Abstract
One of the most consistent findings in schizophrenia is the decreased expression of the GABA synthesizing enzymes GAD67 and GAD65 in specific interneuron populations. This dysfunction is observed in distributed brain regions including the prefrontal cortex, hippocampus, and cerebellum. In an effort to understand the mechanisms for this GABA deficit, we investigated the effect of the N-methyl-D-aspartate receptor (NMDAR) antagonist phencyclidine (PCP), which elicits schizophrenia-like symptoms in both humans and animal models, in a chronic, low-dose exposure paradigm. Adult rats were given PCP at a dose of 2.58 mg/kg/day i.p. for a month, after which levels of various GABAergic cell mRNAs and other neuromodulators were examined in the cerebellum by RT-qPCR. Administration of PCP decreased the expression of GAD67, GAD65, and the presynaptic GABA transporter GAT-1, and increased GABAA receptor subunits similar to those seen in patients with schizophrenia. Additionally, we found that the mRNA levels of two Golgi cell selective NMDAR subunits, NR2B and NR2D, were decreased in PCP treated rats. Furthermore, we localized the deficits in GAD67 expression solely to these interneurons. Slice electrophysiological studies showed that spontaneous firing of Golgi cells was reduced by acute exposure to low dose PCP, suggesting that these neurons are particularly vulnerable to NMDA receptor antagonism. In conclusion, our results demonstrate that chronic exposure to low levels of PCP in rats mimics the GABAergic alterations reported in the cerebellum of patients with schizophrenia (Bullock et al., Am J Psychiatry 165: 1594-1603, 2008), further supporting the validity of this animal model.
Keywords: phencyclidine, gene expression, cerebellar Golgi cells, GABA, animal model, schizophrenia
Schizophrenia is a chronic and severely debilitating neuropsychiatric disease affecting approximately 1% of the world’s population. Since schizophrenia is a purely human disease, no animal model can fully reproduce the complex pathophysiological mechanisms underlying the spectrum of symptoms in patients. Animal models have, however, proven useful to understand specific aspects of the illness. For example, non-competitive N-methyl-D-aspartate (NMDA) receptor antagonism by compounds such as MK-801 or phencyclidine (PCP) are known to elicit in humans and animal models many of the positive, negative, and cognitive symptoms seen in schizophrenic patients (Javitt and Zukin, 1991, Krystal et al., 1994, Morris et al., 2005). Furthermore, administration of PCP in rodents reproduces many of the molecular changes seen in human post-mortem tissue analysis (Cochran et al., 2003, Lindahl and Keifer, 2004), including alterations in NMDA receptor levels (Akbarian et al., 1996) and deficits in subtypes of markers of γ-aminobutyric acid (GABA) expressing interneurons (Akbarian et al., 1995b, Lewis et al., 2001, Lewis et al., 2005).
It has been proposed that NMDA receptor antagonists produce schizophrenia-like symptoms by selectively blocking NMDA channels located on GABAergic interneurons (Grunze et al., 1996, Rujescu et al., 2006, Homayoun and Moghaddam, 2007). Deficits in specific subpopulations of these cells are some of the most consistent findings reported in post-mortem tissue of patients with schizophrenia (Akbarian and Huang, 2006). Specifically, decreases in the levels of the major isoform of the GABA synthesizing enzyme glutamic acid decarboxylase 67kDa (GAD67) and the presynaptic GABA reuptake transporter GAT-1 have been reported in the prefrontal cortex (PFC) and other brain regions, including the hippocampus and cerebellum (Akbarian et al., 1995a, Guidotti et al., 2000, Heckers et al., 2002, Fatemi et al., 2005, Lewis et al., 2005, Bullock et al., 2008). Considering that GABAergic interneurons modulate excitatory output, deficient activity of specific interneuron populations may account for some of the positive, negative, and cognitive symptoms seen in schizophrenia.
Chronic low dose administration of PCP in rodents has been shown to decrease metabolic activity in the prefrontal cortex, auditory cortex, hippocampus, and reticular nucleus of the thalamus (Cochran et al., 2003), all regions affected in schizophrenia. Along with this decrease in metabolic function, decreases in parvalbumin expression were also seen (Cochran et al., 2003), mirroring the deficits seen in the prefrontal cortex of patients with schizophrenia (Lewis et al., 2001). Additionally, levels of n-acetylaspartate (NAA) and its metabolite n-acetylaspartyl glutamate (NAAG) were also altered by chronic PCP treatment similarly to that seen in humans (Reynolds et al., 2005). Moreover, these animals show disrupted paired-pulse inhibition (PPI) (Egerton et al., 2008), behavioral impairments in working memory (Jentsch et al., 1997a, Jentsch et al., 1997b, Egerton et al., 2008), and social interaction (Sams-Dodd, 1997, Tanaka et al., 2003). Thus, animal models utilizing NMDA receptor blockade through PCP, ketamine, or MK801 may be an effective tool to study some the pathophysiological mechanisms underlying schizophrenia (Krystal et al., 1994, Olney and Farber, 1995, Morris et al., 2005, Kondziella et al., 2007, Mouri et al., 2007).
PCP studies in rodents have focused mainly on regions of the brain traditionally associated with schizophrenia, such as the PFC and limbic regions. However, increasing numbers of reports suggest that the cerebellum is involved in this disease (Andreasen and Pierson, 2008). Besides its role in motor coordination in humans, the cerebellum, through connections to the PFC, is known to contribute to “higher” cognitive function (Schmahmann and Sherman, 1998, Ramnani, 2006). Such function suggests that deficits in cerebellar interneuron activity may contribute to the disease process. Supporting this idea, we have recently found that expression of key GABA neurotransmission components, including synthetic enzymes GAD67 and GAD65 are decreased in lateral cerebellar hemisphere from patients (Bullock et al., 2008). Given that NMDA antagonist administration in rodents replicates some of the molecular and behavioral findings in schizophrenia presumably by inhibiting GABA transmission, here we investigated whether the levels of specific GABAergic markers, NMDA receptor subunits, and neuromodulators were affected in rats chronically exposed to low-dose PCP. Additionally, we sought to identify the specific type(s) of GABAergic interneurons in which GAD67 expression was deficient and electrophysiologically characterized the effect of PCP on spontaneous Golgi cell firing. Our findings indicate that Golgi cells, the interneurons that modulate tonic and phasic activity of granule cells, are particularly vulnerable to the effects of low doses of PCP and suggest that this cell type may contribute to dysfunctional cerebellar output to the prefrontal cortex, contributing to alterations in animal behavior.
