Abstract
While task-irrelevant perceptual learning (TIPL) suggests that perceptual learning of a feature occurs without focused attention to the feature, some kind of attentional involvement was implied by recent findings that TIPL occurred only when a task-irrelevant stimulus was paired with a main task target. Here, during training, two task-irrelevant stimuli with different coherent motion directions were exposed, one on an attended side and the other on an unattended side. We found no performance improvements for the direction on the attended side. These results suggest that while attention facilitates task-relevant learning, it can suppress TIPL.
Keywords: perceptual learning, task-irrelevant perceptual learning, attention, attentional inhibition
The visual system encounters an overwhelming amount of signals from continuously changing environments. While it is not ideal to unconditionally learn all the signals, neither is it desirable to reject all. Thus, the question arises: what to learn and what not to learn? One of the most plausible speculations is that attention gates learning.
This view has been supported by various studies on perceptual learning (PL). Previous studies showed that learning did not transfer between different tasks performed on the same stimuli and thus suggested that PL can only occur with focused attention to the features relevant to a given task (Ahissar & Hochstein, 1993; Schoups, Vogels, Qian & Orban, 2001; Shiu & Pashler, 1992). For example, in the experiment by Ahissar and Hochstein, practicing a task of discriminating the orientation of a global shape did not improve performance in a task of detecting a local element, and vice versa, even though the same visual stimuli were presented in both tasks.
This focused attention hypothesis was challenged by a more recent finding that showed learning of a feature to occur without attention to that feature (Watanabe, Náñez & Sasaki, 2001). In that experiment, learning occurred on a subliminal feature that was irrelevant to the main task; this was called task-irrelevant perceptual learning (TIPL). This finding not only indicated that attention to a feature was not necessary for the feature to be learned, but also complicated the question of attentional selection. In cases where attention does not gate PL, what does?
A subsequent series of studies have addressed this question. Seitz and Watanabe (2003) found that for TIPL to occur, a task-irrelevant feature had to be exposed simultaneously with a target of a task in which a subject was engaged. Subsequently, Seitz and his colleagues tested whether TIPL occurred when a target was presented but not perceived using the attentional blink (AB), in which the second target in a rapid serial visual presentation (RSVP) was hardly recognized when it was presented within half a second after the appearance of the first target (Seitz, Lefebvre, Watanabe & Jolicoeur, 2005). They found that when subjects failed to identify the second target due to AB, learning of a task-irrelevant stimulus temporally paired with the second target also did not occur.
How would these series of research results suggest a role for attention in PL? Note that most plausible explanations of the attentional blink relied on limited attentional resources. For example, the attentional dwell time hypothesis suggested that the attentional blink occurred because attentional resource was insufficient when the second target appeared while the first target was being processed (Ward, Duncan & Shapiro, 1996). Another line of research indicated that task-irrelevant stimuli could be better perceived when a main task was so easy that the attentional load for the task was low (Lavie & Tsal, 1994). Together, these results implied that even irrelevant stimuli could not stop being processed if available resources remained, and therefore task-irrelevant learning could occur. Conversely, but consistently, the failure to learn a task-irrelevant stimulus paired with the “attentionally blinked” second target (Seitz et al., 2005) might be attributed to depletion of limited attentional resources.
In this current study, we tested the hypothesis that TIPL should occur as a result of attention to task-irrelevant features when available attentional resources remained.
During training sessions, two task-irrelevant stimuli were presented simultaneously, one on either side of the screen, with one being exogenously attended to. For task-irrelevant stimuli two circular patches of dynamic random-dot displays (DRDs) were employed at a 5% coherence level (5% of the motion display consisted of coherently moving dots and 95% consisted of randomly moving dots) so that the direction of coherently moving dots could hardly be identified (Watanabe et al., 2001). These two DRDs had different directions relative to each other.
