Abstract
Task-irrelevant perceptual learning (TIPL) has captured a growing interest in the field of perceptual learning. The basic phenomenon is that stimulus features that are irrelevant to a subject’s task (i.e. convey no useful information to that task) can be learned due to their consistent presentation during task-performance. Here we review recent research on TIPL and focus on two key aspects of TIPL; 1) the mechanisms gating learning in TIPL, and, 2) what is learned through TIPL. We show that TIPL is gated by learning signals that are triggered from task processing or by rewards. These learning signals operate to enhance processing of individual stimulus features and appear to result in plasticity in early stages of visual processing. Furthermore, we discuss recent research that demonstrates that TIPL is not in opposition to theories of attention but instead that TIPL operates in concert with attention. Where attentional learning is best to enhance (or suppress) processing of stimuli of known task relevance, TIPL serves to enhance perception of stimuli that are originally inadequately processed by the brain.
How do we select what to learn? For example, for one to hit or catch a baseball, two things must be learned. 1) How to perceive the trajectory of the baseball as precisely as possible, and, 2) how to properly place the bat or glove in the ball’s trajectory. Perceptual learning focuses on the former. There are a number of leading theories regarding mechanisms by which this learning occurs. One possibility is that the visual system itself undergoes no plasticity, however, one can learn by better reading out the signals from the sensory processing stages (Dosher & Lu, 1998, Law & Gold, 2008). Other theories suggest that the sensory systems are plastic and can adapt to better represent the ball’s trajectory (Fahle, 2004, Gilbert, Sigman & Crist, 2001). However, there is debate regarding how this may take place (Seitz & Watanabe, 2005). For example, if one casually observes many games of baseball will their brain adapt to better estimate the ball’s trajectory? Alternatively, perhaps just watching the game isn’t enough but there is a need to attend very closely to the ball’s trajectory to enable learning to occur? Then again, maybe something more is required to learn? For example, perhaps one needs to actually play the game and actually practice hitting and catching the ball. It may be that the engagement in these tasks and reinforcement from successful hits and catches is important for learning. However, one may still ask, does that observer need to be consciously aware of the details of the trajectory of the ball to learn them, or can the observer learn implicitly due to contingent stimulation and reinforcement? Perhaps without being consciously aware of the ball’s detailed trajectory the observer can learn to represent pitches just through feedback given by the announcer of whether the ball was in the strike box and of what type of pitch was given?
These questions regarding the mechanisms that guide the process of perceptual learning are central to understanding learning and have been the focus of significant controversy. Theories of the learning process are far ranging. At one extreme is the view that sensory systems are fixed after developmental critical periods and that after this point only changes in readout from these structures can account for perceptual changes (Hubel & Wiesel, 1964). At the other extreme are those who argue that the perceptual system is constantly, and passively, updating its apparatus to meet the constraints of the currents sensory environment (Dinse, Ragert, Pleger, Schwenkreis & Tegenthoff, 2003, Fiser & Aslin, 2001, Seitz, Kim, van Wassenhove & Shams, 2007, Seitz & Dinse, 2007). In-between theories suggest that such passive learning does not allow for complete restructuring of sensory areas but that attentional and/or reinforcement gating systems can serve to allow or to restrict the learning process (Ahissar & Hochstein, 1993, Ahissar & Hochstein, 2004, Seitz & Watanabe, 2005, Seitz & Dinse, 2007, Seitz & Watanabe, 2003). While these theories differ in their view of the gating process, there is an evolving consensus that some degree of plasticity does occur in the adult perceptual systems and that these changes are adaptive not to the statistics of the overall environment but more specifically to the behavioral environment. Thus stimuli that are important to the organism will produce more learning than those that are not.
There has been an active debate regarding the nature of these gating processes. For example, some theories argue that learning is restricted to those stimuli to which the system “chooses” to process (i.e. attentional selection of learning), whereas others have argued for a weaker form of restriction where learning occurs for stimuli that are correlated with important events whether or not these stimuli have been deemed “relevant” to the behavior. There is good evidence for both viewpoints. Thus we suggest that these are complementary mechanisms that can operate in parallel rather than arguing that only one of these viewpoints is correct.
Here we review the recent literature on task-irrelevant perceptual learning (TIPL), which has lately shown how learning can occur for visual stimuli that are subliminal to the learner. Research on TIPL has given an important insight both into when learning occurs (mechanisms gating learning) and also regarding the stages of visual processing that are changed through the course of learning (what is learned).
Task-Irrelevant Perceptual Learning (TIPL)
In the last few years the phrase task-irrelevant perceptual learning (TIPL) has been coined to refer to a learning paradigm in which subjects learn to better discriminate stimuli to which they are exposed but upon which they perform no task (Gutnisky, Hansen, Iliescu & Dragoi, 2009, Ludwig & Skrandies, 2002, Nishina, Seitz, Kawato & Watanabe, 2007, Paffen, Verstraten & Vidnyanszky, 2008, Seitz & Watanabe, 2005, Seitz, Nanez, Holloway, Koyama & Watanabe, 2005b, Seitz, Nanez, Holloway & Watanabe, 2006, Seitz & Watanabe, 2003, Tsushima, Seitz & Watanabe, 2008, Watanabe, Nanez & Sasaki, 2001, Watanabe, Nanez, Koyama, Mukai, Liederman & Sasaki, 2002). This phenomenon was first observed by Watanabe et al. (2001), when they exposed subjects to a motion direction stimulus while the subjects performed a rapid serial visual presentation task (RSVP). It was found that after many days of exposure to this procedure the subjects became better at discriminating and detecting the exposed motion direction. This result was particularly intriguing given that the motion stimulus was presented at a subliminal signal level. Despite being below the threshold of visibility and being irrelevant to the subjects’ central task, the repetitive exposure improved performance specifically for the direction of the exposed motion when tested in a subsequent supra-threshold test. This finding has provided a significant level of controversy and posed a challenge for attentional accounts of perceptual learning and raises the question, how can a subject learn to better discriminate a stimulus without attention to, or even notice of, that stimulus?
