman at 37 weeks’ gestation became increasingly confused and forgetful. She slept 12 hours daily, mistook her apartment for previous residences, and forgot her children’s names. Her husband helped her eat and walk. She presented to the obstetrical service fully dilated after 2 days of leaking vaginal fluid, and delivered a healthy baby girl. A few hours later, she did not remember giving birth. She was transferred to the neurology service for evaluation.
She had had a febrile seizure at age 4, and several brief convulsions as a teenager. A sleep-deprived EEG had been negative. She took phenytoin for a year, then stopped prior to her first pregnancy. She had no further convulsions.
This was her fifth pregnancy. She had 2 healthy children, 1 abruption at 23 weeks, and 1 elective abortion. Her maternal grandmother had died from a ruptured cerebral aneurysm.
She had no complaints but could not explain why she was in the hospital. She was afebrile with normal blood pressure. She appeared well and had a normal postpartum abdominal examination. She was inattentive and abulic with sparse but fluent speech. She recalled 2 of 3 words at 5 minutes, but had no memory for recent events, including her delivery. She could not describe cocktail ingredients, despite working as a bartender, but correctly recited old addresses. Cranial nerves were normal. Both optic discs had sharp margins by bedside funduscopic examination. Strength was full. Reflexes were brisk, with 3 beats of clonus at her right ankle. Toes were equivocal on the right and downgoing on the left. Sensation and coordination were normal. Gait was narrow-based and slightly unsteady, but she did not fall.
Questions for consideration:
What can cause subacute mental status changes in the peripartum state?
What studies would you pursue?
SECTION 2
This 28-year-old peripartum woman has subacute onset encephalopathy with memory loss and abulia, as well as long tract signs. Encephalopathy suggests a process affecting large areas of the brain bilaterally due to metabolic derangements or diffuse structural injury to gray and/or white matter. Focal insults to structures responsible for memory or attention, such as the thalamus, hippocampus, and medial temporal lobe, may present similarly. Linking encephalopathy with the focal upper motor neuron sign of right leg hyperreflexia suggests a multifocal process.
The differential diagnosis includes emergent peripartum conditions, such as dural sinus thrombosis, metastatic choriocarcinoma, and postpartum angiopathy, a form of reversible cerebral vasoconstriction syndrome.1 Other emergent conditions should be considered, including viral encephalitis (particularly herpesviruses), infectious meningoencephalitis, substance abuse (especially cocaine), complex partial seizures, and intracerebral hemorrhage. Subacute processes, such as demyelinating diseases and paraneoplastic processes, should also be considered.
The evaluation should be broad, including bloodwork, brain imaging, EEG, and CSF examination.
Serum chemistries were normal except for low total protein (5.6 g/dL), albumin (3 g/dL), and calcium (7.8 mg/dL). A complete blood count showed an elevated white blood cell count (14,000 per mm3). Erythrocyte sedimentation rate (ESR) was 30 mm/hour and C-reactive protein 33.9 mg/L. Lumbar puncture revealed a protein of 121 mg/dL, normal glucose, 3 white blood cells/mm3, and 23 red blood cells/mm3. Urine toxicology was positive for marijuana. The combined herpes simplex virus (HSV) titer was high, the HSV immunoglobulin M slightly above normal, and the CSF HSV PCR negative. The CSF albumin ratio was high at 30.2 (normal 0–9.0). Additional infectious, coagulation, endocrine, cardiac, lipid, and immunologic studies were unrevealing.