EXPERIMENTAL PROCEDURES
PCP Treated Rats
All animal procedures were performed in accordance to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and approved by the University of New Mexico Institutional Animal Care and Use Committee (IACUC). Pair-housed adult male Long-Evans rats (n=20) were injected intraperitoneally with 2.58 mg/kg/day of either PCP dissolved in saline or with saline alone as described by Cochran et al., 2003. Briefly, after an acclimation period of 7 days the rats were injected once a day for the first five days, then injected on days 8, 10, 12, 15, 17, 19, 22, 24, and 26. On day 29, rats were anesthetized using isoflurane and sacrificed. For reverse-transcription quantitative real time PCR (RT-qPCR) experiments, the lateral hemisphere of the cerebellum was removed, frozen on dry ice and stored at −80°C.
For in situ hybridization and immunohistochemistry studies, a separate group of rats (n=10) were injected as described above. The cerebellum was removed and flash frozen in isopentane that was cooled at −40°C using methanol/dry ice. Samples were stored at −80°C. Frozen rat cerebellar tissue was cut to 10 μm thick sections on a cryostat and placed on a glass slide. Each slide had two coronal sections, one from a PCP-treated rat and one from its pair-housed saline control rat. Slides were stored at −80°C until the time of use.
qRT-PCR
qRT-PCR was performed as previously described (Bullock et al., 2008). Briefly, rat cerebellar tissue was homogenized using a Polytron homogenizer (Brinkmann Instruments, Inc.; Westbury, NY) and total RNA was isolated using TriReagent (Sigma; St. Louis, MO). The integrity of samples was validated using a Bioanalyzer 2100 (Agilent Technologies; Santa Clara, CA) and used only if the RNA integrity number (RIN) was >8.0. cDNA was synthesized using M-MLV reverse transcriptase (Promega; Madison, WI). qRT-PCR reactions were run on an Applied Biosystems 7300 or 7500 Fast qRT-PCR machine. Gene expression levels in all samples were examined using SYBR® Green (Applied Biosystems; Foster City, CA) with the exception of GABAA receptor subunits α6 and δ, which were analyzed using TaqMan® Assays-on-Demand probes (Applied Biosystems), all according to the manufacturer’s protocols. Exon spanning primer pairs (Operon; Huntsville, AL) specific to GABAergic markers GABAA-β3, GAD67, GAD65, and GAT-1; NMDA receptor subunits NR1, NR2A, NR2B, NR2C, and NR2D; kainate receptor subunits GluR6 and KA2; the metabotropic glutamate receptors mGluR2, mGluR3; and neuronal nitric oxide synthase (nNOS) were designed with Primer Express 3.0 (Applied Biosystems) (Supplemental Table 1). Dissociation curves of all SYBR Green primer pairs revealed no evidence of dimerization. All primer pairs for genes of interests were validated against β-actin and found to be within optimal amplification values (validation curve slopes <|0.1|). mRNA levels were normalized to β-actin because previous studies (Bullock et al., 2008) demonstrated that the expression of this housekeeping gene did not change under several experimental conditions. Samples were run in triplicate in three separate plates and compared to β-actin on the same plate. After correcting the levels of expression of each mRNA by β-actin, the relative levels of transcripts in PCP vs. saline treated rats were calculated using the 2-ΔΔCt method (Livak and Schmittgen, 2001).
Quantitative In Situ Hybridization (qISH) and Immunohistochemistry (IHC)
GAD67 cRNA sense and antisense transcripts with incorporated 35S-UTP were synthesized from cloned plasmids generously provided by Dr. Niranjala Tillakaratne (Department of Physiological Science, UCLA). Riboprobes were transcribed using T3 RNA polymerase (Promega; Madison, WI), purified using RNeasy (Qiagen; Valencia, CA) and kept at −80°C until slides were treated.
ISH was performed according to methods developed by Simmons, et al. (1989) as described by Bolognani, et al. (2006). Briefly, slides were fixed for 20 min in 4% paraformaldehyde prior to hybridization. Slides were incubated with 1.5 × 106 cpm of 35S-UTP labeled antisense probes in a volume of 75 μL hybridization buffer at 55°C for 16 hours. After hybridization, slides were treated with RNase A and then washed in decreasing SSC (20X stock solution: 0.3M sodium citrate, pH 7.0, 3M NaCl) concentrations. Slides were then exposed to film for 24 hours to monitor adequate hybridization and to determine proper exposure time to emulsion.
Following ISH, slides were preincubated for 1 hour in 10% normal horse serum (NHS) in TBS (50 mM Tris Base, pH 7.4, 0.9% NaCl), then incubated with mGluR2 monoclonal antibodies (AbCam; Cambridge, MA) at a 1:750 dilution in 1% NHS in TBS for 16 hours at 4°C. Slides were washed and then incubated for 2 hours with biotinylated horse-anti-mouse antibodies (Vector Labs; Burlingame, CA) at a 1:400 dilution in 1% NHS in TBS at room temperature. After another wash, the slides were treated with Vector ABC Elite kit (Vector Labs; Burlingame, CA) according to manufacturer’s directions and developed using DAB as the chromogen.
Following ISH and IHC, slides were dipped in Kodak NTB2 emulsion (Kodak; Rochester, NY) and exposed for 6 days at 4°C. Slides were then developed using Kodak D-19 developer and fixer.
ISH Data Analysis
After development of emulsion slides half of the slides were counterstained using hematoxylin and eosin. Slides were photographed using an Olympus DP71 camera (Olympus America Inc.; Center Valley, PA) attached to an Olympus BX60 microscope. To count the number of cells per area, images were acquired using either a 10X objective (for Golgi cell and Purkinje cell measurements) or at 20X (for basket/stellate cell measurements). Images were acquired using a 60X objective to measure the number of grains per cell.
All images were analyzed using ImagePro® Plus 4.0 (Media Cybernetics; Bethesda, MD). Cell number per area was determined by manually defining the region of interest and counting the cells within that region. Area covered by grains per cell was determined by creating a circle with a predetermined diameter, placing the circle over the cell of interest, and quantitating the area covered by grains within the area of the circle. Diameters were set at 13 μm for basket/stellate cells, 25 μm for Golgi cells, and 30 μm for Purkinje cells. All images were analyzed blind by at least two different observers and measurements were averaged per slide and per condition.
Statistical Analysis of Expression Data
Results from qRT-PCR and qISH experiments were averaged independently and entered into Prism 4.0 (GraphPad Software; San Diego, CA) and analyzed using t-tests with a p<0.05 considered significant. All values were expressed as a ratio of PCP/Saline (P/S).
Brain Slice Preparations
Parasagittal vermis cerebellar slices were prepared from 3 different male Sprague-Dawley rats (23-24 day-old; Harlan, Indianapolis, IN). Briefly, animals were euthanized by rapid decapitation under deep anesthesia with ketamine (250 mg/kg I.P.) and 200 μm thick slices were prepared with a vibratome (Technical Products International, St. Louis, MO). Slices were cut in cold solution containing (in mM) 220 sucrose, 26 NaHCO3, 10 glucose, 6 MgSO4, 2 KCl, 1.25 NaH2PO4, 0.2 CaCl2 and 0.43 ketamine; this solution was pre-equilibrated with 95% O2 plus 5% CO2. Immediately after this procedure, slices were transferred to a chamber containing artificial cerebrospinal fluid (ACSF) and allowed to recover at 35-36°C for 35 min, followed by storage at room temperature. ACSF contained (in mM): 126 NaCl, 2 KCl, 1.25 NaH2PO4, 1 MgSO4, 26 NaHCO3, 2 CaCl2, and 10 glucose equilibrated with 95% O2 plus 5% CO2. After storage for 1-8 hrs, slices were transferred to a recording chamber perfused with ACSF at a rate of 2-3 ml/min and maintained at 32-33°C.