Does TIPL occur simply as a result of passive exposure to a stimulus?
Recently, Seitz and Watanabe (2003) found that TIPL occurred as the result of temporal-pairing between the presentation of a subliminal, task-irrelevant, motion stimulus and a task-target. In this experiment, four different directions of motion were presented an equal number of times during the exposure stage, but a single direction of interest was consistently paired (temporally preceded and then overlapped) with the task-targets. Learning was found only for the motion-direction that was paired with the task-targets, not for the other motion-directions. Similar results were obtained when the luminance contrast of the dots (100% coherence) was made so low that the subjects did not notice the presentation of the motion stimuli (Seitz et al., 2005b). These results suggest that TIPL does not occur as a result of purely passive exposure, but that learning for the irrelevant feature was related to task performance. These results led to a model based on the idea that plasticity is gated by confluence between a spatially diffusive task-related signal and a task-irrelevant feature (Seitz & Watanabe, 2005).
A key aspect of the model of Seitz and Watanabe (2005) was that processing of the targets of the letter task is sufficient to trigger the learning signals that gates PL. To clarify the level of processing in the RSVP task that is required to release of learning signals, Seitz et al. (Seitz, Lefebvre, Watanabe & Jolicoeur, 2005a) adapted the procedure of the attentional blink (AB) (Weichselgartner & Sperling, 1987) to TIPL. The AB refers to the observation of a “blink” in attentional processing that occurs when subjects must concurrently process two task-targets and results in a deficit of processing for the second target. This “blink” is thought to result from a bottleneck in high-level processing (such as decision making and memory encoding) (Duncan, Ward & Shapiro, 1994, Jolicoeur, 1999, Joseph, Chun & Nakayama, 1997, Vogel, Luck & Shapiro, 1998, Weichselgartner & Sperling, 1987). Thus, using the AB, Seitz et al. (2005a) addressed whether TIPL is gated by a learning signal that is released from a relatively low-level target-processing or whether TIPL is gated by a later stage reinforcement system (note that even if the learning signal is released from a high-level, the stage at which neural plasticity takes place can be low-level). They found that while subjects obtained performance improvements for subthreshold motion stimuli presented outside of the time-window of the attentional blink, no learning occured for stimuli presented during the AB. A control study showed that this lack of TIPL during the AB was not due to a deficit of sensory processing during the AB.
Together these results imply that the learning signal involved in TIPL results from relatively high-level processing of the RSVP task targets and against the notion that TIPL results from passive processing of the learned motion stimuli. While these findings were novel to the area of perceptual learning they share common elements to theories of reinforcement learning and in this regard have a high degree of ecological validity. Namely, that learning is gated by behaviorally relevant events (rewards, punishment, novelty, etc). At these times reinforcement signals are released to better learn aspects of the environment (even those for which the organism is not consciously aware) that are predictive or co-vary with the event (Dalley, McGaughy, O’Connell, Cardinal, Levita & Robbins, 2001, Dayan & Balleine, 2002, Dayan & Yu, 2003, Doya, Samejima, Katagiri & Kawato, 2002, Schultz, 2000). For example, in a natural environment a target (e.g., a predator) to which one needs to direct attention is usually presented in the same or similar context. Thus, the need to gain higher sensitivity to features in such a context may lead one to better perceive the environment in which a target tends to appear. This learning of the sensory features of the context can proceed without the subject’s awareness (e.g. Chun, 2000).
Is Reward Sufficient to cause TIPL?
While studies discussed thus far suggest that TIPL may be due to a reinforcement process, evidence for this conclusion was rather indirect. More recently, Seitz, Kim and Watanabe (2009) examined whether TIPL actually requires engagement in a task or whether a simple reinforcement paradigm, like classical conditioning, could result in learning. For example, going back to our example of baseball, does the observer actually need to be playing the game or would just the visual stimulation of a series of throws and pitches associated with an appropriate reinforcement schedule be sufficient to produce learning?
To address this question, they examined whether pairing visual stimuli with a liquid reward (in humans) would result in a performance improvement in discriminating that stimulus (Seitz, Kim & Watanabe, 2009). Using a classical conditioning paradigm, they presented every 500-ms a different sinusoidal noise background that filled the display. At random intervals, an oriented sinusoidal grating was superimposed on the noise background. For each subject, one of two oriented gratings was paired with liquid-delivery (C+) and the other orientation was presented without reward (C-). Subjects were asked to refrain from eating or drinking for five hours prior to each experimental session. Furthermore, continuous flash suppression (Tsuchiya & Koch, 2005) was used to render the C+ and C- orientations imperceptible throughout the 20-days of conditioning.
Results of this experiment show learning for the C+ orientation and that this improvement of performance did not transfer to the untrained eye. No learning was observed for the C- orientation in either eye. Furthermore, this learning only occurred when subjects were deprived of food and water prior to the conditioning sessions (and in consequence rated the liquid delivery to be pleasant). Without deprivation, liquid delivery was not pleasant and no learning occurred for the orientation paired with that liquid. Thus the conjunction of a stimulus with a reward is sufficient to cause TIPL, even when there is no awareness of the learned stimulus or the stimulus-reward contingencies.
While the results presented above suggest that TIPL is not purely a passive process, we cannot deny the possibility that passive learning occurs. Behaviorally, there is a growing literature of statistical learning that suggests that statistical regularities in the sensory environment can be passively picked up and learned by the sensory systems (Fiser & Aslin, 2001, Saffran, Johnson, Aslin & Newport, 1999, Seitz et al., 2007). Haptic coactivation studies demonstrate that perceptual learning leading to greater sensitivity in subjects’ digits can arise from passive stimulation (Dinse, Kleibel, Kalisch, Ragert, Wilimzig & Tegenthoff, 2006, Godde, Stauffenberg, Spengler & Dinse, 2000). Complementing these behavioral findings, there are numerous neuroscientific studies that show that sensory plasticity can be driven by passive stimulation (for review see Seitz & Dinse, 2007). Given these results, Seitz and Dinse (2007) suggested that while typical sensory inputs are insufficient to cause learning in adults, that reinforcement, attention, coactivation, among others, are processes by which sensory activation can be boosted over a learning threshold and result in perceptual learning. We suggest that the visual system does not at first know which sensory features are and are not relevant to ones goals and that thus until unambiguous evidence of goal relevance is achieved the visual system must reply upon a variety of heuristics (such as attention, reinforcement, coactivation, etc; see Seitz and Dinse 2007 for a review) to determine what should be learned. Achieving a better understanding of these learning heuristics is key to understanding perceptual learning.
