Abstract
The somatostatin receptor subtype 2 (sst2) behaves as a tumor suppressor when expressed and stimulated by its ligand somatostatin in pancreatic cancer. We reveal a mechanism underlying oncosuppressive action of sst2, whereby this inhibitory receptor upregulates the expression of the secreted angioinhibitory factor thrombospondin-1 (TSP-1), as demonstrated in exocrine BxPC-3 and endocrine BON pancreatic cancer cells. The sst2-dependent upregulation of TSP-1 occurs through the inhibition of the PI3K pathway. It depends on transcriptional and translational events, involving a previously undescribed IRES in the 5′-UTR of TSP-1 mRNA. Chick chorioallantoic membrane was used as an in vivo model to demonstrate that TSP-1 is a critical effector of the inhibitory role of sst2 on the neoangiogenesis and oncogenesis induced by pancreatic cancer cells. TSP-1 reduced in vitro tubulogenesis of endothelial cells when grown in conditioned medium from pancreatic cancer cells expressing sst2, as compared to those expressing the control vector. TSP-1 inhibited tumor cell-induced neoangiogenesis by directly sequestering the proangiogenic factor VEGF, and inactivating the angiogenesis initiated by VEGFR2 phosphorylation in endothelial cells. Using human pancreatic tissue-microarrays, the expression of both sst2 and TSP-1 was shown to be correlated during the pancreatic neoplastic program. Both proteins are nearly undetectable in normal exocrine pancreas and in most invasive cancer lesions, but their expression is strikingly upregulated in most preinvasive cancer-adjacent lesions. The upregulation of both sst2 and TSP-1 tumor suppressors may function as an early negative feedback to restrain pancreatic carcinogenesis.
Keywords: angiogenesis, chick chorioallantoic membrane model, IRES-dependent translation
Somatostatin (SRIF, Somatotropin Release-Inhibiting Factor) is a neuropeptide with broad inhibitory effects on endocrine and exocrine secretion of pituitary, pancreatic, and gastrointestinal hormones, as well as on intestinal motility, absorption of nutrients and ions, and vascular contractility. SRIF also functions as a neurotransmitter produced by normal endocrine, gastrointestinal, immune, and neuronal cells, and by certain tumors (1). Attention has recently focused on the role of SRIF in the progression and control of neoplastic disease. SRIF displays potent antitumor activity in several human cancers in vitro and in vivo (2). It acts directly on tumor cells (inhibiting their survival and/or invasiveness), or indirectly on normal cells of the host affecting tumor microenvironment (2). The biological activities of SRIF are mediated through five different high affinity G protein-coupled receptor subtypes (sst1–5) whose expression is cell- and organ-specific. Most importantly, ssts, and especially sst2, are overexpressed in a large variety of tumors (3). Because SRIF has a short half-life, numerous stable derivatives have been synthesized (1). Two decades of medical use have documented that SRIF octapeptide analogs, which are predominantly sst2-preferring binding peptides, are excellent agents for diagnostic evaluation and tumor-localization by scintigraphy. These analogs, as therapeutic molecules, can often cure hormonal symptoms associated with pituitary and endocrine tumors, and concurrently induce marked shrinkage of the tumors (2). We and others have shown that anti-neoplastic activity of SRIF is not restricted to endocrine tumors, but also occurs in pancreatic ductal adenocarcinoma (PDAC), indicating that sst2 behaves as a tumor suppressor for PDAC (4–8). The expression of sst2 is lost in 90% of PDAC and their metastases (7). Its re-expression in pancreatic cancer cells results in an autocrine loop whereby sst2 induces the expression of SRIF, which in turn constitutively activates the sst2 receptor (4). Apoptosis is thus induced, and pancreatic cancer cell proliferation, tumorigenesis, metastasis, and angiogenesis are inhibited (4–6, 9, 10).
Tumor angiogenesis is essential for tumor growth, invasion, and metastasis (11). SRIF and SRIF analogs inhibit the proliferation and migration of endothelial cells by interacting with sst2 in vitro and in vivo (2). Sst3 and sst5 could also be involved. An upregulation of sst2 expression has been observed during the angiogenic switch from resting to proliferating endothelium (12, 13). This suggests a critical inhibitory role for SRIF during the initial steps of cancer progression. The overexpression of peritumoral vascular somatostatin receptors, mostly sst2, has been reported in some human carcinomas and malignant lymphomas (13, 14). This could represent a host defense mechanism against tumor angiogenesis. This angioinhibitory action of SRIF on tumors also relies on its ability to abrogate the secretion and/or activity of angiogenic factors, including the main proangiogenic factor, VEGF, as has been primarily observed in vivo using intratumor sst2 gene transfer (9). In this study, we document a mechanism underlying the angioinhibitory action of SRIF in tumors involving a sst2-dependent upregulation of expression of the potent inhibitor of angiogenesis thrombospondin-1 (TSP-1). TSP-1 acts by sequestering and consequently inactivating angiogenic activity of VEGF. TSP-1 is therefore identified here as a critical effector of sst2 tumor-suppressive activity on pancreatic tumor growth and angiogenesis.
Results
SRIF-Activated sst2 Upregulates the Expression of the Angioinhibitory Factor Thrombospondin-1 (TSP-1).
