Abstract
Seizures are the result of a sudden and temporary synchronization of neuronal activity, the reason for which is not clearly understood. Astrocytes participate in the control of neurotransmitter storage and neurotransmission efficacy. They provide fuel to neurons, which need a high level of energy to sustain normal and pathological neuronal activities, such as during epilepsy. Various genetic or induced animal models have been developed and used to study epileptogenic mechanisms. Methionine sulfoximine induces both seizures and the accumulation of brain glycogen, which might be considered as a putative energy store to neurons in various animals. Animals subjected to methionine sulfoximine develop seizures similar to the most striking form of human epilepsy, with a long pre-convulsive period of several hours, a long convulsive period during up to 48 hours and a post convulsive period during which they recover normal behavior. The accumulation of brain glycogen has been demonstrated in both the cortex and cerebellum as early as the pre-convulsive period, indicating that this accumulation is not a consequence of seizures. The accumulation results from an activation of gluconeogenesis specifically localized to astrocytes, both in vivo and in vitro. Both seizures and brain glycogen accumulation vary when using different inbred strains of mice. C57BL/6J is the most “resistant” strain to methionine sulfoximine, while CBA/J is the most “sensitive” one. The present review describes the data obtained on methionine sulfoximine dependent seizures and brain glycogen in the light of neurotransmission, highlighting the relevance of brain glycogen content in epilepsies.
Keywords: Epilepsy, methionine sulfoximine, chemically-induced epilepsy, glycogen, astrocytes, glycogenesis, gluconeogenesis.
1. INTRODUCTION
Glutamate and γ-aminobutyric acid (GABA) are the most abundant neurotransmitters in the central nervous system (CNS). The action potential transmission operated by the excitatory amino-acid glutamate is mediated through two kinds of receptors: (i) ionotropic receptors and (ii) metabotropic receptors. Three classes of ionotropic receptors to glutamate are commonly described, i.e. AMPA (α-amino-3-hydroxy-5-methylisoazol-4-propionate), NMDA (N-methyl-D-aspartate) and kainate (kainic acid) receptors. The activation of these receptors induces a K+ ion outward and a Na+ ion inward transport, which in turn induces activation of action potential. Two types of the GABA receptors have been described: GABAA and GABAB. The activation of GABAA receptors induces an inward entry of chloride ions, while that of GABAB receptors induces an outflow of K+ ions, both actions lead to an inhibition of neuronal activity. These two complementary and contradictory neurotransmission pathways constitute the targets for most antiepileptic drugs. Besides neurons, glial cells constitute the majority of brain cells and are of four types: (i) ependymocytes, (ii) microgliocytes, (iii) oligodendrocytes and (iv) astrocytes. Astrocytes are the more abundant glial cells, and they have numerous functions including their contribution to neurogenesis [1-3] and synaptogenesis [3-8]. Moreover, the end-feet of astrocytes are also involved in various functions.
Epilepsy is characterized by many symptoms which are manifestations of the various clinical forms of the condition. The World Health Organization (WHO) recognizes at least 40 forms [9-14], which correspond to a sudden and temporary synchronization of neuron activities whose origins are not well understood [15-20]. The balance between excitatory and inhibitory neurons, basically the equilibrium between glutamatergic and GABAergic neurons, controls temporary neuron synchronization. The knowledge of this disequilibrium is one of the keys to a cure for epilepsies. Searches for epileptic mechanisms are based upon clinical approaches, and the need to know where the epileptic foci are localized. Such localization depends on both EEG and medical imageries. The latter are based upon various mechanisms, and the uptake of glucose analogs represents one of them [21-23]. As humans cannot constitute an adequate experimental model for studying the mechanisms responsible for seizures various animal models have been developed and used for this purpose.
The present review briefly describes human epilepsies and animal models developed to study this disease, as well as data obtained for methionine sulfoximine (MSO) dependent seizures and brain glycogen accumulation. The review highlights the relevance of brain glycogen content in epilepsies. Brain glycogen will be determined in vivo in the near future, either in animals or in humans, and might therefore be used as a diagnostic tool as well as a therapeutic target.
2. EPILEPSY IN HUMAN AND ANIMALS
Epilepsy is a neurological disorder characterized by sudden and temporary bursts of a group of neurons for focal epilepsy, or of “whole” cortex when the crisis is generalized. When febrile epilepsies are excluded, epilepsy affects up to 1% of the population [24]. The most striking form of human epilepsy, the former so-called “grand-mal”, has enormous consequences. It can lead to a difficult life in affected patients, some of who may die from status epilepticus. The reasons why a group of neurons suddenly and temporarily synchronize their activity are not well understood, and need clarification. A better understanding of the condition will provide neuroscientists and physicians with crucial knowledge, which will enable the development of new therapeutic approaches with benefits to patients. Such advances are being based on studies of specific forms of human epilepsies; and although research on human epilepsies is limited, for deontological reasons, it is needed in order to fit the physiological conditions of this disease. Animal models of experimental epilepsies have therefore been developed and widely used, and are essentially classified into three groups: (i) genetic models, (ii) models in which crisis is induced by external stimuli, such as chemical or physical agents and (iii) models in which the responses to external stimuli depend on the genetic background.
Animal models mimicking human epilepsies are peculiar, and each animal model most likely concerns a specific aspect, or a mixture of some aspects of the variety of human epilepsies. Nonetheless these models have high utility and are an important prerequisite for research into epilepsy, as they allow us to approach specific point(s) of the human pathology experimentally. They are also powerful, making it possible to pinpoint a specific aspect of epilepsy and to analyze it in a “simpler” context than in human pathology.
3. EPILEPTOGENESIS
The understanding of the process by which a normal brain becomes epileptic, epileptogenesis, may help to identify molecular targets for drugs that could prevent epilepsy, as the current pharmacological therapy is only symptomatic and never cures the illness. The basic structure and functioning of the neuronal network is well understood, and below we take the example of the network of the cerebral cortex.
3.1. EEG Formation
The pyramidal cells are the major projection neurons of the cerebral cortex, and they project their axons into other areas of the brain and the spinal cord. They are excitatory neurons and principally use glutamate as a neurotransmitter. These cells exhibit a large number of collaterals that spread inside the cerebral cortex, and their dendrites often cross several layers of the cerebral cortex and are oriented perpendicular to the surface of the brain. A second kind of cell in the cerebral cortex is the interneural stellate cells that spread their axons vertically in the plane of the cortical column. Stellate cells get action potentials from the thalamus, and convey them to other interneurons or to pyramidal cells in the same column. A third kind of cerebral cortex cells are the basket cells, that are interneurons, which horizontally oriented their axons toward neuron somas. The latter axons envelop the somas and are able to inhibit them using the neurotransmitter GABA. These preceding three kinds of neurons are the main substratum of EEG genesis through the so-called theory of volume conduction [25-28]. The recorded potential from a large number of neurons is of course the sum of the electric activity of individual neurons. The excitatory post-synaptic potential of an individual neuron is the result of an inward current, and it spreads as an extracellular voltage outside the neuron membrane. Its magnitude (microvolt range) is too small to be recorded on the scalp, but when many neurons are taken into account, with large EEG electrodes, such a recording becomes possible. Because of the orientation described above, it is believed that EEG principally comes from pyramidal neurons, since the contribution of the other kinds of cell is almost negligible. It is not easy to distinguish an EEG coming solely from the pyramidal cells, or from a modulation of these pyramidal activities by other neurons, as pyramidal neurons are connected to the thalamus that synchronously activates a large number of these neurons. The main component of EEG is surely extracellular potentials deriving from excitatory post-synaptic potentials, since the latter are slow and can summate [29, 30]. The contribution of action potentials may exist but may be negligible, because its rapidity makes it difficult to summate.