Attentional selection can modulate TIPL
Numerous studies have reported that learning does not occur on stimulus features that are irrelevant to a subject’s task (Ahissar & Hochstein, 1993, Shiu & Pashler, 1992). An important question is, why is TIPL found in some studies and not in others? A study by Tsushima et al. (Tsushima et al., 2008) helped answer this question by systematically exploring the relation between signal strength of the motion stimuli used during training and the resultant magnitude of TIPL. The results showed that performance improvements only occurred for the motion-stimuli trained at low, perithreshold, coherence levels. These results provide a parsimonious explanation of why TIPL is found in some studies, but not in other studies. Namely that weak task-irrelevant signals fail to be “noticed”, and to be suppressed, by the attention system and thus are learned while stronger stimulus signals are detected, and suppressed (Tsushima, Sasaki & Watanabe, 2006), and are not learned.
Further evidence for how attention can suppress TIPL is shown in a paper contained in this issue (Choi, Seitz & Watanabe, in press). In this work, an exogenous attention paradigm was employed in concert with TIPL. Subjects performed a task reporting the direction of arrows, which were presented above the fixation point. These arrows served as exogenous attentional cues directing subjects’ attention towards the location to which the arrows pointed. Task-irrelevant dynamic random dot (DRD) motion stimuli where displayed at the left and right of fixation at subthreshold levels of motion coherence. One direction of motion was always presented at the location to which the arrows pointed (attended DRD) and another direction of motion was presented at the location away from which the arrows pointed (unattended DRD). TIPL was found for the unattended DRD, but not for the attended DRD. These results suggest that while both DRDs were present at the time that the arrow targets were processed, and thus were temporally proximal with the learning signals, attention suppressed learning of the attended DRD.
A recent study by Gutnisky et al. (2009) provides additional evidence of how attention can restrict what is learned. While this study uses very different methods as compared to the standard TIPL procedure, they do find learning that is task-irrelevant. In this study subjects performed a contrast detection task at one point in the visual field, while similar stimuli were exposed at another location. While more learning was found for stimuli that were presented at the attended location, significant learning was also observed at the unattended location. Notably, the extent of transfer to other orientations (and to more complex stimuli such as natural scenes) was greater for stimuli presented at the unattended location than for those at the attended location. The authors suggested that the more restricted learning at the attended location may be due to feature based attention inhibiting cells tuned to angles nearby trained orientation. While the task-irrelevant learning found by Gutnisky et al. (2009) generalizes across features, the learning found in standard TIPL procedures has been largely stimulus specific. Further research needs to be conducted to better understand the relationship between standard studies of TIPL, which are reinforcement based, and the learning found by Gutnisky et al. (2009), in which the mechanisms leading to learning are less clear.
Nishina et al. (2007) investigated the spatial profile of TIPL. During the training period, participants performed an attentionally demanding RSVP task at one location while subthreshold, static, Gabor patches, which were masked in noise, were presented at different locations in the visual field. The largest improvement was found for the Gabors presented in closest spatial proximity to the task. These data indicate that the learning of the task-irrelevant visual feature depends significantly on the task location, with a gradual attenuation according to the spatial distance between them. These results, combined with the previous findings, indicate that TIPL is spatiotemporally regulated by brain activity related to successful detection of task targets. However, it is not clear what brain mechanisms underlie this spatial relationship between the task locus and the task-irrelevant perceptual learning. While the results showed that there is a clear spatial gradient of the learning, a more extensive investigation is necessary to clarify the overall shape of this learning function and whether it is due to a single mechanism with a narrow spatial profile or an interaction between a broad learning signal and a spatially narrow attentional mechanism. This latter possibility would be in agreement with the results of Tsushima et al. (2008) and Choi et al. (2009), which found that attentional suppression can restrict TIPL.
The possible role of neuromodulatory signals in TIPL
The above results suggest that TIPL involves interactions between learning and selective attentional signals in the brain. We have previously postulated that TIPL is driven by neuromodulatory signals in the brain (Seitz & Watanabe, 2005, Seitz & Watanabe, 2003) and that some of the same neuromodulatory signals that have been implicated in aspects of reinforcement learning (Dayan & Balleine, 2002, Dayan & Yu, 2003, Doya et al., 2002) have also been implicated in different aspects of attention (Fan, Fossella, Sommer, Wu & Posner, 2003, Fossella, Sommer, Fan, Wu, Swanson, Pfaff & Posner, 2002). Accordingly, we suggested that potentially disparate accounts of TIPL via attentional or reinforcement-learning signals may be reconciled by the observation that attention is not a singular process, but instead consists of multiple systems that have different spatial and temporal profiles (Seitz & Watanabe, 2005).
