Abstract
Z- and E-phosphonate analogues 12 and 13 derived from cyclopropavir and the corresponding cyclic phosphonates 14 and 15 were synthesized and their antiviral activity was investigated. The 2,2-bis(hydroxymethylmethylenecyclopropane acetate (17) was transformed to tetrahydropyranyl acetate 18. Deacetylation gave intermediate 19 which was converted to bromide 20. Alkylation with diisopropyl methylphosphonate afforded after protecting group exchange (21 to 22) acetylated phosphonate intermediate 22. Addition of bromine gave the dibromo derivative 16 which was used in the alkylation-elimination procedure with 2-amino-6-chloropurine to give Z- and E-isomers 23 and 24. Hydrolytic dechlorination coupled with removal of all protecting groups gave the guanine phosphonates 12 and 13. Cyclization afforded the cyclic phosphonates 14 and 15. Z-Phosphonate 12 was a potent and non-cytotoxic inhibitor of human and murine cytomegalovirus (HCMV and MCMV) with EC50 2.2-2.7 and 0.13 μM, respectively. It was also an effective agent against Epstein-Barr virus (EBV, EC50 3.1 μM). The cyclic phosphonate 14 inhibited HCMV (EC50 2.4-11.5 μM) and MCMV (EC50 0.4 μM) but it was ineffective against EBV. Both phosphonates 12 and 14 were as active against two HCMV Towne strains with mutations in UL97 as they were against wild-type HCMV thereby circumventing resistance due to such mutations. Z-Phosphonate 12 was a moderate inhibitor of replication of herpes simplex virus types 1 and 2 (HSV-1 and HSV-2) but it was a potent agent against varicella zoster virus (VZV, EC50 2.9 μM). The cyclic phosphonate 14 lacked significant potency against these viruses. E-isomers 13 and 15 were devoid of antiviral activity.
Keywords: Methylenecyclopropanes, Phosphonates, Alkylation-eliminatiom, Antiviral agents, Cyclopropavir, UL97 phosphotransferase
1. Introduction
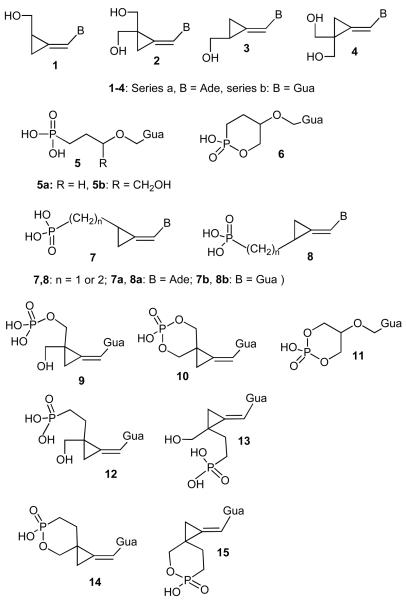
Methylenecyclopropane analogues of nucleosides are established antiviral agents, particularly effective against herpesviruses such as cytomegalovirus (CMV), Epstein-Barr virus (EBV), human herpes virus 6 and 8 (HHV-6 and HHV-8).1 The most potent are the Z-isomers of purine nucleosides 1 and 2 although the individual E-isomers 3 and 4 (Chart 1) are active against EBV, especially in the series of fluorinated analogues.2,3 The Z-guanine analogue 2b, cyclopropavir, is under preclinical development as a potential drug against human cytomegalovirus (HCMV) infections.4-6 It is accepted that methylenecyclopropane analogues follow the intracellular activation process (monophosphate - diphosphate - triphosphate) generally established for nucleosides and their analogues. In several cases, metabolically stable mimics of nucleoside phosphates, phosphonates, have yielded antiviral agents.7,8 These include phosphonate derivatives9-12 of antiherpetic drugs acyclovir (Zovirax), ganciclovir (Cytovene) 5a, 5b. and the cyclic phosphonate 6. By contrast, phosphonates of methylenecyclopropanes 7 and 8 did not exhibit antiviral potency13 with a single exception of compound 7b (n = 1) which was a moderate inhibitor of replication of varicella zoster virus (VZV).
Chart 1.
Whereas the cyclopropavir phosphate (9) is an effective prodrug of the parent compound 2b, the pattern of antiviral activity of the cyclic phosphate 10 is different.14 Although it had limited potency against HCMV in Towne strain of the virus, it was effective against AD169 strain with efficacy comparable to the cyclic phosphate of ganciclovir 11. Compound 10 also exhibited potent activity against hepatitis B virus (HBV). It was therefore of interest to synthesize phosphonate analogues 12 - 15 and investigate their antiviral activity.
2. Results and discussion
2.1. Synthesis
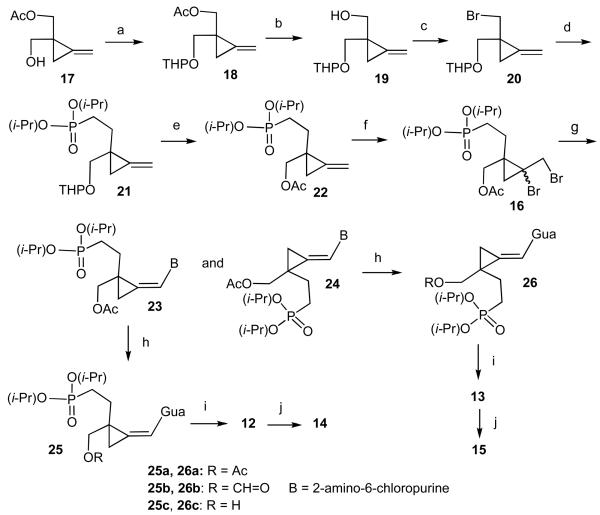
Alkylation-elimination method which had been successfully exploited13 for synthesis of phosphonates 7 and 8 formed also the basis of our approach to analogues 12 - 15. The suitably protected reagent 16 for alkylation-elimination was prepared as follows (Scheme 1). The previously described15 monoacetate 17 was converted to tetrahydropyranyl (THP) derivative 18 in 82% yield using 3,4-dihydro-2H-pyran in CH2Cl2 under acid catalysis. Ammonolysis afforded intermediate 19 (98%). The latter was converted to bromide 20 in 76% yield by CBr3-triphenylphosphine reagent. This reagent is not compatible16 with acid-labile groups like THP but inclusion of triethylamine in the reaction mixture successfully removed this obstacle. This modification may significantly expand use of the reagent. Reaction of 20 with lithium salt of diisopropyl methylphosphonate in THF gave the phosphonate intermediate 21 (81%). The presence of THP group was considered a potential liability for the bromination step and, therefore, the THP was replaced with acetyl in a single step17 using acetyl chloride in CH2Cl2 to give acetate 22 in 92% yield. Addition of bromine using pyridinium perbromide in CH2Cl2 was uneventful to provide dibromo derivative 16 as a mixture of cis,trans isomers (92%).
Scheme 1.