3.2. Genesis of Epileptic Waves
It is possible to observe a large amplitude in the EEG in alpha, theta, and delta waves, which is due to a synchronization of the activities of groups of neurons [31-33], after which a normal EEG is observed. In the case of epilepsy a temporary synchronization occurs, although its level is high and it generates large spikes and spikes and waves. Moreover, the synchronization is recurrent. The actual cause of such a synchronization is not well understood [15-20]. In experiments using a topic application of penicillin it was shown that GABA inhibition is blocked. Action potentials are concomitant with the EEG spikes when the individual neuron activities are recorded at the same time. Each neuron makes its own action potential when its threshold of depolarization is reached in a normal brain, but the fundamental question of epileptogenesis is why neighboring neurons all make their action potential at the same time? The current believe of a decrease in inhibition of pyramidal neurons is not convincing. Indeed, if such a decreased inhibition is favorable for an increased firing of a given individual neuron, it does not explain why a group of thousands of neurons make their action potentials simultaneously. Some speculations have been proposed [15-20]. A pyramidal cell does not excite its neighboring pyramidal cell in a normal situation, but in epileptic foci such an excitation may occur [34], although the mechanism of this excitation remains unclear. One possibility is an increase in the extracellular potentials described above. In the epileptic situation, extracellular potential at the level of the soma of a given pyramidal cell could be too high, and it could excite the neighbor pyramidal cells, but this possibility is not tenable without a solid experimental demonstration. The soma of the pyramidal cells is “enveloped” in glial cells, principally in non-myelinating oligodendrocytes and astrocytes, so that the spread of extracellular potentials up to the adjacent pyramidal cells is improbable, unless abnormalities exist in the enveloping glial cells. This observation poses the question of a possible involvement of glial cells in epileptogenesis [35-43].
4. NEURAL CELLS, NEUROTRANSMISSION, ENERGY METABOLISM
Glial cells are no longer regarded as the “glue” between neurons, but have many roles necessary to sustain brain functions. In 2003 Ben Barres [44] claimed: “…virtually every aspect of brain development and function involves a neuron-glial partnership. It is no longer acceptable to consider glia as passive support cells.” Indeed, oligodendrocytes and Schwann cells are necessary for high-speed neurotransmission by ensheathing axons and generating nodes of Ranvier. These cells provide insulation and trophic factors to neurons. Moreover, the most abundant glial cells, the astrocytes, have come to be considered as an important partner in neurotransmission, and also in terms of regulators of energy provided to neurons. Many roles in fact are now attributed to astrocytes [44, 45].
4.1. Astrocytes as Partners in Neurotransmission
The relevance of glial cells in controlling neurogenesis and synaptogenesis has only recently been acknowledged, presumably because the alteration in the preceding processes during development could explain childhood epilepsies. Furthermore, the end-feet of astrocytes surround the capillaries, and therefore contribute to the maintenance of the BBB [46, 47]. They also ensheath the synaptic cleft, and thus are now considered as the third part of the synapses [44, 48]. The end-feet of astrocytes participate in the control of synaptic neurotransmitters and ion concentrations, such as glutamate and potassium, and therefore of the neurotransmission. Moreover, glial cells, particularly astrocytes, control the fate specification of adult neural stem cells. Indeed, astrocytes from hippocampus are capable of regulating neurogenesis by instructing the stem cells to adopt a neural fate [2]. Differentiation of neuronal stem cells into astrocytes or neurons is highly relevant in maintaining brain function. The question of the issues of neuronal/glial cell identity, and neuronal-glial interactions, in the context of adult neural stem cells biology, and their implication in neurogenesis during development and adulthood, was recently reviewed [49-52].
Astrocytes are also involved in the control of synaptogenesis [4, 6, 53-56]. In the mouse CNS this effect varies according to the brain regions and the type of neurons [57]. Astrocyte-derived cholesterol has been demonstrated to be responsible for an effect of astrocytes on synaptogenesis [3, 58-60]. Astrocyte-derived estrogen increases synaptogenesis through the estrogen receptor-alpha in primary rat cortical neurons in culture [61]. Neurothrophins [62, 63], which are widely expressed in the developing and mature CNS and are well known for their role in promoting neuron survival and differentiation, and thrombospondins [64], which induce ultrastructurally normal synapses that are presynaptically active, are involved in the astrocyte-control of synaptogenesis. The astrocyte-control of synaptogenesis involving numerous mechanisms therefore seems to be more complex than expected, more likely bilateral rather than unilateral.
Astrocytes participate in neurotransmission by regulating concentrations of ions and neurotransmitters in the synaptic cleft, thereby controlling synaptic efficacy [5, 44, 65-67]. The synaptic concentration of neurotransmitters depends primarily upon presynaptic activity. However, the end-feet of astrocytes regulate this concentration, as was unambiguously demonstrated for glutamatergic and GABAergic synapses which are often involved in epilepsy [68, 69]. Astrocytes are responsible for the major part of glutamate uptake after its release from glutamatergic neurons [70]. The processes of astrocytes contain glutamate transporters (GLAST) that are more active than those of neural cells. Glutamate, after entering the astrocytes, is transformed into glutamine by glutamine synthetase (GS), an enzyme that is essentially localized to astrocytes and not to neurons [71]. Glutamine is therefore given to neurons in which it is metabolized either to glutamate or GABA (Fig. (1)). Moreover, the K+ concentration of the synaptic cleft is also regulated by the end-feet of astrocytes [72-75]. In addition, the active roles of extrasynaptic neurotransmitter receptors and their relevance to neurovascular coupling, and of exocytosis of neurotransmitters from astrocytes, have recently been reviewed [67, 76-78], and the implication of these roles in epilepsy have been evoked [79-81]. Astrocytes contribute to the control of neurotransmission using additional mechanisms: (i) they synthesize a glia-derived soluble acetylcholine-binding protein (AChBP), which is a naturally occurring analog of the ligand-binding domains of the nicotinic acetylcholine receptors (nAChRs) [7]. This lure, or fake, receptor contributes to the regulation of acetylcholine content in the cholinergic synapses, by capturing acetylcholine. (ii) astrocytes produce a protein, the tumor necrosis factor alpha (TNFα), which enhances synaptic efficacy by increasing surface expression of AMPA receptors [8]. All these events may influence neuronal excitability and thus epileptogenesis.
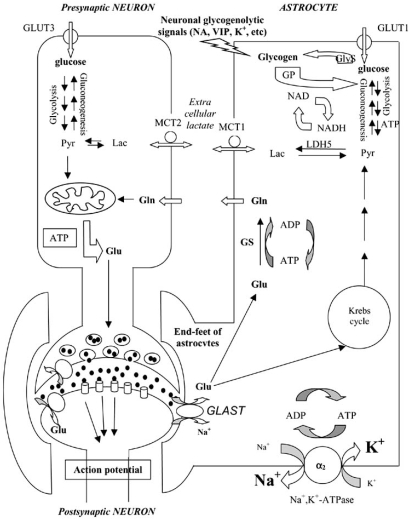
Fig. (1).
Schematic representation of the glucose-lactate shuttle between neurons and astrocytes (adapted from Pellerin [119], and Cloix [144]). Gln: glutamine, GlyS: glycogen synthase, GP: glycogen phosphorylase, GS: glutamine synthetase, GLAST, GLT-1: Na+-glutamate co-transporter, Glu: glutamate, Lac: lactate, LDH5: Lactate dehydrogenase 5, MCT: monocarbohydrate transporter, NA: noradrenaline, Pyr: pyruvate, VIP: vasointestinal peptide.
Glial cells, particularly astrocytes, should therefore be regarded as active partners of both the neuron and the synapse in terms of regulators of their synthesis and efficacy. These cells participate in neurotransmission through their effects on neurons and synapses, and through their processes of information via the astrocyte network. The complexity of neural information and brain organization is reinforced by new considerations that we have to take into account for the most abundant cells in the CNS, namely glial cells.
4.2. Astrocytes as Energy Providers to Neurons
It is now thought that astrocytes also largely contribute to fuel neurons during pathological conditions, such as during periods of intense neural activity when energy demand exceeds the supply of glucose from the blood. This idea might also apply during normal conditions. Indeed, two possibilities might exist to fuel neurons [82, 83]. Firstly, glucose enters astrocytes where it is metabolized down to lactate that is given to neurons to supply their energy demands. This is the astrocytes-neuron lactate shuttle hypothesis (ANLSH) [84-88]. Secondly, glucose enters neurons where it is utilized as the main source of energy. This is the conventional hypothesis.