Loose-patch cell-attached electrophysiological recordings of Golgi cell firing
Neurons were visualized using infrared-differential interference contrast microscopy and recordings performed with a Multiclamp 700B amplifier (Molecular Devices, Sunnyvale, CA). Golgi cells were primarily identified on the basis of their location in the granule cell layer, larger size when compared to granule cells, and the presence of spontaneous action potential firing. In all cases, each slice was exposed once to a single PCP concentration and the duration of PCP exposure was limited to 10 min. The loose-patch cell-attached configuration (seal resistance = 8–30 MΩ) was action currents. The patch pipettes (tip resistance = 2-5 MΩ) were h ACSF filled and the holding potential was 0 mV; it should be noted that the holding potential in loose-cell attached experiments is unlikely to significantly affect the Golgi cell resting membrane potential because most of the current generated by the amplifier will leak across the loose seal rather than passing through the patch.
Electrophysiology Data Analyses
Data were filtered at 2 kHz and digitized at 5-50 kHz with 1322A pClamp-9 (Molecular Devices, Sunnyvale, CA) and analyzed with Clampfit-9 (Molecular Devices) and MiniAnalysis-6.0.3. (Synaptosoft, Decatur, GA). Data were statistically analyzed with Prism 4 (GraphPad, San Diego, CA) and are presented as mean ± S.E.M.
RESULTS
GABAergic Marker Expression in the Cerebellum of PCP-treated Rats
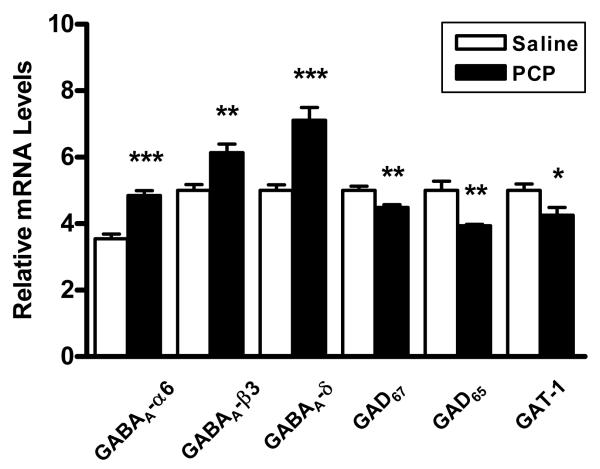
Given the evidence that PCP elicits many of the behaviors seen in schizophrenia (Javitt and Zukin, 1991, Jentsch and Roth, 1999, Morris et al., 2005), we characterized gene expression changes in GABAergic interneurons from the cerebella of rats chronically treated with relatively low levels of this NMDA receptor antagonist. Expression of six markers of GABA function in the lateral cerebellar hemisphere of PCP treated rats and paired saline control rats were initially examined using qRT-PCR. We found that mRNAs levels of presynaptic GABA transmission markers GAD67, GAD65 and GAT-1 were significantly decreased in PCP rats versus controls (Figure 1, Table 1). In contrast, the GABAA receptor subunits α6, β3, and δ were significantly increased in the PCP rats. The overall GABAergic expression profile in the cerebellum was therefore consistent with deficient release of this neurotransmitter and a compensatory increase in extrasynaptic GABAA receptor expression.
Figure 1.
Expression of GABAergic markers in the cerebellum of PCP treated rats versus saline controls. Postsynaptic GABA receptors are significantly increased in PCP rats while GABA synthesizing enzymes and the GAT-1 reuptake transporter are significantly decreased. Expression levels normalized to β-actin. *p<0.05, **p<0.01, ***p<0.001.
TABLE 1.
Summary of Gene Expression Changes in PCP Treated Rats
| GABAergic Markers |
NMDA Receptor Subunits |
Neuromodulators |
||||||
|---|---|---|---|---|---|---|---|---|
| Gene of Interest |
P/S Ratio | p value | Gene of Interest |
P/S Ratio | p value | Gene of Interest |
P/S Ratio | p value |
| GABAA-α6 | 1.55*** | 0.0001 | NR1 | 0.95 | 0.1324 | GluR6 | 0.84** | 0.0014 |
| GABAA-β3 | 1.28** | 0.0024 | NR2A | 1.00 | 0.0952 | KA2 | 1.39*** | <0.0001 |
| GABAA-δ | 1.55*** | 0.0002 | NR2B | 0.78** | 0.0049 | mGluR2 | 1.37 | 0.0610 |
| GAD65 | 0.82** | 0.0023 | NR2C | 1.09 | 0.6822 | mGluR3 | 0.94 | 0.3612 |
| GAD67 | 0.89** | 0.0060 | NR2D | 0.74** | 0.0076 | nNOS | 1.15 | 0.1862 |
| GAT-1 | 0.83* | 0.0242 | ||||||
Decreases in mRNA levels of GAD65 and GAD67 are seen in the cerebellum of PCP treated rats versus saline controls as shown by PCP/Saline expression ratios. All other gene changes are involved in GABAergic transmission between Golgi cells and granule cells. Differences in expression determined by t test
p<0.05
p<0.01
p<0.0001
N= 10 animals per group.
NMDA Receptor Subunit Expression in the Cerebellum of PCP-treated Rats
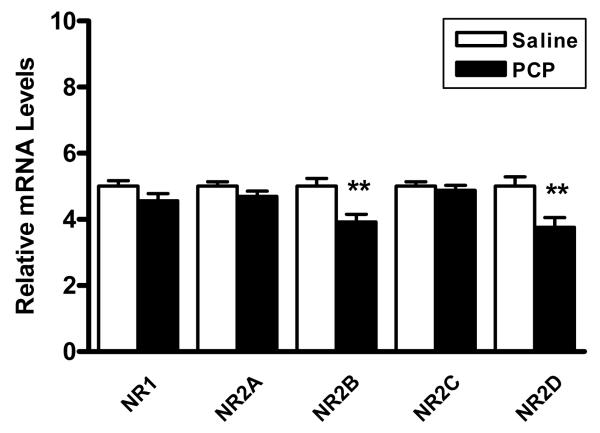
PCP acts as an open channel NMDA receptor antagonist and preferentially antagonizes receptors on GABAergic interneurons (Grunze et al., 1996, Rujescu et al., 2006, Homayoun and Moghaddam, 2007). To determine the effects of PCP on NMDA receptor expression in the cerebellum, we evaluated expression levels of NMDA receptor subunits. We found that the mRNA levels of two subunits, NR2B and NR2D were decreased by the treatment. Interestingly, NR2B and NR2D are colocalized to Golgi cells (Brickley et al., 2003), suggesting that these cells may be preferentially affected by PCP. In contrast, the mRNA levels of the obligatory NR1 subunit, or the NR2A, and NR2C subunits were not significantly altered (Figure 2, Table 1).