Reagents and conditions: (a) 3,4-Dihydro-2H-pyran, MeSO3H, CH2Cl2; (b) NH3, MeOH, Δ; (c) CBr4, Ph3P, NEt3, CH2Cl2; (d) CH3P(O)(i-PrO)2, BuLi, THF; (e) AcCl, CH2Cl2; (f) Pyridine.HBr3, CH2Cl2; (g) 1. B-H, Cs2CO3, DMF, Δ. 2. Chromatography; (h) 80% HCO2H, Δ; (i) 1. Me3SiBr, DMF. 2. NH4OH. 3. Chromatography; (j) N,N′-Dicyclohexyl-4-mopholinecarboxamidine, DCC, pyridine. 2. NH4OH. 3. Dowex 50 (H(+)).
Alkylation-elimination of 2-amino-6-chloropurine with 16 (Cs2CO3, DMF, 75 °C, 20 h) furnished Z- and E-isomers 23 and 24 which were separated by column chromatography on silica gel in 31 and 30% yield, respectively. Hydrolytic dechlorination of the Z-isomer 23 by 80% formic acid afforded after chromatographic separation a mixture of acetyl and formyl esters 25a + 25b in the ratio of 4 : 1 and 89% yield. A smaller amount (4%) of deacylated phosphonate 25c was also obtained. In this case, conversion to guanine moiety was accompanied by a partial deacetylation followed by formylation. Formylation of hydroxy groups in the course of this procedure was observed before.19 Dealkylation of 25a + 25b with trimethylsilyl bromide in DMF followed by ammonolysis, chromatography on DEAE Sephadex in NH4HCO3 buffer and then Dowex 1 (HCO2(−)) in formic acid gave phosphonate 12 in 81% yield as a free acid. Cyclization was performed using a protocol previously employed for the corresponding cyclic phosphate1410 with N,N′-dicyclohexyl-4-morpholinecarboxamidine and N,N′-dicyclohexylcarbodiimide (DCC) in pyridine. Cyclic phosphonate 14 was obtained as a free acid with the aid of Dowex 50 (H(+)) in 85% yield. In a similar fashion, hydrolytic dechlorination of the E-isomer 24 furnished a mixture of acetyl and formyl esters 26a + 26b in the ratio of 4 : 1 and 88% yield as well as deacetylated product 26c (5%). Dealkylation, ammonolysis and work-up employed for the Z-isomer 12 gave the E-phosphonate 13 (80%). Cyclization as described above then afforded the cyclic E-phosphonate 15 in 82% yield.
2.2. The Z- and E-isomeric Assignment
The UV spectra of intermediates 23, 24 (lmax 311 nm) and final products 12, 13 (lmax 267-268 nm) have indicated that the phosphonylated side-chain is attached in the 9 position of the purine base as found also for phosphonates13 7 and 8. As far as the Z/E isomerism is concerned, a general trend13 that Z-isomers are less polar (moving faster on silica gel) than E-isomers was also observed with compounds 23 and 24. In addition, the C3′ chemical shifts of the Z-isomers are more shielded than those of E-isomers (Table 1). This was also found in analogues 7 and 8 and their derivatives.13 However, the reversed pattern of the C4′ chemical shifts found in analogues 7 and 8 was not followed possibly because of a quarternary character of C4′. Final confirmation of the Z- and E-assignment came from the NOE experiments with compounds 23 and 24 (Table 2). In the Z-isomer 23, the NOE enhancements were observed between the cis-related atoms such as the H8 of the heterocyclic base in an anti-like conformation and H4′′ and H5′ and between the H1′ and H3′. As expected, the strongest interactions in the E-isomer 22 were observed between the H8 and H3′ as well as H1′ and H4′′, H6′, H5′. A weaker NOE enhancements were found between the H1′ and CH3 of the acetyl and isopropyl groups.
Table 1.
Comparison of the C3′ 13C NMR chemical shifts of isomeric methylenecyclopropane phosphonates
Table 2.
NOE data of the Z- and E-isomers 23 and 24 (500 MHz, CDCl3)
 | |||||
|---|---|---|---|---|---|
| Compound | Hirr | d | □obs | □ | .□□□ |
| 23 | H8 | 8.10 | CH3 of Ac | 1.97 | 1.50, 2.66a |
| H8 | 8.10 | H5′ | 2.20 | 1.81 | |
| H8 | 8.10 | H4″ | 3.83 | 0.84 | |
| H5′ | 2.20 | H8 | 8.10 | 3.85, 4.73a | |
| H4″ | 3.83 | H8 | 8.10 | 0.85, 0.95a | |
| H3′ | 1.46 | H1′ | 7.26 | 2.43, 4.24a | |
| H3′ | 1.39 | H1′ | 7.26 | 1.47, 2.89a | |
| H1′ | 7.26 | H3′ | 1.46 | 0.55 | |
| H1′ | 7.26 | H3′ | 1.39 | 0.51 | |
| H1′ | 7.26 | H3′ | 1.39 + 1.46 | 0.64 | |
| 24 | H8 | 8.17 | H3′ | 1.66 | 1.54 |
| H8 | 8.17 | H3′ | 1.58 | 1.37 | |
| H3′ | 1.66 | H8 | 8.17 | 2.16, 3.82a | |
| H3′ | 1.58 | H8 | 8.17 | 2.28, 3.16a | |
| H1′ | 7.46 | H4″ | 4.16 | 0.04 | |
| H1′ | 7.46 | H4″ | 4.03 | 0.28 | |
| H1′ | 7.46 | H6′, H5′ | 1.84 | 1.10 | |
| H1′ | 7.46 | CH3 of Ac | 2.09 | 0.17 | |
| H1′ | 7.46 | CH3 of i-Pr | 1.30 | 0.21 | |
| CH3 of Ac | 2.09 | H1′ | 7.46 | 0.13 | |
Two different scans.
2.3. Antiviral Activity
All phosphonates were tested against the following viruses: Human cytomegalovirus (HCMV), murine cytomegalovirus (MCMV), herpes simplex virus types 1 and 2 (HSV-1, HSV-2), varicella zoster virus (VZV), Epstein-Barr virus (EBV), hepatitis B virus (HBV) and hepatitis C virus (HCV). As mentioned at the outset, the first series of phosphonates of methylenecyclopropanes 7 and 8 designed as analogues of acyclovir phosphonates 5a lacked any significant antiviral activity.13 It is therefore surprising (and rewarding) that among analogues 12 - 15 new potent antivirals were found. Thus, the Z-isomeric phosphonate 12 and the corresponding cyclic derivative 14 which can be regarded as mimics of ganciclovir phosphonate 5b and cyclic phosphonate 6, are potent and non-cytotoxic inhibitors of replication of HCMV comparable to ganciclovir (Table 3). Analogues 12 and 14 were virtually equipotent against the Towne strain of HCMV with EC50's 2.2 and 2.4 μM, respectively. Against the AD169 strain, phosphonate 12 was somewhat more effective (EC50 2.7 μM) than the cyclic derivative 14 (EC50 11.6 μM). In comparison with the cyclic phosphate14 10, cyclic phosphonate 14 was significantly more effective in Towne strain and somewhat less potent in AD169 strain. Both phosphonates 12 and 14 inhibited murine cytomegalovirus (MCMV) with EC50 0.13 and 0.4 μM, respectively, surpassing the cyclic phosphate14 10. Comparison with cyclopropavir4 (2b) reveals reduced potency of the phosphonates 12 and 14 against HCMV in vitro but against MCMV the efficacy was about the same (Table 3). A distinct advantage of phosphonates 12 and 14 is circumventing the first step of activation (phosphorylation) of cyclopropavir (2b) most likely catalyzed by HCMV UL97 phosphotransferase.5 Therefore, analogues 12 and 14 may overcome resistance of cyclopropavir (2b) caused by mutations in this enzyme.19,20 To test this hypothesis, cyclopropavir (2b) and its phosphonates 12 and 14 were assayed in two strains of Towne HCMV with mutations in UL97 that are known to produce resistance to cyclopropavir.19,20 Strain E8 has two point mutations19 in UL97 whereas strain 2696r has most of UL97 sequence deleted.20 In both strains, cyclopropavir (2b) was 13 to 47 times less active than in wild-type Towne strain (Table 4). In contrast, phosphonates 12 and 14 were as active against both mutant strains as they were against wild-type Towne strain of HCMV. We conclude, therefore, that 12 and 14 do circumvent the necessity of UL97 to phosphorylate cyclopropavir (2b) to produce their activity.
