Abstract
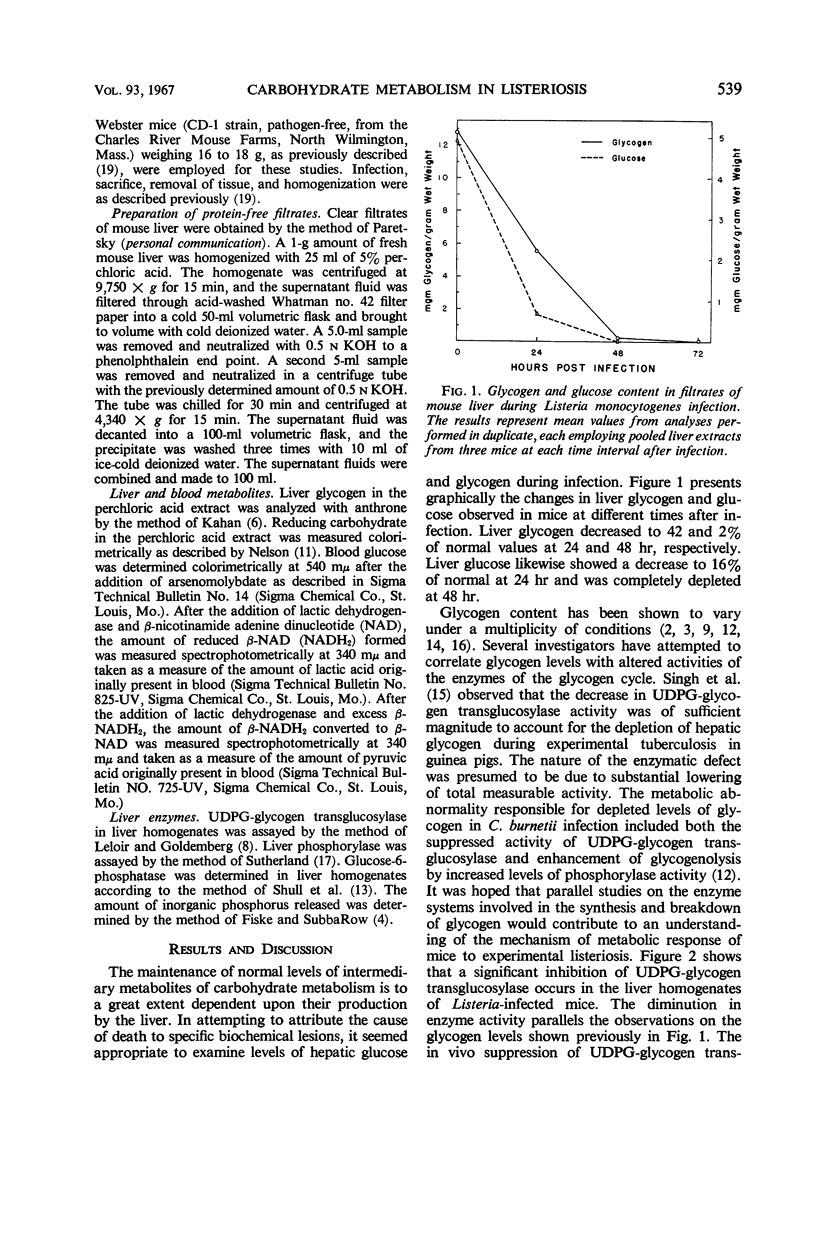
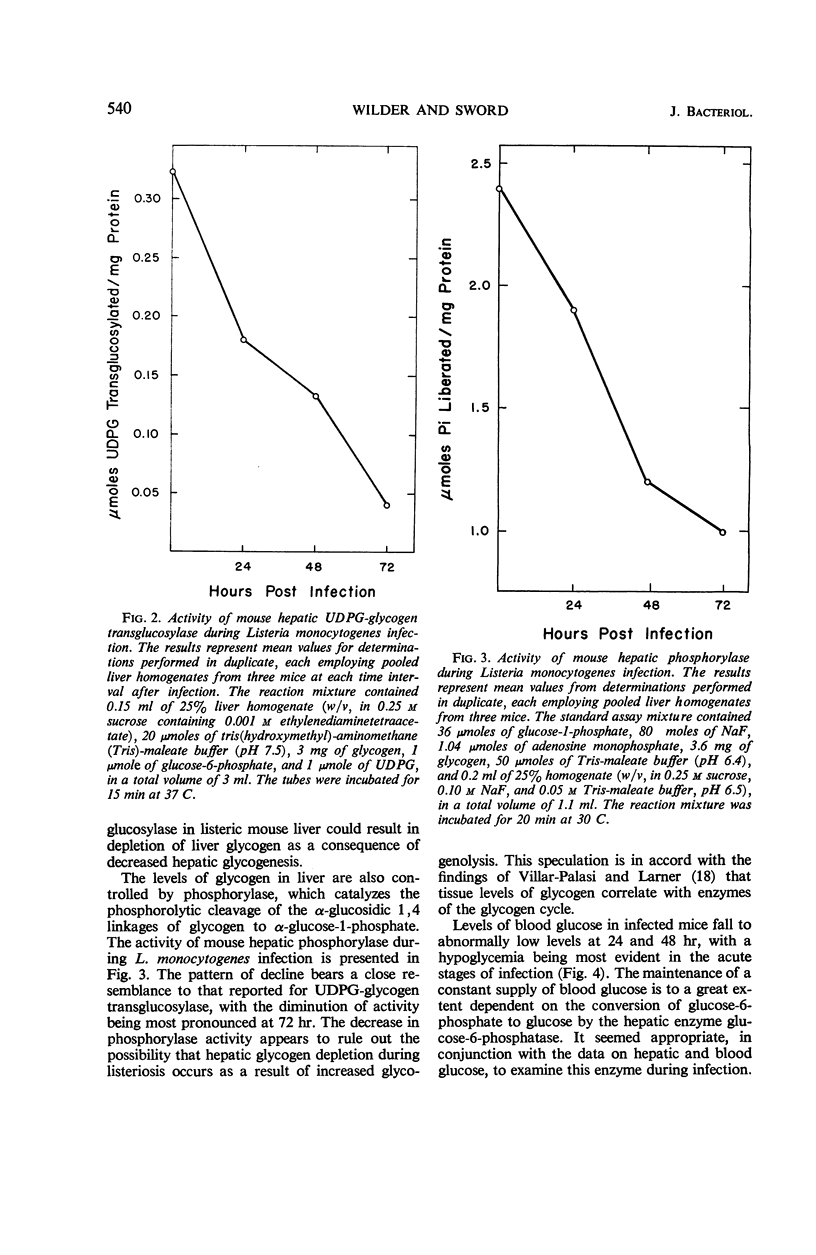
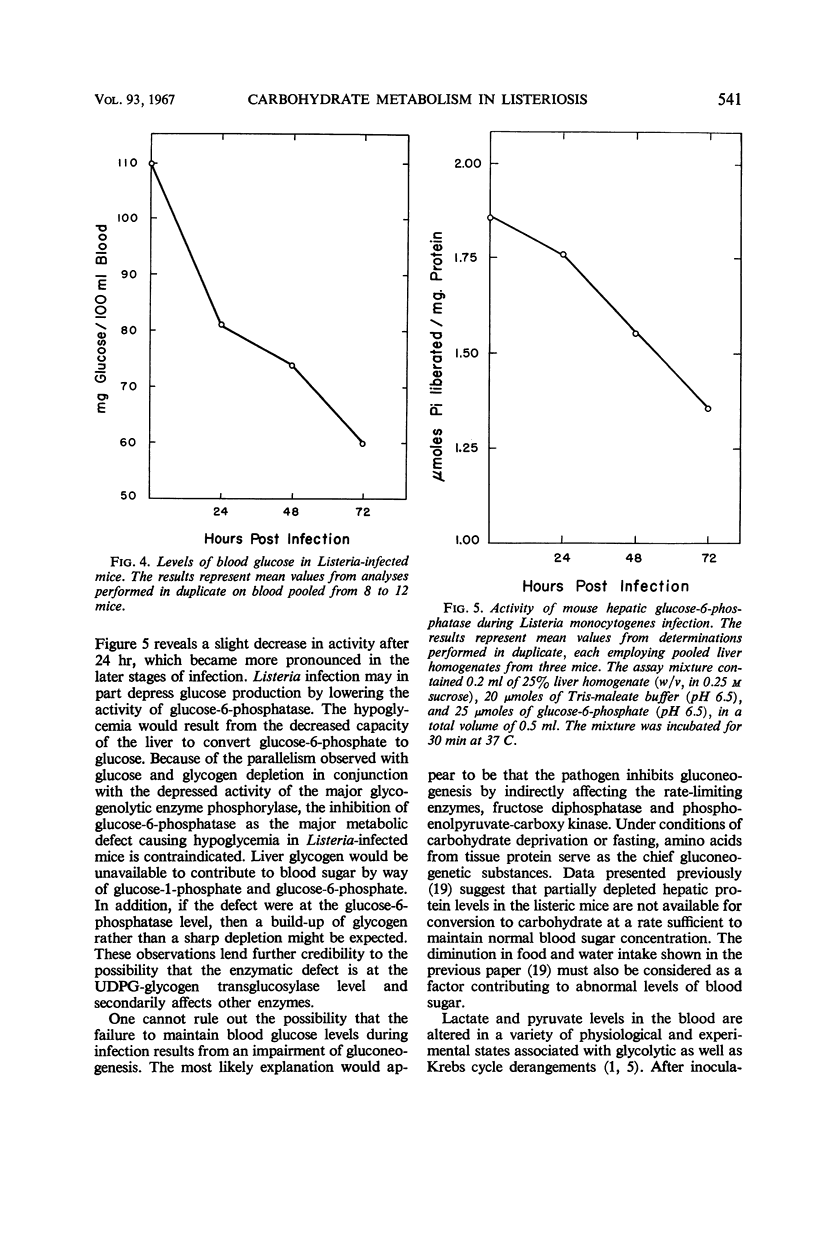
Several enzymes and metabolites concerned with carbohydrate metabolism were examined in mice infected with Listeria monocytogenes. Liver glycogen and glucose decreased parallel to severity of infection. The concentration of glucose in the blood fell to abnormally low levels with a hypoglycemia being most evident at 72 hr. There was a significant decrease in the activity of hepatic uridine diphosphate glucose-glycogen transglucosylase. This decrease in enzymatic activity correlated with the rate of glycogen depletion. Phosphorylase activity declined in a similar fashion, contraindicating enhanced glycogenolysis as the mechanism responsible for glycogen depletion. Although glucose-6-phosphatase decreased throughout the infection period, it did not appear to be the major metabolic defect causing hypoglycemia in Listeria-infected mice. Further distortion of carbohydrate metabolism was indicated by findings of increased levels of pyruvate and lactate in the blood of infected animals.