4.2.1. Glucose Entering the Brain
Glucose is the main source of brain energy. Blood glucose has therefore first to cross the endothelial cell capillaries whose tight junctions constitute the BBB, then the intercellular area and the membranes of end-feet of astrocytes, and lastly the neuron membranes. GLUT proteins deliver glucose from the circulatory system to the brain. The microvascular endothelial cells and astrocytes contain GLUT1, while neurons contain both GLUT1 and GLUT3. Under normal conditions there is tightly limited in vivo expression of 45 kDa GLUT1 in neurons [89-92]. GLUT3 was cloned from a variety of mammalian brain cDNA libraries, and in the brain it has been localized almost exclusively to neurons [93-96]. As glucose has been detected in extracellular spaces of the brain [97-101], it is quite possible that neurons are capable of taking it up and metabolizing it. Indeed, Simpson et al. [102] calculated that GLUT3 has both a higher affinity for glucose than GLUT1, and at least a fivefold greater transport capacity than GLUT1. They concluded that as there are approximately the same number of GLUT1 on glial and endothelial cells, the combination of lower Km and higher capacity enables the neurons preferential access to available glucose. In addition to this glucose uptake by neurons, it may be possible that astrocyte metabolism contributes to feeding neurons according to the ANLSH (Fig. (1)). Indeed, it seems likely that glucose enters astrocytes, in which it is metabolized to lactate through the glycolysis pathway, and that astrocytes provide neurons with lactate as fuel [87, 103-113]. Neurons are therefore provided with a metabolite that can be rapidly used by the Krebs cycle (Fig. (1)). To fuel neurons, the ANLSH might be directly involved with glucose in parallel with the conventional hypothesis. Indeed, it is highly conceivable that these two hypotheses are not mutually exclusive to each other [114, 115], and the conventional hypothesis would be the prevalent one in the normal state, while the ANLSH might be favored in pathological conditions. Putatively, in pathologies such as epilepsy other forms of abnormal carbohydrate metabolism may also occur (see below).
In addition to glucose transporters the glycolytic product of glucose metabolism, lactate, is transported in and out of neural cells by monocarboxylate transporters (MCT), MCT1 in capillaries [116] and MCT2 in neurons [116-118]. The specific localization of these various transporters is in agreement with the ANLSH between astrocytes and neurons [87, 104-108, 111, 119] as summarized in Fig. (1).
4.2.2. Glycogen Synthesis and Degradation
The synthesis of glycogen inside the brain is essentially due to astrocytes [120] and microgliocytes and not to neurons, as the latter do not contain glycogen synthase (GlyS) [103], while the two former cells have this enzyme. Nevertheless neurons, which are capable of gluconeogenesis [121-123], are unable to accumulate glycogen [103, 124-126]. Inside astrocytes, glycogen synthesis might be performed using G-6-P coming either from glucose or from gluconeogenesis. Glycogen is therefore accumulated inside astrocytes [110, 111, 124, 127], and when energy is in high demand by neurons astrocytic glycogen might be metabolized down to lactate [88, 109, 119, 128, 129]. Astrocytic glycogen could therefore be considered as an energy-store for neurons [130-133], in addition to lactate-derived from blood glucose through glycolysis (conventional hypothesis), this latter being possible both in neurons and astrocytes [106, 124, 134-136], although during convulsions in which neurons have a high-energy demand, obtaining glucose via the blood stream might not be sufficient. The astrocytic energy-store might therefore be used through glycogen degradation that is performed by glycogen phosphorylase (GP), which is essentially expressed in astrocytes [106, 124]. Moreover, astrocytes contain receptors for glycogenolytic neurotransmitters, such as noradrenaline [108, 137] or vasointestinal peptide [104, 138-140]. K+ ions also have an acute glycogenolytic action on astrocytes [128, 141]. In fine, the preceding neurotransmitter and ion effects, with their glycogenolytic potencies [105, 109-112, 142, 143], contribute to the production of lactate. Astrocytes therefore represent a source of energy to neurons [144], and they perform this role by various means, such as glycogenolysis and glycolysis.
4.2.3. In Vivo Glycogen as Brain Function Marker
Brain glycogen content is lower than that of liver and muscle. Nevertheless, the glycogen content of the CNS is nowadays also regarded as relevant in brain functions. Glycogen synthesis and breakdown in the brain are beginning to be considered as relevant in normal brain functions, and in brain pathologies. Targeting research on brain glycogen pathways will be promising for both diagnosis and therapy. However, in terms of diagnosis, the in vivo measurement of brain glycogen content and synthesis is a challenge for the future, although the use of injection of [1-13]C-glucose has made possible the non-invasive measurement of brain glycogen content and turnover in both humans [145, 146] and rats [132, 133, 147-149]. These advances in the determination of brain glycogen will create a new understanding of its functions, roles and metabolism in both normal and in pathological conditions [110, 111, 130, 131, 150]. Once this difficult step has been overcome new tools will emerge to challenge brain glycogen in brain pathologies, and to target it as a treatment.
5. MSO-INDUCED EPILEPSY
Among the various epileptic or seizure-induced models, one of them is associated with both crisis and a specific increase in brain glycogen content [124, 125, 151-156]. This model corresponds to seizures induced by a derivative of methionine, namely methionine sulfoximine (MSO).
5.1. Discovery of MSO
MSO has been described as the chemical responsible for seizures induced in various animals after consumption of agenized flour, during the mid-1950s [157-159]; and it was therefore used to induce seizures in different animals, essentially rats and mice with characterized EEG alterations before and during seizures [124, 154, 160, 161]. Moreover, some plants of the Cnestis genus, from Madagascar and Asia, were used to kill running dogs by causing severe convulsions. The active compound in the seeds of Cnestis palada [162], and in the roots of Cnestis glabra and Cnestis polyphylla [163], was identified as MSO.
5.2. MSO as an Epileptogenic Molecule
Animals develop seizures after intraperitoneal administration of MSO, and such seizures mimic the human “grand mal”. They are characterized by disequilibrium, fits, running, myoclony and myotony, with EEG modifications. Animals developed epileptiform seizures 6-8 hours after MSO dosing (pre-convulsive period). The seizures were recurrent over 24-48 hours (convulsive or ictal period), and the animals then recovered normal behavior (post-convulsive period) [124, 151, 164]. During this long pre-convulsive period, the animals present ataxia, akinesia, and equilibrium disorders.
MSO strongly resembles glutamate, and it has been suggested that it can induce seizures by mimicking the effect of this excitatory amino-acid [165, 166]. Indeed, if MSO can replace glutamate in the synaptic cleft, it could stimulate neurotransmission and therefore induce seizures, but this mechanism of action is still not clearly understood. In addition, MSO has also been described as an irreversible inhibitor of GS [166]. Such an inhibition might therefore induce a putative increase of glutamate, which has been suggested as being responsible for the MSO-dependent convulsions [166]. Nonetheless, some reports [167-170] describe a decrease in brain glutamate content after the administration of a convulsive dose of MSO in rats, rather than the expected increase; but chronic inhibition of brain GS by MSO did not induce seizures in mice [171]. The involvement of GS inhibition by MSO in the MSO-dependent convulsions is therefore controversial, and may not even be responsible for MSO-dependent seizures.
6. MSO AS A GLYCOGENIC MOLECULE
Searches are based on clinical approaches, with the need to know where the epileptic foci are localized. Such localization depends on both EEG and medical imageries. The latter are based on various mechanisms; the uptake of glucose analogs represents one of them [21-23]. This allowed the demonstration that epileptic foci are hypermetabolic during crisis [23], and hypometabolic during the interictal period [22]. Such a hypometabolism is a reflection of either an intrinsic decrease of cellular glucose uptake, or an intrinsic high level of capacity of brain production of energy, or both. The intrinsic increase in brain energy capacity may be due to various cell alterations, such as glycolysis, gluconeogenesis or glycogenesis; the latter resulting in accumulation of glycogen. Such an increase in glycogen content is difficult to demonstrate in humans, although it has been shown that brain biopsies obtained from the hippocampus of epileptic patients contained high glycogen content in comparison with grey and white matter [172]. Such a high level of glycogen could contribute to high-energy demands by neurons during synchronization of their activities. Indeed, during seizures when neurons need a lot of energy, as they are starved by their high activities, this energy might come from different sources: blood supply, neuron glycolysis and astrocyte source. When the first two sources are depleted the astrocyte glycogen might be mobilized, and astrocyte glycogenolysis could then generate lactate, which could be transferred to the neurons.