Table 3.
Inhibition of human and murine cytomegalovirus (HCMV and MCMV) and Epstein-Barr virus (EBV) replication by methylenecyclopropane phosphonates
| EC50/CC50 (μM) |
||||
|---|---|---|---|---|
| HCMV/HFF |
||||
| Compound | Townea,b | AD169c,d | MCMV/MEFa | EBV/Akatae |
| 2b | 0.46/>100f | 0.49/>380a,f | 0.27/>380f | 0.22/46g |
| 10 | 20/>100h | 6.0/>301a,h | 7.2/>301h | 0.96/150h,i |
| 12 | 2.2/>100 | 2.7/>300a | 0.13/100 | 3.1/>100 |
| 13 | >100/>100 | >300/>300 | - | >100/>100 |
| 14 | 2.4/>100 | 11.6/>300a | 0.4/>100 | >100/>100 |
| 15 | >100/>100 | >300/>300 | - | >100/>100 |
| Control | 2.9/>100j | 0.09/>100a,j | 2.6/>100j | 8.4/>100k |
Plaque reduction assay in HFF cultures.
Visual cytotoxicity of HFF's in cells unaffected by virus.
Cytopathic effect (CPE) assay.
Cytotoxicity by neutral red uptake.
DNA hybridization assay.
Ref 4.
Ref. 21.
Ref. 14.
Daudi cells, viral capsid antigen (VCA) assay. The EC50/CC50 in H-1 cells (DNA hybridization assay) was >20/>100 μM, cytotoxicity was determined in CEM cells.
Ganciclovir.
Acyclovir.
Table 4.
Activity of Phosphonates 12 and 14 against Drug Resistant HCMV
The cyclic phosphonate 14 may be either either a prodrug of 12 or have an entirely different mechanism of action. It is interesting that against EBV in Akata cells, only the Z-isomeric phosphonate 12 was effective (EC50 3.1 μM) whereas the corresponding cyclic phosphonate 14 was inactive. This precludes that the latter analogue can function as a prodrug of phosphonate 12 under the conditions of this assay. Interestingly, the cyclic phosphate 10 was active against EBV although the assays used were different.14
Against α-herpes viruses, the most potent activity was found for analogue 12 in varicella zoster virus (VZV), EC50 2.9 μM whereas cyclic phosphonate 14 and cyclopropavir4 (2b) were ineffective (Table 5). Interestingly, phosphonate 7b (n = 1) was also effective against VZV but to a much lesser extent13 (EC50 24 μM) than 12. Similar to other methylenecyclopropane analogues,1 only moderate activity was found against herpes simplex virus types 1 and 2 (HSV-1 and HSV-2). Phosphonate 12 was the most effective against HSV-1 in a plaque assay in BSC-1 cells (EC50 15 μM) but it was less potent against HSV-1 and HSV-2 in a cytopathic effect (CPE) inhibition assay in HFF cultures. Regardless, we hypothesize that the activity of 12 against the α-herpes viruses is the result of delivering this monophosphate analogue into virus-infected cells thereby circumventing the necessity of an initial phosphorylation step by a viral-specified kinase. This would explain the activity of 12 against the α-herpes viruses compared to the inactivity of 2b.
Table 5.
Inhibition of herpes simplex viruses types 1 and 2 (HSV-1 and HSV-2) and varicella zoster virus (VZV) replication by methylenecyclopropane phosphonates
| EC50/CC50 (μM) |
||||
|---|---|---|---|---|
| Compound. | HSV-1/BSC-1a | HSV-1/HFFb,c | HSV-2/HFFb,d | VZV/HFFb,d |
| 2b | >100/>100e | >380/>380e | >380e | >380e |
| 10 | 20/>100f | >301/>301f | 242f | >301f |
| 12 | 15/>100 | 59.4/>300 | 76.5 | 2.9g |
| 13 | 100/>100 | >300/>300 | >300 | 191 |
| 14 | 40/>100 | >300/>300 | >300 | >300 |
| 15 | 100/>100 | >300/>300 | >300 | >300 |
| Acyclovir | 0.3 | 1.3/>300 | 1.2 | 4.4g |
ELISA in BSC-1 cells was used for compounds 2b and 12; other compounds were assayed by plaque reduction in BSC-1 cells. Cytotoxicity was determined in replicating KB cells.
Cytopathic effect (CPE) assay.
Cytotoxicity by neutral red uptake.
For cytotoxicity see HSV-1.
Ref. 4.
Ref. 14.
Plaque reduction assay.
All analogues including the cyclic phosphonate 14 were inactive against HBV and HCV. By contrast, the cyclic phosphate 10 is an effective anti-HBV agent.14 The E-isomers 13 and 15 were devoid of potency against all tested viruses.
3. Conclusion
Phosphonates 12, 13, 14 and 15 were synthesized and they were evaluated for antiviral activity. The Z-phosphonates 12 and 14 were effective inhibitors of replication of HCMV and MCMV in HFF and MEF culture. Compounds 12 and 14 also inhibited two Towne strains of HCMV with mutations in UL97. Phosphonate 12 was effective against EBV in Akata cells and VZV in HFF culture whereas cyclic phosphonate 14 was inactive. Analogue 12 was a moderate inhibitor of HSV-1 and HSV-2. The E-isomers 13 and 15 were devoid of antiviral activity.
4. Experimental
4.1. General Methods
The UV spectra were measured in ethanol and NMR spectra were determined on Varian instruments at 300, 400 or 500 MHz (1H), 75 or 100 MHz (13C) and 121 or 161 MHz (31P) in CDCl3 unless stated otherwise. Mass spectra were determined in electrospray ionization (ESI-MS) mode using methanol - sodium acetate or by negative ESI-MS.