Figure 2.
Levels of NMDA receptor subunit mRNAs in the cerebellum of PCP treated rats versus saline controls. NR2B and NR2D are significantly decreased in PCP treated rats versus saline controls. Expression levels normalized to β-actin. **p<0.01.
Neuromodulator Expression in the Cerebellum of PCP-treated Rats
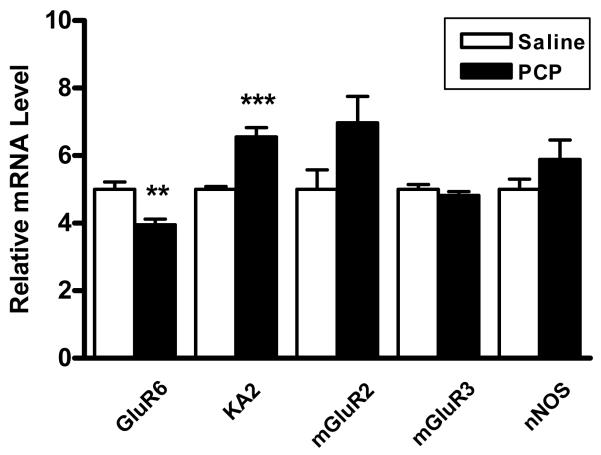
The changes in expression of GABAergic markers and NMDA receptor subunits seen in our samples are consistent with deficits in Golgi-granule cell communication. To confirm this idea, subsequent studies examined the expression levels of major modulatory components involved in GABAergic transmission to granule cells. We observed a significant decrease in the mRNA for kainate receptor subunit GluR6, which is present in both Golgi and granule cells (Porter et al., 1997, Bureau et al., 2000), and a contrasting increase in mRNA for the granule cell specific KA2 subunit (Porter et al., 1997, Pemberton et al., 1998). We also found a trend for an increased expression (p=0.061) in the metabotropic glutamate receptor mGluR2, which is present presynaptically in Golgi cells (Berthele et al., 1999, Mitchell and Silver, 2000, Watanabe and Nakanishi, 2003), and no change in mGluR3, which is mainly glial (Berthele et al., 1999). Additionally, nNOS levels were also unchanged (Figure 3, Table 1).
Figure 3.
Expression levels of cerebellar neuromodulators in PCP treated rats versus saline controls. Granule and Golgi cell kainate receptor subunit GluR6 is significantly decreased in PCP treated rats while granule cell specific KA2 is significantly increased. Expression levels normalized to β-actin. **p<0.01, ***p<0.005.
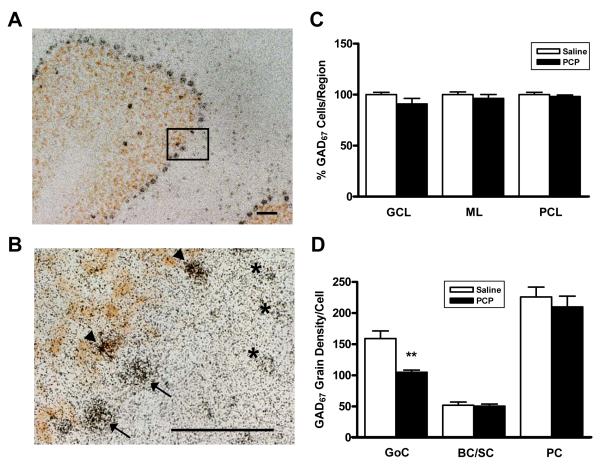
GAD67 Expression Decreased in Golgi Cells of the Cerebellum
Since many of the alterations seen in cerebellar transcript levels implicate deficient Golgi cell to granule cell neurotransmission, we performed quantitative in situ hybridization to localize the changes in GAD67 expression to a particular cell type. Analysis of Golgi cells dually stained with GAD67 riboprobe and mGluR2 antibody, a Golgi cell specific marker (Figure 4 A,B), showed no changes in Golgi cell numbers in PCP-treated rats. Also, we did not find any alterations in the numbers of basket/stellate cells of the molecular layer or in Purkinje cells (Figure 4C). In contrast, analysis of the area covered by grains per cell type showed that PCP-treated rats expressed significantly less GAD67 in Golgi cells (P/S=0.66; p=0.0058), but remained unchanged in basket/stellate cells (P/S=0.97; p=0.8319) and in Purkinje cells (P/S=0.93; p=0.5191) (Figure 4D).
Figure 4.
Determination of GAD67 levels in cerebellar interneurons by quantitative in situ hybridization (ISH). A: 10X magnification of a cerebellar folium showing GAD67 ISH (black grains) and mGluR2 IHC (brown) to stain Golgi cells. Golgi cells are visualized in the granule cell layer, Purkinje cells in the Purkinje cell layer, and basket and stellate cells in the molecular layer. Bar represents 50 μm. B: Higher magnification representing inset in panel A. Distinction can be made between Golgi cells (arrowheads) and Purkinje cells (arrows). Basket and stellate cells are also shown (asterisks). Bar also represents 50 μm. C: Percentage of GAD67 expressing cells in the granule cell layer (GCL), molecular layer (ML), and Purkinje cell layer (PCL) of the cerebellum. All values normalized to those of saline-treated animals. D: Density of GAD67 silver grains in cerebellar Golgi (GoC) cells, basket and stellate (BC/SC) cells, and Purkinje (PC) cells. Results were analyzed by a one way ANOVA followed by post-hoc unpaired t-tests between PCP and saline values for each of the 3 regions examined. **p<0.01.
