Abstract
Anxiety disorders are a common focus of clinical concern and certain forms of anxiety may be conceptualized as disorders of emotional learning. Behavior therapies effective in the treatment of anxiety are modeled on extinction training as a means of reducing pathological anxiety. The present understanding of human anxiety has been informed by preclinical research using rodent models to study the acquisition and extinction of fear. Glutamate appears to have a central role in both of these processes. The authors review this literature and discuss novel applications of D-cycloserine, a partial N-methyl-D-aspartate agonist, for the treatment of anxiety.
Needs Assessment
The neurotransmitter, glutamate, has a central role in the process of learning. Anxiety disorders may be thought of as disorders of emotional learning that are remediated by therapeutic learning in a clinical context. The role of glutamate in these processes is reviewed in this article.
Learning Objectives
At the end of this activity, the participant should be able to:
Differentiate among the different anxiety disorders, recognizing that some of them can be classified as disorders of fear dysregulation.
Appreciate the role of preclinical data in support of the importance of glutamate in the acquisition of associative fear condition.
Appreciate the role of glutamate in the extinction of fear.
Understand the potential implications of the adjunctive use of the partial N-methyl-d-aspartate receptor agonist D-cycloserine during the exposure-based treatment of anxiety.
Target Audience
Neurologists and psychiatrists
Accreditation Statement
Mount Sinai School of Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide Continuing Medical Education for physicians.
Mount Sinai School of Medicine designates this educational activity for a maximum of 3.0 Category 1 credit(s) toward the AMA Physician’s Recognition Award. Each physician should claim only those credits that he/she actually spent in the educational activity. Credits will be calculated by the MSSM OCME and provided for the journal upon completion of agenda.
It is the policy of Mount Sinai School of Medicine to ensure fair balance, independence, objectivity and scientific rigor in all its sponsored activities. All faculty participating in sponsored activities are expected to disclose to the audience any real or apparent discussion of unlabeled or investigational use of any commercial product or device not yet approved in the United States.
This activity has been peer-reviewed and approved by Eric Hollander, MD, professor of psychiatry, Mount Sinai School of Medicine. Review Date: August 22, 2005.
To Receive Credit for This Activity
Read this article, and the two CME-designated accompanying articles, reflect on the information presented, and then complete the CME quiz found on pages 840 and 841. To obtain credits, you should score 70% or better. Termination date: October 31, 2007. The estimated time to complete this activity is 3 hours.
INTRODUCTION
Fear is a basic emotion that facilitates adaptation to threat in the environment and is based on the motivation to be the consumer and not the consumed. This adaptation comes in the form of anxiety that may be thought of as a cognitive association that relates the experience of fear to events, their outcome, and their meaning.1 Anxiety disorders are characterized by the presence of maladaptive anxiety or fear and are among the most prevalent and comorbid psychiatric illnesses.2,3 Certain anxiety disorders seem to be based, in part, on associative learning that occurs in conjunction with a fear-provoking experience or the mental representation of such a real or imagined experience. This includes posttraumatic stress disorder (PTSD), panic disorder, social anxiety disorder, and specific phobias. The common thread linking these four disorders is fear of a specific object, activity, place, or circumstance that triggers excessive fear and is managed primarily by inappropriate avoidance. What is not clear, however, is whether these disorders result from excess in the neural processes that facilitate acquisition of a fearful memory, deficits in the neural systems that inhibit expression of fear memories, or both. Given that these deficit or excess states relate to learning, these anxiety disorders may be conceptualized as disorders of emotional learning.
Adaptive fear exists along a continuum that eventually merges with pathological fear and is characterized by allostatic changes that facilitate behaviors, which permit the evasion or confrontation of threat.4 After the threat has passed, the allostatic changes that permitted evasion or confrontation of threat return to a normal state and some memory of the experience is stored so that similar future threats may be dealt with more effectively. Alternatively, pathological fear is characterized by fear that is inappropriately triggered, excessive in proportion to the threat, or unable to be dampened once the threat has passed or reevaluated as being not dangerous.
This review will examine the role of glutamate in the acquisition of fear in animals and the extinction of fear in animals and humans. To that end, we will selectively review the preclinical literature on associative fear conditioning in rodents with an emphasis on the role of glutamate in the acquisition of fear memories and the inhibition of fearful behavior by extinction. We will conclude with an examination of how this work may be applied to the treatment of pathological anxiety in humans.
EMOTIONAL LEARNING, NEUROPLASTICITY, AND GLUTAMATE
Emotional Learning
The formation of memories associated with particular emotional experiences is valuable because it permits an individual to anticipate the recurrence of similar events in the future. In general, such anticipation is useful because it facilitates approach behavior to resources and avoidance behavior of hazards and thus requires effective discrimination between safety signals and danger signals to be adaptive. However, such a system can also cause great harm if approach behavior or avoidance behavior becomes deficient, excessive, or inappropriately linked with contingencies of the external environment or the internal milieu of the individual.
Insight into the formation of emotional memory has been provided in part by the study of classical fear conditioning in animals. Classical fear conditioning is a form of associative learning, whereby an initially neutral conditioned stimulus, such as a tone, gains emotional significance after being paired with an aversive unconditioned stimulus, such as a foot shock. The experience of the aversive unconditioned stimulus in conjunction with the conditioned stimulus results in a collection of behavioral and physiological responses, known as the conditioned response, that enable an animal to escape from or adapt to the unconditioned stimulus. After the conditioned stimulus-unconditioned stimulus pairing is made, the conditioned stimulus acquires the capacity to function as a cue independently capable of eliciting the conditioned response, which serves as an observable representation of the emotional memory that was established. Similarly, the environmental context in which the initial conditioning occurred also becomes able to elicit the conditioned response.
The neural system that mediates associative fear learning and the expression of fear behavior is a distributed system whose coordinating center is the amygdala.5-9 Functional brain imaging studies in humans10,11 are consistent with this hypothesis and demonstrate enhanced activation of the amygdala in healthy volunteers during the acquisition phase of associative fear conditioning. Similarly, functional imaging studies of the brain in patients with anxiety disorders across a variety of experimental paradigms12,13 repeatedly demonstrate excessive activation of the amygdala in response to a variety of provocative stimuli suggesting a role for the amygdala in the etiology of pathological anxiety.