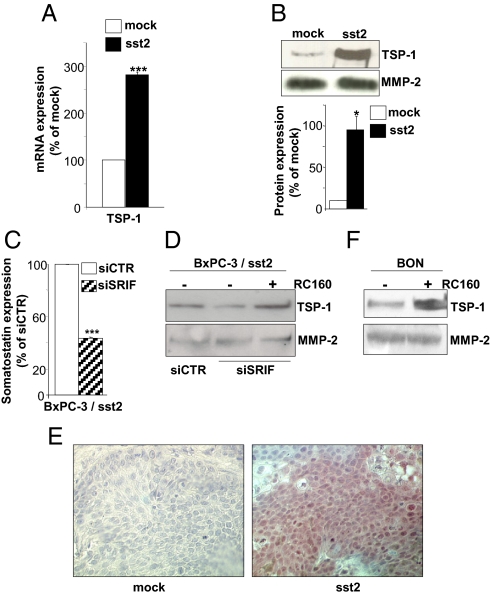
From differential gene profile analysis in the human pancreatic cancer BxPC-3 cells expressing sst2 (BxPC-3/sst2) or not (BxPC-3/mock), we have shown an upregulation by 3.38 ± 0.44-fold of the mRNA for the angioinhibitory factor thrombospondin-1 (TSP-1). This result was confirmed by real-time quantitative RT-PCR as well as by Western blot, showing a 2.81 ± 0.05 and a 9.40 ± 2.82 fold-increase in TSP-1 mRNA (Fig. 1A) and its protein (Fig. 1B) expression, respectively. BxPC-3/sst2 cells exhibit an autocrine loop whereby the production and secretion of SRIF continuously activates sst2 independently of addition of exogenous SRIF (4). Blocking this autocrine loop by transfecting BxPC-3/sst2 cells with a specific siRNA targeting SRIF downregulated SRIF expression by 57 ± 1% (Fig. 1C), and decreased secreted TSP-1 protein expression, which was rescued when cells were treated with 10 nM somatostatin analog RC-160 (Fig. 1D). This indicates that TSP-1 is directly regulated by the autocrine sst2-SRIF loop. Consistently, tumors resulting from BxPC-3/sst2 cell s.c. xenografts in athymic mice, presented a significant decrease in volume progression, as previously described (4, 15), and a potent upregulation of TSP-1 expression, as shown by immunohistochemistry (Fig. 1E), compared to BxPC-3/mock-derived tumors. The regulation of TSP-1 protein expression by SRIF-activated sst2 was then extended to another human cell model, the endocrine pancreatic cancer BON, which endogenously expresses the sst2 receptor. Strikingly, challenging these cells with 10 nM RC-160 also upregulated TSP-1 protein expression by 1.82 ± 0.44-fold (Fig. 1F).
Fig. 1.
sst2 upregulates the expression of the angioinhibitory factor TSP-1.(A) Quantification by qRT-PCR of TSP-1 mRNA in BxPC-3/mock and/sst2 cells, as normalized with mRNA quantified in mock cells. (B, D, F) Immunoblots with an anti-TSP-1 (Upper), or an anti- MMP-2 antibody (Lower, loading control) using CM from BxPC-3/mock and/sst2 cells, and densitometric analyses (B, bottom), or from BxPC-3/sst2 cells transfected with a control (siCTR) or SRIF (siSRIF) siRNA and treated or not with 10−8 M RC-160 for 72 h (D), or from BON cells treated or not with 10−8 M RC-160 for 72 h (F). Expression of MMP-2 was used as an internal loading control. (C) SRIF expression assessed by ELISA in CM from BxPC-3/sst2 cells transfected with siCTR or siSRIF, and normalized with SRIF quantified in siCTR-transfected BxPC-3/sst2 cells. (E) Immunohistochemistry using an anti-TSP-1 antibody on mock- or sst2-expressing tumors issued from the s.c. xenograft of corresponding cells in athymic mice. Results are representative of three independent experiments, and are presented as the mean ± SEM.
Molecular Mechanisms for sst2-Dependent Upregulation of TSP-1 Expression.
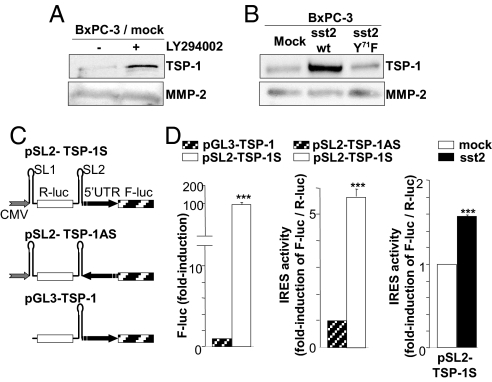
Excess activation of the Ras/PI3K pathway is a hallmark of pancreatic cancer (16). We have previously demonstrated that sst2 inhibits PI3K activity through a mechanism depending on a direct interaction between sst2 and the regulatory p85 subunit of PI3K (15). Upon treatment with SRIF, the sst2/p85 association is disrupted resulting in a subsequent inactivation of PI3K activity (15). The possible involvement of PI3K in TSP-1 regulation was therefore investigated in BxPC-3 cells either treated with the PI3K inhibitor LY294002, or transfected with the wild-type (wt) or, mutated Y71F, sst2. Mutating Y71 of sst2 to F impedes the direct interaction between sst2 and p85, thereby abrogating sst2-mediated inhibition of PI3K activity (15). Interestingly, LY294002-mediated inhibition of PI3K activity in BxPC-3/mock cells stimulated TSP-1 expression, therefore mimicking sst2 action (Fig. 2A). More importantly, reverting sst2-dependent inhibition of PI3K activity in BxPC-3/sst2-Y71F cells decreased TSP-1 expression, as compared in BxPC-3/sst2wt cells (Fig. 2B), demonstrating that sst2 increases TSP-1 expression by inhibiting the PI3K pathway.