In MSO-dependent seizures the accumulation of brain glycogen is observable during the pre-convulsive period before crisis, demonstrating that such an accumulation is not a consequence of seizures [124-126, 151, 164]. This high level of glycogen and its highest augmentation are specifically localized in the cortex and cerebellum, and more precisely in astrocytes (Fig. (2)) [124-126]. Moreover, the action of MSO on alterations in metabolism is also demonstrable in cultured rat and mouse astrocytes, again showing that such metabolic effects are not consequences of seizures [124, 151, 155, 164]. Conversely, a decrease of glycogen content is also observed in other models of induced seizures, for example seizures induced by homocysteic acid [173, 174], indoklon/flurothyl [175], PTZ [176], hypoglycemia [177], bicuculine [178, 179], and maximal electroshock [177]. The accumulation of glycogen is essentially due to astrocyte metabolism, although in progressive myoclonus epilepsy inclusion Lafora bodies, resembling abnormal glycogen, accumulate in this disease [180]. In addition, and contrary to previous data, it has been recently reported that mouse neurons have enzymatic machinery for synthesizing glycogen [181]. Nonetheless, the accumulation of glycogen in MSO-dependent seizures is specific to astrocytes, and might be relevant in the onset of seizures and in the maintenance of crisis [151, 164].
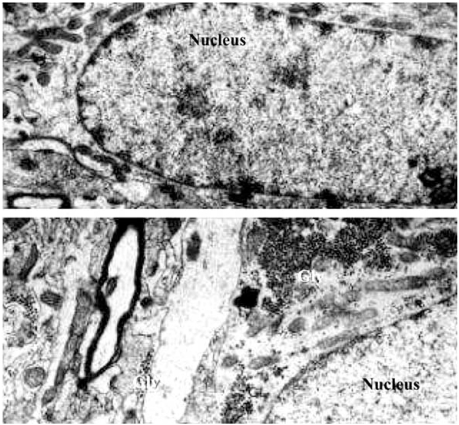
Fig. (2).
Electron micrographs of sections made in the parietal cerebral cortex of mice after the administration of methionine sulfoximine. Swiss mice were intraperitoneally injected with 100 mg/kg of methionine sulfoximine (B) or with saline as control (A). After intracardiac perfusion of glutaraldehyde, the brain tissue were taken off, washed in buffered sucrose, post-fixed in osmium tetroxide, dehydrated in graded alcohol and propylene oxide, and embedded in Effapoxy. The sections were made and stained with uranyl acetate and lead citrate and then, examined in an electron microscope. In control, beta glycogen particles are rare. After methionine sulfoximine administration, glycogen as alpha and beta particles considerably increases. Gly: glycogen; magnification A and B: x 15000.
7. MSO EPILEPTOGENESIS IN RELATION WITH NEUROTRANSMISSION
As the excitability of neurons is largely monitored by neurotransmitters many investigations have been devoted to abnormalities in neurotransmission during epilepsy [182-184]; yet despite a large number of experiments we still have no clear explanation on the involvement of neurotransmission in epilepsy. The general believe is that of a defect in the inhibitory neuronal circuits related to GABA, and/or an enhancement of excitatory circuits related to glutamate. The difficulty with this idea is that many epileptogenic situations do not involve defects in either GABA or in glutamate neurotransmissions. One illustration is the model created by organophosphoric compounds such as soman [185-187]. It is well established that soman principally targets cholinergic neurotransmission, and generates typical epileptic seizures involving EEG and convulsions. Moreover, many other models clearly involve catecholamine and indoleamine neurotransmissions [182-184]. The impairment of different neurotransmission systems is thus surely able to generate a variety of epileptic seizure models in animals, and probably different kinds of epilepsies in human as well. We looked for changes in the content of many neurotransmitters in the brain of rodents developing seizures after an intraperitoneal administration of the drug MSO. The aim was to discover any possible relationship between the impairment in neurotransmission and the impairment in the brain carbohydrate metabolism, and MSO-dependent convulsions. Briefly, MSO induced a decrease in dopamine and serotonin levels and an increase in acetylcholine, while the noradrenaline level showed only a small change [188-191]. Using both agonists and antagonists of these neurotransmitters we were able to modulate the seizures, except in the case of acetylcholine. At the same time MSO induced a large increase in glycogen content in all areas of the brain, as previously described [124-126, 151, 154, 164, 178].
The question now arises as to what neurotransmission impairment has to do with glycogen content in epileptogeny? Experiments in vivo or in vitro [192, 193], and our unpublished data, show that the glycogen content in the brain is insensitive to changes in acetylcholine. So the involvement of a cholinergic system in glycogen accumulation induced by MSO in the brain is improbable. GABA may not cause MSO epileptic-like activity in rodents, nor a glycogen level increase, because the level of this neurotransmitter is insensitive to the convulsant [188]. Biogenic amines, such as catecholamines, indolamines and histamine, have been reported to elicit glycogenolysis in the brains of various species, including mammals [194-197]. As glycogen accumulation is restricted to astrocytes in the brain and neurotransmitter release to neurons, it is obvious that interactions between neurons and astrocytes are responsible for changes in the metabolism of glycogen and of catecholamines and indoleamines. In the case of MSO, the observed decrease in dopamine and in serotonin levels is in a good agreement with the increase in glycogen content in the brain. Does the neurotransmitter decrease therefore trigger the glycogen accumulation, or does glycogen accumulation induce changes in the preceding biogenic neurotransmission system? At present our experimental data does not allow us to clearly identify the primer of change. One can speculate that MSO decreases dopamine and serotonin levels, and thus causes an imbalance between GlyS and GP activities, and that this change results in the glycogen level increase. We have also speculated on the possible sequestration of glucose by astrocytes under the effect of MSO. Primarily, astrocytes may incorporate molecules of glucose into glycogen. Moreover, over the same period of time, via an enhanced gluconeogenesis, lactate and pyruvate that astrocytes can normally send to neurons as fuel are converted to G-1-P through gluconeogenesis pathway, which is polymerized as glycogen. Gluconeogenesis and glycogen accumulation in astrocytes deprive neurons of energy, and this may alter neuronal function which then results in epileptic-like activity [125].. We now need to find a robust experimental design that will able us to discriminate an epileptogenesis event primarily triggered by changes in glycogen content, or by changes in neurotransmission under the effect of MSO.
8. RELATIONSHIP BETWEEN MSO EFFECTS
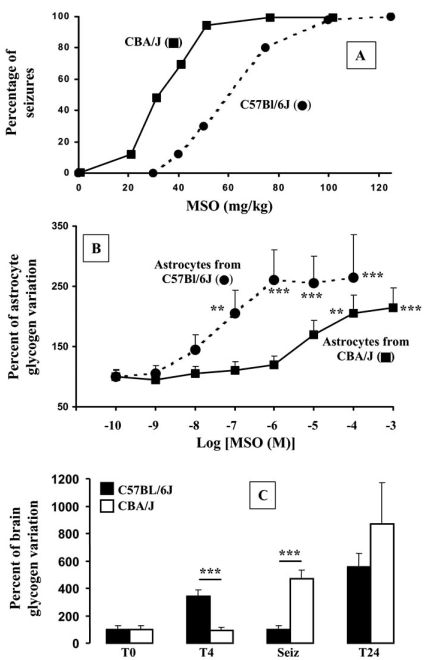
After MSO administration glycogen content remains high during the convulsive period, and up to the post-convulsive period. We have demonstrated that both seizure latency and glycogen accumulation are dependent on genotype in mice [151, 164]. Using various inbred strains of mice [151, 164], we observe that the latency to MSO-dependent seizures varies (Fig. (3)). The strain C57BL/6J presents the longest latency (13.03 ± 0.45 h, n=92), and the lowest susceptibility toward MSO (ED50=60 mg/kg), while CBA/J presents the fastest latency (5.95 ± 0.06 h, n=63) and the highest susceptibility toward MSO (ED50=30 mg/kg). The CBA/J strain is also unable to accumulate glycogen inside the brain during the preconvulsive period, as opposed to C57BL/6J that does. This accumulation of brain glycogen is specifically localized to astrocytes, as previously described [124-126]. Indeed, MSO induces an activation of gluconeogenesis by a specific increase in activity of the last irreversible gluconeogenic enzyme, fructose-1,6-bisphosphatase (FBPase). Increases in both FBPase and glycogen deposits are specifically localized to astrocytes [124-126, 155, 191]. In addition, primary cultures of astrocytes from these two strains demonstrate that MSO induces accumulation of glycogen only in cultured astrocytes from C57BL/6L, which is mediated by the specific activation of gluconeogenesis [151].
Fig. (3).