Cue-specific information about the conditioned stimulus is relayed by sensory afferents via the thalamus to the lateral nucleus of the amygdala and the basolateral complex of the amygdala by two neural circuits operating in parallel. These circuits consist of a direct subcortical pathway, whereby sensory information is relayed directly to the lateral nucleus of the amygdala from the dorsal thalamic nuclei without preprocessing by the sensory cortices, and an extended pathway that involves processing of sensory information by intervening cortical areas. Information about the unconditioned stimulus also converges on the amygdala nuclei. Expression of fear, manifest as the conditioned response, is mediated predominantly by the central amygdala and its extensive system of efferent projections.5,14
Coincident stimulation of neurons within the amygdala by the conditioned stimulus and unconditioned stimulus is thought to be the critical event in associative fear learning that leads to the induction of the cellular and molecular events responsible for neuroplasticity. Calcium elevation within neurons of the lateral nucleus of the amygdala/basolateral complex of the amygdala appears to be required for the induction of neuroplasticity leading to the formation of fear memories. This rise in calcium comes from the entry of extracellular calcium through glutamate receptors15,16 and L-type voltage-gated calcium channels17 and is thought to result in activation of calcium ions/calmodulin-dependent protein kinase II as well as the cyclic adenosine 3’,5’-cyclic monophosphate-dependent protein kinase pathway and the mitogen-activated protein kinase pathway. These signaling cascades act to convert unstable short-term memory into a lasting long-term memory through changes in gene activity, which subsequently alter the structural and physiological milieu of the synapse.18-20
Glutamate and Fear Learning
Glutamate is the major excitatory neurotransmitter within the central nervous system and is critically involved in learning and memory formation through both conventional neurotransmission and the regulation of synaptic plasticity. These effects are mediated by the large variety of receptors for glutamate that have been identified through electrophysiological and pharmacologic methods in various regions of the brain. They include three major groups of excitatory ionotropic receptors: the N-methyl-d-aspartate (NMDA) receptor, the α-amino-3-hydroxy-5-methylisoxazole-4-proprionic acid (AMPA), kainate acid receptors and a group of metabotropic receptors (mGluR). A central role for glutamate in the acquisition of fear memories has been demonstrated by a variety of rodent studies21 showing that disruption of glutamatergic transmission in the amygdala, involving all major classes of glutamate receptor, impairs fear conditioning.
N-methyl-d-aspartate Receptors and Fear Conditioning
Miserendino and colleagues22 provided the first evidence of a role for NMDA receptors in associative fear conditioning. Microinjection of the NMDA receptors antagonist, DL-2-amino-5-phosphonovalerate, into the basolateral complex of the amygdala of rats prior to training with light-shock pairings impaired the acquisition but not expression, of fear-potentiated startle. Subsequent research using auditory23 and olfactory24 cues for fear conditioning has replicated the primary findings of this study and other researchers25-28 have similarly found that blockade of amygdalar NMDA receptors reduces the acquisition of fear conditioning.
Recently reported data using ifenprodil, a selective antagonist of the NMDA receptor NR2B subunit,29 have further supported these results.26 Systemic administration or microinjection of ifenprodil into the lateral nucleus of the amygdala blocks the acquisition of auditory fear conditioning but does not alter expression of preexisting fear memory. Converging lines of data30 suggest that the NR2B subunit contributes significantly to the induction of synaptic plasticity and that experimental blockade of this subunit has negligible effects on conventional synaptic transmission consonant with the hypothesis that the effects observed following antagonism of the NR2B subunit are representative of an effect on memory acquisition. Finally, it was recently found that administration of the NMDA receptors antagonist +/--3-(2-carboxypiperazin-4-yl) propyl-1-phosphonic acid prior to training blocked the acquisition of conditioned fear, associative spike firing in the lateral amygdala, and the induction of long-term potentiation in the amygdala.31 These data lend further credence to the hypothesis that activation of amygdalar NMDA receptors during fear conditioning supports the induction of neuroplasticity leading to the acquisition of fear memories.
α-amino-3-hydroxy-5-methylisoxazole-4-proprionic acid Receptors and Fear Conditioning
AMPA receptors are excitatory ionotropic glutamate receptors. They are located postsynaptically and play a major role in facilitating the membrane depolarization required for NMDA receptors function. AMPA receptors appear to have a significant role in the induction and maintenance of synaptic plasticity and aid in the acquisition of fear memories.32-34 Using an associative learning paradigm, McKernan and colleagues35 identified an AMPA-mediated potentiation of activity within the amygdala in rats. In this study, rats were divided into groups receiving either paired tone and shock, unpaired tone and shock, or remained experimentally naïve. Fear-potentiated startle, measured as an indicator of fear acquisition, was observed only in rats receiving paired tone and shock. Subsequent whole cell recordings of amygdala neurons from animals in each of the three experimental group found enhanced firing in response to stimulation of the internal capsule, which contains fibers delivering auditory information from the thalamus, only in rats that had received paired tone-shock presentations. Isolation of the AMPA component of this firing was achieved by application of the NMDA receptors antagonist DL-2-amino-5-phosphonovalerate and shown to be AMPA specific by application of the AMPA receptors antagonist, GYKI 52466, which blocked firing entirely. These data suggest that the acquisition of fear memory occurred at least in part as a result of plastic changes in AMPA activity within the amygdala as a consequence of fear conditioning. Similarly, Rumpel and colleagues,34 employing a novel gene delivery technique, found that associative fear conditioning increases the density of AMPA receptors within the lateral nucleus of the amygdala and that interference with this process impaired fear learning. These two studies suggest that upregulation of AMPA receptors within the lateral nucleus of the amygdala is an essential component of the plastic changes involved in the acquisition of fear memory.
Kainate Receptors and Fear Conditioning
Kainate receptors are excitatory ionotropic glutamate receptors. Kainate receptors have been previously shown to contribute to synaptic transmission within the amygdala.36 Subsequent data, also reported by Li and colleagues,37 demonstrated a role for kainate receptors in both homosynaptic and heterosynaptic potentiation within the amygdala that appears to be GluR5 dependent.37 Follow-up data from this same group38 found that application of GluR5 agonists in vitro resulted in complex bidirectional effects on the firing of local GABAergic interneurons suggesting that two types of GluR5 subunits are expressed on terminals of GABAergic neurons within the lateral nucleus of the amygdala that have differing agonist affinities and opposing effects on neuronal activity.