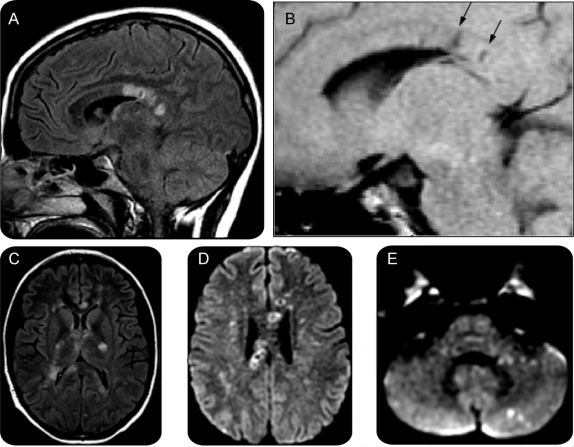
EEG showed generalized slowing with superimposed bursts of left frontotemporal maximal slowing. Head CT found mild diffuse cerebral atrophy and deep frontal white matter lucencies. Brain MRI revealed multiple T2-hyperintense lesions in the cerebellum and cerebral white matter. Many lesions were hypointense on T1-weighted imaging and some demonstrated restricted diffusion. There were multiple lesions in the corpus callosum, many with a rim of T2 hyperintensity around a center of T1 hypointensity (figure 1). There was no abnormal enhancement. Magnetic resonance angiography showed caliber changes in the distal branches of both middle cerebral arteries. Magnetic resonance venography was normal.
Figure 1 MRI on the day of presentation
(A) T2 fluid-attenuated inversion recovery (FLAIR) parasagittal view shows corpus callosal lesions, some with a ring of increased signal and a darker center. (B) T1 parasagittal view, similar cut, shows areas of T1 hypointensity (arrows) corresponding to the T2 bright lesions. (C) T2 FLAIR axial views show additional lesions throughout the internal capsule, and the genu, splenium, and tapetum of the corpus callosum. (D) Diffusion-weighted imaging (DWI) (1000b) axial view through the superior extent of the lateral ventricles shows several lesions with restricted diffusion through the central fibers of the corpus callosum, many with bright rings and dark centers. (E) DWI (1000b) axial view of the cerebellum and pons shows pinpoint lesions in the middle cerebellar peduncle and cerebellar cortex.
The patient’s husband, who had been unavailable initially, reported that for several weeks she had had headaches, hearing difficulties, and episodic visual loss, occasionally losing vision in 1 eye for 30 minutes. Subtle memory problems had begun 1 month prior.
Questions for consideration:
How does this information change your differential diagnosis?
What additional information would you like?
SECTION 3
The widespread distribution of MRI lesions suggests a multifocal process affecting primarily the white matter. The normal CSF glucose and low CSF cell count argue against an infectious process. The high CSF protein, serum C-reactive protein, and ESR suggest an inflammatory or autoimmune process. The negative CSF HSV PCR and noninfectious CSF cell count rule out HSV encephalitis.
CNS vasculopathy, primary or secondary, could explain the distal caliber changes in the middle cerebral arteries. Postpartum angiopathy, a reversible cerebral vasoconstriction syndrome, is a parsimonious diagnosis linking her headache, pregnancy, elevated CSF protein, MRI findings, and encephalopathy.2,3 However, postpartum angiopathy typically follows delivery, rather than precedes it, and often presents with vomiting and/or seizures.
Demyelinating disorders, such as multiple sclerosis (MS) or acute disseminated encephalomyelitis (ADEM), could explain the MRI findings, albuminocytologic dissociation, change in mental status, and visual disturbances (i.e., optic neuritis). However, encephalopathy and the high CSF protein are unusual for MS. Pregnancy tends to protect against flares, especially in the third trimester. Optic neuritis worsens over hours to days, and lasts days to weeks, rather than 30 minutes. Finally, in the setting of acute symptoms, MS lesions often enhance on MRI and rarely have limited diffusion. ADEM is typically preceded by a viral illness or immunization. Additionally, the lesions in ADEM are usually larger, at the grey–white junction and deep nuclei, and often confluent.
Multiple emboli could explain multifocal restricted diffusion on MRI. Postpartum deep venous thrombosis (DVT), for example, could cause paradoxical embolization to the brain through a patent foramen ovale (PFO). However, the absence of cortical lesions, the predominance of corpus callosum lesions, and the high CSF protein argue against embolism.