Fig. 2.
PI3K- and IRES-dependent translational upregulation of TSP-1 by sst2. (A and B) Immunoblots with an anti-TSP-1 (Upper), or anti- MMP-2 antibody (Lower) using CM from BxPC-3/mock cells treated or not with 25 μM LY294002 (A), or from BxPC-3/mock,/wt sst2 or/mutated sst2-Y71F cells (B). (C) Bicistronic constructs containing the two luciferase reporters, Renilla (R-luc) and firefly (F-luc) are described (Left). The gray arrow represents the CMV promoter and the black arrow the TSP-1 5′ UTR cloned forward (TSP-1S) or backward (TSP-1AS). (D) Quantification of F-luc activity in BxPC-3/mock cells transfected with either pGL3-TSP-1 or pSL2-TSP-1S (Left); quantification of TSP-1 IRES activity as the ratio of F-luc/R-luc activities in BxPC-3/mock cells transfected with pSL2-TSP-1AS or pSL2-TSP-1S (Middle), or in BxPC-3/mock and/sst2 cells transfected with pSL2-TSP-1S (Right), and normalized with the F-luc/R-luc ratio quantified in mock cells with pSL2-TSP-1AS (Middle), or with pSL2-TSP-1S (Right).
Surprisingly, sst2 regulated TSP-1 mostly posttranscriptionally since TSP-1 protein was upregulated to a greater extent by sst2 than mRNA for TSP-1 (9.40 ± 2.82-fold vs. 2.81 ± 0.05-fold, respectively) (Fig. 1 A and B). We have previously demonstrated that the inhibitor of cap-dependent translation 4E-BP1 is activated in BxPC-3/sst2 cells, sst2 increasing both its transcriptional expression, and its activity by repressing PI3K activity (17, 18). Furthermore, treatment of BxPC-3 cells with the mTOR inhibitor, rapamycin, which inhibits 4E-BP1 activity, does not affect TSP-1 expression, suggesting a cap-independent process in the control of TSP-1 mRNA translation. In the search for the presence of a cap-independent internal ribosome entry site (IRES) in TSP-1 mRNA, its 5′ UTR has been inserted between two reporter genes (Renilla and firefly luciferases) of a bicistronic CMV-based expression vector in a sense (pSL2-TSP-1S) or antisense (pSL2- TSP-1AS) orientation, as described in Fig. 2C. To exclude the possibility that the TSP-1 5′ UTR possesses an intrinsic promoter activity that could induce a bias in data interpretation, an additional bicistronic vector carrying the TSP-1 5′ UTR, but without CMV promoter (pGL3-TSP-1), was also created. No significant firefly activity was detected with the pGL3-TSP-1 vector (lacking CMV promoter) as compared with pSL2-TSP-1S (containing CMV promoter), indicating that TSP-1 5′ UTR does not contain a cryptic promoter (Fig. 2D, Left). A 5.7 ± 0.3-fold increase of the normalized firefly/Renilla luciferase activity was then observed after transfecting BxPC-3/mock cells with the pSL2-TSP-1S, as compared with the pSL2-TSP-1AS, vector (Fig. 2D, Middle), indicating the existence of an IRES in the TSP-1 5′ UTR which is active in BxPC-3 cells. Interestingly, IRES activity in TSP-1 5′ UTR was enhanced (1.56 ± 0.04-fold) in cells that express sst2 as compared to mock cells (Fig. 2D, Right).
Upregulation of TSP-1 Expression Is Required for SRIF-Activated sst2 to Inhibit Pancreatic Tumor Growth and Angiogenesis.
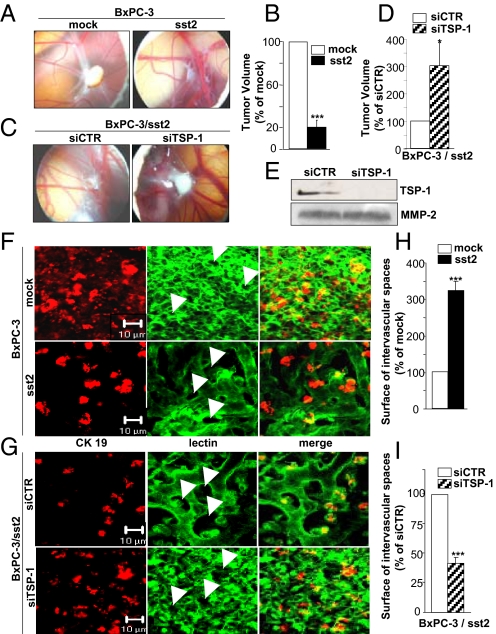
To investigate the role for TSP-1 in tumor-suppressive and angioinhibitory activity of sst2, the chick chorioallantoic membrane (CAM) was used as an experimental in vivo model (19). Upon xenografting tumor cells onto the CAM, the chick host provides the essential living environment for cancer cells, including pancreatic, to induce neoangiogenesis and to form tumors (19, 20). BxPC-3/mock cells spread onto the CAM survived and proliferated to form a solid cellular mass, while expression of sst2 abrogated tumor growth by 81 ± 7% (Fig. 3 A and B), confirming tumor suppressor activity of sst2 in this animal model. Strikingly, CAM surrounding the BxPC-3/mock-derived tumors showed a dense, tortuous and tumor-like capillary network, as evidenced by lectin immunostaining, where BxPC-3 cells form proangiogenic nodules, as visualized by anti-CK19 immunostaining (Fig. 3F, Upper). The density of this tumor-derived capillary network was dramatically reduced when sst2 is expressed in BxPC-3 cells, as quantified using the mean of the intervascular space surfaces, indicated by white arrows, which was increased by 323 ± 24% (Fig. 3 F Lower, and H). This result indicated that the expression of sst2 in pancreatic cancer cells potently inhibited tumor-induced angiogenesis in this in vivo model. To explore the role for TSP-1 in sst2 angio-inhibitory action, BxPC-3/sst2 cells were transfected with a siRNA targeting TSP-1 (siTSP-1), which abrogated TSP-1 expression in BxPC-3/sst2 cell CM, as compared to control siRNA (siCTR) (Fig. 3E). The extinction of TSP-1 partially reversed sst2-mediated inhibition of tumor growth, as evidenced by an increase of sst2-expressing tumor growth onto the CAM by 303 ± 83% (Fig. 3 C and D). Moreover, abrogating TSP-1 in BxPC-3/sst2 cells also completely re-established tumor angiogenesis of the CAM surrounding sst2-expressing tumors where the surface of the intervascular spaces was decreased by 59 ± 5% (Fig. 3 G and I). These results indicated that TSP-1 is critical for tumor suppressor activity of sst2 with both autocrine inhibitory effect on pancreatic cancer cell growth and paracrine angio-inhibitory activity.