In order to manipulate a subject's attention to a task-irrelevant stimulus, arrows were employed as an exogenous orienting cue. Previous studies showed that arrows could trigger attentional orienting such that a subject's attention was automatically directed to the place where the arrowhead pointed (e.g. Tipples, 2002). During training sessions subjects were asked to report the orientation of the arrows in the center of the screen. (This task was easy, requiring low attentional load.) The task-irrelevant stimuli were selectively presented according to where the arrowhead pointed: a DRD with a specific motion direction was consistently presented on the side where the arrowhead pointed, the attended side; the other patch with a different motion direction was on the other side, the unattended side (see Figure 1).
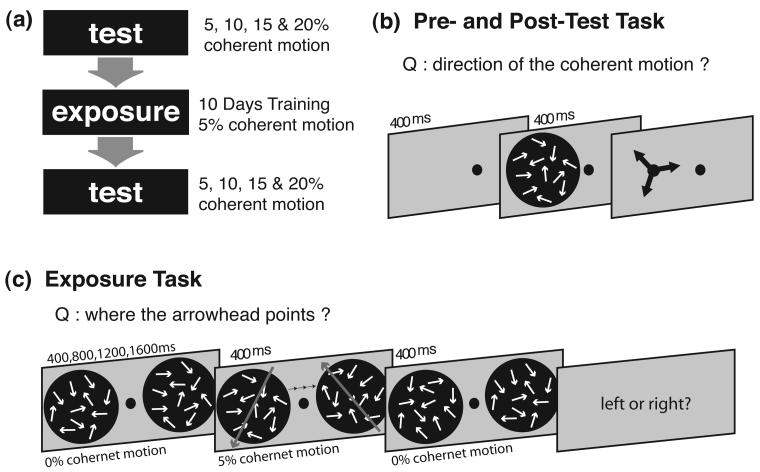
Figure 1.
Illustration of the general experimental procedure. (a) Outline: experiment consisted of a pre-test session, ten training sessions, and a post-test session, in that order. (b) Procedure of the task in pre- and post-test sessions. In each trial, dynamic random dot (DRD) displays were presented on the right or left side of the screen. Some of these dots moved coherently in one of three directions — 15°, 135° and 255°. 400ms later, this display was replaced with three arrows. Subjects selected the arrow that best matched their perceived direction. Their performance was measured in four coherence levels — 5,10,15 and 20%. (c) Procedure of the task in training sessions. In each trial, two DRD displays with 0% coherence level were presented on both sides of the screen (one on each side). After a period of 400, 800, 1200 or 1600ms, a row of five arrows appeared above or below a fixation point for 400ms. (For convenience, only three arrows appear in this figure.) While the arrows were presented 5% of the dots in lateral DRD displays moved coherently in directions different from each other. (The big grey arrow in the figure represents the direction of coherent motion.) As soon as the arrows disappeared, all dots of the DRD displays moved randomly again. Subjects were asked to report the orientation of the arrows. The row of arrows played two roles. It was the task-relevant stimulus of the main task: subjects had to report its orientation; it also worked as an exogenous attentional orienting cue. Arrows could automatically trigger attentional orienting, so that subjects would pay attention involuntarily to where the arrow pointed.
If TIPL occurs due to residual available attention directed to the task-irrelevant feature, stronger TIPL should occur for the motion direction of the DRD patch presented on the attended side.
Method
Participants
Ten university students participated for monetary payment. All subjects had normal or corrected-to-normal acuity and were naïve as to the purpose of the study.
Apparatus
The whole experiment was constructed using Psychophysics Toolbox (Brainard, 1997; Pelli, 1997) for MATLAB (The MathWorks, Natick, MA) on a Macintosh G5 computer. All displays were presented on a 19″ CRT monitor, with a resolution of 1280 × 1024 pixels and a refresh rate of 85 Hz. Subjects were positioned approximately 56 cm from the monitor, so that the display subtended 36 by 27 deg of visual angle. In order to maintain the subject's head position, a chin rest was used. The experiment was conducted in a darkened room.