There is only one study examining the role of kainate receptors in associative fear conditioning. Ko and colleagues39 found that mice lacking the GluR6 subunit of the kainate receptors exhibited impaired acquisition of fear memory using an associative fear-conditioning paradigm consisting of paired tone and footshock in comparison to mice lacking the GluR5 subunit of the kainate receptors or littermate controls. In addition, these mice also showed complete blockade of synaptic potentiation following fear as measured by whole-cell recording from lateral nucleus of the amygdala neurons in comparison with littermate controls following the above tone-shock pairings. These data are consistent with a contribution from kainate receptors in the induction of fear-related neuroplasticity.
Collectively, these data suggest a complex role for kainate receptors on the acquisition of fear learning within the amygdala. The differences in the electrophysiological findings as reported by Ko and colleagues39 and Li and colleagues,37 most likely reflect differences in experimental methodology exaggerated by the presence of multiple forms of GluR5 subunits within the lateral nucleus of the amygdala.
Metabotropic Glutamate Receptors and Fear Conditioning
Metabotropic glutamate receptors (mGluRs) differ fundamentally from other glutamate receptors by virtue of being directly coupled to second messenger regulation by G proteins. There are a number of pre-synaptic and postsynaptic mGluRs and they may be placed into three subtype groups based on function. These include group I (mGluR I and mGluR5), group II (mGluR2 and mGluR3), and group III (mGluR4, mGluR7, mGluR8). Several lines of data suggest that group II agonists and group I antagonists have an important role in learning and expressing fear.
Activation of group II mGluRs appears to have significant anxiolytic effects on fear acquired through associative learning. Pretesting infusions of the group II mGluR agonist, LY354740 into the amygdala disrupted the expression of fear-potentiated startle and this effect was blocked by coadministration of the group II mGluR antagonist, LY341495.40 Similar effects have been found with LY354740 using a fear-potentiated startle paradigm in humans41 and in mice with the elevated plus maze.42 More recently, it has been reported that blockade of consolidation of fear-potentiated startle training and depotentiation of lateral nucleus of the amygdala neurons occurs following administration of an group II mGluR agonist.43
Blockade of group I mGluRs using selective antagonists also has anxiolytic effects on associatively learned fear similar to those observed with use of mGluR II agonists. Pretraining infusions of the mGluR5 antagonist, 2-methyl-6-(phenylethynl) pyridine hydrochloride impairs the acquisition and formation of short-term memory of associative fear conditioning,44 whereas posttraining infusions of 2-methyl-6-(phenylethynl) pyridine hydrochloride do not alter the acquisition of short-term fear memory or its consolidation into long-term memory.44,45 More recently, it was reported46 that administration of the mGluR5 antagonist, 3-[(2-methyl-1,3-thiazol-4-yl)ethynl]pyridine, prior to behavioral testing, reduced fear-potentiated startle and increased punished responding in a modified Geller-Seifter conflict model.46 Collectively, these data support a varied and complex role for mGluRs in acquisition, maintenance, and expression of fear learning.
EXTINCTION AND THE REDUCTION OF FEAR
Pavlov47 provided the first report of extinction in his studies of digestive physiology in dogs. He observed that the conditioned salivation of dogs in response to an external food-signaling cue slowly decreased and eventually disappeared when the cue was presented repeatedly in the absence of food. The inhibition of fear acquired by associative learning has been studied in both animals and humans.48 Following the pairing of an aversive unconditioned stimulus to a neutral conditioned stimulus a conditioned fear response is established. If the neutral conditioned stimulus is then repeatedly presented in the absence of the unconditioned stimulus, a procedure known as extinction training, the result is the loss of the conditioned fear to the neutral conditioned stimulus. From an operational perspective, extinction may thus be defined as “a reduction in the strength or probability of a conditioned fear response as a consequence of repeated presentation of the conditioned stimulus in the absence of the unconditioned stimulus.”48
A variety of behavioral observations support the hypothesis that extinction is a form of learning and not “unlearning” or the forgetting of a conditioned association.49,50 The most nonspecific of these observations is the phenomenon of spontaneous recovery. This refers to the reappearance over time of a conditioned fear that had been previously extinguished through extinction training. An additional characteristic of extinction is context specificity, a phenomenon known as renewal. This means that extinction seems to be specific for the context in which the extinction training occurs and that the previously extinguished conditioned fear will return if tested in a new context. Finally, the phenomenon of reinstatement occurs when unsignaled presentations of the unconditioned stimulus or other stressor interrupt extinction and lead to re-emergence of the previously diminished conditioned fear response. Collectively, these data suggest that extinction is a labile form of learning that is specific with respect to environmental and temporal context and is vulnerable to degradation by stress and the passage of time.
Glutamate and the Neurobiology of Extinction
The neurobiology of extinction has not been studied to the same degree as that of fear learning. Data obtained with rodents51,52 indicate that extinction appears to be dependent on events occurring within, and interactions between, the prefrontal cortex and the amygdala. Functional brain imaging studies in humans53 are consistent with this hypothesis and demonstrate engagement of the amygdala and ventral medial prefrontal cortex during acquisition training and early extinction.
Several lines of data suggest that glutamate has a central role in this process. Virtually all of this work has focused on the role of the NMDA receptor in extinction and will thus form the focus for this portion of our review. Like associative fear conditioning, extinction is dependent upon activation of NMDA receptors and the induction of neuroplasticity to form new patterns of connectivity that facilitate alternative forms of behavior in response to a previously conditioned stimulus. Administration of NMDA receptor antagonists either systemically54,55 or by direct infusion into the basolateral complex of the amygdala25,56 prior to extinction training blocks the extinction of fear memories. In addition, other investigators have found that blockade of NMDA receptors after extinction training also impairs extinction suggesting that NMDA receptors participate in the consolidation of extinction memories.57 Finally, bilateral infusions of the mitogen-activated protein kinase inhibitor PD58089 into the basolateral complex of the amygdala impair extinction of previously acquired fear-potentiated startle,58 suggesting that the effects of extinction are dependent on the induction of neuroplasticity.