Effect of various doses of MSO, both in vivo (A, C) and in vitro (B) using two different strains of mice, C57BL/6J and CBA/J. A: Induction of seizures expressed as the percentage of convulsing mice as a function of MSO doses. B: Variation of glycogen determined in cultured astrocytes as a function of MSO doses. C: Variation of glycogen content of mice cortices sacrificed at either predetermined time T4 and T24) or at the onset of seizures (Seiz). Adapted from Bernard-Hélary et al. [151].
The question of a causal link between glycogen accumulation and seizures remains open. We have demonstrated that at the onset of MSO-dependent seizures the glycogen that was accumulated in cortices before convulsions returns to the basal level (Fig. (3)). Such a normalization of glycogen content occurred only in C57BL/6J mice and not in CBA/J ones [151]. These data suggested (i) a mobilization of the high level of brain glycogen at the onset of seizures, which is observed in mice having a long latency to MSO-dependent convulsions, and (ii) that the accumulated glycogen in brain might be used as energy supply when neurons are in a convulsive state. We therefore hypothesized that these two MSO effects, i.e. the elevation of glycogen and its mobilization during seizures, might constitute a way to postpone seizures [151, 164]. Bi-directional selection of MSO sensitive and MSO resistant mice is under investigation, in order to verify such a hypothesis. Preliminary results suggest that glycogen can accumulate after MSO dosing to a greater extent in cortices of MSO resistant mice than in MSO sensitive ones (unpublished data), confirming our hypothesis that high levels of glycogen could delay MSO-induced seizures [151, 164].
9. CONCLUSIONS: POTENTIAL OF MSO-DEPENDENT SEIZURES MODEL
The MSO-dependent seizures model presents some advantages compared with other chemically-induced seizures. Our two most important concerns are as follows. (i) The existence of a long preconvulsive period during which it is possible to easily observe changes before the onset of seizures might not be a consequence of crisis. (ii) This model associates an accumulation of brain glycogen with induction of seizures, and therefore allows the analyses of the relationship between seizures and brain glycogen content. This consideration is relevant, as high brain glycogen content has sideration is relevant, as high brain glycogen content has been described in human epilepsy [172]. Moreover, in MSO dependent seizures glycogen accumulates in the brain before the onset of seizures, and remains high during the convulsive period. Nevertheless, such a high brain glycogen content is mobilized at the onset of seizures and returns to high level thereafter. We therefore propose, that such an elevation of brain glycogen could contribute to the postponement of seizures [151, 164], by mechanisms such as astrocyte glycogen mobilization, which provides neuron with energy. In the very near future brain glycogen will be regarded as a diagnostic tool, as well as a therapeutic target [144]. Furthermore, in vivo MRI analyses have been performed in various animal models of epilepsy demonstrating that such a tool is valuable and informative, and might provide a clarification of the mechanisms involved in epilepsy [198, 199].
10. CONCLUDING REMARKS
Knowing all the mechanisms and biological events responsible for the various forms of human epilepsies still has a long way to go. Nevertheless, the numerous researches performed using human approaches present a doorway to the subject, which is now fully open due to the experimental models developed to study this neuropathology. Even if much is still unknown, new developments are on the way to giving us a deeper insight into the underlying mechanisms. For example, the use of transgenic mice could provide us with a useful tool for the genetic “dissection” of various aspects of the pathology, and may eventually lead to a “full” understanding of epilepsies. In any event such goals depend upon a better knowledge of brain physiology, which in itself needs the development of new approaches, technologies and ideas. Concerning this new knowledge, the brain glycogen content might be considered as highly relevant, and its in vivo measurement of content and variation in humans during normal and pathological conditions will be soon possible [145, 146]. Such a determination will be highly useful for analyzing the potency and relevance of brain glycogen content, and its mobilization in patients during pathological conditions.
ACKNOWLEDGEMENTS
This work was supported by the Ministère de l’Education Nationale, de la Recherche et de l’Enseignement Supérieur, the Centre National de la Recherche Scientifique (C.N.R.S.), the Région Centre, La ligue contre le Cancer via its regional council, and by the Conseil Général du Loiret. The authors are also indebted to Philippe Moreau for his skilful technical assistance.
ABBREVIATIONS
- AMPA
= α-amino-3-hydroxy-5-méthyl-4 isoazole propionic acid
- ANLSH
= Astrocytes-neuron lactate shuttle hypothesis
- BBB
= Blood-brain barrier
- CNS
= Central Nervous System
- EEG
= Electroencephalography
- FBPase
= Fructose-1,6-bisphosphatase
- G-1-P
= Glucose-1-phosphate
- G-6-P
= Glucose-6-phosphate
- GABA
= γ-aminobutyric acid
- GLAST
= Na+-glutamate co-transporter
- Gln
= Glutamine
- Glu
= Glutamate
- GLUT
= Glucose transporter, facilitative type
- GlycS
= Glycogen synthase
- GP
= Glycogen phosphorylase
- GS
= Glutamine synthetase
- kDa
= kilo Dalton
- Lac
= Lactate
- LDH5
= Lactate dehydrogenase, type 5
- MCT
= Monocarboxylate transporters
- MSO
= Methionine sulfoximine
- NMDA
= N-methyl-D-aspartate
- Pyr
= Pyruvate
- VIP
= Vasointestinal peptide
- WHO
= World Health Organization
REFERENCES
- 1.Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- 2.Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- 3.Pfrieger FW. Role of cholesterol in synapse formation and function. Biochim. Biophys. Acta. 2003;1610:271–280. doi: 10.1016/s0005-2736(03)00024-5. [DOI] [PubMed] [Google Scholar]
- 4.Ullian EM, Sapperstein SK, Christopherson KS, Barres BA. Control of synapse number by glia. Science. 2001;291:657–661. doi: 10.1126/science.291.5504.657. [DOI] [PubMed] [Google Scholar]
- 5.Pfrieger FW, Barres BA. Synaptic efficacy enhanced by glial cells in vitro. Science. 1997;277:1684–1687. doi: 10.1126/science.277.5332.1684. [DOI] [PubMed] [Google Scholar]
- 6.Pfrieger FW. Role of glia in synapse development. Curr. Opin. Neurobiol. 2002;12:486–490. doi: 10.1016/s0959-4388(02)00358-6. [DOI] [PubMed] [Google Scholar]
- 7.Smit AB, Syed NI, Schaap D, van Minnen J, Klumperman J, Kits KS, Lodder H, van der Schors RC, van Elk R, Sorgedrager B, Brejc K, Sixma TK, Geraerts WP. A glia-derived acetylcholine-binding protein that modulates synaptic transmission. Nature. 2001;411:261–268. doi: 10.1038/35077000. [DOI] [PubMed] [Google Scholar]
- 8.Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC. Control of synaptic strength by glial TNFalpha. Science. 2002;295:2282–2285. doi: 10.1126/science.1067859. [DOI] [PubMed] [Google Scholar]
- 9.Everitt AD, Sander JW. Classification of the epilepsies: time for a change? A critical review of the International Classification of the Epilepsies and Epileptic Syndromes (ICEES) and its usefulness in clinical practice and epidemiological studies of epilepsy. Eur. Neurol. 1999;42:1–10. doi: 10.1159/000008061. [DOI] [PubMed] [Google Scholar]