This suggestion is in accord with research of Posner and colleagues who have suggested that alerting, orienting and executive function are triply dissociable attentional subsystems (Fan, McCandliss, Sommer, Raz & Posner, 2002, Posner & Petersen, 1990). The alerting system controls a nonspecific arousal state, the orienting system directs resources to a specific spatial cue or feature, and the executive system is involved in solving tasks involving conflict. The orienting and executive systems are thought to be selective to regions of space (spatial attention), individual features (feature-based attention) or objects (object-based attention) regarded to be task-relevant items. Notably, task-irrelevant features can also cause orienting, even without awareness (Zhaoping, 2008), however how this plays a role in TIPL is unclear. Whereas alerting is a temporally phasic but featurally nonspecific signal that increases general processing at times important stimuli are thought to be present (temporal attention). Each of these attention subsystems has been linked with different neuromodulatory signals (Fan et al., 2002); orienting with the acetylcholine system (Davidson & Marrocco, 2000), alerting with the norepinephrine system (Coull, Frith, Frackowiak & Grasby, 1996, Marrocco, Witte & Davidson, 1994, Witte & Marrocco, 1997) and executive with dopamine (Fossella et al., 2002). Notably, acetylcholine, norepinephrine, and dopamine are known to be involved in learning (Dalley et al., 2001, Schultz, 2000) and have been proposed to have distinct roles in reinforcement learning (Dayan & Balleine, 2002, Dayan & Yu, 2003, Doya et al., 2002).
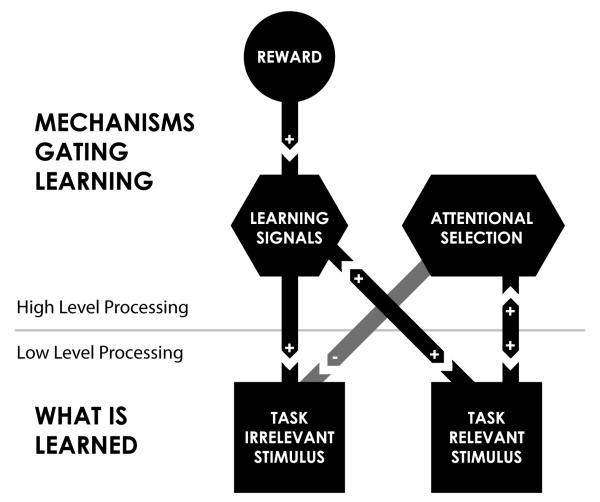
These findings suggest that some attentional and learning signals may be subserved by the same substrate. If this is indeed the case, then the important question in evaluating the findings of TIPL is not whether attention or reinforcement-learning signals are responsible for the restricted spatial-temporal profile of learning, but rather what types of attentional/reinforcement signals are responsible and how they may interact in shaping TIPL? Given that there are many different types of attentional and learning signals it is worth understanding how each of them contribute to the process of perceptual learning. The results discussed thus far give a partial answer to this question. TIPL occurs for stimuli temporally paired with rewards or task-targets and accordingly, we have hypothesized that alerting (and norepinephrine) may be the learning signal involved in TIPL (Seitz and Watanabe 2005). More recent results showing that TIPL is disrupted by exogenous spatial attention (Choi et al., in press) and task-directed attention (Tsushima et al., 2006, 2008) leads us to hypothesize that orienting and executive signals may serve to suppress TIPL. A schematic indicating the mechanisms that gate learning is shown in Figure 1. Future research will be required to further test these hypotheses.
Figure 1.
Schematic of mechanisms found to be involved in TIPL. TIPL consists of mechanisms gating learning (top), which are thought to be high-level stages of processing, and, changes in the sensory processing (bottom), which are thought to be low-level stages of processing. A task-relevant stimulus can trigger the release of learning signals that promote learning of both task-relevant and task-irrelevant stimuli (c.f. Seitz and Watanabe, 2003). Task-relevant stimulus processing can also trigger attentional selection that inhibits learning of task-irrelevant stimuli and promotes learning for task-relevant stimuli (c.f. Tsushima, Seitz and Watanabe, 2008; Chou, Seitz and Watanabe, in press). Learning signals can also be released through other processes such as delivery of rewards (c.f. Seitz, Kim and Watanabe, 2009).
What is learned in TIPL?
So far in this article we have focused on the mechanisms gating learning, a complementary question regarding TIPL is, what is learned? An important characteristic of TIPL is that the learning takes place for stimuli to which the subject is not attending (and is typically unaware of) and that the learning is difficult to explain as an effect of subjects discovering the distinguishing features by which to perform the testing tasks. Furthermore TIPL transfers to a variety of testing tasks including direction detection, discrimination and identification tasks (Watanabe et al., 2001) and even transfers to aspects of perception that relate to motion processing such as critical flicker fusion thresholds (Seitz, Nanez, Holloway & Watanabe, 2005c, Seitz et al., 2006). TIPL has also been shown for orientation discrimination (Nishina et al., 2007) and for auditory stimuli (Vlahou, Seitz & Protopapas, 2009). Furthermore, performance benefits resulting from TIPL have been shown to last months to years (Seitz et al., 2006, Watanabe et al., 2002).
A study by Watanabe et al. (2002) sought to determine the stage of motion processing at which TIPL occurs. To do this they exposed subjects to dynamic random dot (DRD) motion stimuli in which the local dot motion was varied while the direction of global motion was kept constant. In one of the displays (i.e. “center missing”) individual dot motion angles between 5° and 30° and between −5° and −30° were employed to produce the perception of global motion direction of 0° . Thus, the global motion direction was not represented in the distribution of local motion directions. Sensitivity tests that were conducted before and after this TIPL training found that learning occurred only for the local motion direction to which the subject was exposed and that no learning occurred in the direction of the global motion percept (i.e. 0°). A related recent study by Pilly, Seitz and Grossberg (2009) used polarity-specific DRDs based upon a technique by Wehrhahn and Rapf (2001) that was devised to selectively activate ON or OFF cells by ensuring that the two spatially offset flashes that constitute an apparent motion stimulus have different onset but simultaneous offset times. The result of this study indicated that TIPL is specific to the contrast polarity of the trained DRD. The results of these studies demonstrated that the learning resulting from TIPL can take place at an early motion processing stage at which local, but not global, motion directions are processed and that is contrast polarity specific.