Extinction can be Enhanced with Partial N-methyl-d-aspartate Agonists
In contrast to previous experiments showing extinction to be dependent on the functional integrity of NMDA receptors or neuroplasticity pathways, Walker and colleagues59 tested the reciprocal hypothesis that enhancing neurotransmission at NMDA receptors would facilitate extinction. Because administration of full agonists at the NMDA receptor is associated with excitotoxic effects on neurons,60 the partial NMDA agonist D-cycloserine was used. D-cycloserine acts at the strychnine-insensitive glycine recognition site of the NMDA receptor complex to enhance NMDA receptor activity and has previously been used in humans for the treatment of tuberculosis. The central findings of this study were that systemic administration of D-cycloserine dose-dependently enhanced extinction of previously conditioned fear-potentiated startle but did not influence fear-potentiated startle in rats that had not received extinction training. Similar effects on extinction were found when D-cycloserine was given by infusion into the basolateral complex of the amygdala and appear to be pharmacologically specific as co-administration of the NMDA glycine recognition site antagonist, HA966, with D-cycloserine blocked the effects of D-cycloserine on extinction. The general findings of this study have been replicated by studies61-63 that have used a cue-conditioned freezing paradigm showing that extinction occur in a time-dependent manner suggesting an effect on consolidation. In addition, this same group has demonstrated that post-extinction training administration of D-cycloserine interferes with reinstatement of conditioned fear63 and recently reported that extinction training enhanced by D-cycloserine appears to result in generalized extinction such that fear behavior elicited by a nonextinguished conditioned stimulus is reduced.61 Collectively, the data from these rodent studies suggest that D-cycloserine, a drug already shown to be safe for use in humans, may have significant potential use in the facilitation of extinction-based therapies for human anxiety disorders.
Glutamatergic Therapies for the Treatment of Anxiety in Humans
Preclinical data regarding the properties of associative learning and extinction have enriched our understanding of the etiology of anxiety disorders and have been applied to the treatment of pathological anxiety in humans. From a therapeutic standpoint, the behavior therapies for different anxiety disorders generally involve some form of extinction training.48 This involves graded exposure to the feared object or event in the absence of any likely actual harm or where there is a small chance that harm may occur. This exposure may be imaginal in nature, wherein a narrative is read or listened to by the patient or in vivo where the feared stimulus is directly encountered by the patient. Additional variables are the within-session duration of the exposure and the number of sessions. Goals of this form of therapy are reductions in the frequency and intensity of the reflexive conditioned response (fear response) as the conditioned stimulus (fear stimulus) is repeatedly encountered without adverse effect.
Considering the similarity between extinction training in rodents and exposure therapy for anxiety disorders in humans, we were interested in finding novel ways to integrate pharmacotherapy with psychotherapy. Historically, there has been hope to combine these two approaches into a treatment more effective than either alone; unfortunately this has not been achieved.64,65 In fact, sometimes combining pharmacotherapy with psychotherapy can make a bad situation worse.66,67 However, extinction-based therapies for anxiety may be an exception to this trend. We believe that the preclinical literature on the neurobiology of extinction provides valuable insight into mechanisms, whereby behavior therapy approaches utilizing exposure protocols might be made more effective with adjunctive use of medication.
D-cycloserine Enhances Extinction of Fear of Heights in Patients with Acrophobia
Recently, Ressler and colleagues68 demonstrated that D-cycloserine facilitates psychotherapy for the treatment of specific phobia in humans. We wished to examine the ability of D-cycloserine to enhance emotional learning in humans using the most optimally controlled form of psychotherapeutic learning available. Virtual reality exposure (VRE) therapy is ideal for clinical research assessment because exposure and testing is identical between patients, is well controlled by the therapist, and occurs within the spatial and temporal confines of the limited therapy environment.69 This method has proven to be successful for the treatment of specific phobias and PTSD.69-71 With VRE for fear of heights we used a virtual glass elevator, in which participants stood while wearing a VRE helmet and were able to peer over a virtual railing. Previous research69 has shown improvements on all acrophobia outcome measures for treated as compared with untreated groups after 7 weekly therapy sessions.
To examine whether D-cycloserine would enhance the learning that occurs during exposure therapy for humans with specific phobia, Ressler and colleagues68 enrolled 28 volunteers who were diagnosed with clinical criteria acrophobia. Participants were randomly assigned to three treatment groups, placebo + VRE therapy, or D-cycloserine + VRE Therapy at two different doses of D-cycloserine (50 mg/day or 500 mg/day). Treatment condition was double-blinded, such that the subjects, therapists, and assessors were not aware of assigned study medication condition. Although Ressler and colleagues68 used two different doses of D-cycloserine, preliminary analysis of our data indicated that there were no significant differences between the 50 mg/day and 500 mg/day drug groups for the primary outcome measures of acrophobia. Therefore, Ressler and colleagues68 combined the two drug groups for analysis.
Participants underwent two therapy sessions, which is a suboptimal amount of exposure therapy for acrophobia.69 They were instructed to take a single pill of study medication 2–4 hours before each therapy session, such that only two pills were taken for the entire study. A posttreatment assessment was performed within 1 week following the two therapy sessions, and an additional follow-up assessment was performed 3 months after the therapy.
At 1–2 weeks and 3 months posttreatment, subjects that received D-cycloserine in conjunction with VRE therapy had significantly enhanced decreases in fear within the virtual environment (Figure 1, P<.05). Furthermore, within the virtual environment, skin conductance fluctuations, a psychophysiological measure of anxiety, was significantly decreased in the group that received D-cycloserine in conjunction with therapy.
FIGURE 1. Acrophobia within the virtual environment is improved with D-Cycloserine68.
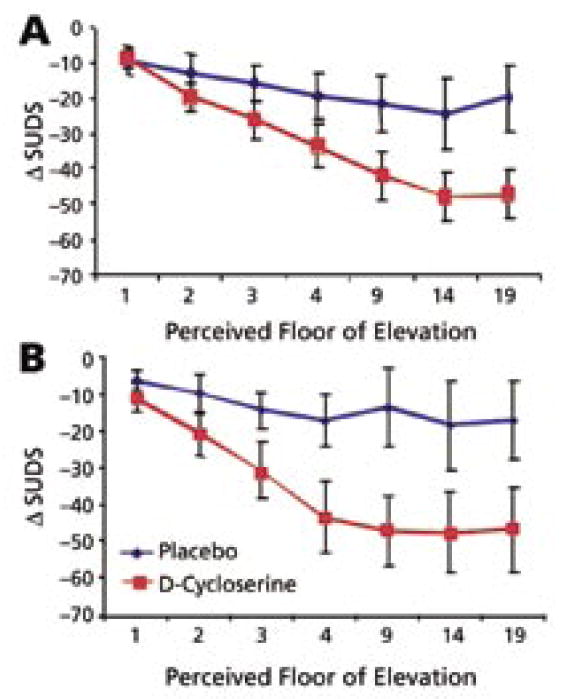
A. Change in SUDS from pre- to post-test following two therapy sessions that occurred ~1 week prior to this short-term follow-up assessment. Decrease in SUDS level (y axis) is shown for each floor (1–19) of the virtual glass elevator. Overall ANOVA was performed using pre-post difference and floor as within subjects variables and drug group as between subjects variable. Significant overall pre-post changes were seen: F(1,25)=38, P<.001. Significant effect of floor was found: F(6,150)=89, P<.001. Most importantly, significant effect of pre-post X floor X drug interaction was found: F(6,150)=3.8, P<.001.