- 10.Proposal for revised clinical and electroencephalographic classification of epileptic seizures. From the Commission on Classification and Terminology of the International League Against Epilepsy. Epilepsia. 1981;22:489–501. doi: 10.1111/j.1528-1157.1981.tb06159.x. [DOI] [PubMed] [Google Scholar]
- 11.Proposal for classification of epilepsies and epileptic syndromes. Commission on Classification and Terminology of the International League Against Epilepsy. Epilepsia. 1985;26:268–278. [PubMed] [Google Scholar]
- 12.Proposal for revised classification of epilepsies and epileptic syndromes. Commission on Classification and Terminology of the International League Against Epilepsy. Epilepsia. 1989;30:389–399. doi: 10.1111/j.1528-1157.1989.tb05316.x. [DOI] [PubMed] [Google Scholar]
- 13.Dreifuss FE. Classification of the epilepsies: influence on management. Rev. Neurol. (Paris) 1987;143:375–380. [PubMed] [Google Scholar]
- 14.Wieser HG, Blume WT, Fish D, Goldensohn E, Hufnagel A, King D, Sperling MR, Luders H, Pedley TA. ILAE Commission Report. Proposal for a new classification of outcome with respect to epileptic seizures following epilepsy surgery. Epilepsia. 2001;42:282–286. [PubMed] [Google Scholar]
- 15.Rampp S, Stefan H. Fast activity as a surrogate marker of epileptic network function? Clin. Neurophysiol. 2006;117:2111–2117. doi: 10.1016/j.clinph.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 16.Johnston MV. Developmental aspects of epileptogenesis. Epilepsia. 1996;37(Suppl 1):S2–9. doi: 10.1111/j.1528-1157.1996.tb06018.x. [DOI] [PubMed] [Google Scholar]
- 17.Dudek FE, Patrylo PR, Wuarin JP. Mechanisms of neuronal synchronization during epileptiform activity. Adv. Neurol. 1999;79:699–708. [PubMed] [Google Scholar]
- 18.McCormick DA, Contreras D. On the cellular and network bases of epileptic seizures. Annu. Rev. Physiol. 2001;63:815–846. doi: 10.1146/annurev.physiol.63.1.815. [DOI] [PubMed] [Google Scholar]
- 19.Uhlhaas PJ, Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron. 2006;52:155–168. doi: 10.1016/j.neuron.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 20.Scharfman HE. The neurobiology of epilepsy. Curr. Neurol. Neurosci. Rep. 2007;7:348–354. doi: 10.1007/s11910-007-0053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sitoh YY, Tien RD. Neuroimaging in epilepsy. J. Magn. Reson. Imaging. 1998;8:277–288. doi: 10.1002/jmri.1880080207. [DOI] [PubMed] [Google Scholar]
- 22.Engel J Jr, Brown WJ, Kuhl DE, Phelps ME, Mazziotta JC, Crandall PH. Pathological findings underlying focal temporal lobe hypometabolism in partial epilepsy. Ann. Neurol. 1982;12:518–528. doi: 10.1002/ana.410120604. [DOI] [PubMed] [Google Scholar]
- 23.Engel J Jr, Kuhl DE, Phelps ME, Rausch R, Nuwer M. Local cerebral metabolism during partial seizures. Neurology. 1983;33:400–413. doi: 10.1212/wnl.33.4.400. [DOI] [PubMed] [Google Scholar]
- 24.Anderson VE, Hauser WA, Rich SS. Genetic heterogeneity and epidemiology of the epilepsies. Adv. Neurol. 1999;79:59–73. [PubMed] [Google Scholar]
- 25.Niedermeyer E. Dipole theory and electroencephalography. Clin. Electroencephalogr. 1996;27:121–131. doi: 10.1177/155005949602700305. [DOI] [PubMed] [Google Scholar]
- 26.Henderson JA, Phillips AJ, Robinson PA. Multielectrode electroencephalogram power spectra: theory and application to approximate correction of volume conduction effects. Phys. Rev. E. Stat. Nonlin. Soft. Matter. Phys. 2006;73:051918. doi: 10.1103/PhysRevE.73.051918. [DOI] [PubMed] [Google Scholar]
- 27.Onton J, Westerfield M, Townsend J, Makeig S. Imaging human EEG dynamics using independent component analysis. Neurosci. Biobehav. Rev. 2006;30:808–822. doi: 10.1016/j.neubiorev.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 28.Onton J, Makeig S. Information-based modeling of event-related brain dynamics. Prog. Brain Res. 2006;159:99–120. doi: 10.1016/S0079-6123(06)59007-7. [DOI] [PubMed] [Google Scholar]
- 29.Fountain NB, Bear J, Bertram EH 3rd, Lothman EW. Responses of deep entorhinal cortex are epileptiform in an electrogenic rat model of chronic temporal lobe epilepsy. J. Neurophysiol. 1998;80:230–240. doi: 10.1152/jn.1998.80.1.230. [DOI] [PubMed] [Google Scholar]
- 30.Hallez H, Vanrumste B, Grech R, Muscat J, De Clercq W, Vergult A, D'Asseler Y, Camilleri KP, Fabri SG, Van Huffel S, Lemahieu I. Review on solving the forward problem in EEG source analysis. J. Neuroeng. Rehabil. 2007;4:46. doi: 10.1186/1743-0003-4-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lazarev VV. On the intercorrelation of some frequency and amplitude parameters of the human EEG and its functional significance. Communication. I: Multidimensional neurodynamic organization of functional states of the brain during intellectual, perceptive and motor activity in normal subjects. Int. J. Psychophysiol. 1998;28:77–98. doi: 10.1016/s0167-8760(97)00068-8. [DOI] [PubMed] [Google Scholar]
- 32.Lazarev VV. On the intercorrelation of some frequency and amplitude parameters of the human EEG and its functional significance. Communication II: neurodynamic imbalance in endogenous asthenic-like disorders. Int. J. Psychophysiol. 1998;29:277–289. doi: 10.1016/s0167-8760(98)00008-7. [DOI] [PubMed] [Google Scholar]
- 33.Liberson WT. Reciprocal relationship between the major EEG rhythms and latencies of evoked potential intermediate components. A tentative explanation. Electromyogr. Clin. Neurophysiol. 1989;29:425–432. [PubMed] [Google Scholar]
- 34.Martin JH. The collective electrical behavior of cortical neurons: The electroencephalogram and the mechanism of epilepsy. In: Kandel EC, Schwartz JH, Jessell TM, editors. Principles of Neural Science. 3rd. 1991. pp. 777–791. [Google Scholar]
- 35.D'Ambrosio R. The role of glial membrane ion channels in seizures and epileptogenesis. Pharmacol. Ther. 2004;103:95–108. doi: 10.1016/j.pharmthera.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 36.Steinhauser C, Seifert G. Glial membrane channels and receptors in epilepsy: impact for generation and spread of seizure activity. Eur. J. Pharmacol. 2002;447:227–237. doi: 10.1016/s0014-2999(02)01846-0. [DOI] [PubMed] [Google Scholar]
- 37.McNamara JO, Huang YZ, Leonard AS. Molecular signaling mechanisms underlying epileptogenesis. Sci. STKE. 2006;2006:re12. doi: 10.1126/stke.3562006re12. [DOI] [PubMed] [Google Scholar]
- 38.Grisar T, Lakaye B, Thomas E, Bettendorf L, Minet A. The molecular neuron-glia couple and epileptogenesis. Adv. Neurol. 1999;79:591–602. [PubMed] [Google Scholar]
- 39.Khurgel M, Ivy GO. Astrocytes in kindling: relevance to epileptogenesis. Epilepsy Res. 1996;26:163–175. doi: 10.1016/s0920-1211(96)00051-4. [DOI] [PubMed] [Google Scholar]
- 40.Khurgel M, Switzer RC 3rd, Teskey GC, Spiller AE, Racine RJ, Ivy GO. Activation of astrocytes during epileptogenesis in the absence of neuronal degeneration. Neurobiol. Dis. 1995;2:23–35. doi: 10.1006/nbdi.1995.0003. [DOI] [PubMed] [Google Scholar]
- 41.Chang FL, Hawrylak N, Greenough WT. Astrocytic and synaptic response to kindling in hippocampal subfield CA1. I. Synaptogenesis in response to kindling in vitro. Brain Res. 1993;603:302–308. doi: 10.1016/0006-8993(93)91252-n. [DOI] [PubMed] [Google Scholar]
- 42.Hawrylak N, Chang FL, Greenough WT. Astrocytic and synaptic response to kindling in hippocampal subfield CA1. II. Synaptogenesis and astrocytic process increases to in vivo kindling. Brain Res. 1993;603:309–316. doi: 10.1016/0006-8993(93)91253-o. [DOI] [PubMed] [Google Scholar]
- 43.Vezzani A, Ravizza T, Balosso S, Aronica E. Glia as a source of cytokines: implications for neuronal excitability and survival. Epilepsia. 2008;49(Suppl 2):24–32. doi: 10.1111/j.1528-1167.2008.01490.x. [DOI] [PubMed] [Google Scholar]