Low dose PCP decreases the firing frequency of cerebellar Golgi neurons
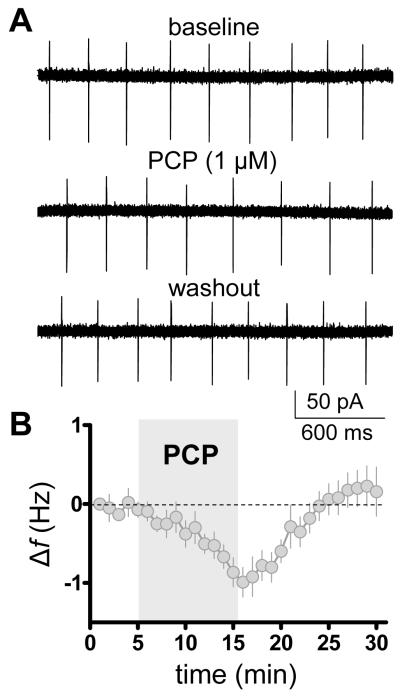
These results described above point to Golgi cells as the interneuron primarily affected by chronic intermittent exposure to low dose PCP. In order to assess if PCP had a direct effect on Golgi cell pacemaker activity, cerebellar slices were prepared from juvenile rats and treated with 1 μM PCP for 10 min. This dose was used because, based upon previous work (Kalinichev et al., 2008), it is near the brain concentrations that are achieved after an acute i.p. injection of 2.58 mg/kg. As shown in Figure 5, PCP (1 μM) reversibly decreases spontaneous firing of Golgi cells. On average, PCP decreased firing frequency by 0.8± 0.1 Hz (p < 0.001 by one-sample t-test vs. zero, n = 8). Baseline firing frequency was 4.1 ± 0.7 Hz. The percent frequency change from baseline was −21.85 ± 3.8 % (p < 0.0001 by one-sample t-test vs. zero, n = 8).
Figure 5.
PCP decreases the spontaneous action potential firing frequency (f) of cerebellar Golgi cells. A: Representative traces of loose-patched cell-attached recordings obtained in absence and presence of PCP (1 μM). B: Time course of the effect of PCP. Firing f was normalized with respect to frequency obtained at time point 0 (bin size, 1 min; n = 8).
DISCUSSION
An increasing number of studies have characterized GABAergic gene expression deficits in patients with schizophrenia (Lewis et al., 2005, Akbarian and Huang, 2006), and some of these deficits have been reproduced in an animal model of chronic low dose PCP exposure (Cochran et al., 2003). In the current study, we demonstrated a decreased expression of markers of GABA mediated neurotransmission in the cerebellum of this animal model that mimic the alterations found in the same markers in patients with schizophrenia (Bullock et al., 2008). Additionally, we localized the decrease in GAD67 expression to a specific subtype of GABAergic interneuron, the Golgi cell, and demonstrated a direct effect of PCP on the intrinsic activity of these interneurons. Since Golgi cells are responsible for modulating excitatory granule cell firing through negative feedforward and feedback mechanisms, decreased Golgi cell activity may disinhibit granule cells, ultimately leading to disrupted cerebellar output to other brain regions, such as the PFC. Indeed, it was recently demonstrated that Golgi cells are extensively connected via electrical synapses that drive low frequency oscillatory synchronization and rhythmic inhibition of granule cells and computer modeling suggests that Golgi cells can lock efficiently onto rhythmic input patterns that may be involved in cortico-cerebellar coherence (Dugue et al., 2009).
Golgi cells provide GABAergic input to granule cells making them a high signal-to-noise filtering unit. This GABAergic input provides both tonic and phasic inhibition (De Schutter et al., 2000, Rossi et al., 2003). Tonic inhibition originates from activation of extrasynaptic high-affinity GABAA receptors by low levels of GABA derived from spillover of synaptically released transmitter as well as by ambient GABA levels (Hamann et al., 2002, Geurts et al., 2003, Fritschy and Panzanelli, 2006). Extrasynaptic cerebellar GABAA receptors contain α6βxδ subunits, which are potently activated by GABA and display relatively little desensitization. In vivo and in vitro studies have shown that Golgi cells are pacemaker neurons that tonically fire action potentials and this generates the spillover component of the tonic current (Brickley et al., 1996, Carta et al., 2004, Forti et al., 2006). These processes occur in a unit called the cerebellar glomerulus where Golgi cell and mossy fiber terminals synapse onto granule cell dendrites (De Schutter et al., 2000). Here, extracellular GABA concentrations are regulated by the neuronal presynaptic transporter GAT-1, which also effectively ends phasic GABA transmission (Morara et al., 1996). Our findings of decreased levels of GAD67 and GAD65 expression (Figure 1, Table 1, Figure 4), along with decreases in spontaneous Golgi cell firing in the presence of low dose of PCP (Figure 5), point to aberrant GABAergic neurotransmission between Golgi cells and granule cells. Furthermore, the observed decreases in GAT-1 expression and increased levels of GABAA receptor subunits α6, β3, and δ (Figure 1, Table 1) indicate an attempt to compensate for decreased GABA neurotransmission. Insufficient GABA tone at this synapse is known to decrease the threshold of activation for granule cells, allowing for increased granule cell activity (Chadderton et al., 2004) and increased expression of activity dependent proteins, such as GABAA-δ (Salonen et al., 2006) and the granule cell specific kainate receptor subunit KA2 (Feligioni et al., 2006).
Regarding phasic inhibition, Golgi cells receive excitatory input from granule cell parallel fibers and mossy fibers, which ultimately results in feedback and feedforward inhibition of granule cells, respectively (Dieudonne, 1998, De Schutter et al., 2000, Kanichay and Silver, 2008). Release of glutamate triggered by high frequency stimulation of mossy fibers excites granule cells while inhibiting GABA release from Golgi cells through presynaptic binding of mGluR2/3 receptors (Geurts et al., 2003). Dendritic mGluR2 activation by high-frequency glutamate release from parallel fibers to Golgi neurons also transiently inhibits downstream GABA release (Watanabe and Nakanishi, 2003). Further inhibition of GABA neurotransmission takes place when granule cells release nitric oxide (NO), synthesized by the activity dependent nNOS (Wall, 2003), decreasing GABA release from Golgi cells in a retrograde manner. Hence, simultaneous granule cell activation by glutamate and/or inhibition of Golgi cell GABA release increases granule cell activity (Chadderton et al., 2004).
While patients with schizophrenia showed apparent compensatory decreases in mGluR2 and nNOS (Bullock et al., 2008), the PCP rat model failed to replicate these deficits. This could be, in part, a consequence of increased NO production due to PCP administration (Wiley, 1998, Wass et al., 2006). However, decreased levels of GluR6 (Figure 3, Table 1), a Golgi and granule cell selective kainate receptor forming functional homomeric channels (Bureau et al., 2000) and modulating GABA release presynaptically (Mathew et al., 2008), suggest alternative methods of compensation for decreased GABA release and further implicate Golgi cells as dysfunctional.
The sensitivity of Golgi cells to parallel fiber input is low (Dieudonne, 1998), suggesting that parallel fibers are unlikely to provide the main excitatory drive of Golgi cells in vivo and that Golgi cell’s main function may be to provide tonic rather than phasic inhibition of granule cells (Rossi et al., 2003). When glutamate is released onto Golgi cells, it acts in part by activating NMDA receptors. Golgi cells express NR1/NR2B subunit containing receptors at synaptic sites (Misra et al., 2000). However, at extrasynaptic sites, there is evidence that NMDARs are composed of NR1/NR2D or heterotetramers containing NR1 and both NR2B and NR2D (Brickley et al., 2003). We found decreased expression of both NR2B and NR2D subunits (Figure 2, Table 1), suggesting preferential antagonism of Golgi cell NMDA receptors. In agreement with our observations, Lindahl and Keifer (2004) also found decreases in NR2B protein levels in the cerebellar cortices of rats chronically treated with a higher dose (10 mg/kg/day) of PCP for one month. Furthermore, these results are consistent with previous reports indicating that GABAergic interneurons in the hippocampus and PFC are selectively affected by PCP administration (Grunze et al., 1996, Homayoun and Moghaddam, 2007). Molecular layer basket and stellate interneurons also express NR2D (Berthele et al., 1999), but do not show deficits in GAD67 expression (Figure 4D) and thus, are not likely affected by low dose PCP administration.