B. Change in SUDS from pre to post-test at the 3-month long-term follow-up assessment. Statistics were performed as above. Significant overall pre-post changes were seen: F(1,17)=21, P<.001. Significant effect of floor was found: F(6,102)=81, P<.001. Most importantly, significant effect of pre-post X floor X drug interaction was found: F(6,102)=2.4, P<.05.
Ressler KJ, Rothbaum BO, Tannenbaum L, et al. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61:136-144.
SUDS=subjective units of discomfort; ANOVA=analysis of variance.
One of the cardinal features of extinction in animal models is the context specificity of the extinction environment. However, Ledgerwood and colleagues61 have demonstrated that D-cycloserine enhancement of extinction in animal models appears to lead to generalization across contexts. Ressler and colleagues,68 therefore, wondered if the decreased fear of heights found within the virtual environment would generalize to other settings. This question was assessed in two ways: first by asking questions related to the subject’s fear of heights in the real world, and secondly by assessing how much the subjects had decreased their avoidance of heights since the treatment. We found that all measures of fear were decreased at the early assessment (not shown) and the 3 month assessment (Figure 2A and 2B). The researchers also found that subjects’ self-exposure to heights in the “real world” had increased, suggesting decreased avoidance (Figure 2C). Finally, subjects that received D-cycloserine in conjunction with therapy felt that they had improved significantly compared to the placebo group in their overall acrophobia symptoms (Figure 2D).
FIGURE 2. Reduction in acrophobia in the real world with DCS augmentation of virtual reality therapy. Assessment scores of acrophobia measures are shown at 3-month follow-up58.
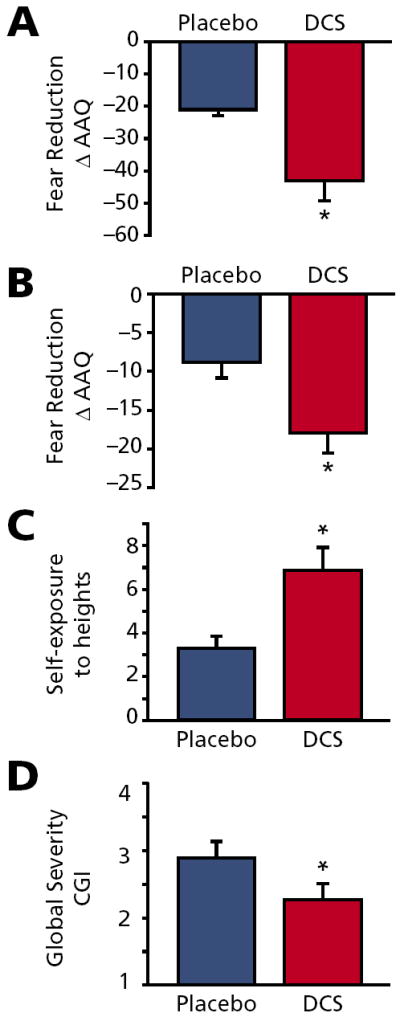
A. AAQ Pre-post difference score, t(19)=2.4; P<.05
B. AAVQ Pre-post difference score, t(19)=2.4; P<.05
C. Self-report of number of in vivo exposures to heights since the treatment, t(17)=3.0; P<.01
D. Self-report of CGI (scale: “1”=very much improved, “4”=no change) t(19)=2.3; P<.05
* P<.05
Ressler KJ, Rothbaum BO, Tannenbaum L, et al. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61:136-144.
AAQ=Acrophobia Anxiety Questionnaire; DCS=D-cycloserine; AAVQ=Acrophobia Avoidance Questionnaire; CGI=Clinical Global Improvement.
Our data indicate that participants receiving D-cycloserine experienced no increase in anxiety or fear during the exposure paradigm so that the enhancement of extinction is not due simply to enhanced intensity of exposure. Participants in the D-cycloserine group showed some evidence of enhanced extinction after only a single dose of medication and therapy. Following two doses of medication and therapy, they showed significant reductions in levels of fear to the specific exposure environment. Finally, we found that 3 months following the two treatment sessions, the D-cycloserine participants showed significant improvements on all acrophobia outcome measures, their own self-exposures in the real world, and their impression of clinical self-improvement.
Although it is possible that D-cycloserine somehow specifically enhances extinction, the current literature would suggest that it enhances learning in general, and thus enhances extinction as a form of learning. The specific evidence that D-cycloserine is enhancing extinction in a learning-specific way again comes from preclinical evidence in rodents. When combined with the conditioned stimulus, the D-cycloserine-treated animals showed accelerated extinction. However, this reduction was not seen when the animals were simply placed back in the fear conditioning context in the absence of the conditioned stimulus. Thus, D-cycloserine did not reduce fear by itself, but only facilitated the specific process of extinction of fear in combination with the exposure.59,62
CONCLUSION
It is important to appreciate that the emotion of fear can be clearly acquired through learning mechanisms. This review has focused predominantly on the acquisition of fear through associative learning and the mechanisms of extinction of such learning. This is not to imply that associative learning is the primary means that fear is acquired. Rather, it is related to the particular volume of work specifically examining the contribution of glutamate to fear learning using associative rather than instrumental experimental paradigms and the basis of current behavioral treatments for anxiety on extinction. There is an extensive literature across a variety of neurotransmitter and hormonal systems examining the acquisition of fear using instrumental learning approaches.9,72 It appears likely that more than one neural system for fear exists.73
We have also reviewed recent work from our group examining the use of D-cycloserine, a partial agonist at the NMDA receptor, to enhance the emotional learning that takes place with certain forms of psychotherapy.68 Although specific phobia provides the most easily testable disorder that is amenable to behavioral exposure therapy, this form of therapy is also the mainstay of treatment for other anxiety disorders such as panic disorder, obsessive-compulsive disorder, social anxiety disorder, and PTSD. It is important to note that the efficacy rates of exposure therapy for these different conditions differ considerably, which may in part be due to different rates of anxiety and fear reduction between these conditions. An advanced understanding of the learning mechanisms underlying exposure therapy will be critical to enhancing treatment of these disorders.