Stimulus
In the pre- and post-training tests a circular patch of DRD was employed. The DRD patch subtended 14 deg in diameter with a dot density of 0.85 dots per deg squared. The traveling speed of the dots was approximately 14.2 deg/s. From one frame to another, a certain proportion of the dots moved coherently in one direction and speed, whereas the other dots moved in random directions and speeds. The coherence level of the DRD patch was determined by the proportion of coherently moving dots; in the 5% coherence level display 5% of the dots moved coherently and 95% moved randomly. In the test four coherence levels were used — 5, 10, 15 and 20%. The DRDs were constructed to mimic a display originally developed by Newsome and Pare (1988), which did not allow for a long dot lifetime and required subjects to integrate the local motion directions to determine the global motion direction. In each trial, one motion patch was presented on either side of the screen, and the inner edge of the patch was 1.4 deg from the fixation point located in the center of the screen.
In the training sessions, a row of five arrows was presented as a stimulus in the main task. The length of each arrow was 26 pixels, which corresponded to 0.7 deg, and each arrow was separated by 0.08 deg from the nearest arrow. All arrows pointed to one side (either left or right). The row of arrows was presented above or below the central fixation point, such that there was always 1.3 deg of vertical blank space between the arrows and the fixation point. As task-irrelevant stimuli, two DRDs with 5% coherence level were employed. The patches were positioned identically to the placements in the pre- and post-tests. Whereas only one DRD was presented in the pre- and post-tests, two DRDs were presented simultaneously, one on either side of the screen, in the training sessions.
Procedure
A whole experiment consisted of one pre-test session, ten training sessions, and one post-test session, in that order. The experiment was completed in 12 days, with only one session being completed per day (see Figure 1a).
1. Pre- and post- tests
The procedure of the pre- and post- tests was identical. Subjects started each trial by pressing a key. After the fixation point (a red dot) was presented for 400ms, a DRD patch was presented for 400ms on the right or left side of the screen. Subjects reported their perception of the DRD's coherent motion direction by selecting the arrow representing that motion direction with the mouse cursor. Four coherence levels (5, 10, 15 and 20%) were employed. The coherently moving dots of the DRD moved in one of three directions: 1) the attended direction, the motion direction of the DRD to which the arrowhead pointed during training sessions, 2) the unattended direction, the motion direction of the DRD from which the arrowhead pointed away during training sessions, and 3) the control direction, presented only in the pre- and post-tests to avoid response bias and not exposed during training sessions. The employed directions were 15°, 135° and 255° and counterbalanced across subjects.
Subjects first completed a small number of practice trials, data of which was excluded from analysis. These practice trials familiarized subjects with each of the conditions employed in this experiment. However, in order to minimize the possibility that learning could occur during practice trials, these stimuli had a higher coherent level (at least 30%) compared to levels in the actual test. Subjects then completed 40 trials in each of the conditions for a total of 480 trials presented in a random order, unblocked.
2. Training
As in the pre- and post-tests, each trial began by subjects pressing a key. First, two motion patches were presented, one on either side of the screen, with a fixation point at the center. The motion patches did not have any coherent motion (i.e. all dots moved in random directions). This display was presented for a period of 400, 800, 1200 or 1600ms, with periods being equally and randomly distributed throughout. Then, a row of five arrows appeared above or below the fixation point for 400ms. While the row of arrows was presented, 5% of dots in lateral DRD moved coherently. The motion directions of the two DRDs were different from each other: the DRD with one direction was always placed where the arrowhead pointed, and the DRD with the other direction was always on the opposite side of the screen. After the arrows disappeared, all dots of the lateral DRDs moved randomly again and that display was presented for 400ms. Subjects were asked to report the direction of the arrows by pressing a key.
In each training session, subjects completed 1280 trials, which took approximately an hour to complete. Only one training session was conducted in a day, such that ten days were required for the whole training. Subjects were not allowed to suspend sessions for longer than two successive days.