Fig. 3.
TSP-1 inhibits the growth and angiogenesis of pancreatic tumors xenografted onto the chick CAM. (A–D and F–I). Xenograft on the CAM of BxPC-3/mock or/sst2 cells (A and F) or of BxPC-3/sst2 cells transfected with siCTR or siTSP-1 (C and G). CAM pictures at day 4 using a stereomicroscope (A and C). Histograms of tumor volumes 4 days postimplantation (B and D). Results are expressed as normalized with volumes quantified in mock cells (A and B), or in BxPC-3/sst2 cells transfected with siCTR (C and D). Confocal microscope analyses of the peritumoral CAM immunostained with a CK19 antibody or a SNA-lectin recombinant protein. White arrows point to the intercapillary spaces (F and G). Histograms of intercapillary space surfaces (H and I). Results are expressed as normalized to the intervascular space surfaces quantified in mock cells (F and H), or in BxPC-3/sst2 cells transfected with siCTR (G and I). (E) Immunoblot of TSP-1 using CM of BxPC-3/sst2 cells transfected with siCTR or siTSP-1. Results are presented as the mean ± SEM of at least three independent experiments.
TSP-1 Inhibits Pancreatic Cancer Cell-Induced Angiogenesis by Sequestering VEGF.
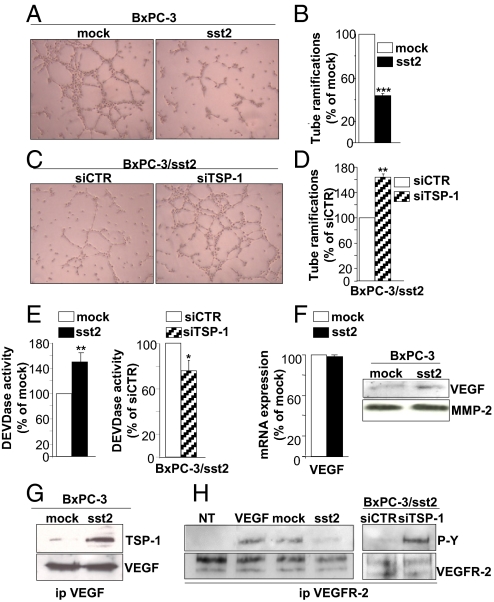
TSP-1 is known to inhibit endothelial cell migration and/or to induce endothelial cell apoptosis, putatively acting by affecting the bioavailability of proangiogenic factors including VEGF in the extracellular matrix (21). Tubulogenesis assays, using the human microvascular endothelial HMEC cells, showed a potent reduction (54 ± 2%) of HMEC cell tube formation when grown in conditioned media (CM) from sst2-, as compared to mock-, expressing cells (Fig. 4 A and B). Conversely, abrogating TSP-1 expression in BxPC-3/sst2 cells partially reversed sst2-mediated inhibition of HMEC cell tube formation, increasing cell tubulogenesis by 163 ± 5% (Fig. 4 C and D). Apoptosis was then quantified at the level of the executioner caspase-3 activation. A significant increase of HMEC cell apoptotic activity (by 151 ± 16%) was observed when these cells were grown in the presence of CM from sst2-, as compared to mock-, expressing BxPC-3 cells, which was then decreased (by 25 ± 8%) while TSP-1 expression was abrogated in BxPC-3/sst2 cells (Fig. 4E). Interestingly, BxPC-3 cells express and secrete VEGF, but its mRNA and protein expression is not affected by sst2 in these cells (Fig. 4F). However TSP-1, secreted in BxPC-3/sst2 cell CM, coimmunoprecipitates with VEGF (Fig. 4G), suggesting that TSP-1 might inhibit VEGF-induced angiogenesis by sequestering this angiogenic factor. HMEC VEGFR2 tyrosine phosphorylation, which reflects its level of activation, was consistently increased when HMEC cells were grown in CM, as compared to non-CM, from BxPC-3/mock cells (Fig. 4H). This was seen also in HMEC cells treated with 30 ng/mL of VEGF, indicating that BxPC-3-secreted VEGF is active on HMEC cells. More importantly, treatment of HMEC cells with CM from sst2-expressing BxPC-3 cells, as compared to mock-expressing, resulted in a decrease of tyrosine phosphorylation of VEGFR2. This was completely reversed when TSP-1 expression was silenced in BxPC-3/sst2 cells. These results demonstrated that TSP-1 is a critical effector of angioinhibitory action of sst2 acting by decreasing bioavailability of VEGF.