Susac syndrome is a microvasculopathy due to endothelial damage, which links encephalopathy, hearing loss, and visual changes. The distinctive corpus callosum lesions in this patient are like the “snowball” lesions that are characteristic of this disease, and high CSF protein is common.4,5 It is not a known complication of pregnancy.
Question for consideration:
What further testing would help distinguish among these diagnoses?
SECTION 4
Transthoracic echocardiogram with bubble contrast found a small PFO, no evidence of thrombus or vegetation, and normal ejection fraction. Lower extremity Doppler studies found no DVT. Digital subtraction angiography found generalized small caliber arteries intracranially, but no morphologic changes consistent with a large vessel vasculopathy as would be expected in postpartum angiopathy.
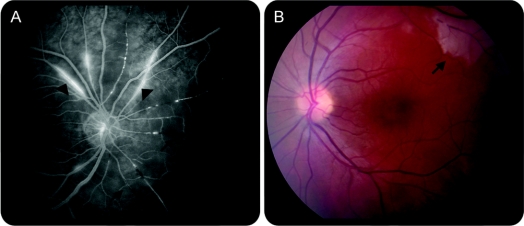
To evaluate for Susac syndrome, ophthalmologic and audiologic evaluations were performed. Bedside dilated funduscopic examination revealed bilateral branch retinal artery occlusions with retinal infarcts. Fluorescein angiography found bilateral retinal infarcts, retinal artery branch occlusions, and arteriolar hyperfluorescence, suggesting a retinal vasculopathic process (figure 2). Audiologic evaluation found low frequency sensorineural hearing loss.
Figure 2 Ophthalmologic imaging 4 days after presentation
(A) Fluorescein angiogram of the right eye shows retinal artery branch occlusions (black vessels, arrows). Note also hyperfluorescence of the arteriolar walls (arrowheads). (B) Retinography of the left eye shows retinal infarct (arrow).
Muscle biopsy and additional serum tests to look for evidence of endothelial damage were obtained. Antiendothelial antibody tests were weakly positive, and factor VIII levels were elevated (319%, reference 50–150%). Factor VIII is synthesized and released by endothelial cells, and may rise if they are damaged. A muscle biopsy, including electron microscopy, was normal.
We diagnosed Susac syndrome, or retinocochleocerebral vasculopathy, based on the pathognomonic triad of encephalopathy, branch retinal artery occlusions, and hearing loss. Elevated CSF protein supports vasculopathy, but the affected vessels were too small to be detected by angiography.
Pregnancy was a cognitive distracter in this case. We initially focused on postpartum angiopathy as our leading diagnosis. Only after an unrevealing evaluation for stroke did we learn of the visual and hearing loss. Also of note, initial bedside funduscopic examination found sharp disc margins, but missed the retinal infarcts. Once we considered the rare diagnosis of Susac syndrome, ophthalmologic examination confirmed the branch retinal artery occlusions. This case underscores the importance of the history in an encephalopathic patient and the utility of a broad differential diagnosis.