Related studies by Seitz et al. (Seitz et al., 2005c, Seitz et al., 2006) examined how other aspects of perception that related to motion processing might be affected by TIPL. To do this they measured critical flicker fusion thresholds (CFFTs) of subjects during the course of TIPL. The idea behind these studies was that cells in magnocellular brain areas, which are specialized for processing specific motion-directions, respond well to stimuli of high temporal frequencies (Colby, Duhamel & Goldberg, 1993, Lisberger & Movshon, 1999). In addition, lesion studies in non-human primates indicate that the magnocellular visual pathway (Merigan, Byrne & Maunsell, 1991, Schiller, Logothetis & Charles, 1991) and occipital lobe processing (Halstead, 1947, Mishkin & Weiskrantz, 1959) are required to detect relatively high-frequency flickering stimuli. Given this, it seemed likely that if the sensitivity of these cells were increased through TIPL on motion then this learning may also transfer to other read-outs from these cells, such as CFFT. The result of these studies confirmed this hypothesis. Subjects who showed processing benefits for motion-direction discrimination also showed dramatic changes in their CFFTs (on average 30% change), in that flickering lights were now fused at a greater frequency. Control tasks demonstrate that CFFT changes were tightly coupled with improvements in discriminating the direction of motion stimuli and that this plasticity is long-lasting and is retained for at least one year after training.
Ocular specific learning has been argued to be evidence of plasticity of early, monocular, stages of visual processing (Fahle, Edelman & Poggio, 1995, Karni & Sagi, 1991, Lu, Chu, Dosher & Lee, 2005, Schoups, Vogels & Orban, 1995). Seitz, Kim and Watanabe (2009) found TIPL to be largely specific to both the stimulus orientation and to the eye of training. While it is intriguing to conclude that the learning effect is indicative of plasticity in an early, monocular, stage of visual processing (Nishida, Ashida & Sato, 1994, Paradiso, Shimojo & Nakayama, 1989, von der Heydt, Peterhans & Baumgartner, 1984), such as in V1, some degree of ocular specificity remains in higher visual areas (although at lower incidence; Uka et al., 2000) and thus some learning may be occurring at later processing stages as well (Ahissar and Hochstein 1997). Also, previous results regarding monocularity of learning have been inconsistent (for example Karni and Sagi, 1991 found monocular learning while Schoups and Orban, 1996 found transfer of learning between eyes with a very similar task) and there is still a degree of controversy regarding findings of monocular learning. However, physiological studies conducted with a similar procedure in awake behaving macaques indicates that the learning effect is taking place at V4 or earlier (Franko, Seitz & Vogels, in press) and provides further evidence that TIPL involves plasticity in early stages of visual processing.
How does the magnitude of TIPL compare to other type of perceptual learning? We made an attempt to address this question by compared learning slopes (SlopeL) from TIPL to results of task-relevant perceptual learning (TRPL) of basic features as reported by Fine and Jacobs (2002). We examined studies of TIPL for which an initial data point had d’ between .5 and 1 and for which training lasted for at most 20 days (e.g. Nishina et al., 2007, Seitz et al., 2005a, Seitz et al., 2009, Seitz et al., 2005b, Seitz & Watanabe, 2003, Tsushima et al., 2008, Watanabe et al., 2001). In these studies the mean value of SlopeL was 0.179 (range 0.085 – 0.32), which is highly comparable to SlopeL for TRPL on basic features (studies 1-11 as reported by Fine and Jacobs, 2002), which had a mean of 0.173 (range 0.001 – 0.381). While this analysis shows that learning for task-relevant and task-irrelevant perceptual learning is comparable, it is important to realize that we are comparing TIPL to a select group of TRPL studies and that our analysis mostly shows that TIPL falls within the distribution of learning produced by TRPL, not that TIPL is equivalent to TRPL. Future research needs to make more direct comparisons between these two types of learning using identical stimuli and tests, and similar training regimes to better understand how TIPL and TRPL compare in the magnitude and the quality of what is learned.
As a whole, these studies are consistent with the suggestion that TIPL involves plasticity in the earliest stages of sensory processing. We suggest that TIPL is a particularly good paradigm by which to identify low-level effects of perceptual learning. Recent research has suggested that many results of perceptual learning may take place at late stages of sensory processing or at decision stages (Dosher and Lu, 1998; Smirnakis et al., 2005; Law & Gold, 2008; Xiao et al., 2008). However, a key advantage of TIPL over other perceptual learning paradigms is that TIPL dissociates what is being learned (i.e., the task-irrelevant stimulus) from the decision processes that correspond to the training task, and thus TIPL cannot easily be explained by learning in the decision processes. While it is premature (and likely incorrect) to conclude that TIPL is fundamentally different from other forms of perceptual learning, TIPL seems to provide better evidence for a low level basis of perceptual learning.
Is TIPL really task-irrelevant?
A key question is what is TIPL good for? For example, is there a way in which learning of the task-irrelevant stimuli can actually benefit performance of the main task? A recent study directly addressed the question of whether TIPL is truly task-irrelevant (Seitz & Watanabe, 2008). To test the hypothesis that associations that are beneficial to task-performance may develop between the task-relevant and task-irrelevant stimuli, or the task-responses and the task-irrelevant stimuli, a new procedure was developed where correlations were introduced between the presentation of task-irrelevant motion stimuli and the identity of task-targets or task-responses. The results of this study showed no evidence that associations develop between the learned (task-irrelevant) motion stimuli and the task-targets, or responses types, of the letter identification task. Surprisingly, the conditions that had the greatest correlations between stimulus and response showed the least amount of TIPL. The greatest learning was found in conditions of greatest response uncertainty and with the greatest processing requirements for the task-relevant stimuli. This is in line with the previously published model that suggests that task-irrelevant stimuli benefit from the spill-over of learning signals that are released due to processing of task-relevant stimuli (Seitz & Watanabe, 2005).
What use is TIPL if it doesn’t provide direct performance benefits to the subjects’ task? We suggest that the brain has evolved mechanisms of learning and perception that work exceedingly well in most situations but which are not always beneficial (Chun & Marois, 2002, Seitz et al., 2005b). TIPL may be a general mechanism for the enhancement of stimulus processing for features that are consistently presented at behaviorally relevant times. As these stimuli become more salient they can be brought in to the “awareness” of the individual and become accessible to attentional and decision processes that can more directly determine the “task-relevance” of the learned stimuli. The published studies of TIPL are conducted in rather artificial laboratory scenarios in which the exposed stimulus features provide very little information that can benefit subjects’ task performance. However, in real world settings exposed subthreshold stimuli may be truly task-relevant and may be enhanced, rather than be suppressed, by attentional processes and this learning may provide task benefits.