Recently, it was demonstrated that Mg2+ regulates the sensitivity of NMDA receptors to channels blockers such as memantine and ketamine (Leveille et al., 2008, Kotermanski and Johnson, 2009). Inhibition of NMDARs containing NR1/2B subunits and NR1/2D subunits was decreased ~20-fold and ~3-fold by Mg2+, respectively (Kotermanski and Johnson, 2009). These findings suggest that under physiological concentrations of Mg2+ (i.e. 1 mM, which is the concentration used in our electrophysiological studies), the main targets of channel blockers would be the NR2D containing receptors. Therefore, it is likely that PCP decreased Golgi cell firing by blocking extrasynaptic NMDARs. Future studies should assess whether these extrasynaptic NMDARs are tonically activated in Golgi cells, as it has been demonstrated in other neuronal populations (Sah et al., 1989, Le Meur et al., 2007).
Granule cells ultimately synapse on Purkinje cells, the sole source of output from the cerebellar cortex. Purkinje cells are GABAergic projection neurons that integrate multiple inputs from parallel fibers, basket/stellate interneurons, and climbing fibers, and send their output signal to deep cerebellar nuclei (Sastry et al., 1997). Our results suggest Purkinje cells are not directly affected by PCP administration, as seen by the unchanged levels of Purkinje cell selective NR2A (Figure 2, Table 1) and GAD67 (Figure 4D), and the decreased expression of GAT-1 (Figure 1, Table 1), which is not expressed in Purkinje cells (Takayama and Inoue, 2005). However, Purkinje cell output may be indirectly affected by PCP due to increased granule cell activity resulting from deficient Golgi cell firing. These deficits may desynchronize Purkinje cell activity, ultimately leading to aberrant cerebellar output.
In conclusion, our results demonstrate that chronic low-dose administration of PCP in rats models the GABA neurotransmission deficits seen in patients with schizophrenia (Bullock et al., 2008) and that these changes are localized to Golgi cells, a subset of cerebellar inhibitory interneurons critical for controlling granule cell output to Purkinje cells. Furthermore, comparison of the profile of gene expression changes in PCP-treated rats and patients with schizophrenia suggest that GABAergic neurotransmission mediated by Golgi cells may be altered in this illness. These findings, along with our previous study, suggest that aberrant cerebellar physiology, through its connections to the PFC, may contribute to the cognitive deficits seen in the patients.
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported by a grant from the Mental Illness and Neuroscience Discovery (MIND) Research Network to N.P.-B. and by RO1-AA014973 to C.F.V.
Footnotes
DISCLOSURE/CONFLICT OF INTEREST: The authors declare that, except for income received from their primary employer, no financial support or compensation has been received from any individual or corporate entity over the past 3 years for research or professional service, and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Akbarian S, Huang HS. Molecular and cellular mechanisms of altered GAD1/GAD67 expression in schizophrenia and related disorders. Brain Res Brain Res Rev. 2006;52:293–304. doi: 10.1016/j.brainresrev.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Huntsman MM, Kim JJ, Tafazzoli A, Potkin SG, Bunney WE, Jr., Jones EG. GABAA receptor subunit gene expression in human prefrontal cortex: comparison of schizophrenics and controls. Cereb Cortex. 1995a;5:550–560. doi: 10.1093/cercor/5.6.550. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE, Jr., Jones EG. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995b;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Sucher NJ, Bradley D, Tafazzoli A, Trinh D, Hetrick WP, Potkin SG, Sandman CA, Bunney WE, Jr., Jones EG. Selective alterations in gene expression for NMDA receptor subunits in prefrontal cortex of schizophrenics. J Neurosci. 1996;16:19–30. doi: 10.1523/JNEUROSCI.16-01-00019.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, Pierson R. The role of the cerebellum in schizophrenia. Biol Psychiatry. 2008;64:81–88. doi: 10.1016/j.biopsych.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthele A, Platzer S, Laurie DJ, Weis S, Sommer B, Zieglgansberger W, Conrad B, Tolle TR. Expression of metabotropic glutamate receptor subtype mRNA (mGluR1-8) in human cerebellum. Neuroreport. 1999;10:3861–3867. doi: 10.1097/00001756-199912160-00026. [DOI] [PubMed] [Google Scholar]
- Bolognani F, Tanner DC, Merhege M, Deschenes-Furry J, Jasmin B, Perrone-Bizzozero NI. In vivo post-transcriptional regulation of GAP-43 mRNA by overexpression of the RNA-binding protein HuD. J Neurochem. 2006;96:790–801. doi: 10.1111/j.1471-4159.2005.03607.x. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J Physiol. 1996;497(Pt 3):753–759. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Misra C, Mok MH, Mishina M, Cull-Candy SG. NR2B and NR2D subunits coassemble in cerebellar Golgi cells to form a distinct NMDA receptor subtype restricted to extrasynaptic sites. J Neurosci. 2003;23:4958–4966. doi: 10.1523/JNEUROSCI.23-12-04958.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock WM, Cardon K, Bustillo J, Roberts RC, Perrone-Bizzozero NI. Altered expression of genes involved in GABAergic transmission and neuromodulation of granule cell activity in the cerebellum of schizophrenia patients. Am J Psychiatry. 2008;165:1594–1603. doi: 10.1176/appi.ajp.2008.07121845. [DOI] [PubMed] [Google Scholar]
- Bureau I, Dieudonne S, Coussen F, Mulle C. Kainate receptor-mediated synaptic currents in cerebellar Golgi cells are not shaped by diffusion of glutamate. Proc Natl Acad Sci U S A. 2000;97:6838–6843. doi: 10.1073/pnas.97.12.6838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta M, Mameli M, Valenzuela CF. Alcohol enhances GABAergic transmission to cerebellar granule cells via an increase in Golgi cell excitability. J Neurosci. 2004;24:3746–3751. doi: 10.1523/JNEUROSCI.0067-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadderton P, Margrie TW, Hausser M. Integration of quanta in cerebellar granule cells during sensory processing. Nature. 2004;428:856–860. doi: 10.1038/nature02442. [DOI] [PubMed] [Google Scholar]