Fig. 4.
Mechanisms for TSP-1-mediated inhibition of angiogenesis. (A–D) Endothelial cell tubulogenesis assay using HMEC cells seeded on top of a matrigel and incubated with CM from BxPC-3/mock or/sst2 cells (A), or from BxPC-3/sst2 cells transfected with siCTR or siTSP-1 (C). Histograms as quantified by counting number of HMEC cell tube branchings, and as normalized to the number of branchings quantified in mock cells (A and B), or in BxPC-3/sst2 cells transfected with siCTR (C and D). (E) Executioner caspase activity assayed in HMEC cells treated with CM from BxPC-3/mock- or/sst2 cells (Left), or from BxPC-3/sst2 cells transfected with siCTR or siTSP-1 (Right), and as normalized with caspase activity measured in mock cells (Left), or in BxPC-3/sst2 cells transfected with siCTR (Right). (F) Expression of VEGF mRNA (Left) or protein (Right) in BxPC-3/mock or/sst2 cells assessed by qRT-PCR or immunoblot using an anti-VEGF antibody, and as normalized to VEGF mRNA quantified in mock cells (Left). (G) Co-IP of TSP-1, assessed by Western blot using an anti-TSP-1 antibody, with VEGF in anti-VEGF antibody immunoprecipitates using CM from BxPC-3/mock or/sst2 cells (Upper). VEGF immunoblot to control equal VEGF IP (Lower). (H) VEGFR-2 tyrosine phosphorylation analysis by Western blot using an anti-tyrosine phosphorylation antibody in anti-VEGFR-2 antibody immunoprecipitates from HMEC cells treated with VEGF, or with non-CM (NT), or with CM from BxPC-3/mock or/sst2 cells, or from BxPC-3/sst2 cells transfected with siCTR or siTSP-1 (Upper). VEGFR-2 immunoblot to control equal VEGFR-2 IP (Lower). Results are presented as the mean ± SEM and are representative of three independent experiments.
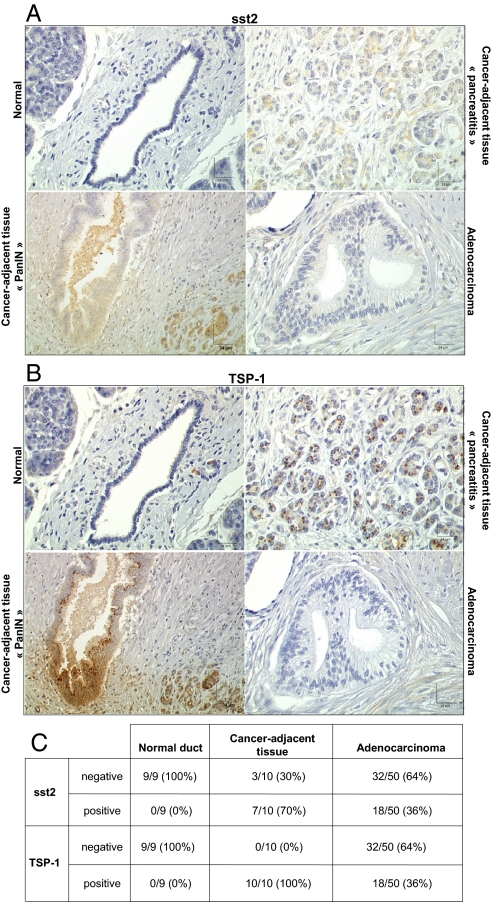
Correlation and Biphasic Pattern of sst2 and TSP-1 Expression during the Pancreatic Neoplastic Process.
After identifying TSP-1 as a critical effector of sst2 tumor-suppressive activity in human pancreatic cancer cells, we explored expression of both proteins during human pancreatic neoplastic process. A good correlation between sst2 and TSP-1 expression was observed during the evolution of the cancerous lesions, as demonstrated using pancreatic tissue-microarrays (Fig. 5 A–C). Both sst2 and TSP-1 are not expressed in ducts and are only faintly detectable in normal pancreatic acinar cells (n = 9), whereas they are strongly expressed in Langerhans islets. Interestingly, both proteins are strongly expressed in nearly all cancer-adjacent pancreatic tissues (70–100%), but absent in most of pancreatic adenocarcinomas (64%), from involved patients (n = 60). The pancreatic cancer-adjacent tissues present lesions of chronic pancreatitis and precancerous lesions of PanIN (pancreatic intraepithelial neoplasia), which stained positive for both sst2 and TSP-1. These results suggest a biphasic pattern of sst2 and TSP-1 expression during the pancreatic neoplastic process with an increase in the expression during the early precancerous stages, and then a loss of expression at the late stages of in situ or invasive carcinoma.
Fig. 5.
Expression of sst2 and TSP-1 proteins. (A–C) Immunohistochemistry using anti-sst2 (A) or anti-TSP-1 (B) antibody on high-density pancreatic tissue-microarrays including triplicate cores of 60 cases of pancreatic tumors and of nine cases of normal pancreas.