4.2. 2-Acetoxymethyl-2-(tetrahydropyranyloxy)methyl-1-methylenecyclopropane (18)
To a solution of monoacetate17 17 (5.0 g, 32.04 mmol) and 3,4-dihydro-2H-pyran (7.31 mL, 80.1 mmol) in CH2Cl2 (50 mL) was added methanesulfonic acid (0.03 mL, 0.46 mmol) in CH2Cl2 (2 mL) dropwise with stirring at 0 °C. The stirring was continued for 6 h. The reaction was quenched with triethylamine (0.07 mL, 0.50 mmol), the solvent was evaporated, the residue was dissolved in ethyl acetate (60 mL). The organic phase was washed with saturated aqueous NaHCO3 (3 × 20 mL) and brine (3 × 20 mL) whereupon it was dried (MgSO4) and the solvent was evaporated. The crude product was chromatographed on a silica gel column using ethyl acetate-hexane (0.5 : 10) to furnish compound 18 (6.3 g, 82%) as a sirup. 1H NMR (400 MHz) δ 5.52, 5.48 (2t, J = 2-3 Hz, 1H), 5.41 (s, 1H, CH2=), 4.63, 4.61 (2t, J = 3-4 Hz, 1H, CHO of THP), 4.17-4.05 (m, 2H, CH2OAc), 3.81 (m, 1H), 3.68 (t, J = 9.6 Hz, 1H, CH2OTHP), 3.46 (m, 1H), 3.39 (dd, J = 10.0, 3.4 Hz. 1H, CH2O of THP), 2.05 (s, 3H, CH3), 1.8-1.5 (cluster of m, 6H, 3×CH2 of THP), 1.29-1.26 (m, 2H, H3). 13C NMR (100 MHz) 171.3 (C=O), 135.5, 135.1 (C=), 105.11, 105.05 (CH2=), 98.6, 98.2 (CHO of THP), 69.2, 68.9, 66.51, 66.48, 62.3, 61.9 (CH2O), 30.8, 30.7, 25.68, 25.65, 19.6, 19.3 (3×CH2 of THP), 23.9, 23.8 (C2), 21.2 (CH3), 14.1, 13.9 (C3). ESI-MS 241 (7.4, M + H), 263 (100.0, M + Na). Anal. Calcd for C13H20O4: C, 64.98; H, 8.39. Found: C, 65.08; H, 8.35.
4.3. 2-Hydroxymethyl-2-(tetrahydropyranyloxy)methyl-1-methylenecyclopropane (19)
A solution of compound 18 (5.7 g, 23.74 mmol) in NH3/MeOH (30%, 100 mL) was stirred at 0 °C for 30 min and at room temperature for 16 h. The volatile components were evaporated and the product 19 obtained as a sirup (4.6 g, 98%) was used directly in the next step. For analysis, a sample of 19 was chromatographed on a silica gel column using ethyl acetate - hexane (1 : 5). 1H NMR (400 MHz) δ 5.50, 5.48 (2t, J = 2.4 Hz, 1H), 5.40 (poorly resolved t, 1H, CH2=), 4.63 (2 overlapped t, 1H, CHO of THP), 3.94-3.36 (cluster of m, 6H, CH2O), 2.69, 2.61 (2t, J = 6 Hz, 1H, OH), 1.84-1.52 (cluster of m, 6H, 3×CH of THP), 1.31-1.20 (m, 2H, H3). 13C NMR (100 MHz) 135.9, 135.5 (C=), 104.5, 104.4 (CH2=), 99.22, 99.15 (CHO of THP), 72.2, 71.9, 67.29, 67.27, 62.81, 62.7 (CH2O), 30.83, 30.78, 25.5, 19.9, 19.8 (3×CH2 of THP), 26.6, 26.5 (C2), 14.1, 14.0 (C3). ESI-MS 199 (5.0, M + H), 221 (100.0, M + Na). Anal. Calcd for C11H18O3: C, 66.64; H, 9.15. Found: C, 66.34, H, 9.38.
4.4. 2-Bromomethyl-2-(tetrahydropyranyloxy)methyl-1-methylenecyclopropane (20)
Triphenylphosphine (25.15 g, 95.9 mmol) in CH2Cl2 (25 mL) was added to a solution of CBr4 (31.8 g, 95.9 mmol) in CH2Cl2 (75 mL) at −5 °C with stirring which continued for another 10 min. Triethylamine (16.04 mL, 0.12 mol) was added, followed by a dropwise addition of compound3 19 (3.8 g, 19.18 mmol) in CH2Cl2 (15 mL) over a period of 10 min. Reaction was complete in 30 min. The reaction mixture was diluted with hexane (130 mL), the insoluble portion was filtered and it was washed with hexane (50 mL). The filtrate was concentrated and the crude product was chromatographed on a silica gel column using ethyl acetate - hexane (0.2 : 10) to furnish compound 20 (3.79 g, 76%) as a sirup. 1H NMR (400 MHz) δ 5.56, 5.54 (2t, J = 2.8-3.0 Hz, 1H), 5.37 (poorly resolved d, 1H, CH2=), 4.66, 4.64 (partially overlapped 2t, J = 3.6 Hz, 1H, CHO of THP), 3.89-3.47 (cluster of m, 6H, CH2Br, CH2O ), 1.89-1.49 (m, 6H, 3×CH2 of THP), 1.43-1.42 (m, 1H), 1.31-1.28 (2 poorly resolved t, 1H, H3).
13C NMR (100 MHz) 137.6, 137.5 (C=), 104.83, 104.79 (CH2=), 98.9, 98.5 (CHO of THP), 69.1, 68.9, 62.5, 62.1 (CH2O), 39.1 (CH2Br), 30.8, 30.7, 25.67, 25.65, 19.7, 19.4 (3×CH2 of THP), 26.1, 26.0 (C2), 17.2, 17.1 (C3). ESI-MS 283, 285 (98.8, 100.0, M + Na). Anal. Calcd for C11H17BrO2×0.05 H2SiO3: C, 49.84; H, 6.50. Found: C, 49.90; H, 6.47.
4.5. 2-[(2-Diisopropylphosphono)ethyl]-2-(tetrahydropyranyloxymethyl-1-methylenecyclopropane (21)
1-Butyllithium (1.6 M in hexanes, 12.1 mL, 19.29 mmol) was added to a solution of diisopropyl methylphosphonate (3.35 mL, 18.15 mmol) in THF (25 mL) at −78 °C with stirring which was continued for 30 min. Compound 20 (2.95 g, 11.34 mmol) in THF (15 mL) was then slowly added over a period of 10 min and the reaction mixture was stirred for 2 h at −78 °C. The reaction mixture was then allowed to warm to room temperature and, after 30 min, it was quenched with saturated aqueous NH4Cl (15 mL). Ethyl acetate (80 mL) was added, the organic phase was washed with brine (3 × 30 mL), saturated aqueous NaHCO3 (3 × 30 mL) and brine (3 × 30 mL). After drying (MgSO4), the solvent was evaporated and the crude product was chromatographed on a silica gel column using ethyl acetate - hexane (2 : 1) to furnish phosphonate 21 (3.3 g, 81%) as a sirup. 1H NMR δ 5.44, 5.41 (poorly resolved 2t, 1H), 5.35 (s, 1H, CH2=), 4.70-4.57 (m, 3H, CH of i-PrO, CHO of THP), 3.82 (m, 1H), 3.47 (m, 1H, CH2O of THP), 3.66, 3.22 and 3.59, 3.32 (2AB's, J = 10.4 and 10.6 Hz, 2H, CH2OTHP), 1.93-1.49 (cluster of m, 10H, CH2 of THP, H4 and H5), 1.30-1.28 (2 poorly resolved d, 12H, CH3), 1.17- 1.04 (m, 2H, H3). 13C NMR 138.3, 137.7 (C=), 104.0, 103.8 (CH2=), 98.6, 98.1 (CHO of THP), 71.3, 70.7 (CH2O of THP), 70.0 (d, J = 6.7 Hz, CH of i-PrO), 62.4, 62.0 (CH2OTHP), 30.8, 30.7, 25.68, 25.65, 19.7, 19.4 (CH2 of THP), 26.84, 26.80 (2 overlapped d, J = 4.5 Hz, C4), 24.58, 24.54 (2d, J = 140.2 Hz, C5), 24.57, 24.36 (C2), 24.3 (d, J = 3.8 Hz, CH3), 15.1, 14.7 (C3). 31P NMR (161 MHz) 31.24. ESI-MS 361 (6.3, M + H), 283 (100.0, M + Na). Anal. Calcd for C18H33O5P: C, 59.98; H, 9.23. Found: C, 60.24, H, 9.44.