CONCLUSION
TIPL has emerged as an important mechanism of perceptual learning. This learning can occur without awareness of the learned stimuli and even outside of the context of a task. Furthermore, the learning has been shown to be consistent with plasticity of low-level visual processes. However, this learning is not purely passive and requires the release of a learning (reinforcement) signal that can be triggered by important task events or an external reward. Furthermore, attentional signals directed towards the task-irrelevant stimuli can disrupt TIPL. These finding suggest that TIPL results through the interaction of bottom-up stimulus processing top-down learning and attentional signals (see Figure 1).
TIPL has been shown to occur most consistently for learning of things that are below the perceptual threshold. This nature of TIPL may be important for allowing the system to better respond to subtle cues whose incidences are implicitly correlated with a given task and may lead to higher performance on the task. However, at least in the laboratory, TIPL is also a way to trick the system into better perceiving stimuli that are irrelevant. We suggest that TIPL and attentional learning processes operated together. TIPL serves to enhance processing of unattended or unperceived stimuli and attention serves to select which stimuli should be processed (and learned). Presumably, once task-irrelevant stimuli rise above the perceptual threshold they will become more accessible to attentional processes, which in turn will suppress features deemed to be irrelevant and to enhance features deemed to be relevant to the subject. Thus we suggest that TIPL represents an important process of learning that operates in concert with other forms of learning.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahissar M, Hochstein S. Attentional control of early perceptual learning. Proc Natl Acad Sci U S A. 1993;90(12):5718–5722. doi: 10.1073/pnas.90.12.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahissar M, Hochstein S. The reverse hierarchy theory of visual perceptual learning. Trends Cogn Sci. 2004;8(10):457–464. doi: 10.1016/j.tics.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Choi H, Seitz A, Watanabe T. When Attention Interrupts Learning: Inhibitory Effects of Attention on TIPL. Vision Res. doi: 10.1016/j.visres.2009.07.004. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun MM. Contextual cueing of visual attention. Trends Cogn Sci. 2000;4(5):170–178. doi: 10.1016/s1364-6613(00)01476-5. [DOI] [PubMed] [Google Scholar]
- Chun MM, Marois R. The dark side of visual attention. Curr Opin Neurobiol. 2002;12(2):184–189. doi: 10.1016/s0959-4388(02)00309-4. [DOI] [PubMed] [Google Scholar]
- Colby CL, Duhamel JR, Goldberg ME. Ventral intraparietal area of the macaque: anatomic location and visual response properties. J Neurophysiol. 1993;69(3):902–914. doi: 10.1152/jn.1993.69.3.902. [DOI] [PubMed] [Google Scholar]
- Coull JT, Frith CD, Frackowiak RS, Grasby PM. A fronto-parietal network for rapid visual information processing: a PET study of sustained attention and working memory. Neuropsychologia. 1996;34(11):1085–1095. doi: 10.1016/0028-3932(96)00029-2. [DOI] [PubMed] [Google Scholar]
- Dalley JW, McGaughy J, O’Connell MT, Cardinal RN, Levita L, Robbins TW. Distinct changes in cortical acetylcholine and noradrenaline efflux during contingent and noncontingent performance of a visual attentional task. J Neurosci. 2001;21(13):4908–4914. doi: 10.1523/JNEUROSCI.21-13-04908.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson MC, Marrocco RT. Local infusion of scopolamine into intraparietal cortex slows covert orienting in rhesus monkeys. J Neurophysiol. 2000;83(3):1536–1549. doi: 10.1152/jn.2000.83.3.1536. [DOI] [PubMed] [Google Scholar]
- Dayan P, Balleine BW. Reward, motivation, and reinforcement learning. Neuron. 2002;36(2):285–298. doi: 10.1016/s0896-6273(02)00963-7. [DOI] [PubMed] [Google Scholar]
- Dayan P, Yu AJ. Uncertainty and learning. IETE Journal of Research. 2003;49:171–182. [Google Scholar]
- Dinse HR, Kleibel N, Kalisch T, Ragert P, Wilimzig C, Tegenthoff M. Tactile coactivation resets age-related decline of human tactile discrimination. Ann Neurol. 2006;60(1):88–94. doi: 10.1002/ana.20862. [DOI] [PubMed] [Google Scholar]
- Dinse HR, Ragert P, Pleger B, Schwenkreis P, Tegenthoff M. Pharmacological modulation of perceptual learning and associated cortical reorganization. Science. 2003;301(5629):91–94. doi: 10.1126/science.1085423. [DOI] [PubMed] [Google Scholar]
- Dosher BA, Lu ZL. Perceptual learning reflects external noise filtering and internal noise reduction through channel reweighting. Proc Natl Acad Sci U S A. 1998;95(23):13988–13993. doi: 10.1073/pnas.95.23.13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doya K, Samejima K, Katagiri K, Kawato M. Multiple model-based reinforcement learning. Neural Computation. 2002;14:1347–1369. doi: 10.1162/089976602753712972. [DOI] [PubMed] [Google Scholar]
- Duncan J, Ward R, Shapiro K. Direct measurement of attentional dwell time in human vision. Nature. 1994;369(6478):313–315. doi: 10.1038/369313a0. [DOI] [PubMed] [Google Scholar]
- Fahle M. Perceptual learning: a case for early selection. J Vis. 2004;4(10):879–890. doi: 10.1167/4.10.4. [DOI] [PubMed] [Google Scholar]