- Cochran SM, Kennedy M, McKerchar CE, Steward LJ, Pratt JA, Morris BJ. Induction of metabolic hypofunction and neurochemical deficits after chronic intermittent exposure to phencyclidine: differential modulation by antipsychotic drugs. Neuropsychopharmacology. 2003;28:265–275. doi: 10.1038/sj.npp.1300031. [DOI] [PubMed] [Google Scholar]
- De Schutter E, Vos B, Maex R. The function of cerebellar Golgi cells revisited. Prog Brain Res. 2000;124:81–93. doi: 10.1016/s0079-6123(00)24009-0. [DOI] [PubMed] [Google Scholar]
- Dieudonne S. Submillisecond kinetics and low efficacy of parallel fibre-Golgi cell synaptic currents in the rat cerebellum. J Physiol. 1998;510(Pt 3):845–866. doi: 10.1111/j.1469-7793.1998.845bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugue GP, Brunel N, Hakim V, Schwartz E, Chat M, Levesque M, Courtemanche R, Lena C, Dieudonne S. Electrical coupling mediates tunable low-frequency oscillations and resonance in the cerebellar Golgi cell network. Neuron. 2009;61:126–139. doi: 10.1016/j.neuron.2008.11.028. [DOI] [PubMed] [Google Scholar]
- Egerton A, Reid L, McGregor S, Cochran SM, Morris BJ, Pratt JA. Subchronic and chronic PCP treatment produces temporally distinct deficits in attentional set shifting and prepulse inhibition in rats. Psychopharmacology (Berl) 2008;198:37–49. doi: 10.1007/s00213-008-1071-5. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Stary JM, Earle JA, Araghi-Niknam M, Eagan E. GABAergic dysfunction in schizophrenia and mood disorders as reflected by decreased levels of glutamic acid decarboxylase 65 and 67 kDa and Reelin proteins in cerebellum. Schizophr Res. 2005;72:109–122. doi: 10.1016/j.schres.2004.02.017. [DOI] [PubMed] [Google Scholar]
- Feligioni M, Holman D, Haglerod C, Davanger S, Henley JM. Ultrastructural localisation and differential agonist-induced regulation of AMPA and kainate receptors present at the presynaptic active zone and postsynaptic density. J Neurochem. 2006;99:549–560. doi: 10.1111/j.1471-4159.2006.04087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forti L, Cesana E, Mapelli J, D’Angelo E. Ionic mechanisms of autorhythmic firing in rat cerebellar Golgi cells. J Physiol. 2006;574:711–729. doi: 10.1113/jphysiol.2006.110858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschy JM, Panzanelli P. Molecular and synaptic organization of GABAA receptors in the cerebellum: Effects of targeted subunit gene deletions. Cerebellum. 2006;5:275–285. doi: 10.1080/14734220600962805. [DOI] [PubMed] [Google Scholar]
- Geurts FJ, De Schutter E, Dieudonne S. Unraveling the cerebellar cortex: cytology and cellular physiology of large-sized interneurons in the granular layer. Cerebellum. 2003;2:290–299. doi: 10.1080/14734220310011948. [DOI] [PubMed] [Google Scholar]
- Grunze HC, Rainnie DG, Hasselmo ME, Barkai E, Hearn EF, McCarley RW, Greene RW. NMDA-dependent modulation of CA1 local circuit inhibition. J Neurosci. 1996;16:2034–2043. doi: 10.1523/JNEUROSCI.16-06-02034.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, Di-Giorgi-Gerevini V, Dwivedi Y, Grayson DR, Impagnatiello F, Pandey G, Pesold C, Sharma R, Uzunov D, Costa E. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- Hamann M, Rossi DJ, Attwell D. Tonic and spillover inhibition of granule cells control information flow through cerebellar cortex. Neuron. 2002;33:625–633. doi: 10.1016/s0896-6273(02)00593-7. [DOI] [PubMed] [Google Scholar]
- Heckers S, Stone D, Walsh J, Shick J, Koul P, Benes FM. Differential hippocampal expression of glutamic acid decarboxylase 65 and 67 messenger RNA in bipolar disorder and schizophrenia. Arch Gen Psychiatry. 2002;59:521–529. doi: 10.1001/archpsyc.59.6.521. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci. 2007;27:11496–11500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Redmond DE, Jr., Elsworth JD, Taylor JR, Youngren KD, Roth RH. Enduring cognitive deficits and cortical dopamine dysfunction in monkeys after long-term administration of phencyclidine. Science. 1997a;277:953–955. doi: 10.1126/science.277.5328.953. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Roth RH. The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1999;20:201–225. doi: 10.1016/S0893-133X(98)00060-8. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Tran A, Le D, Youngren KD, Roth RH. Subchronic phencyclidine administration reduces mesoprefrontal dopamine utilization and impairs prefrontal cortical-dependent cognition in the rat. Neuropsychopharmacology. 1997b;17:92–99. doi: 10.1016/S0893-133X(97)00034-1. [DOI] [PubMed] [Google Scholar]
- Kalinichev M, Robbins MJ, Hartfield EM, Maycox PR, Moore SH, Savage KM, Austin NE, Jones DN. Comparison between intraperitoneal and subcutaneous phencyclidine administration in Sprague-Dawley rats: a locomotor activity and gene induction study. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:414–422. doi: 10.1016/j.pnpbp.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Kanichay RT, Silver RA. Synaptic and cellular properties of the feedforward inhibitory circuit within the input layer of the cerebellar cortex. J Neurosci. 2008;28:8955–8967. doi: 10.1523/JNEUROSCI.5469-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondziella D, Brenner E, Eyjolfsson EM, Sonnewald U. How do glial-neuronal interactions fit into current neurotransmitter hypotheses of schizophrenia? Neurochem Int. 2007;50:291–301. doi: 10.1016/j.neuint.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Kotermanski SE, Johnson JW. Mg2+ imparts NMDA receptor subtype selectivity to the Alzheimer’s drug memantine. J Neurosci. 2009;29:2774–2779. doi: 10.1523/JNEUROSCI.3703-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr., Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Le Meur K, Galante M, Angulo MC, Audinat E. Tonic activation of NMDA receptors by ambient glutamate of non-synaptic origin in the rat hippocampus. J Physiol. 2007;580:373–383. doi: 10.1113/jphysiol.2006.123570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leveille F, El Gaamouch F, Gouix E, Lecocq M, Lobner D, Nicole O, Buisson A. Neuronal viability is controlled by a functional relation between synaptic and extrasynaptic NMDA receptors. Faseb J. 2008;22:4258–4271. doi: 10.1096/fj.08-107268. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Cruz DA, Melchitzky DS, Pierri JN. Lamina-specific deficits in parvalbumin-immunoreactive varicosities in the prefrontal cortex of subjects with schizophrenia: evidence for fewer projections from the thalamus. Am J Psychiatry. 