DISCUSSION: SUSAC SYNDROME
Susac syndrome is an autoimmune endotheliopathy, pathophysiologically akin to dermatomyositis, targeting arterioles under 100 μm in the cochlea, retina, and brain (rather than muscle and skin, as in dermatomyositis). Evidence supporting this etiology includes high serum antiendothelial antibodies, elevated factor VIII (released by damaged endothelium), and tissue pathology with endothelial cell necrosis, basement membrane thickening, and C3d and C4d deposition in vessel walls.4,6–8
The current literature only describes about 100 patients with Susac syndrome, but the disease is underappreciated and may be more common. Women outnumber men 3:1. Age at onset is 20 to 40 years, ranging from 9 to 58. The clinical triad may not present together. Months to years may separate the initial symptom from the development of the others.9 Headache, often migrainous, frequently precedes the onset of encephalopathy, and progresses to confusion, memory loss, behavioral changes, dysarthria, and mutism.4,5
CSF typically shows a mild lymphocytic pleocytosis (less than 20) and markedly elevated protein. Oligoclonal bands and elevated immunoglobulin G index may falsely suggest MS.4
Brain MRI reveals multiple small (1–7 mm) white matter lesions in the cerebral hemispheres.10 Many show restricted diffusion, suggesting they represent small infarcts.10 Deep gray, cerebellar, brainstem, and gadolinium-enhancing lesions are common. Leptomeningeal enhancement is occasionally seen. The characteristic callosal lesions in Susac syndrome are frequently misdiagnosed as demyelinating disease. However, their central location, “snowball” appearance on T2-weighted imaging, and evolution into pathognomonic T1-hypointensities are atypical of MS lesions, which are smaller and involve the callosal-septal interface.5 The size of the affected arterioles is below the resolution of angiography, which is typically normal.
Branch retinal artery occlusions present as flashes of light, black spots, scintillating scotoma, or occasionally monocular amaurosis fugax.4 Fluorescein angiography shows retinal artery branch occlusions with hyperfluorescence of the arterial wall and late dye leakage.11 Hearing loss may be gradual, fluctuating, or sudden. Low frequencies are typically lost first, as the apex of the cochlea, which transduces lower frequencies, is more susceptible to infarction.4
With no controlled therapeutic trials in Susac syndrome, treatment recommendations are based upon clinical experience.6,9 Rennebohm and Susac6 outline a detailed aggressive regime, including low-dose aspirin, high-dose IV corticosteroids followed by a prolonged oral taper, monthly IV immunoglobulin, and consideration of cyclophosphamide or mycophenolate mofetil based on disease severity.
Only 7 pregnancies in 6 patients with Susac syndrome have been reported. Two developed symptoms during pregnancy, in 1 symptoms abated with pregnancy, and 3 had recurrent encephalopathy postpartum.9 Rennebohm and Susac’s6 analogy to inflammatory myopathy may be instructive for care: pregnant women with inflammatory myopathy often respond to steroids alone, and may flare postpartum.12
The disease is usually self-limited, lasting 2 to 4 years, and most patients eventually return to work. Most are left with bilateral hearing impairment, some (35%–50%) have residual cognitive dysfunction, and as many as 1/3 have relapse of encephalopathy. Asymptomatic visual field defects are more common than symptomatic visual loss.9,13
FOLLOW-UP
The patient was treated with daily aspirin, pulse steroids followed by an oral steroid taper, and IV immunoglobulin. Mycophenolate mofetil was added after a week, as she had not significantly improved, and the disease severity warranted additional immunosuppression.6 Mycophenolate mofetil was chosen over cyclophosphamide to be less detrimental to fertility.
Follow-up MRI 2 weeks postpartum showed new lesions, suggesting continuing disease activity. A repeat fluorescein angiogram showed decreased arterial wall hyperfluorescence. However, the patient improved clinically with fluent spontaneous speech and improved attention and memory. On discharge, 3 weeks postpartum, she demonstrated right visual field deficits, brisk reflexes, and clonus at both ankles, right more than left. Seven months postpartum, she continues to take mycophenolate mofetil, and is slowly tapering prednisone. She still complains of short-term memory problems, right eye visual problems, and poor hearing in her left ear. She fatigues easily, but manages household chores and childcare on her own.