- 44.Barres BA. What is a glial cell? Glia. 2003;43:4–5. doi: 10.1002/glia.10252. [DOI] [PubMed] [Google Scholar]
- 45.Barres BA. New roles for glia. J. Neurosci. 1991;11:3685–3694. doi: 10.1523/JNEUROSCI.11-12-03685.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol. Dis. 2004;16:1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 47.Ueno M. Molecular anatomy of the brain endothelial barrier: an overview of the distributional features. Curr. Med. Chem. 2007;14:1199–1206. doi: 10.2174/092986707780597943. [DOI] [PubMed] [Google Scholar]
- 48.Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- 49.Ihrie RA, Alvarez-Buylla A. Cells in the astroglial lineage are neural stem cells. Cell Tissue Res. 2008;331:179–191. doi: 10.1007/s00441-007-0461-z. [DOI] [PubMed] [Google Scholar]
- 50.Gritti A, Bonfanti L. Neuronal-glial interactions in central nervous system neurogenesis: the neural stem cell perspective. Neuron Glia Biol. 2007;3:309–323. doi: 10.1017/S1740925X0800001X. [DOI] [PubMed] [Google Scholar]
- 51.Jordan JD, Ma DK, Ming GL, Song H. Cellular niches for endogenous neural stem cells in the adult brain. CNS Neurol. Disord. Drug Targets. 2007;6:336–341. doi: 10.2174/187152707783220866. [DOI] [PubMed] [Google Scholar]
- 52.Wang DD, Bordey A. The astrocyte odyssey. Prog. Neurobiol. 2008 doi: 10.1016/j.pneurobio.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nagler K, Mauch DH, Pfrieger FW. Glia-derived signals induce synapse formation in neurones of the rat central nervous system. J. Physiol. 2001;533:665–679. doi: 10.1111/j.1469-7793.2001.00665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.He F, Sun YE. Glial cells more than support cells? Int. J. Biochem. Cell Biol. 2007;39:661–665. doi: 10.1016/j.biocel.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 55.Ullian EM, Christopherson KS, Barres BA. Role for glia in synaptogenesis. Glia. 2004;47:209–216. doi: 10.1002/glia.20082. [DOI] [PubMed] [Google Scholar]
- 56.Slezak M, Pfrieger FW. New roles for astrocytes: regulation of CNS synaptogenesis. Trends Neurosci. 2003;26:531–535. doi: 10.1016/j.tins.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 57.Steinmetz CC, Buard I, Claudepierre T, Nagler K, Pfrieger FW. Regional variations in the glial influence on synapse development in the mouse CNS. J. Physiol. 2006;577:249–261. doi: 10.1113/jphysiol.2006.117358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goritz C, Mauch DH, Nagler K, Pfrieger FW. Role of glia-derived cholesterol in synaptogenesis: new revelations in the synapse-glia affair. J. Physiol. Paris. 2002;96:257–263. doi: 10.1016/s0928-4257(02)00014-1. [DOI] [PubMed] [Google Scholar]
- 59.Pfrieger FW. Outsourcing in the brain: do neurons depend on cholesterol delivery by astrocytes? Bioessays. 2003;25:72–78. doi: 10.1002/bies.10195. [DOI] [PubMed] [Google Scholar]
- 60.Mauch DH, Nagler K, Schumacher S, Goritz C, Muller EC, Otto A, Pfrieger FW. CNS synaptogenesis promoted by glia-derived cholesterol. Science. 2001;294:1354–1357. doi: 10.1126/science.294.5545.1354. [DOI] [PubMed] [Google Scholar]
- 61.Hu R, Cai WQ, Wu XG, Yang Z. Astrocyte-derived estrogen enhances synapse formation and synaptic transmission between cultured neonatal rat cortical neurons. Neuroscience. 2007;144:1229–1240. doi: 10.1016/j.neuroscience.2006.09.056. [DOI] [PubMed] [Google Scholar]
- 62.Elmariah SB, Hughes EG, Oh EJ, Balice-Gordon RJ. Neurotrophin signaling among neurons and glia during formation of tripartite synapses. Neuron Glia Biol. 2005;1:1–11. doi: 10.1017/S1740925X05000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Elmariah SB, Oh EJ, Hughes EG, Balice-Gordon RJ. Astrocytes regulate inhibitory synapse formation via Trk-mediated modulation of postsynaptic GABAA receptors. J. Neurosci. 2005;25:3638–3650. doi: 10.1523/JNEUROSCI.3980-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, Barres BA. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 65.Nadkarni S, Jung P, Levine H. Astrocytes optimize the synaptic transmission of information. PLoS Comput. Biol. 2008;4:e1000088. doi: 10.1371/journal.pcbi.1000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nadkarni S, Jung P. Modeling synaptic transmission of the tripartite synapse. Phys. Biol. 2007;4:1–9. doi: 10.1088/1478-3975/4/1/001. [DOI] [PubMed] [Google Scholar]
- 67.Tritsch NX, Bergles DE. Defining the role of astrocytes in neuromodulation. Neuron. 2007;54:497–500. doi: 10.1016/j.neuron.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 68.Oliet SH, Piet R, Poulain DA. Control of glutamate clearance and synaptic efficacy by glial coverage of neurons. Science. 2001;292:923–926. doi: 10.1126/science.1059162. [DOI] [PubMed] [Google Scholar]
- 69.Schousboe A. Role of astrocytes in the maintenance and modulation of glutamatergic and GABAergic neurotransmission. Neurochem. Res. 2003;28:347–352. doi: 10.1023/a:1022397704922. [DOI] [PubMed] [Google Scholar]
- 70.Danbolt NC. Glutamate uptake. Prog. Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 71.Derouiche A. Non-Neuronal Cells of the Nervous System: Function and Dysfunction (Hertz, L., ed) Amsterdam: Elsevier; 2004. The perisynaptic astrocyte process as a glial compartment: immunolabeling for glutamine synthatase and other glial markers; pp. 147–163. [Google Scholar]
- 72.Lian XY, Stringer JL. Astrocytes contribute to regulation of extracellular calcium and potassium in the rat cerebral cortex during spreading depression. Brain Res. 2004;1012:177–184. doi: 10.1016/j.brainres.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 73.Walz W. Role of glial cells in the regulation of the brain ion microenvironment. Prog. Neurobiol. 1989;33:309–333. doi: 10.1016/0301-0082(89)90005-1. [DOI] [PubMed] [Google Scholar]
- 74.Walz W. Role of astrocytes in the clearance of excess extracellular potassium. Neurochem. Int. 2000;36:291–300. doi: 10.1016/s0197-0186(99)00137-0. [DOI] [PubMed] [Google Scholar]
- 75.Walz W, Hertz L. Functional interactions between neurons and astrocytes. II. Potassium homeostasis at the cellular level. Prog. Neurobiol. 1983;20:133–183. doi: 10.1016/0301-0082(83)90013-8. [DOI] [PubMed] [Google Scholar]
- 76.Montana V, Malarkey EB, Verderio C, Matteoli M, Parpura V. Vesicular transmitter release from astrocytes. Glia. 2006;54:700–715. doi: 10.1002/glia.20367. [DOI] [PubMed] [Google Scholar]
- 77.Chen XK, Xiong YF, Zhou Z. "Kiss-and-run" exocytosis in astrocytes. Neuroscientist. 2006;12:375–378. doi: 10.1177/1073858406291588. [DOI] [PubMed] [Google Scholar]
- 78.Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol. Rev. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- 79.Nadkarni S, Jung P. Spontaneous oscillations of dressed neurons: a new mechanism for epilepsy? Phys. Rev. Lett. 2003;91:268101. doi: 10.1103/PhysRevLett.91.268101. [DOI] [PubMed] [Google Scholar]
- 80.Halassa MM, Fellin T, Haydon PG. The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol. Med. 2007;13:54–63. doi: 10.1016/j.molmed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 81.Halassa MM, Fellin T, Takano H, Dong JH, Haydon PG. Synaptic islands defined by the territory of a single astrocyte. J. Neurosci. 2007;27:6473–6477. doi: 10.1523/JNEUROSCI.1419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chih CP, Roberts Jr EL. Energy substrates for neurons during neural activity: a critical review of the astrocyte-neuron lactate shuttle hypothesis. J. Cereb. Blood Flow Metab. 2003;23:1263–1281. doi: 10.1097/01.WCB.0000081369.51727.6F. [DOI] [PubMed] [Google Scholar]