Discussion
We have herein revealed a mechanism underlying sst2 oncosuppressive and angioinhibitory actions in pancreatic cancer involving the upregulation of a critical inhibitor of angiogenesis, TSP-1. Knocking-down TSP-1 consistently abrogated in vivo sst2 antineoplastic activity.
Tumor progression is a multistep process that requires that tumor cells acquire sustained neoangiogenesis. The net balance of angiogenic factors versus inhibitors defines the final angiogenic phenotype of the tumor. A disturbance in this dynamic balance determines whether the angiogenic switch does or does not occur in the tumor microenvironment. Interestingly, downregulation of TSP-1 expression represents a common event characterizing the switch of several dormant tumors to fast-growing angiogenic tumors (22), highlighting the critical role of this multifunctional matrix protein in tumor progression. TSP-1 is a 450-kDa homotrimeric glycoprotein and the first naturally occurring angiogenic inhibitor to be discovered (21). TSP-1 acts directly on endothelial cells, through CD36, CD47, and/or integrin receptors. It also acts indirectly by affecting the bioavailability of angiogenic factors, including VEGF, in the extracellular matrix, subsequently inhibiting or inducing endothelial cell migration or apoptosis, respectively (21). Our results clearly demonstrate that, by upregulating TSP-1 expression, SRIF-activated sst2 affects the organization of endothelial cell into a capillary network and apoptosis. We also showed that TSP-1 acts by sequestering VEGF secreted by tumor cells (21). These findings reveal an indirect mechanism for SRIF-mediated inhibition of VEGF action, in addition to its previously described direct effect on the inhibition of VEGF expression (9).
The demonstration of increased TSP-1 expression induced by SRIF-activated sst2 was shown in two human pancreatic cancer cell models expressing sst2 either endogenously (BON cells) or upon transfection (BxPC-3 cells). Because BON cells also endogenously express sst3 and sst5, which present high binding-affinities (nM range) for RC-160, we cannot exclude that TSP-1 expression is increased through activation of one or both of these other ssts, in addition to sst2. Surprisingly, sst2-dependent upregulation of TSP-1 expression occurs through partially transcriptional but mostly translational events, involving a previously undescribed IRES in the 5′-UTR of the TSP-1 mRNA. IRES-dependent translation may represent an alternate mechanism for sst2 to upregulate expression of antiangiogenic or growth-inhibiting proteins. These may include TSP-1 and connexins, as sst2 blocks cap-dependent translation (17, 18). Interestingly, posttranscriptional TSP-1 gene regulation has been previously demonstrated to depend on RNA turnover of TSP-1 (23), thus emphasizing the diversity of posttranscriptional mechanisms that can control TSP-1 expression. In addition to sst2-dependent upregulation of TSP-1, TSP-1 expression is negatively or positively regulated by numerous oncogenes or tumor suppressor genes, including Ras, Myc, c-jun, p53, PTEN, and Smad4, thus further strengthening its role as an inhibitor of tumor growth (21). In addition, our demonstration that sst2-dependent inhibition of PI3K activity is required for sst2 to upregulate TSP-1 is consistent with the dependence on PI3K activation of Ras to inhibit TSP-1 (21). By inhibiting PI3K activity (15), sst2 may affect both transcriptional- and/or IRES translational-dependent regulation of TSP-1.
The observation that sst2 and TSP-1 expression is simultaneously upregulated in epithelial pancreatic cells during the preneoplastic stages of human pancreatic cancer development, and is then lost in most cases of PDAC, reveals a good correlation of expression of both tumor suppressor proteins during pancreatic carcinogenesis. This could be interpreted as a mechanism of host defense against tumor neoangiogenesis and metastasis. Recently, the tumor-suppressive role of TSP-1 has also been demonstrated to rely, in cervical carcinogenesis, on the inhibition of the fibroblastic stroma reaction (24), thus creating a permissive environment for tumor progression. High TSP-1 levels in early PDAC lesions could therefore impede the dialogue between epithelial cancer and stromal cells, thus preventing the development of a stromal reaction favorable to tumor development. A biphasic pattern of TSP-1 expression has been also described in skin carcinogenesis, with a high TSP-1 expression in early neoplastic lesions, followed by the loss of TSP-1 expression, observed in malignant invasive cutaneous squamous cell carcinoma, resulting in tumor vascularization and malignant progression. Knock-out of TSP-1 has been consistently shown to accelerate dysplasic changes during early stages of tumor initiation in a genetic intestinal carcinogenesis model (21). In addition, an upregulation of sst2 expression has been observed in peritumoral veins (3). This may also represent a negative feedback against tumor neoangiogenesis and metastasis. Loss of sst2 and TSP-1 expression in epithelial cancer cells in late stages of pancreatic carcinogenesis most probably results from genetic and epigenetic events, which have been specifically described in PDAC, and include sst2 promoter hypermethylation (25), and a gain/loss of function of several oncogenes/tumor suppressors that regulate TSP-1 expression [including Kras/p53 or Smad4 (21), respectively]. Interestingly, TSP-1 has been shown here to be poorly expressed in the abundant fibroblastic reaction that surrounds PDAC (Fig. 5B). Consistently, fibroblast-derived expression of TSP-1 has been demonstrated to be strongly decreased when fibroblasts are exposed to the soluble factors derived from epithelial cancer cells that specifically harbor an activating mutation of Kras (26). This provides an elegant mechanism whereby cancer cells modulate the properties of their adjacent normal stroma to foster neoangiogenesis. Kras is mutated in 90% of pancreatic cancer cells (16), which may explain our observation that TSP-1 expression is absent in PDAC stroma.