- Fahle M, Edelman S, Poggio T. Fast perceptual learning in hyperacuity. Vision Res. 1995;35(21):3003–3013. doi: 10.1016/0042-6989(95)00044-z. [DOI] [PubMed] [Google Scholar]
- Fan J, Fossella J, Sommer T, Wu Y, Posner MI. Mapping the genetic variation of executive attention onto brain activity. Proc Natl Acad Sci U S A. 2003;100(12):7406–7411. doi: 10.1073/pnas.0732088100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. J Cogn Neurosci. 2002;14(3):340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- Fiser J, Aslin RN. Unsupervised statistical learning of higher-order spatial structures from visual scenes. Psychological Science. 2001;12(6):499–504. doi: 10.1111/1467-9280.00392. [DOI] [PubMed] [Google Scholar]
- Fossella J, Sommer T, Fan J, Wu Y, Swanson JM, Pfaff DW, Posner MI. Assessing the molecular genetics of attention networks. BMC Neurosci. 2002;3(1):14. doi: 10.1186/1471-2202-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franko E, Seitz AR, Vogels R. Dissociable neural effects of long term stimulus-reward pairing in macaque visual cortex. Journal of Cognitive Neuroscience. doi: 10.1162/jocn.2009.21288. (in press) [DOI] [PubMed] [Google Scholar]
- Gilbert CD, Sigman M, Crist RE. The neural basis of perceptual learning. Neuron. 2001;31(5):681–697. doi: 10.1016/s0896-6273(01)00424-x. [DOI] [PubMed] [Google Scholar]
- Godde B, Stauffenberg B, Spengler F, Dinse HR. Tactile coactivation-induced changes in spatial discrimination performance. J Neurosci. 2000;20(4):1597–1604. doi: 10.1523/JNEUROSCI.20-04-01597.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutnisky DA, Hansen BJ, Iliescu BF, Dragoi V. Attention alters visual plasticity during exposure-based learning. Curr Biol. 2009;19(7):555–560. doi: 10.1016/j.cub.2009.01.063. [DOI] [PubMed] [Google Scholar]
- Halstead WC. Brain and intelligence; a quantitative study of the frontal lobes. University of Chicago Press; Chicago: 1947. p. xiii.p. 206. [Google Scholar]
- Hubel DH, Wiesel TN. Effects of Monocular Deprivation in Kittens. Naunyn Schmiedebergs Arch Pharmacol. 1964;248:492–497. doi: 10.1007/BF00348878. [DOI] [PubMed] [Google Scholar]
- Jolicoeur P. Restricted attentional capacity between sensory modalities. Psychon Bull Rev. 1999;6(1):87–92. doi: 10.3758/bf03210813. [DOI] [PubMed] [Google Scholar]
- Joseph JS, Chun MM, Nakayama K. Attentional requirements in a ‘preattentive’ feature search task. Nature. 1997;387(6635):805–807. doi: 10.1038/42940. [DOI] [PubMed] [Google Scholar]
- Karni A, Sagi D. Where practice makes perfect in texture discrimination: evidence for primary visual cortex plasticity. Proc Natl Acad Sci U S A. 1991;88(11):4966–4970. doi: 10.1073/pnas.88.11.4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law CT, Gold JI. Neural correlates of perceptual learning in a sensory-motor, but not a sensory, cortical area. Nat Neurosci. 2008;11(4):505–513. doi: 10.1038/nn2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisberger SG, Movshon JA. Visual motion analysis for pursuit eye movements in area MT of macaque monkeys. J Neurosci. 1999;19(6):2224–2246. doi: 10.1523/JNEUROSCI.19-06-02224.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu ZL, Chu W, Dosher BA, Lee S. Independent perceptual learning in monocular and binocular motion systems. Proc Natl Acad Sci U S A. 2005;102(15):5624–5629. doi: 10.1073/pnas.0501387102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig I, Skrandies W. Human perceptual learning in the peripheral visual field: sensory thresholds and neurophysiological correlates. Biol Psychol. 2002;59(3):187–206. doi: 10.1016/s0301-0511(02)00009-1. [DOI] [PubMed] [Google Scholar]
- Marrocco RT, Witte EA, Davidson MC. Arousal systems. Curr Opin Neurobiol. 1994;4(2):166–170. doi: 10.1016/0959-4388(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Merigan WH, Byrne CE, Maunsell JH. Does primate motion perception depend on the magnocellular pathway? J Neurosci. 1991;11(11):3422–3429. doi: 10.1523/JNEUROSCI.11-11-03422.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishkin M, Weiskrantz L. Effects of cortical lesions in monkeys on critical flicker frequency. J Comp Physiol Psychol. 1959;52:660–666. doi: 10.1037/h0038577. [DOI] [PubMed] [Google Scholar]
- Nishida S, Ashida H, Sato T. Complete interocular transfer of motion aftereffect with flickering test. Vision Res. 1994;34(20):2707–2716. doi: 10.1016/0042-6989(94)90227-5. [DOI] [PubMed] [Google Scholar]
- Nishina S, Seitz AR, Kawato M, Watanabe T. Effect of spatial distance to the task stimulus on task-irrelevant perceptual learning of static Gabors. Journal of Vision. 2007;7(13):1–10. doi: 10.1167/7.13.2. [DOI] [PubMed] [Google Scholar]
- Paffen CL, Verstraten FA, Vidnyanszky Z. Attention-based perceptual learning increases binocular rivalry suppression of irrelevant visual features. J Vis. 2008;8(4):25, 21–11. doi: 10.1167/8.4.25. [DOI] [PubMed] [Google Scholar]
- Paradiso MA, Shimojo S, Nakayama K. Subjective contours, tilt aftereffects, and visual cortical organization. Vision Res. 1989;29(9):1205–1213. doi: 10.1016/0042-6989(89)90066-7. [DOI] [PubMed] [Google Scholar]
- Pilly P, Seitz AR, Grossberg S. Where in the motion pathway does task-irrelevant perceptual learning occur? Vision Sciences Society (Naples. 2009 [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Saffran JR, Johnson EK, Aslin RN, Newport EL. Statistical learning of tone sequences by human infants and adults. Cognition. 1999;70(1):27–52. doi: 10.1016/s0010-0277(98)00075-4. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Logothetis NK, Charles ER. Parallel pathways in the visual system: their role in perception at isoluminance. Neuropsychologia. 1991;29(6):433–441. doi: 10.1016/0028-3932(91)90003-q. [DOI] [PubMed] [Google Scholar]