4.6. 2-(Acetoxymethyl)-2-[2-(diisopropylphosphono)ethyl]-1-methylenecyclopropane (22)
Acetyl chloride (8.9 mL, 0.13 mol) was added to a solution of phosphonate 21 (3.0 g, 8.33 mmol) in CH2Cl2 (50 mL). The reaction mixture was stirred at room temperature for 6 h, it was concentrated to a half of its original volume and the reaction was quenched with saturated aqueous NaHCO3 (30 mL). Ethyl acetate (50 mL) was then added and the organic layer was washed with saturated aqueous NaHCO3 (3 × 25 mL) and brine (3 × 25 mL). After drying (MgSO4), the solvents were evaporated and the crude product was chromatographed on a silica gel column using ethyl acetate - hexane (1 : 1) to give acetate 22 (2.44 g, 92%) as a sirup. 1H NMR (400 MHz) δ 5.47, 5.40 (2 poorly resolved t, 2H, CH2=), 4.67 (m, 2H, CH of i-PrO), 3.97 (s, 2H, CH2OAc), 2.06 (s, 3H, CH3 of Ac), 1.92-1.62 (cluster of m, 4H, H4 and H5), 1.30 (d, J = 2.4 Hz), 1.29 (d, 1.6 Hz, 12H, CH3 of i-PrO), 1.20, 1.11 (poorly resolved t of AB, JAB = 10 and 9.2 Hz, 2H, H3). 13C NMR (100 MHz) 171.2 (C=O), 136.7 (C=), 104.8 (CH2=), 70.1 (d, J = 6.7 Hz, CHO of i-PrO), 68.0 (CH2OAc), 26.6 (d, J = 4.5 Hz, H4), 24.4 (d, J = 140.9 Hz, H5), 24.2 (d, J = 4.4 Hz, CH3 of i-PrO), 23.5 (d, J = 21.5 Hz, C2), 21.1 (CH3 of Ac), 14.9 (C3). 31P NMR (161 MHz) 30.57. ESI-MS 319 (13.3, M + H), 341 (100.0, M + Na). Anal. Calcd for C15H27O5P: C, 56.59; H, 8.55. Found: C, 56.56, H, 8.64.
4.7. (cis,trans)-2-(Acetoxymethyl)-2-[2-(diisopropylphosphono)ethyl]-1-bromo-1-bromomethylcyclopropane (16)
Pyridinium hydrobromide perbromide (3.32 g, 10.37 mmol) was added in three portions to a solution of acetate 22 (2.2 g, 6.92 mmol) in CH2Cl2 (50 mL) at-20 °C. Reaction mixture was stirred for 1 h whereupon it was diluted with diethyl ether (50 mL). The precipitate was filtered off and it was washed with diethyl ether (25 mL). The organic phase was washed with saturated aqueous Na2S2O3 (3 × 25 mL), water (3 × 25 mL) and brine (3 × 25 mL). The solvents were evaporated to give dibromophosphonate 16 (3.03 g, 92%) as a sirup. 1H NMR (400 MHz) δ 4.69 (m, 2H, CH of i-PrO), 4.43-3.68 (4 overlapped AB's, 4H, CH2Br, CH2OAc), 2.10, 2.08 (2s, 3H, CH3 of Ac), 2.27-2.19, 2.04-1.67 (cluster of m, 4H, H4 and H5), 1.24-1.36 (cluster of d, CH3 of i-PrO), 1.12 (d, J = 7.6 Hz, part of H3 obscured by CH3, total 14H). 31P NMR (161 MHz) 29.55, 29.44. ESI-MS 477, 479, 481 (M + H, 9.6, 16.7, 7.4), 499, 501, 503 (M + Na, 47.5, 100.0, 49.4), Anal. Calcd for C15H27Br2O5P: C, 37.68; H, 5.69. Found: C, 37.72, H, 5.73.
4.8. (Z)- and (E)-2-Amino-6-chloro-9-{[2-(2-diisopropylphosphonoethyl)-2-(acetoxymethyl)cyclopropylidene]methyl}purine (23 and 24)
A mixture of dibromophosphonate 16 (2.7 g, 5.67 mmol), 2-amino-6-choropurine (0.96 g, 5.67 mmol) and Cs2CO3 (9.24 g, 28.36 mmol) in DMF (30 mL) was stirred at room temperature for 5 h and at 75 °C for 20 h. After cooling, the insoluble portion was filtered off and it was washed with DMF (10 mL). The solvent was evaporated in vacuo and the crude product was chromatographed on a silica gel, using methanol - CH2Cl2 (0.3 : 10) to obtain the Z-isomer 23 (850 mg, 31%) followed by E-isomer 24 (820 mg, 30%).
Z-isomer 23
Mp 174-176 °C. UV λmax 311 nm (ε 7,700), 231 (ε 26,700). 1H NMR (300 MHz, CD3SOCD3) δ 8.16 (s, 1H, H8), 7.28 (s, 1H, H1′), 7.05 (bs, 2H, NH2), 4.46 (m, 2H, CH of i-PrO), 4.27, 4.02 (AB, J = 11.7 Hz, 2H, H4″), 2.05-1.74 (m, 2H, H6′, overlapped with 1.89 (s, CH3 of Ac, 3H), 1.68-1.42 (m, H5′ partially overlapped with split AB, J = 9.2 Hz, 4H, H3′), 1.19-1.12 (m, 12H, CH of i-PrO). 13C NMR (75 MHz) 170.6 (C=O, Ac), 160.8, 153.2, 150.4, 140.9, 123.9 (purine), 120.6 (C2′), 112.4 (C1′), 70.0 (d, J = 6 Hz, CH of i-PrO), 68.2 (C4″), 26.5 (d, J = 22.2 Hz, C5′), 25.2 (d, J = 2.8 Hz, C4′), 24.4-24.3 (2 overlapped d, J = 4.0 Hz, CH3 of i-PrO), 22.8 (d, J = 140.1 Hz, C6′), 21.0 (CH3 of Ac), 12.3 (C3′). 31P NMR (121 MHz) 30.57. ESI-MS 486, 488 (M + H, 14.4, 3.8). 508, 510 (M + Na, 100.0, 33.7). Anal. Calcd for C20H29ClN5O5P: C, 49.44; H, 6.02; N, 14.41. Found: C. 49.62; H, 6.05; N, 14.33.