Our hypothesis is that early genetic alterations and inflammation that occur in preneoplastic pancreatic epithelial and stroma cells, respectively, result in upregulation of the expression of sst2, as it has previously been reported in endothelial cells and monocytes upon activation (12, 27), and subsequently of its target TSP-1. This may serve as negative feedback to restrain carcinogenesis. Similarly, a potent upregulation of sst2 expression by estrogens has been observed in breast cancer that express estrogen receptor, which has been interpreted as a negative regulatory control on estrogen-induced cell growth (28). An understanding of how inflammatory processes and key cancer pathways, including sst2 and its targets, may shape tumor stroma ontogeny in preinvasive lesions of pancreatic cancer may reveal therapeutic options for this deadly malignancy. Furthermore, identifying the TSP-1/VEGF axis as a target of sst2 offers a rationale for the use of sst2-preferring analogs and also of SRIF-analogs for the treatment of angiogenic tumors that express sst2, including pancreatic endocrine tumors.
Materials and Methods
Capillary-Like Structure Formation.
Tubulogenesis was assessed in Matrigel (BD Biosciences) using HMEC cells grown (18 h) in the presence of CM collected from BxPC-3 cell cultures. Angiogenic activity was quantified by counting the number of endothelial cell tube branchings in each well.
Executioner Caspase Activity Assay.
Executioner caspase activity was assayed using HMEC cells grown (24 h) in the presence of CM collected from BxPC-3 cell cultures with the Quantipak kit (Biomol International).
CAM Assay.
Fertilized chicken eggs (Morizeau, France) were incubated at 37 °C and 80% humidified atmosphere. On day 4 of development, a window was cut out in the eggshell. On embryonic day 10, 2 × 106 cells in 30 μL were deposited onto the CAM. On day 14, tumor volumes were estimated by the equation V = 4/3r3, with r = 1/2. CAMs were fixed in situ with paraformaldehyde, and areas surrounding tumors were cut out for analysis by immunohistochemistry. Pictures of CAM were taken under a stereomicroscope (Nikon SMZ800) using a digital camera (Nikon Coolpix 950).
Immunohistochemistry.
Fixed CAMs were incubated successively with biotinylated lectin SNA (Vector Laboratories), cytokeratin-19 antibody (Abcam), and, finally secondary streptavidin Alexa Fluor 488 and 647 antibodies (Molecular Probes). Sections were examined under a Zeiss laser scanning confocal microscope LSM510. TMA contain triplicate cores of 60 cases of pancreatic tumors and nine cases of normal pancreas (US Biomax). Tumor xenografts were started with the s.c. inoculation of BxPC-3/mock or/sst2 cells into athymic female mice. Deparaffinized tissue sections of TMA or of tumor xenografts were placed in citrate buffer, pH 6.0, heated in microwave oven for 3 × 5 min, probed with TSP-1 (NeoMarkers) or sst2 antibody (generated in our laboratory), and then with horseradish peroxidase-conjugated secondary antibody (Dako). A solution of 3-amino-9-ethylcarbazole (AEC) was used as chromogen (Dako), and sections were counterstained with hematoxylin.
For a list of reagents and sources, and methodological details, see SI Text.
Supplementary Material
Acknowledgments.
This work was supported by Association pour la Recherche Contre le Cancer Grant 3899, Ligue Nationale Contre le Cancer Grant RAB09006BBA, Canceropole Grand Sud-Ouest Grant RMA04002BPA, Agence Nationale pour la Recherche Project R06423BS, and University Paul Sabatier of Toulouse Grant CR27 A01BQR-2007. H.L. is recipient of a fellowship from INSERM-Région Midi-Pyrénées. S.L. is recipient of a fellowship from LNCC.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0908674106/DCSupplemental.