The use of partial agonists at the NMDA receptor for acute augmentation of the emotional learning within psychotherapy represents a novel and potentially very powerful new therapeutic tool. Glutamate receptors clearly serve to mediate the learning events that take place during the acquisition of fear. Recognition that treatment of anxiety disorders also includes new emotional learning—in the form of extinction—that may be facilitated with augmentation at the NMDA receptor, provides for an exciting set of opportunities for the treatment of these often refractory disorders.
Acknowledgments
Funding/Support: This work was supported by National Science Foundation grant IBN-987675 for the Science and Technology Center Program, Center for Behavioral Neuroscience, Yerkes National Primate Research Center, and by National Institutes of Health grant MH069884.
Footnotes
Disclosure: Dr. Gillespie does not have an affiliation with or financial interest in any organization that might pose a conflict of interest. Dr. Ressler has co-submitted with Michael Davis, PhD, a patent for the use of D-cyloserine for the specific enhancement of learning during psychotherapy. SyneurRx, LLC, has licensed this technology. Dr. Ressler is entitled to royalties derived from SyneurRx’s sale of products related to this research. The terms of this arrangement have been reviewed and approved by Emory University in accordance with its conflict of interest policies.
Contributor Information
Charles F. Gillespie, Department of Psychiatry and Behavioral Sciences at Emory University in Atlanta, Georgia.
Kerry J. Ressler, Department of Psychiatry and Behavioral Sciences at Emory University.
References
- 1.Izard CE. Basic emotions, relations among emotions, and emotion-cognition relations. Psychol Rev. 1992;99:561–565. doi: 10.1037/0033-295x.99.3.561. [DOI] [PubMed] [Google Scholar]
- 2.Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 3.Greenberg PE, Sisitsky T, Kessler RC, et al. The economic burden of anxiety disorders in the 1990s. J Clin Psychiatry. 1999;60:427–435. doi: 10.4088/jcp.v60n0702. [DOI] [PubMed] [Google Scholar]
- 4.McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Horm Behav. 2003;43:2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- 5.Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- 6.Davis M. The role of the amygdala in conditioned and unconditioned fear and anxiety. In: Aggleton JP, editor. The Amygdala. Oxford, England: Oxford University Press; 2000. pp. 213–287. [Google Scholar]
- 7.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 8.Fanselow MS, LeDoux JE. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron. 1999;23:229–232. doi: 10.1016/s0896-6273(00)80775-8. [DOI] [PubMed] [Google Scholar]
- 9.McGaugh JL, Cahill L, Roozendaal B. Involvement of the amygdala in memory storage: interaction with other brain systems. Proc Natl Acad Sci U S A. 1996;93:13508–13514. doi: 10.1073/pnas.93.24.13508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- 11.Phelps EA, O’Connor KJ, Gatenby JC, Gore JC, Grillon C, Davis M. Activation of the left amygdala to a cognitive representation of fear. Nat Neurosci. 2001;4:437–441. doi: 10.1038/86110. [DOI] [PubMed] [Google Scholar]
- 12.Whalen PJ, Shin LM, McInerney SC, Fischer H, Wright CI, Rauch SL. A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion. 2001;1:70–83. doi: 10.1037/1528-3542.1.1.70. [DOI] [PubMed] [Google Scholar]
- 13.Rauch SL, Whalen PJ, Shin LM, et al. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry. 2000;47:769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- 14.Pitkanen A. Connectivity of the rat amygdaloid complex. In: Aggleton JP, editor. The Amygdala Second Edition: A Functional Analysis. New York, NY: Oxford University Press; 2000. pp. 31–116. [Google Scholar]
- 15.Bading H, Segal MM, Sucher NJ, Dudek H, Lipton SA, Greenberg ME. N-methyl-D-aspartate receptors are critical for mediating the effects of glutamate on intracellular calcium concentration and immediate early gene expression in cultured hippocampal neurons. Neuroscience. 1995;64:653–664. doi: 10.1016/0306-4522(94)00462-e. [DOI] [PubMed] [Google Scholar]
- 16.Mahanty NK, Sah P. Calcium-permeable AMPA receptors mediate long-term potentiation in interneurons in the amygdala. Nature. 1998;394:683–687. doi: 10.1038/29312. [DOI] [PubMed] [Google Scholar]
- 17.Bauer EP, Schafe GE, LeDoux JE. NMDA receptors and L-type voltage-gated calcium channels contribute to long-term potentiation and different components of fear memory formation in the lateral amygdala. J Neurosci. 2002;22:5239–5249. doi: 10.1523/JNEUROSCI.22-12-05239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ressler KJ, Paschall G, Zhou XL, Davis M. Regulation of synaptic plasticity genes during consolidation of fear conditioning. J Neurosci. 2002;22:7892–7902. doi: 10.1523/JNEUROSCI.22-18-07892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schafe GE, LeDoux JE. Memory consolidation of auditory pavlovian fear conditioning requires protein synthesis and protein kinase A in the amygdala. J Neurosci. 2000;20:RC96. doi: 10.1523/JNEUROSCI.20-18-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schafe GE, Nader K, Blair HT, LeDoux JE. Memory consolidation of Pavlovian fear conditioning: a cellular and molecular perspective. Trends Neurosci. 2001;24:540–546. doi: 10.1016/s0166-2236(00)01969-x. [DOI] [PubMed] [Google Scholar]
- 21.Walker DL, Davis M. The role of amygdala glutamate receptors in fear learning, fear-potentiated startle, and extinction. Pharmacol Biochem Behav. 2002;71:379–392. doi: 10.1016/s0091-3057(01)00698-0. [DOI] [PubMed] [Google Scholar]