E-isomer 24
Mp 86 °C. UV λmax 311 (ε 7,400), 229 nm (ε 28,600). 1H NMR (400 MHz, CD3SOCD3) δ 8.42 (s, 1H, H8), 7.43 (s, 1H, H1′), 7.01 (bs, 2H, NH2), 4.51 (m, 2H, CH of i-PrO), 4.11, 3.97 (AB, J = 11.2 Hz, 2H, H4″), 2.03 (s, 3H, CH3 of Ac), 1.82-1.59 (s + cluster of m, 6H, H5′ and H6′ overlapped with H3′), 1.20 (d, J = 6 Hz, 12H, CH of i-PrO). 13C NMR (100 MHz) 171.0 (C=O), 160.8, 153.3, 150.3, 140.2, 123.8 (purine), 120.0 (C2′), 111.8 (C1′), 69.9 (d, J = 5.9 Hz), 67.2 (C4″), 26.6 (d, J = 5 Hz, C4′), 24.5, 24.4, 24.2 (2 overlapped d, C5′ and CH3 of i-PrO), 23.9 (d, J = 138.7 Hz, C6′), 21.4 (CH3 of Ac), 17.0 (C3′). 31P (161 MHz) 30.21. ESI-MS 486, 488 (M + H, 19.1, 5.0). 508, 510 (M + Na, 100.0, 19.8, 32.3). Anal. Calcd for C20H29ClN5O5P: C, 49.44; H, 6.02; N, 14.41. Found: C, 49.34; H, 5.99; N, 14.38.
4.9. (Z)-9-{[2-(2-diisopropylphosphono)ethyl)-2-(acetoxy/formyloxy/methyl)cyclopropylidene]methyl}guanine (25a + 25b) and (Z)-9-{[2-(2-diisopropylphosphono)ethyl)-2-(hydroxymethyl)cyclopropylidene]methyl}guanine (25c)
A solution of compound 23 (600 mg, 1.24 mmol) in formic acid (80%, 30 mL) was heated at 70 ° for 6 h. Formic acid was evaporated in vacuo, the residue was dissolved in water and the solution was lyophilized. The crude product was chromatographed on a silica gel column using methanol - CH2Cl2 (0.5 : 10) to obtain a mixture of acetate and formate 25a + 25b (510 mg, 89 %). The ratio 25a/25b determined from the acetyl and formyl 1H NMR signals was 4 : 1. Further elution of the column using methanol - CH2Cl2 (1 : 5) gave hydroxymethyl phosphonate 25c. Rechromatography in methanol - CH2Cl2 (0.5 : 10) afforded 27c (21 mg, 4%).
Z-isomers 25a + 25b
UV λmax 271, 229 nm. 1H NMR (300 MHz, CD3SOCD3) δ 10.70 (s, 1H, NH), 8.25 (s, 0.25H, CH=O), 7.78, 7.77 (2 overlapped s, 1H, H8), 7.19, 7.17 (2 overlapped s, 1H, H1′), 6.56 (s, 2H, NH2), 4.47-4.45 (m, 2H, CH of i-PrO), 4.30, 3.98 (2 overlapped AB's, J = 11.4 Hz, 2H, H4″), 2.01, 1.95 (2 overlapped s, 2.25H, CH3 of Ac), 1.72-1.19 (cluster of m, 6H, H5′, H6′, H3′), 1.19-1.13 (cluster of d, 12H, CH3 of i-PrO). 13C NMR (100 MHz) 170.7 (C=O of Ac), 162.7 (CH=O), 157.3, 154.7, 150.6, 134.7, 118.9, 118.3, 117.1, 112.6, 112.4 (guanine, C2′, C1′), 71.0, 70.0 (2 overlapped d, J = 5.2 Hz, CH of i-PrO), 68.1, 67.6 (C4″), 26.1 (2 overlapped d, J = 21.6 and 22.1 Hz, C5′), 25.3 (poorly resolved d, C4′), 24.4, 24.36, 24.6 (2 overlapped d, CH3 of i-PrO), 23.0 (d, J = 141.9 Hz), 22.9 (d, J = 140.4 Hz, C6′), 21.1 (CH3 of Ac), 12.4 (C3′). 31P NMR (121 MHz) 30.29, 30.19. ESI-MS 454 (M + H, 25b, 30.0), 468 (M + H, 25a, 100.0), 476 (M + Na, 25b, 32.3), 490 (M + Na, 25a, 100.0).
Z-Isomer 25c
Mp 232-234 °C. UV λmax 273 (ε 12,300), 231 nm (ε 30,100). 1H NMR (300 MHz, CD3SOCD3) δ 10.66 (bs, 1H, NH), 8.21 (s, 1H, H8), 7.09 (s, 1H, H1′), 6.57 (s, 2H, NH2), 5.25 (poorly resolved t, 1H, OH), 4.45 (m, 2H, CH of i-PrO), 3.80, 3.22 (poorly resolved split AB, J = 12.0 Hz, 2H, H4”), 2.01-1.94 and 1.69-1.32 (2 poorly resolved m, 4H, H5′, H6′), 1.31, 1.25 (AB, J = 8.7 Hz, 2H, H3′), 1.17-1.12 (poorly resolved m, 12H, CH3 of i-PrO). 13C NMR (75 MHz) 157.3, 154.7, 150.4, 134.5, 120.1 (guanine), 117.0 (C2′), 111.1 (C1′), 69.9 (d, J = 4.0 Hz, CH of i-PrO), 65.4 (C4″), 28.7 (d, J =21.1 Hz, C5′), 24.9 (d, J = 3.8 Hz, C4′), 24.4 (d, J = 4.0 Hz, CH3 of i-PrO), 23.1 (d, J = 141.0 Hz), 11.9 (C3′). 31P NMR (121 MHz) 30.57. ESI-MS 426 (M + H, 100.0), 448 (M + Na, 30.9). Anal. Calcd for C18H28N5O5P×0.2 H2O: C, 50.38; H, 6.99; N, 16.33. Found: C, 50.38; H, 6.65; N, 16.12.
4.10. (E)-9-{[2-(2-diisopropylphosphonoethyl)-2-(acetoxy/formyloxy/methyl)cyclopropylidene]methyl}guanine (26a + 26b) and (E)-9-{[2-(2-diisopropylphosphono)ethyl)-2-(hydroxymethyl)cyclopropylidene]methyl}guanine (26c)
The procedure described above for the Z-isomers 25a + 25b and 25c was repeated with the E-isomer 24 (600 mg, 1.24 mmol) to give 26a + 26b (502 mg, 88 %) and 26c (26 mg, 5%). The ratio 26a/26b determined as described above for 25a/25b was 4 : 1.
E-Isomers 26a + 26b
UV λmax 271, 229 nm. 1H NMR (300 MHz, CD3SOCD3) δ 10.67 (bs, 1H, NH), 8.29 (s, 0.2H, CH=O), 8.03 (s, 1H, H8), 7.33 (poorly resolved t, 1H, H1′), 6.53 (s, 2H, NH2), 4.52 (m, 2H, CH of i-PrO), 4.20, 4.12 and 4.11, 3.97 (2AB, J = 11.6 Hz, 2H, H4″), 2.04 (s, 2.4H, CH3 of Ac), 1.9-1.5 (m, 6H, H5′, H6′, H3′), 1.21, 1.20 (2 partly overlapped d, J = 6-6.3 Hz, CH of i-PrO).31P NMR (121 MHz, CD3SOCD3) 30.21. ESI-MS 454 (M + H of 26b, 11.8), 468 (M + H of 26a, 39.1), 476 (M + Na of 26b, 12.1), 490 (M + Na of 26a, 100.0).