Results
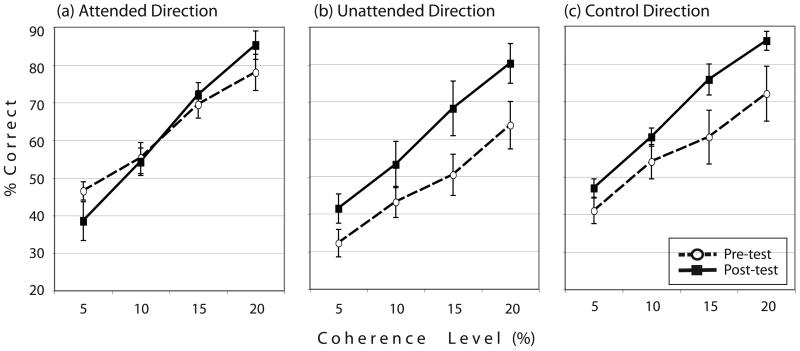
The performance of subjects in the pre- and post-tests is shown in Figure 2. Contrary to expectations that TIPL occurs as a result of attention to task-irrelevant features when available attentional resources remain, subjects' performances in the post-test did not significantly improve for the attended direction when compared with performances in the pre-test (F(1,9) = 0.007, p = .937). In the case of the 5% coherence level, especially, the performance of the post-test was marginally worse than the pre-test (t(9) = 1.855, p = .096). For the unattended direction, however, significant improvements were found (F(1,9) = 9.474, p = .0132); significant or marginal improvements were found in all coherence levels (at 5%, t(9) = 2.924, p = .017; at 10%, t(9) = 1.800, p = .105; at 15%, t(9) = 3.229, p = .010; at 20%, t(9) = 2.316, p = .045). Even for the control direction, significant improvements were found (F(1,9) = 17.876, p = .002); significant or marginal improvements were found in all coherence level (at 5%, t(9) = 2.167, p = .058; at 10%, t(9) = 2.248, p = .051; at 15%, t(9) = 3.759, p = .004; at 20%, t(9) = 4.168, p = .002). Additionally, as in previous studies of TIPL that used coherent motion stimuli, there was a significant effect of coherence level in all directions (in the attended direction, F(3,27) = 97.313, p <.001; in the unattended direction, F(3,27) = 57.452, p <.001; in the control direction, F(3,27) = 62.804, p < .001). There was a significant interaction between coherence-level and tests (pre- vs. post-tests) for the attended direction (F(3,27) = 6.723, p = .001) and the control direction (F(3,27) = 3.666, p = .025).
Figure 2.
Mean correct percentage (a) for a direction presented on an attended side during training sessions, (b) for a direction on an unattended side, and (c) for a direction never exposed. The learning effect was found only for the unattended direction and the control direction, and not for the attended direction.
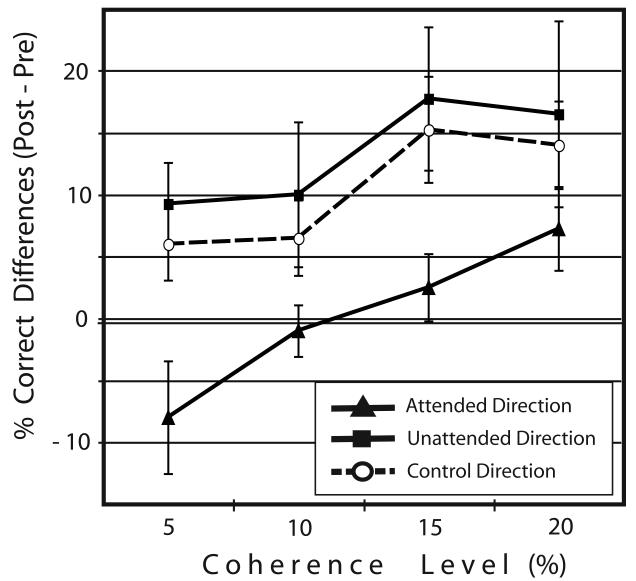
The learning effects in the tested directions were compared more directly. Figure 3 depicts performance improvement, determined by the subtraction of the performances in the pre-test from the performances in the post-test for each direction. The performance improvement for the attended direction was significantly smaller than for the unattended direction (F(1,9) = 6.258, p = .034) and, surprisingly, even smaller than for the control direction (F(1,9) = 9.126, p = .015).
Figure 3.