- 22.Miserendino MJ, Sananes CB, Melia KR, Davis M. Blocking of acquisition but not expression of conditioned fear-potentiated startle by NMDA antagonists in the amygdala. Nature. 1990;345:716–718. doi: 10.1038/345716a0. [DOI] [PubMed] [Google Scholar]
- 23.Campeau S, Miserendino MJ, Davis M. Intra-amygdala infusion of the N-methyl-Daspartate receptor antagonist AP5 blocks acquisition but not expression of fear-potentiated startle to an auditory conditioned stimulus. Behav Neurosci. 1992;106:569–574. doi: 10.1037//0735-7044.106.3.569. [DOI] [PubMed] [Google Scholar]
- 24.Walker DL, Paschall GY, Davis M. Glutamate receptor antagonist infusions into the basolateral and medial amygdala reveal differential contributions to olfactory vs. context fear conditioning and expression. Learn Mem. 2005;12:120–129. doi: 10.1101/lm.87105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee H, Kim JJ. Amygdalar NMDA receptors are critical for new fear learning in previously fear-conditioned rats. J Neurosci. 1998;18:8444–8454. doi: 10.1523/JNEUROSCI.18-20-08444.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodrigues SM, Schafe GE, LeDoux JE. Intra-amygdala blockade of the nr2b subunit of the nmda receptor disrupts the acquisition but not the expression of fear conditioning. J Neurosci. 2001;21:6889–6896. doi: 10.1523/JNEUROSCI.21-17-06889.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fanselow MS, Kim JJ. Acquisition of contextual Pavlovian fear conditioning is blocked by application of an NMDA receptor antagonist D,L-2-amino-5-phosphonovaleric acid to the basolateral amygdala. Behav Neurosci. 1994;108:210–212. doi: 10.1037//0735-7044.108.1.210. [DOI] [PubMed] [Google Scholar]
- 28.Maren S, Fanselow MS. Synaptic plasticity in the basolateral amygdala induced by hippocampal formation stimulation in vivo. J Neurosci. 1995;15:7548–7564. doi: 10.1523/JNEUROSCI.15-11-07548.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chenard BL, Menniti FS. Antagonists selective for NMDA receptors containing the NR2B subunit. Curr Pharm Des. 1999;5:381–404. [PubMed] [Google Scholar]
- 30.Tang YP, Shimizu E, Dube GR, et al. Genetic enhancement of learning and memory in mice. Nature. 1999;401:63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- 31.Goosens KA, Maren S. NMDA receptors are essential for the acquisition, but not expression, of conditional fear and associative spike firing in the lateral amygdala. Eur J Neurosci. 2004;20:537–548. doi: 10.1111/j.1460-9568.2004.03513.x. [DOI] [PubMed] [Google Scholar]
- 32.Isaac JT, Crair MC, Nicoll RA, Malenka RC. Silent synapses during development of thalamocortical inputs. Neuron. 1997;18:269–280. doi: 10.1016/s0896-6273(00)80267-6. [DOI] [PubMed] [Google Scholar]
- 33.Poncer JC, Esteban JA, Malinow R. Multiple mechanisms for the potentiation of AMPA receptor-mediated transmission by alpha-Ca2+/calmodulin-dependent protein kinase II. J Neurosci. 2002;22:4406–4411. doi: 10.1523/JNEUROSCI.22-11-04406.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rumpel S, LeDoux J, Zador A, Malinow R. Postsynaptic receptor trafficking underlying a form of associative learning. Science. 2005;308:83–88. doi: 10.1126/science.1103944. [DOI] [PubMed] [Google Scholar]
- 35.McKernan MG, Shinnick-Gallagher P. Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature. 1997;390:607–611. doi: 10.1038/37605. [DOI] [PubMed] [Google Scholar]
- 36.Li H, Rogawski MA. GluR5 kainate receptor mediated synaptic transmission in rat basolateral amygdala in vitro. Neuropharmacology. 1998;37:1279–1286. doi: 10.1016/s0028-3908(98)00109-9. [DOI] [PubMed] [Google Scholar]
- 37.Li H, Chen A, Xing G, Wei ML, Rogawski MA. Kainate receptor-mediated heterosynaptic facilitation in the amygdala. Nat Neurosci. 2001;4:612–620. doi: 10.1038/88432. [DOI] [PubMed] [Google Scholar]
- 38.Braga MF, Aroniadou-Anderjaska V, Xie J, Li H. Bidirectional modulation of GABA release by presynaptic glutamate receptor 5 kainate receptors in the basolateral amygdala. J Neurosci. 2003;23:442–452. doi: 10.1523/JNEUROSCI.23-02-00442.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ko S, Zhao MG, Toyoda H, Qiu CS, Zhuo M. Altered behavioral responses to noxious stimuli and fear in glutamate receptor 5 (GluR5)- or GluR6-deficient mice. J Neurosci. 2005;25:977–984. doi: 10.1523/JNEUROSCI.4059-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walker DL, Rattiner LM, Davis M. Group II metabotropic glutamate receptors within the amygdala regulate fear as assessed with potentiated startle in rats. Behav Neurosci. 2002;116:1075–1083. doi: 10.1037//0735-7044.116.6.1075. [DOI] [PubMed] [Google Scholar]
- 41.Grillon C, Cordova J, Levine LR, Morgan CA., 3 Anxiolytic effects of a novel group II metabotropic glutamate receptor agonist ( LY354740) in the fear-potentiated startle paradigm in humans. Psychopharmacology (Berl) 2003;168:446–454. doi: 10.1007/s00213-003-1444-8. [DOI] [PubMed] [Google Scholar]
- 42.Linden AM, Shannon H, Baez M, Yu JL, Koester A, Schoepp DD. Anxiolytic-like activity of the mGLU2/3 receptor agonist LY354740 in the elevated plus maze test is disrupted in metabotropic glutamate receptor 2 and 3 knock-out mice. Psychopharmacology (Berl) 2005;179:284–291. doi: 10.1007/s00213-004-2098-x. [DOI] [PubMed] [Google Scholar]
- 43.Lin CH, Lee CC, Huang YC, Wang SJ, Gean PW. Activation of group II metabotropic glutamate receptors induces depotentiation in amygdala slices and reduces fear-potentiated startle in rats. Learn Mem. 2005;12:130–137. doi: 10.1101/lm.85304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodrigues SM, Bauer EP, Farb CR, Schafe GE, LeDoux JE. The group I metabotropic glutamate receptor mGluR5 is required for fear memory formation and long-term potentiation in the lateral amygdala. J Neurosci. 2002;22:5219–5229. doi: 10.1523/JNEUROSCI.22-12-05219.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fendt M, Schmid S. Metabotropic glutamate receptors are involved in amygdaloid plasticity. Eur J Neurosci. 2002;15:1535–1541. doi: 10.1046/j.1460-9568.2002.01988.x. [DOI] [PubMed] [Google Scholar]