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARAONA E., SALINAS A., NAVIA E., ORREGO H. ALTERATIONS OF AMMONIA METABOLISM IN THE CEREBRAL CORTEX OF RATS WITH HEPATIC DAMAGE INDUCED BY CARBON TETRACHLORIDE. Clin Sci. 1965 Apr;28:201–208. [PubMed] [Google Scholar]
- BERNELLI-ZAZZERA A., GAJA G. SOME ASPECTS OF GLYCOGEN METABOLISM FOLLOWING REVERSIBLE OR IRREVERSIBLE LIVER ISCHEMIA. Exp Mol Pathol. 1964 Aug;17:351–368. doi: 10.1016/0014-4800(64)90007-3. [DOI] [PubMed] [Google Scholar]
- HAUGAARD E. S., HAUGAARD N. DIABETIC METABOLISM. I. CARBOHYDRATE UTILIZATION AND HIGH ENERGY PHOSPHATE FORMATION IN HEART HOMOGENATES FROM NORMAL AND ALLOXAN-DIABETIC RATS. J Biol Chem. 1964 Mar;239:705–709. [PubMed] [Google Scholar]
- KAHAN J. A rapid photometric method for the determination of glycogen. Arch Biochem Biophys. 1953 Dec;47(2):408–418. doi: 10.1016/0003-9861(53)90477-9. [DOI] [PubMed] [Google Scholar]