2001;158:1411–1422. doi: 10.1176/appi.ajp.158.9.1411. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Lindahl JS, Keifer J. Glutamate receptor subunits are altered in forebrain and cerebellum in rats chronically exposed to the NMDA receptor antagonist phencyclidine. Neuropsychopharmacology. 2004;29:2065–2073. doi: 10.1038/sj.npp.1300485. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mathew SS, Pozzo-Miller L, Hablitz JJ. Kainate modulates presynaptic GABA release from two vesicle pools. J Neurosci. 2008;28:725–731. doi: 10.1523/JNEUROSCI.3625-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra C, Brickley SG, Farrant M, Cull-Candy SG. Identification of subunits contributing to synaptic and extrasynaptic NMDA receptors in Golgi cells of the rat cerebellum. J Physiol. 2000;524(Pt 1):147–162. doi: 10.1111/j.1469-7793.2000.00147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SJ, Silver RA. Glutamate spillover suppresses inhibition by activating presynaptic mGluRs. Nature. 2000;404:498–502. doi: 10.1038/35006649. [DOI] [PubMed] [Google Scholar]
- Morara S, Brecha NC, Marcotti W, Provini L, Rosina A. Neuronal and glial localization of the GABA transporter GAT-1 in the cerebellar cortex. Neuroreport. 1996;7:2993–2996. doi: 10.1097/00001756-199611250-00039. [DOI] [PubMed] [Google Scholar]
- Morris BJ, Cochran SM, Pratt JA. PCP: from pharmacology to modelling schizophrenia. Curr Opin Pharmacol. 2005;5:101–106. doi: 10.1016/j.coph.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Mouri A, Noda Y, Enomoto T, Nabeshima T. Phencyclidine animal models of schizophrenia: approaches from abnormality of glutamatergic neurotransmission and neurodevelopment. Neurochem Int. 2007;51:173–184. doi: 10.1016/j.neuint.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry. 1995;52:998–1007. doi: 10.1001/archpsyc.1995.03950240016004. [DOI] [PubMed] [Google Scholar]
- Pemberton KE, Belcher SM, Ripellino JA, Howe JR. High-affinity kainate-type ion channels in rat cerebellar granule cells. J Physiol. 1998;510(Pt 2):401–420. doi: 10.1111/j.1469-7793.1998.401bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter RH, Eastwood SL, Harrison PJ. Distribution of kainate receptor subunit mRNAs in human hippocampus, neocortex and cerebellum, and bilateral reduction of hippocampal GluR6 and KA2 transcripts in schizophrenia. Brain Res. 1997;751:217–231. doi: 10.1016/s0006-8993(96)01404-7. [DOI] [PubMed] [Google Scholar]
- Ramnani N. The primate cortico-cerebellar system: anatomy and function. Nat Rev Neurosci. 2006;7:511–522. doi: 10.1038/nrn1953. [DOI] [PubMed] [Google Scholar]
- Reynolds LM, Cochran SM, Morris BJ, Pratt JA, Reynolds GP. Chronic phencyclidine administration induces schizophrenia-like changes in N-acetylaspartate and N-acetylaspartylglutamate in rat brain. Schizophr Res. 2005;73:147–152. doi: 10.1016/j.schres.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Hamann M, Attwell D. Multiple modes of GABAergic inhibition of rat cerebellar granule cells. J Physiol. 2003;548:97–110. doi: 10.1113/jphysiol.2002.036459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rujescu D, Bender A, Keck M, Hartmann AM, Ohl F, Raeder H, Giegling I, Genius J, McCarley RW, Moller HJ, Grunze H. A pharmacological model for psychosis based on N-methyl-D-aspartate receptor hypofunction: molecular, cellular, functional and behavioral abnormalities. Biol Psychiatry. 2006;59:721–729. doi: 10.1016/j.biopsych.2005.08.029. [DOI] [PubMed] [Google Scholar]
- Sah P, Hestrin S, Nicoll RA. Tonic activation of NMDA receptors by ambient glutamate enhances excitability of neurons. Science. 1989;246:815–818. doi: 10.1126/science.2573153. [DOI] [PubMed] [Google Scholar]
- Salonen V, Kallinen S, Lopez-Picon FR, Korpi ER, Holopainen IE, Uusi-Oukari M. AMPA/kainate receptor-mediated up-regulation of GABAA receptor delta subunit mRNA expression in cultured rat cerebellar granule cells is dependent on NMDA receptor activation. Brain Res. 2006;1087:33–40. doi: 10.1016/j.brainres.2006.02.104. [DOI] [PubMed] [Google Scholar]
- Sams-Dodd F. Effect of novel antipsychotic drugs on phencyclidine-induced stereotyped behaviour and social isolation in the rat social interaction test. Behav Pharmacol. 1997;8:196–215. [PubMed] [Google Scholar]
- Sastry BR, Morishita W, Yip S, Shew T. GABA-ergic transmission in deep cerebellar nuclei. Prog Neurobiol. 1997;53:259–271. doi: 10.1016/s0301-0082(97)00033-6. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121(Pt 4):561–579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- Simmons DM, Arriza JL, Swanson LW. A complete protocol for in situ hybridization of messenger RNAs in brain and other tissues with radio-labeled single-stranded RNA probes. J Histotechnology. 1989;12:169–181. [Google Scholar]
- Takayama C, Inoue Y. Developmental expression of GABA transporter-1 and 3 during formation of the GABAergic synapses in the mouse cerebellar cortex. Brain Res Dev Brain Res. 2005;158:41–49. doi: 10.1016/j.devbrainres.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Suzuki M, Sumiyoshi T, Murata M, Tsunoda M, Kurachi M. Subchronic phencyclidine administration alters central vasopressin receptor binding and social interaction in the rat. Brain Res. 2003;992:239–245. doi: 10.1016/j.brainres.2003.08.050. [DOI] [PubMed] [Google Scholar]
- Wall MJ. Endogenous nitric oxide modulates GABAergic transmission to granule cells in adult rat cerebellum. Eur J Neurosci. 2003;18:869–878. doi: 10.1046/j.1460-9568.2003.02822.x. [DOI] [PubMed] [Google Scholar]
- Wass C, Archer T, Palsson E, Fejgin K, Klamer D, Engel JA, Svensson L. Effects of phencyclidine on spatial learning and memory: nitric oxide-dependent mechanisms. Behav Brain Res. 2006;171:147–153. doi: 10.1016/j.bbr.2006.03.036. [DOI] [PubMed] [Google Scholar]
- Watanabe D, Nakanishi S. mGluR2 postsynaptically senses granule cell inputs at Golgi cell synapses. Neuron. 2003;39:821–829. doi: 10.1016/s0896-6273(03)00530-0. [DOI] [PubMed] [Google Scholar]
- Wiley JL. Nitric oxide synthase inhibitors attenuate phencyclidine-induced disruption of prepulse inhibition. Neuropsychopharmacology. 1998;19:86–94. doi: 10.1016/S0893-133X(98)00008-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.