Mean performance improvement for each direction, as determined by subtraction of % correct performances in the pre-test from the % correct performances in the post-test.
Discussion
In the present study, the performance improvement was not observed for irrelevant stimuli to which exogenous attention was directed while a tendency for performance improvement was observed for irrelevant stimulus to which exogenous attention was not directed. This finding is at odds with the hypothesis that TIPL occurs as a result of attention being directed to the task-irrelevant features as long as available attentional resources remain.
One of the most plausible explanations for such contradictory results may be that attention inhibits the learning of task-irrelevant stimuli rather than facilitating it. The results of our experiment implied that attention not only caused no learning of the task-irrelevant stimuli, but furthermore may have even worsened the performance for those stimuli. For the attended direction, no performance improvement from the pre- to post-tests was found and, in fact, the performance at the 5% coherence level was marginally worsened after training. This insignificant improvement for the attended direction, furthermore, was contrasted with the significant improvement not only for the unattended direction, but particularly for the control direction which had not been exposed during training. This view is in accordance with the recent finding that perceptual learning on a task-relevant feature inhibits task-irrelevant features (Paffen, Verstraten & Vidnyanszky, 2008).
The idea that attention suppresses the processing of task-irrelevant stimuli has been an important topic in the literature of attention research and supported by various phenomena including negative priming, a slowed response to a stimulus that was previously presented as an ignored distractor (Tipper, 1985). Neuro-imaging studies, in addition, suggested that the lateral prefrontal cortex (LPFC) was involved in the inhibitory attentional control of task-irrelevant stimuli (Knight, Staines, Swick & Chao, 1999).
At first glance, these findings of attentional suppression of task-irrelevant stimuli appear to be inconsistent with results showing task-irrelevant learning to occur only when the subjects are engaged in a task and thus attention is deployed (e.g. Seitz & Watanabe, 2003; Seitz et al., 2005). However, recent findings may reconcile this seeming contradiction. Tsushima, Sasaki, and Watanabe (2006) indicated that subthreshold task-irrelevant motion stimuli interfered more severely with the performance of a main task than suprathreshold stimuli. Their neuro-imaging study suggested that the greater disruption from subliminal stimuli is due to failure of attentional inhibitory control on the stimuli. In their experiment, when the task-irrelevant stimuli (DRDs) were subthreshold (5% coherence level), LPFC, which was thought to be involved in attentional inhibitory control of task-irrelevant stimuli, was not activated. However, MT+, which is known to be highly responsive to motion direction, was highly activated. In contrast, when the task-irrelevant motion stimulus was suprathreshold, LPFC was activated while activation in MT+ was reduced. They suggested that because the threshold of LPFC was higher than that of MT+, the task-irrelevant stimuli with a weak signal activated only MT+. When stimuli were suprathreshold, however, LPFC was activated, which provided inhibitory control on MT+. Further investigations using the DRD coherent motion stimulus demonstrated that TIPL occurred only for coherent motion stimuli around the threshold (5% and 15%), and not for the 50% coherent motion (Tsushima, Seitz & Watanabe, 2008). They suggested that when a task-irrelevant stimulus is weak (including subthreshold conditions), inhibitory control of task-irrelevant stimuli fails and thus learning of the stimuli is allowed. This model is consistent with our present study showing that when attention is directed to a task-irrelevant feature, TIPL does not occur.
An alternative explanation for our results is that the differences of performance improvements among the three tested directions were due to differences in initial performance for each direction. The performance for the attended direction of the pre-test was higher than for the other two directions; even at a 5% coherence level, the level employed during training sessions, subjects showed near 50% accuracy. Because PL has been shown to occur when the signal of task-irrelevant stimuli was not strong, no learning effect on the attended direction could be interpreted as a result of high performance for the attended direction. In most studies of TIPL, 5% subthreshold coherent motion has been used to rule out the possibility that focused attention is directed to the motion direction (e.g. Seitz & Watanabe, 2003; Watanabe et al, 2001). However, this does not necessarily indicate that task-irrelevant learning occurs only with a subthreshold feature. For example, TIPL occurred even when the task-irrelevant stimuli had a 15% coherence level — a level at which subjects showed 59% accuracy in a six alternative forced choice (6AFC) test (Tshushima et al., 2008). Thus, we find it unlikely that high performance for the attended direction inhibits the learning effect on that direction.