- Klein F., Walker J. S., Fitzpatrick D. F., Lincoln R. E., Mahlandt B. G., Jones W. I., Jr, Dobbs J. P., Hendrix K. J. Pathophysiology of anthrax. J Infect Dis. 1966 Apr;116(2):123–138. doi: 10.1093/infdis/116.2.123. [DOI] [PubMed] [Google Scholar]
- LINDELL S. S., SMITH I. M., NELSON J. W., DELLE M., ROUTH J. I. LOCUS OF BIOCHEMICAL CHANGE IN MICE DYING FROM STAPHYLOCOCCAL INFECTION. Nature. 1964 Jan 11;201:185–187. doi: 10.1038/201185b0. [DOI] [PubMed] [Google Scholar]
- MCILWAIN P., EVELETH D. F., DOUBLY J. A. PHARMACOLOGIC STUDIES OF A TOXIC CELLULAR COMPONENT OF LISTERIA MONOCYTOGENES. Am J Vet Res. 1964 May;25:774–781. [PubMed] [Google Scholar]
- PARETSKY D., DOWNS C. M., SALMON C. W. SOME BIOCHEMICAL CHANGES IN THE GUINEA PIG DURING INFECTION WITH COXIELLA BURNETII. J Bacteriol. 1964 Jul;88:137–142. doi: 10.1128/jb.88.1.137-142.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHULL K. H., ASHMORE J., MAYER J. Hexokinase, glucose-6-phosphatase and phosphorylase levels in hereditarily obese-hyperglycemic mice. Arch Biochem Biophys. 1956 May;62(1):210–216. doi: 10.1016/0003-9861(56)90104-7. [DOI] [PubMed] [Google Scholar]