DISCLOSURE
Dr. Grinspan reports no disclosures. Dr. Willey is funded by NIH/NINDS T 32 NS 07153. Dr. Tullman has received research support from Acorda Therapeutics, BioMS, Genentech, and Novartis; and honoraria from Biogen Idec, EMD Serono, Novartis, Pfizer, and Teva Neuroscience. Dr. Elkind serves as Resident and Fellow Section Editor for Neurology®; serves as a consultant to BMS-Sanofi Pharmaceutical Partnership, GlaxoSmithKline, Jarvik Heart, Tethys Bioscience, and Daiichi-Sankyo; serves on the speakers bureaus of Boehringer-Ingelheim, Inc., and BMS-Sanofi Pharmaceutical Partnership; receives research support from diaDexus, Inc., BMS-Sanofi Pharmaceutical Partnership, and NIH/NINDS [K23 NS42912 (PI), R01 NS050724 (PI), NS048134 (PI), P50 NS049060 (Project PI), R37 NS029993 (co-PI), R01 NS55809 (coinvestigator); and has given expert testimony involving Merck & Co., Inc. (Vioxx), Pfizer (Shiley valve and Celebrex/Bextra), and Novartis (Zelnorm and stroke).
Address correspondence and reprint requests to Dr. Zachary M. Grinspan, Division of Pediatric Neurology, Harkness Pavilion, 5th Floor, 180 Fort Washington Ave., New York, NY 10032-3791 zg2126@columbia.edu
Disclosure: Author disclosures are provided at the end of the article.
REFERENCES
- 1.Singhal AB, Bernstein RA. Postpartum angiopathy and other cerebral vasoconstriction syndromes. Neurocrit Care 2005;3:91–97. [DOI] [PubMed] [Google Scholar]
- 2.Bogousslavsky J, Despland PA, Regli F, Dubuis PY. Postpartum cerebral angiopathy: reversible vasoconstriction assessed by transcranial Doppler ultrasounds. Eur Neurol 1989;29:102–105. [DOI] [PubMed] [Google Scholar]
- 3.Ducros A, Boukobza M, Porcher R, Sarov M, Valade D, Bousser MG. The clinical and radiological spectrum of reversible cerebral vasoconstriction syndrome: a prospective series of 67 patients. Brain 2007;130:3091–3101. [DOI] [PubMed] [Google Scholar]
- 4.Susac JO, Egan RA, Rennebohm RM, Lubow M. Susac’s syndrome: 1975–2005 microangiopathy/autoimmune endotheliopathy. J Neurol Sci 2007;257:270–272. [DOI] [PubMed] [Google Scholar]
- 5.Susac JO, Murtagh FR, Egan RA, et al. MRI findings in Susac’s syndrome. Neurology 2003;61:1783–1787. [DOI] [PubMed] [Google Scholar]
- 6.Rennebohm RM, Susac JO. Treatment of Susac’s syndrome. J Neurol Sci 2007;257:215–220. [DOI] [PubMed] [Google Scholar]
- 7.Petty GW, Engel AG, Younge BR, et al. Retinocochleocerebral vasculopathy. Medicine 1998;77:12–40. [DOI] [PubMed] [Google Scholar]
- 8.Magro C, Martin L, Susac JO. Susac’s syndrome: an organ specific autoimmune endothelialitis (in preparation).
- 9.Aubart-Cohen F, Klein I, Alexandra JF, et al. Long-term outcome in Susac syndrome. Medicine 2007;86:93–102. [DOI] [PubMed] [Google Scholar]
- 10.White ML, Zhang Y, Smoker WR. Evolution of lesions in Susac syndrome at serial MR imaging with diffusion-weighted imaging and apparent diffusion coefficient values. AJNR Am J Neuroradiol 2004;25:706–713. [PMC free article] [PubMed] [Google Scholar]
- 11.Martinet N, Fardeau C, Adam R, et al. Fluorescein and indocyanine green angiographies in Susac syndrome. Retina 2007;27:1238–1242. [DOI] [PubMed] [Google Scholar]
- 12.Silva CA, Sultan SM, Isenberg DA. Pregnancy outcome in adult-onset idiopathic inflammatory myopathy. Rheumatology 2003;42:1168–1172. [DOI] [PubMed] [Google Scholar]
- 13.Susac JO. Susac’s syndrome: the triad of microangiopathy of the brain and retina with hearing loss in young women. Neurology 1994;44:591–593. [DOI] [PubMed] [Google Scholar]