A recent study showed the role of attention in exposure-based learning (Gutnisky, Hansen, Illiescu, & Dragoi, 2009). Their results showed that exposure-based learning at an attended location was greater than at an unattended location, which was seemingly inconsistent with our results. A closer comparison of the two studies showed, however, that the experiments examined different aspects of TIPL. In our experiment, both the attended and the unattended stimuli were absolutely independent of the task, while in their study the stimuli at the attended location were also employed in the task that subjects had to conduct during exposure sessions. Although their task during exposure sessions was not identical to the one during test sessions, the stimuli at the attended location were not objects that had to be inhibited for better performances. Another crucial difference between their experiment and ours was that while their irrelevant stimuli were both suprathreshold, ours were weaker and closer to the threshold. One more difference was that while their study used the same feature (identical orientations) for attended and unattended stimuli presented in different locations, our study used different features (different orientations). Thus, it is possible that their learning is a result of feature-based attention that activates not only neurons tuned to a feature at the attended location but also neurons tuned to the same feature at different locations (see Treue & Trujillo, 1999).
While our study focused on attentional inhibitory effects on TIPL, we noticed an unexpected, but significant, performance improvement for the control direction. Because the control direction had not been exposed during training, this improvement could be interpreted as a testing effect that occurs as subjects became accustomed to the experimental setting and stimuli through repeated testing procedures. However, we cannot rule out the possibility that some aspects of this improvement can be from interactions with the facilitating or inhibiting effects on other directions. In a previous study employing the coherent motion display as a main stimulus, PL occurred for a never-exposed motion direction 45° away from the exposed, and thus learned, motion direction (Watanabe et al., 2001). Since this current experiment had three directions employed at separations greater than 45° (120°), it was assumed that there would not be such an interaction. However, transfer of learning across large angular separations had been found in other studies of perceptual learning for visual motion stimuli (Liu, 1999).
We clearly instructed subjects to fixate on the fixation point, but did not monitor eye movements. In the current study, in order to evoke involuntary orienting, arrows had been presented in the center of the screen for 400ms, which was long enough to allow for possible eye movement. This could thus lead to an alternative explanation that eye movements influenced the observed results. However, if this explanation was correct, the results of our experiment should have been opposite to what was found. If, as the alternative explanation might suggest, subjects turned their eyes on the attended DRD during training sessions, the attended DRD would be closer to the fovea and the unattended DRD would be further away from the fovea. In addition, the same tendency would occur in the pre- and post-tests; subjects would move their eyes towards the given DRD. In this case, then, more improvement should be found in the attended direction since PL is very specific to the stimulus position in the visual field (e.g. Beradi & Fiorentini, 1987). Our results, therefore, seem to be independent from the influence of eye movement. However, because of significant ties between eye movement and PL, it is necessary to monitor eye movements with an eye tracker in future studies.
Conclusion
The involvement of attention in PL has long been a controversial topic. The current study has more directly demonstrated the role of attention in TIPL compared to previous studies. Attention may be involved in both facilitating task-relevant signals and inhibiting task-irrelevant signals to form PL, and the failure of attentional inhibition may allow for TIPL.