- SINGH V. N., BHARGAVA U., VENKITASUBRAMANIAN T. A., VISWANATHAN R. Study of glycogen synthesizing and degrading enzymes of guinea pig liver in experimental tuberculosis. Arch Biochem Biophys. 1963 May;101:234–238. doi: 10.1016/s0003-9861(63)80008-9. [DOI] [PubMed] [Google Scholar]
- SINGH V. N., VENKITASUBRAMANIAN T. A., VISWANATHAN R. Study of glucose tolerance and synthesis of hepatic glycogen from glucose and glycine-1-C-14 in tuberculous guinea pigs. Arch Biochem Biophys. 1963 May;101:229–233. doi: 10.1016/s0003-9861(63)80007-7. [DOI] [PubMed] [Google Scholar]
- VILLAR-PALASI C., LARNER J. Levels of activity of the enzymes of the glycogen cycle in rat tissues. Arch Biochem Biophys. 1960 Feb;86:270–273. doi: 10.1016/0003-9861(60)90417-3. [DOI] [PubMed] [Google Scholar]
- Wilder M. S., Sword C. P. Mechanisms of pathogenesis in Listeria monocytogenes infection. II. Characterization of listeriosis in the CD-1 mouse and survey of biochemical lesions. J Bacteriol. 1967 Feb;93(2):531–537. doi: 10.1128/jb.93.2.531-537.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]