- Schoups AA, Vogels R, Orban GA. Human perceptual learning in identifying the oblique orientation: retinotopy, orientation specificity and monocularity. J Physiol. 1995;483(Pt 3):797–810. doi: 10.1113/jphysiol.1995.sp020623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Multiple reward signals in the brain. Nat Rev Neurosci. 2000;1(3):199–207. doi: 10.1038/35044563. [DOI] [PubMed] [Google Scholar]
- Seitz A, Kim R, van Wassenhove V, Shams L. Simultaneous and Independant Aquisition of Multisensory and Unisensory Associations Perception. 2007 doi: 10.1068/p5843. in press. [DOI] [PubMed] [Google Scholar]
- Seitz A, Lefebvre C, Watanabe T, Jolicoeur P. Requirement for high-level processing in subliminal learning. Curr Biol. 2005a;15(18):R753–755. doi: 10.1016/j.cub.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Seitz A, Watanabe T. A unified model for perceptual learning. Trends Cogn Sci. 2005;9(7):329–334. doi: 10.1016/j.tics.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Seitz AR, Dinse HR. A common framework for perceptual learning. Curr Opin Neurobiol. 2007 doi: 10.1016/j.conb.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Seitz AR, Kim D, Watanabe T. Rewards evoke learning of unconsciously processed visual stimuli in adult humans. Neuron. 2009;61(5):700–707. doi: 10.1016/j.neuron.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz AR, Nanez JE, Holloway SR, Koyama S, Watanabe T. Seeing what is not there shows the costs of perceptual learning. Proc Natl Acad Sci U S A. 2005b;102(25):9080–9085. doi: 10.1073/pnas.0501026102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz AR, Nanez JE, Holloway SR, Watanabe T. Visual experience can substantially alter critical flicker fusion thresholds. Hum Psychopharmacol. 2005c;20(1):55–60. doi: 10.1002/hup.661. [DOI] [PubMed] [Google Scholar]
- Seitz AR, Nanez JE, Holloway SR, Watanabe T. Perceptual learning of motion leads to faster flicker perception. PLoS ONE. 2006;1:e28. doi: 10.1371/journal.pone.0000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz AR, Watanabe T. Psychophysics: Is subliminal learning really passive? Nature. 2003;422(6927):36. doi: 10.1038/422036a. [DOI] [PubMed] [Google Scholar]
- Seitz AR, Watanabe T. Is task-irrelevant learning really task-irrelevant? PLoS ONE. 2008;3(11):e3792. doi: 10.1371/journal.pone.0003792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu LP, Pashler H. Improvement in line orientation discrimination is retinally local but dependent on cognitive set. Percept Psychophys. 1992;52(5):582–588. doi: 10.3758/bf03206720. [DOI] [PubMed] [Google Scholar]
- Tsuchiya N, Koch C. Continuous flash suppression reduces negative afterimages. Nat Neurosci. 2005;8(8):1096–1101. doi: 10.1038/nn1500. [DOI] [PubMed] [Google Scholar]
- Tsushima Y, Sasaki Y, Watanabe T. Greater disruption due to failure of inhibitory control on an ambiguous distractor. Science. 2006;314(5806):1786–1788. doi: 10.1126/science.1133197. [DOI] [PubMed] [Google Scholar]
- Tsushima Y, Seitz AR, Watanabe T. Task-irrelevant learning occurs only when the irrelevant feature is weak. Curr Biol. 2008;18(12):R516–517. doi: 10.1016/j.cub.2008.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlahou E, Seitz AR, Protopapas A. Implicit learning of non-native speech stimuli. Acoustical Society of America (Portland, OR. 2009 [Google Scholar]
- Vogel EK, Luck SJ, Shapiro KL. Electrophysiological evidence for a postperceptual locus of suppression during the attentional blink. J Exp Psychol Hum Percept Perform. 1998;24(6):1656–1674. doi: 10.1037//0096-1523.24.6.1656. [DOI] [PubMed] [Google Scholar]
- von der Heydt R, Peterhans E, Baumgartner G. Illusory contours and cortical neuron responses. Science. 1984;224(4654):1260–1262. doi: 10.1126/science.6539501. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Nanez JE, Sasaki Y. Perceptual learning without perception. Nature. 2001;413(6858):844–848. doi: 10.1038/35101601. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Nanez JE, Sr., Koyama S, Mukai I, Liederman J, Sasaki Y. Greater plasticity in lower-level than higher-level visual motion processing in a passive perceptual learning task. Nat Neurosci. 2002;5(10):1003–1009. doi: 10.1038/nn915. [DOI] [PubMed] [Google Scholar]
- Wehrhahn C, Rapf D. Perceptual learning of apparent motion mediated through ON-and OFF-pathways in human vision. Vision Res. 2001;41(3):353–358. doi: 10.1016/s0042-6989(00)00232-7. [DOI] [PubMed] [Google Scholar]
- Weichselgartner E, Sperling G. Dynamics of automatic and controlled visual attention. Science. 1987;238(4828):778–780. doi: 10.1126/science.3672124. [DOI] [PubMed] [Google Scholar]
- Witte EA, Marrocco RT. Alteration of brain noradrenergic activity in rhesus monkeys affects the alerting component of covert orienting. Psychopharmacology (Berl) 1997;132(4):315–323. doi: 10.1007/s002130050351. [DOI] [PubMed] [Google Scholar]
- Zhaoping L. Attention capture by eye of origin singletons even without awareness--a hallmark of a bottom-up saliency map in the primary visual cortex. J Vis. 2008;8(5):1, 1–18. doi: 10.1167/8.5.1. [DOI] [PubMed] [Google Scholar]