References
- 1.Weckbecker G, et al. Opportunities in somatostatin research: Biological, chemical and therapeutic aspects. Nat Rev Drug Discov. 2003;2:999–1017. doi: 10.1038/nrd1255. [DOI] [PubMed] [Google Scholar]
- 2.Pyronnet S, et al. Antitumor effects of somatostatin. Mol Cell Endocrinol. 2008;286:230–237. doi: 10.1016/j.mce.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Susini C, Buscail L. Rationale for the use of somatostatin analogs as antitumor agents. Ann Oncol. 2006;17:1733–1742. doi: 10.1093/annonc/mdl105. [DOI] [PubMed] [Google Scholar]
- 4.Delesque N, et al. sst2 somatostatin receptor expression reverses tumorigenicity of human pancreatic cancer cells. Cancer Res. 1997;57:956–962. [PubMed] [Google Scholar]
- 5.Vernejoul F, et al. Antitumor effect of in vivo somatostatin receptor subtype 2 gene transfer in primary and metastatic pancreatic cancer models. Cancer Res. 2002;62:6124–6131. [PubMed] [Google Scholar]
- 6.Guillermet J, et al. Somatostatin receptor subtype 2 sensitizes human pancreatic cancer cells to death ligand-induced apoptosis. Proc Natl Acad Sci USA. 2003;100:155–160. doi: 10.1073/pnas.0136771100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buscail L, et al. Loss of sst2 somatostatin receptor gene expression in human pancreatic and colorectal cancer. Cancer Res. 1996;56:1823–1827. [PubMed] [Google Scholar]
- 8.Kumar M, et al. Mechanisms of inhibition of growth of human pancreatic carcinoma implanted in nude mice by somatostatin receptor subtype 2. Pancreas. 2004;29:141–151. doi: 10.1097/00006676-200408000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Carrere N, et al. Characterization of the bystander effect of somatostatin receptor sst2 after in vivo gene transfer into human pancreatic cancer cells. Hum Gene Ther. 2005;16:1175–1193. doi: 10.1089/hum.2005.16.1175. [DOI] [PubMed] [Google Scholar]
- 10.Bousquet C, et al. Somatostatin receptors and regulation of cell proliferation. Dig Liver Dis 36 Suppl. 2004;1:S2–7. doi: 10.1016/j.dld.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 12.Adams RL, Adams IP, Lindow SW, Zhong W, Atkin SL. Somatostatin receptors 2 and 5 are preferentially expressed in proliferating endothelium. Br J Cancer. 2005;92:1493–1498. doi: 10.1038/sj.bjc.6602503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woltering EA. Development of targeted somatostatin-based antiangiogenic therapy: A review and future perspectives. Cancer Biother Radiopharm. 2003;18:601–609. doi: 10.1089/108497803322287691. [DOI] [PubMed] [Google Scholar]
- 14.Reubi JC, Waser B, Schaer JC, Laissue JA. Somatostatin receptor sst1-sst5 expression in normal and neoplastic human tissues using receptor autoradiography with subtype-selective ligands. Eur J Nucl Med. 2001;28:836–846. doi: 10.1007/s002590100541. [DOI] [PubMed] [Google Scholar]
- 15.Bousquet C, et al. Direct binding of p85 to sst2 somatostatin receptor reveals a novel mechanism for inhibiting PI3K pathway. EMBO J. 2006;25:3943–3954. doi: 10.1038/sj.emboj.7601279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell PM, et al. K-Ras promotes growth transformation and invasion of immortalized human pancreatic cells by Raf and phosphatidylinositol 3-kinase signaling. Cancer Res. 2007;67:2098–2106. doi: 10.1158/0008-5472.CAN-06-3752. [DOI] [PubMed] [Google Scholar]
- 17.Azar R, Najib S, Lahlou H, Susini C, Pyronnet S. Phosphatidylinositol 3-kinase-dependent transcriptional silencing of the translational repressor 4E-BP1. Cell Mol Life Sci. 2008;65:3110–3117. doi: 10.1007/s00018-008-8418-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lahlou H, Fanjul M, Pradayrol L, Susini C, Pyronnet S. Restoration of functional gap junctions through internal ribosome entry site-dependent synthesis of endogenous connexins in density-inhibited cancer cells. Mol Cell Biol. 2005;25:4034–4045. doi: 10.1128/MCB.25.10.4034-4045.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hagedorn M, et al. Accessing key steps of human tumor progression in vivo by using an avian embryo model. Proc Natl Acad Sci USA. 2005;102:1643–1648. doi: 10.1073/pnas.0408622102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papoutsi M, Sleeman JP, Wilting J. Interaction of rat tumor cells with blood vessels and lymphatics of the avian chorioallantoic membrane. Microsc Res Tech. 2001;55:100–107. doi: 10.1002/jemt.1161. [DOI] [PubMed] [Google Scholar]
- 21.Ren B, Yee KO, Lawler J, Khosravi-Far R. Regulation of tumor angiogenesis by thrombospondin-1. Biochim Biophys Acta. 2006;1765:178–188. doi: 10.1016/j.bbcan.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Almog N, et al. Transcriptional switch of dormant tumors to fast-growing angiogenic phenotype. Cancer Res. 2009;69:836–844. doi: 10.1158/0008-5472.CAN-08-2590. [DOI] [PubMed] [Google Scholar]
- 23.Mazan-Mamczarz K, et al. Post-transcriptional gene regulation by HuR promotes a more tumorigenic phenotype. Oncogene. 2008;27:6151–6163. doi: 10.1038/onc.2008.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu MP, et al. A novel role of thrombospondin-1 in cervical carcinogenesis: Inhibit stroma reaction by inhibiting activated fibroblasts from invading cancer. Carcinogenesis. 2008;29:1115–1123. doi: 10.1093/carcin/bgn077. [DOI] [PubMed] [Google Scholar]
- 25.Torrisani J, et al. Identification of an upstream promoter of the human somatostatin receptor, hSSTR2, which is controlled by epigenetic modifications. Endocrinology. 2008;149:3137–3147. doi: 10.1210/en.2007-1525. [DOI] [PubMed] [Google Scholar]
- 26.Kalas W, et al. Oncogenes and angiogenesis: Downregulation of thrombospondin-1 in normal fibroblasts exposed to factors from cancer cells harboring mutant ras. Cancer Res. 2005;65:8878–8886. doi: 10.1158/0008-5472.CAN-05-1479. [DOI] [PubMed] [Google Scholar]
- 27.van Hagen PM, Dalm VA, Staal F, Hofland LJ. The role of cortistatin in the human immune system. Mol Cell Endocrinol. 2008;286:141–147. doi: 10.1016/j.mce.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Xu Y, Song J, Berelowitz M, Bruno JF. Estrogen regulates somatostatin receptor subtype 2 messenger ribonucleic acid expression in human breast cancer cells. Endocrinology. 1996;137:5634–5640. doi: 10.1210/endo.137.12.8940394. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.