Acknowledgments
This work was supported by NIH (R01EY019466 & R01EY015980-04A2) and NSF (BCS-0549036).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Ahissar M, Hochstein S. Attentional control of early perceptual learning. The Proceedings of the National Academy of Sciences U.S.A. 1993;90:5718–5722. doi: 10.1073/pnas.90.12.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardi N, Fiorentini A. Interhemispheric transfer of visual information in humans: spatial characteristics. J Physiol. 1987;384:633–647. doi: 10.1113/jphysiol.1987.sp016474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997;10(4):433–436. [PubMed] [Google Scholar]
- Gutnisky DA, Hansen BJ, Iliescu BF, Dragoi V. Attention alters visual plasticity during exposure-based learning. Curr Biol. 2009;19(7):555–560. doi: 10.1016/j.cub.2009.01.063. [DOI] [PubMed] [Google Scholar]
- Knight RT, Staines WR, Swick D, Chao LL. Prefrontal cortex regulates inhibition and excitation in distributed neural networks. Acta Psychol (Amst) 1999;101(23):159–178. doi: 10.1016/s0001-6918(99)00004-9. [DOI] [PubMed] [Google Scholar]
- Lavie N, Tsal Y. Perceptual load as a major determinant of the locus of selection in visual attention. Percept Psychophys. 1994;56(2):183–197. doi: 10.3758/bf03213897. [DOI] [PubMed] [Google Scholar]
- Liu Z. Perceptual learning in motion discrimination that generalizes across motion directions. Proc Natl Acad Sci U.S.A. 1999;96(24):14085–14087. doi: 10.1073/pnas.96.24.14085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome WT, Pare EB. A selective impairment of motion perception following lesions of the middle temporal visual area (MT) J Neurosci. 1988;8(6):2201–2211. doi: 10.1523/JNEUROSCI.08-06-02201.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paffen CL, Verstraten FA, Vidnyanszky Z. Attention-based perceptual learning increases binocular rivalry suppression of irrelevant visual features. J Vis. 2008;8(4):25, 1–11. doi: 10.1167/8.4.25. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis. 1997;10(4):437–442. [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Schoups A, Vogels R, Qian N, Orban G. Practising orientation identification improves orientation coding in V1 neurons. Nature. 2001;412(6846):549–553. doi: 10.1038/35087601. [DOI] [PubMed] [Google Scholar]
- Seitz A, Lefebvre C, Watanabe T, Jolicoeur P. Requirement for high-level processing in subliminal learning. Curr Biol. 2005;15(18):R753–755. doi: 10.1016/j.cub.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Seitz A, Watanabe T. Psychophysics: Is subliminal learning really passive? Nature. 2003;422:36. doi: 10.1038/422036a. [DOI] [PubMed] [Google Scholar]
- Seitz A, Watanabe T. A unified model for perceptual learning. Trends Cogn Sci. 2005;9(7):329–334. doi: 10.1016/j.tics.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Shiu LP, Pashler H. Improvement in line orientation discrimination is retinally local but dependent on cognitive set. Percept Psychophys. 1992;52(5):582–588. doi: 10.3758/bf03206720. [DOI] [PubMed] [Google Scholar]
- Tipper SP. The negative priming effect: inhibitory priming by ignored objects. Q J Exp Psychol A. 1985;37(4):571–590. doi: 10.1080/14640748508400920. [DOI] [PubMed] [Google Scholar]
- Tipples J. Eye gaze is not unique: automatic orienting in response to uninformative arrows. Psychon Bull Rev. 2002;9(2):314–318. doi: 10.3758/bf03196287. [DOI] [PubMed] [Google Scholar]
- Treue S, Martinez Trujillo JC. Feature-based attention influences motion processing gain in macaque visual cortex. Nature. 1999;399(6736):575–579. doi: 10.1038/21176. [DOI] [PubMed] [Google Scholar]
- Tsushima Y, Sasaki Y, Watanabe T. Greater disruption due to failure of inhibitory control on an ambiguous distractor. Science. 2006;314(5806):1786–1788. doi: 10.1126/science.1133197. [DOI] [PubMed] [Google Scholar]
- Tsushima Y, Seitz AR, Watanabe T. Task-irrelevant learning occurs only when the irrelevant feature is weak. Curr Biol. 2008;18(12):R516–517. doi: 10.1016/j.cub.2008.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward R, Duncan J, Shapiro K. The Slow Time-Course of Visual Attention. Cognit Psychol. 1996;30(1):79–109. doi: 10.1006/cogp.1996.0003. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Náñez JE, Sasaki Y. Perceptual learning without perception. Nature. 2001;413:844–848. doi: 10.1038/35101601. [DOI] [PubMed] [Google Scholar]