E-Isomer 26c
Mp 154-156 °C. UV λmax 271 nm (ε 11,100), 228 (ε 27,800). 1H NMR (300 MHz, CD3SOCD3) δ 10.77 (bs, 1H, NH), 8.02 (s, 1H, H8), 7.27 (s, 1H, H1′), 6.61 (s, 2H, NH2), 4.85 (t, J = 5.6 Hz, 1H, OH), 4.52 (m, 2H, CH of i-PrO), 3.37 (H4‘’, overlapped with H2O), 1.94-1.58 (m, 4H, H5′, H6′), 1.49 (s, 2H, H3′), 1.22-1.19 (2 poorly resolved d, 12H, CH3 of i-PrO). 13C NMR (75 MHz) 157.4, 154.7, 150.6, 134.4, 120.2 (guanine), 117.0 (C2′), 111.0 (C1′), 69.9 (d, J = 7.0 Hz, CH of i-PrO), 65.0 (C4″), 27.0 (d, J = 20.2 Hz, H5′), 26.4 (d, J = 4.3 Hz, H4′), 24.1 (d, J = 139.0 Hz, H6′), 24.5 (d, J = 3 Hz, CH3 of i-PrO), 15.9 (C3′). 31P NMR (121 MHz) 30.84. ESI-MS 448 (M + Na, 100.0), 426 (M + H, 90.6). Anal. Calcd for C18H28N5O5P×1.2 H2O: C, 48.34; H, 6.71; N, 15.67. Found: C, 47.98; H, 6.46; N, 15.45.
4.11. (Z)-9-{[2-(Hydroxymethy)l-2-(2-phosphonoethyl)cyclopropylidene]methyl}guanine (12)
Bromotrimethylsilane (3.74 mL, 28.90 mmol) was added dropwise to a solution of phosphonate 25a + 25b (450 mg, 0.97 mmol) in DMF (20 mL) at room temperature with stirring which was continued for 24 h. The solvent was evaporated in vacuo and the residue was dissolved in aqueous NH4OH (30%, 30 mL). After stirring for 3 h at room temperature, the volatile components were evaporated and the aqueous solution of the crude product was lyophilized. It was chromatographed on DEAE Sephadex (40-120 mesh, HCO3(−)) column, using a linear gradient of 0 - 0.3 M (500 mL each) NH4HCO3. The fractions containing the compound 12 were lyophilized. The product was loaded on Dowex-1 column (X2, 200 mesh, HCO2(−)) which was eluted first with water (100 mL) followed by formic acid (0.8 M, 800 mL). The fractions containing the compound were lyophilized to give phosphonate 12 (286 mg, 81%) as a white solid, m: >300 °C. UV λ (pH 7) 268 nm (ε 12,800), 230 (ε 29,800). 1H NMR (400 MHz, D2O, sodium salt) δ 7.97 (s, 1H, H8), 7.05 (s, 1H, H1′), 3.80, 3.55 (AB, J = 12.2 Hz, 2H, H5″), 2.06, 1.73 (2m, 2H), 1.43, 1.41 (m overlapped with s, 4H, H5′, H6′, H3′. 13C NMR (100 MHz, CD3SOCD3) 157.3, 154.6, 150.3, 135.0, 120.3, 117.0, 110.8 (guanine, C2′, C1′), 65.4 (C4″), 29.01 (d, J = 20.2 Hz, C5′), 25.5 (d, J = 8.2 Hz, C4′), 24.6 (d, J = 136.5 Hz, C6′ ), 11.8 (C3′). 31P NMR (121 MHz, D2O, sodium salt) 23.83. Negative ESI-MS 340 (M - H). Anal. Calcd for C12H16N5O5P×0.8 H2O: C, 40.52; H 4.99; N, 19.69. Found: C, 40.50; H, 5.01; N, 19.59.
4.12. (E)-9-{[2-(Hydroxymethy)l-2-(2-phosphonoethyl)cyclopropylidene]methyl}guanine (13)
The E-isomer 13 was prepared from a mixture of acetate and formate 26a + 26b (450 mg, 0.98 mmol) as described above for Z-isomer 12 to give phosphonate 13 (279 mg, 80%) as a white solid, mp >300 °C. UV λmax (pH 7) 267 nm (ε 14,500), 230 (ε 38,800). 1H NMR (300 MHz, D2O) δ 8.23 (s, 1H, H8), 7.22 (s, 1H, H1′), 3.56, 3.48 (AB, J = 11.7 Hz, 2H, H4″), 1.84 (m, 1H), 1.68-1.54 (m, 3H, H5′, H6′), 11.47 (s, 2H, H3′). 13C NMR (100 MHz, CD3SOCD3) 157.4, 154.6, 150.5, 134.3, 120.7, 116.9, 110.6 (guanine, C2′, C1′), 65.1 (H4″), 27.5 (d, J = 20.2 Hz, C5′), 27.2 (C4′), 26.2 (d, J = 135.8 Hz, H6′), 16.0 (C3′). 31P NMR (121 MHz, D2O) 26.91. Negative ESI-MS 340 (M - H, 100.0). Anal. Calcd for C12H16N5O5P×0.9 H2O: C, 40.32; H, 5.02; N, 19.59. Found: C, 40.35; H, 5.15; N, 19.61.
4.13. Z-Cyclic Phosphonate 14
A mixture of phosphate 12 (150 mg, 0.44 mmol), DCC (727 mg, 3.52 mmol) and N,N′-dicyclohexyl-4-morpholinecarboxamidine (194 mg, 0.66 mmol) in pyridine (15 mL) was refluxed under N2 with stirring for 12 h. Pyridine was evaporated in vacuo and water (50 mL) was added to the residue. The aqueous layer was extracted with dichloromethane (5 × 25 mL) and it was filtered through a cotton plug. Aqueous NH3 (30%, 10 mL) was added and the resulting reaction mixture was stirred overnight at room temperature. The volatile components were evaporated and the product was absorbed on Dowex-50 column (WX 2, 200 mesh, H(+)) which was eluted with water (600 mL). The appropriate fractions were concentrated to give cyclic phosphonate 14 (121 mg, 85%) as a white solid, mp >300 °C. UV λmax (H2O) 268 nm (ε 12,800), 227 (ε 28,700). 1H NMR (300 MHz, D2O) δ 8.54 (s, 1H, H8), 7.06 (s, 1H, H1′), 4.10, 3.84 (2t, J = 10.8, 11.9 Hz, 2H, H4″), 2.00 (m, 1H), 1.83-1.39 (m, 3H, H5′, H6′), 1.54, 1.45 (AB, J = 9.3 Hz, 2H, H3′ partly overlapped with H5′, H6′). 13C NMR (100 MHz, CD3SOCD3) 157.3, 154.6, 150.3, 135.8, 117.4, 117.2, 113.3 (guanine, C2′, C1′), 73.2 (d, J = 5.0 Hz, H4″), 29.8 (d, J = 8.1 Hz, C4′), 25.4 (d, J = 8.2 Hz, C5′), 23.6 (d, J = 126.9 Hz, C6′), 15.7 (C3′). 31P NMR (121 MHz, D2O) 22.41. Negative ESI-MS 322 (M - H, 100.0). Anal. Calcd for C12H14N5O4P× H2O: C, 42.23; H, 4.73; N, 20.52. Found: C, 42.45; H, 4.83; N, 20.26.