- 46.Busse CS, Brodkin J, Tattersall D, et al. The behavioral profile of the potent and selective mGlu5 receptor antagonist 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine (MTEP) in rodent models of anxiety. Neuropsychopharmacology. 2004;29:1971–1979. doi: 10.1038/sj.npp.1300540. [DOI] [PubMed] [Google Scholar]
- 47.Pavlov I. Conditioned Reflexes. Oxford, England: Oxford University Press; 1927. [Google Scholar]
- 48.Rothbaum BO, Davis M. Applying learning principles to the treatment of posttrauma reactions. Ann N Y Acad Sci. 2003;1008:112–121. doi: 10.1196/annals.1301.012. [DOI] [PubMed] [Google Scholar]
- 49.Bouton ME. Context and behavioral processes in extinction. Learn Mem. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- 50.Myers KM, Davis M. Behavioral and neural analysis of extinction. Neuron. 2002;36:567–584. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- 51.Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- 53.Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 54.Baker JD, Azorlosa JL. The NMDA antagonist MK-801 blocks the extinction of Pavlovian fear conditioning. Behav Neurosci. 1996;110:618–620. doi: 10.1037//0735-7044.110.3.618. [DOI] [PubMed] [Google Scholar]
- 55.Cox J, Westbrook RF. The NMDA receptor antagonist MK-801 blocks acquisition and extinction of conditioned hypoalgesia responses in the rat. Q J Exp Psychol B. 1994;47:187–210. [PubMed] [Google Scholar]
- 56.Falls WA, Miserendino MJ, Davis M. Extinction of fear-potentiated startle: blockade by infusion of an NMDA antagonist into the amygdala. J Neurosci. 1992;12:854–863. doi: 10.1523/JNEUROSCI.12-03-00854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Santini E, Muller RU, Quirk GJ. Consolidation of extinction learning involves transfer from NMDA-independent to NMDA-dependent memory. J Neurosci. 2001;21:9009–9017. doi: 10.1523/JNEUROSCI.21-22-09009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lu KT, Walker DL, Davis M. Mitogen-activated protein kinase cascade in the basolateral nucleus of amygdala is involved in extinction of fear-potentiated startle. J Neurosci. 2001;21:RC162. doi: 10.1523/JNEUROSCI.21-16-j0005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear- potentiated startle in rats. J Neurosci. 2002;22:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Olney J. New mechanisms of excitatory transmitter neurotoxicity. J Neural Transm Suppl. 1994;43:47–51. [PubMed] [Google Scholar]
- 61.Ledgerwood L, Richardson R, Cranney J. D-cycloserine facilitates extinction of learned fear: effects on reacquisition and generalized extinction. Biol Psychiatry. 2005;57:841–847. doi: 10.1016/j.biopsych.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 62.Ledgerwood L, Richardson R, Cranney J. Effects of D-cycloserine on extinction of conditioned freezing. Behav Neurosci. 2003;117:341–349. doi: 10.1037/0735-7044.117.2.341. [DOI] [PubMed] [Google Scholar]
- 63.Ledgerwood L, Richardson R, Cranney J. D-cycloserine and the facilitation of extinction of conditioned fear: consequences for reinstatement. Behav Neurosci. 2004;118:505–513. doi: 10.1037/0735-7044.118.3.505. [DOI] [PubMed] [Google Scholar]
- 64.Otto M. Learning and “unlearning” fears: preparedness, neural pathways, and patients. Biol Psychiatry. 2002;52:917–920. doi: 10.1016/s0006-3223(02)01719-5. [DOI] [PubMed] [Google Scholar]
- 65.Foa EB, Franklin ME, Moser J. Context in the clinic: how well do cognitive-behavioral therapies and medications work in combination? Biol Psychiatry. 2002;52:987–997. doi: 10.1016/s0006-3223(02)01552-4. [DOI] [PubMed] [Google Scholar]
- 66.Barlow DH, Gorman JM, Shear MK, Woods SW. Cognitive-behavioral therapy, imipramine, or their combination for panic disorder: a randomized controlled trial. JAMA. 2000;283:2529–2536. doi: 10.1001/jama.283.19.2529. [DOI] [PubMed] [Google Scholar]
- 67.Marks IM, Swinson RP, Basoglu M, et al. Alprazolam and exposure alone and combined in panic disorder with agoraphobia. A controlled study in London and Toronto. Br J Psychiatry. 1993;162:776–787. doi: 10.1192/bjp.162.6.776. [DOI] [PubMed] [Google Scholar]
- 68.Ressler KJ, Rothbaum BO, Tannenbaum L, et al. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61:136–144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- 69.Rothbaum BO, Hodges LF, Kooper R, Opdyke D, Williford JS, North M. Effectiveness of computer-generated (virtual reality) graded exposure in the treatment of acrophobia. Am J Psychiatry. 1995;152:626–628. doi: 10.1176/ajp.152.4.626. [DOI] [PubMed] [Google Scholar]
- 70.Rothbaum BO, Hodges L, Smith S, Lee JH, Price L. A controlled study of virtual reality exposure therapy for the fear of flying. J Consult Clin Psychol. 2000;68:1020–1026. doi: 10.1037//0022-006x.68.6.1020. [DOI] [PubMed] [Google Scholar]
- 71.Rothbaum BO, Hodges LF, Ready D, Graap K, Alarcon RD. Virtual reality exposure therapy for Vietnam veterans with posttraumatic stress disorder. J Clin Psychiatry. 2001;62:617–622. doi: 10.4088/jcp.v62n0808. [DOI] [PubMed] [Google Scholar]
- 72.McGaugh JL. Memory–a century of consolidation. Science. 200;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 73.Wilensky AE, Schafe GE, LeDoux JE. The amygdala modulates memory consolidation of fear-motivated inhibitory avoidance learning but not classical fear conditioning. J Neurosci. 2000;20:7059–7066. doi: 10.1523/JNEUROSCI.20-18-07059.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]