4.14. E-Cyclic Phosphonate 15
The E-isomer 13 (150 mg, 0.44 mmol) was subjected to the same procedure as Z-isomer 12 (see above) to give cyclic phosphonate 15 (116 mg, 82%) as a white solid, mp >300 °C. UV λmax (H2O) 268 nm (ε 10,400), 229 (ε 28,500). 1H NMR (300 MHz, D2O, sodium salt) δ 7.96 (s, 1H, H8), 7.22 (s, 1H, H1′), 3.94, 3.86 (split AB, J = 12.9 Hz, 2H, H4″), 1.87 (dt, J = 18.9, 6.0 Hz, 2H, H6′), 1.74-1.63 (m, 2H, H5′), 1.57, 1.49 (split AB, 2H, H3′). 13C NMR (100 MHz, CD3SOCD3) 157.4, 154.7, 150.7, 134.3, 118.6, 116.9, 111.4 (guanine, C2′, C1′), 73.3 (d, J = 5.2 Hz), 29.8 (d, J = 7.5 Hz), 24.4 (d, J = 126.9 Hz, C6′), 22.8 (d, J = 8.2 Hz, C4′), 16.5 (C3′). 31P NMR (121 MHz, D2O, sodium salt) 22.52. Negative ESI-MS 322 (M - H, 100.0). Anal. Calcd for C12H14N5O4P×H2O: C, 42.23; H, 4.73; N, 20.52. Found: C, 42.25; H, 4.85; N, 20.17.
4.15. Antiviral Assays
The antiviral assays were performed as described previously.4,15 The HCMV assays were performed using HFF cell culture with two strains of virus, Towne and AD169, in a plaque reduction or cytopathic effect (CPE) inhibition assay. MCMV was assayed in mouse embryonic fibroblasts (MEF) by plaque reduction. The EBV DNA hybridization assay was run in Akata cells. The HSV-1 assays were performed in BSC-1 cells by ELISA and, together with HSV-2, in HFF cells by CPE inhibition assays. The VZV assays were run in HHF culture using CPE or plaque reduction assays. The results are summarized in Table 3 through 5.
Acknowledgments
We thank L. M. Hryhorczuk from the Central Instrumentation Facility, Department of Chemistry, Wayne State University for mass spectra. We also thank Kathy Borysko and Julie Breitenbach of the University of Michigan for performing the antiviral assays. The work described herein was supported by U. S. Public Health Service Grant RO1-CA32779 and contract NO1-AI30049 from the National Institutes of Health, Bethesda, Maryland.
References
- 1.Zemlicka J. In: Advances in Antiviral Drug Design. De Clercq E, editor. Elsevier; Amsterdam: 2007. pp. 113–165. [Google Scholar]
- 2.Zhou S, Kern ER, Gullen E, Cheng Y-C, Drach JC, Matsumi S, Mitsuya H, Zemlicka J. J. Med. Chem. 2004;47:6964. doi: 10.1021/jm040093l. [DOI] [PubMed] [Google Scholar]
- 3.Zhou S, Kern ER, Gullen E, Cheng Y-C, Drach JC, Tamiya S, Mitsuya H, Zemlicka J. J. Med. Chem. 2006;49:6120. doi: 10.1021/jm0607404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou S, Breitenbach JM, Borysko KZ, Drach JC, Kern ER, Gullen E, Cheng Y-C, Zemlicka J. J. Med. Chem. 2004;47:566. doi: 10.1021/jm030316s. [DOI] [PubMed] [Google Scholar]
- 5.Kern ER, Kushner NL, Hartline CB, Williams-Azziz SL, Harden EA, Zhou S, Zemlicka J, Prichard MN. Antimicrob. Agents Chemother. 2005;49:1039. doi: 10.1128/AAC.49.3.1039-1045.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kern ER, Bidanset DJ, Hartline CB, Yan Z, Zemlicka J, Quenelle DC. Antimicrob. Agents Chemother. 2004;48:4745. doi: 10.1128/AAC.48.12.4745-4753.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin JC, editor. Nucleotide Analogues as Antiviral Agents. American Chemical Society; Washington, D. C.: 1989. (ACS Symposium Series 401). [Google Scholar]
- 8.Holy A. In: In Recent Advances in Nucleosides: Chemistry and Chemotherapy. Chu CK, editor. Elsevier; Amsterdam: 2002. pp. 167–238. [Google Scholar]
- 9.Prisbe EJ, Martin JC, McGee DPC, Barker MF, Smee DF, Duke AE, Matthews TR, Verheyden JPH. J. Med. Chem. 1986;29:671. doi: 10.1021/jm00155a015. [DOI] [PubMed] [Google Scholar]
- 10.Reist EJ, Sturm PA, Pong RY, Tanga MJ, Sidwell RW. pp. 17–34. Ref. 7.
- 11.Reist EJ, Bradford WW, III, Ruhland-Frisch BL, Sturm PA, Zaveri NT, Huffman J, Sidwell RW. Nucleosides & Nucleotides. 1994;13:539. [Google Scholar]
- 12.Huffman JH, Sidwell RW, Morrison AG, Coombs J, Reist EJ. Nucleosides & Nucleotides. 1994;13:607. [Google Scholar]
- 13.Guan H-P, Qiu Y-L, Ksebati MB, Kern ER, Zemlicka J. Tetrahedron. 2002;58:6047. and references 5-13 cited therein. [Google Scholar]
- 14.Yan Z, Kern ER, Gullen E, Cheng Y-C, Drach JC, Zemlicka J. J. Med. Chem. 2005;48:391. doi: 10.1021/jm040149b. [DOI] [PubMed] [Google Scholar]
- 15.Li C, Prichard MN, Korba BE, Drach JC, Zemlicka J. Bioorg. Med. Chem. 2008;16:2148. doi: 10.1016/j.bmc.2007.11.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wagner A, Heitz M-P, Mioskowski C. Tetrahedron Lett. 1989;30:557. [Google Scholar]
- 17.Bakos T, Vincze I. Synthetic Commun. 1989;19:523. [Google Scholar]
- 18.Qiu Y-L, Ksebati MB, Ptak RG, Fan BY, Breitenbach JM, Lin J-S, Cheng Y-C, Kern ER, Drach JC, Zemlicka J. J. Med. Chem. 1998;41:1019. doi: 10.1021/jm9705723. [DOI] [PubMed] [Google Scholar]
- 19.Breitenbach JM, Borysko KZ, Zemlicka J, Drach JC. Antiviral Res; 19th International Conference on Antiviral Research; San Juan, Puerto Rico. May 7-11, 2006; 2006. p. A19. Abstract 61. [Google Scholar]
- 20.Borysko KZ, Gentry BG, Breitenbach JM, Zemlicka J, Drach JC. Antiviral Res; 21st International Conference on Antiviral Research; Montreal, Quebec, Canada. April 13-17, 2008; 2008. p. A54. Abstract 98. [Google Scholar]
- 21.Prichard MN, Daily SL, Jefferson GM, Perry AL, Kern ER. J. Virol. Methods. 2007;144:86. doi: 10.1016/j.jviromet.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]