- 83.Chih CP, Lipton P, Roberts EL Jr. Do active cerebral neurons really use lactate rather than glucose? Trends Neurosci. 2001;24:573–578. doi: 10.1016/s0166-2236(00)01920-2. [DOI] [PubMed] [Google Scholar]
- 84.Sibson NR, Dhankhar A, Mason GF, Rothman DL, Behar KL, Shulman RG. Stoichiometric coupling of brain glucose metabolism and glutamatergic neuronal activity. Proc. Natl. Acad. Sci. USA. 1998;95:316–321. doi: 10.1073/pnas.95.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rothman DL, Sibson NR, Hyder F, Shen J, Behar KL, Shulman RG. In vivo nuclear magnetic resonance spectroscopy studies of the relationship between the glutamate-glutamine neurotransmitter cycle and functional neuroenergetics. Philos Trans R Soc. Lond B Biol. Sci. 1999;354:1165–1177. doi: 10.1098/rstb.1999.0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Magistretti PJ, Pellerin L, Rothman DL, Shulman RG. Energy on demand. Science. 1999;283:496–497. doi: 10.1126/science.283.5401.496. [DOI] [PubMed] [Google Scholar]
- 87.Pellerin L, Bonvento G, Chatton J, Pierre K, Magistretti PJ. Role of neuron-glia interactions in brain energy metabolism: implications for neurodegenerative disorders. Diabetes Nutr. Metab. 2002;15:268–273. discussion 273. [PubMed] [Google Scholar]
- 88.Magistretti PJ. Neuron-glia metabolic coupling and plasticity. J. Exp. Biol. 2006;209:2304–2311. doi: 10.1242/jeb.02208. [DOI] [PubMed] [Google Scholar]
- 89.Maher F, Simpson I. Modulation of expression of glucose transporters GLUT3 and GLUT1 by potassium and N-methyl-D-aspartate in cultured cerebellar granule neurons. Mol. Cell Neurosci. 1994;5:369–375. doi: 10.1006/mcne.1994.1044. [DOI] [PubMed] [Google Scholar]
- 90.Wheeler TJ, Simpson IA, Sogin DC, Hinkle PC, Cushman SW. Detection of the rat adipose cell glucose transporter with antibody against the human red cell glucose transporter. Biochem. Biophys. Res. Commun. 1982;105:89–95. doi: 10.1016/s0006-291x(82)80014-4. [DOI] [PubMed] [Google Scholar]
- 91.Lee WH, Bondy CA. Ischemic injury induces brain glucose transporter gene expression. Endocrinology. 1993;133:2540–2544. doi: 10.1210/endo.133.6.8243275. [DOI] [PubMed] [Google Scholar]
- 92.Gerhart DZ, Leino RL, Taylor WE, Borson ND, Drewes LR. GLUT1 and GLUT3 gene expression in gerbil brain following brief ischemia: an in situ hybridization study. Brain Res. Mol. Brain Res. 1994;25:313–322. doi: 10.1016/0169-328x(94)90167-8. [DOI] [PubMed] [Google Scholar]
- 93.Maher F, Simpson IA. The GLUT3 glucose transporter is the predominant isoform in primary cultured neurons: assessment by biosynthetic and photoaffinity labelling. Biochem. J. 1994;301(Pt 2):379–384. doi: 10.1042/bj3010379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nagamatsu S, Sawa H, Kamada K, Nakamichi Y, Yoshimoto K, Hoshino T. Neuron-specific glucose transporter (NSGT): CNS distribution of GLUT3 rat glucose transporter (RGT3) in rat central neurons. FEBS Lett. 1993;334:289–295. doi: 10.1016/0014-5793(93)80697-s. [DOI] [PubMed] [Google Scholar]
- 95.McCall AL, Van Bueren AM, Moholt-Siebert M, Cherry NJ, Woodward WR. Immunohistochemical localization of the neuron-specific glucose transporter (GLUT3) to neuropil in adult rat brain. Brain Res. 1994;659:292–297. doi: 10.1016/0006-8993(94)90896-6. [DOI] [PubMed] [Google Scholar]
- 96.Gerhart DZ, Broderius MA, Borson ND, Drewes LR. Neurons and microvessels express the brain glucose transporter protein GLUT3. Proc. Natl. Acad. Sci. USA. 1992;89:733–737. doi: 10.1073/pnas.89.2.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lowry JP, Demestre M, Fillenz M. Relation between cerebral blood flow and extracellular glucose in rat striatum during mild hypoxia and hyperoxia. Dev. Neurosci. 1998;20:52–58. doi: 10.1159/000017298. [DOI] [PubMed] [Google Scholar]
- 98.Lowry JP, Miele M, O'Neill RD, Boutelle MG, Fillenz M. An amperometric glucose-oxidase/poly(o-phenylenediamine) biosensor for monitoring brain extracellular glucose: in vivo characterisation in the striatum of freely-moving rats. J. Neurosci. Methods. 1998;79:65–74. doi: 10.1016/s0165-0270(97)00171-4. [DOI] [PubMed] [Google Scholar]
- 99.Lowry JP, O'Neill RD, Boutelle MG, Fillenz M. Continuous monitoring of extracellular glucose concentrations in the striatum of freely moving rats with an implanted glucose biosensor. J. Neurochem. 1998;70:391–396. doi: 10.1046/j.1471-4159.1998.70010391.x. [DOI] [PubMed] [Google Scholar]
- 100.Fillenz M, Lowry JP. The relation between local cerebral blood flow and extracellular glucose concentration in rat striatum. Exp. Physiol. 1998;83:233–238. doi: 10.1113/expphysiol.1998.sp004107. [DOI] [PubMed] [Google Scholar]
- 101.Fillenz M, Lowry JP. Studies of the source of glucose in the extracellular compartment of the rat brain. Dev. Neurosci. 1998;20:365–368. doi: 10.1159/000017332. [DOI] [PubMed] [Google Scholar]
- 102.Simpson IA, Dwyer D, Malide D, Moley KH, Travis A, Vannucci SJ. The facilitative glucose transporter GLUT3: 20 years of distinction. Am. J. Physiol. Endocrinol. Metab. 2008;295:E242–253. doi: 10.1152/ajpendo.90388.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pellegri G, Rossier C, Magistretti PJ, Martin JL. Cloning, localization and induction of mouse brain glycogen synthase. Brain Res. Mol. Brain Res. 1996;38:191–199. doi: 10.1016/0169-328x(95)00305-c. [DOI] [PubMed] [Google Scholar]
- 104.Pellerin L, Pellegri G, Martin JL, Magistretti PJ. Expression of monocarboxylate transporter mRNAs in mouse brain: support for a distinct role of lactate as an energy substrate for the neonatal vs. adult brain. Proc. Natl. Acad. Sci. USA. 1998;95:3990–3995. doi: 10.1073/pnas.95.7.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Brown AM, Wender R, Ransom BR. Metabolic substrates other than glucose support axon function in central white matter. J. Neurosci. Res. 2001;66:839–843. doi: 10.1002/jnr.10081. [DOI] [PubMed] [Google Scholar]
- 106.Wiesinger H, Hamprecht B, Dringen R. Metabolic pathways for glucose in astrocytes. Glia. 1997;21:22–34. doi: 10.1002/(sici)1098-1136(199709)21:1<22::aid-glia3>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 107.Medina JM, Tabernero A. Lactate utilization by brain cells and its role in CNS development. J. Neurosci. Res. 2005;79:2–10. doi: 10.1002/jnr.20336. [DOI] [PubMed] [Google Scholar]
- 108.Magistretti PJ, Pellerin L. The contribution of astrocytes to the 18F-2-deoxyglucose signal in PET activation studies. Mol. Psych. 1996;1:445–452. [PubMed] [Google Scholar]
- 109.Brown AM, Ransom BR. Astrocyte glycogen and brain energy metabolism. Glia. 2007;55:1263–1271. doi: 10.1002/glia.20557. [DOI] [PubMed] [Google Scholar]
- 110.Brown AM. Brain glycogen re-awakened. J. Neurochem. 2004;89:537–552. doi: 10.1111/j.1471-4159.2004.02421.x. [DOI] [PubMed] [Google Scholar]
- 111.Brown AM, Baltan Tekkok S, Ransom BR. Energy transfer from astrocytes to axons: the role of CNS glycogen. Neurochem. Int. 2004;45:529–536. doi: 10.1016/j.neuint.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 112.Brown AM, Tekkok SB, Ransom BR. Glycogen regulation and functional role in mouse white matter. J. Physiol. 2003;549:501–512. doi: 10.1113/jphysiol.2003.042416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Brown AM, Sickmann HM, Fosgerau K, Lund TM, Schousboe A, Waagepetersen HS, Ransom BR. Astrocyte glycogen metabolism is required for neural activity during aglycemia or intense stimulation in mouse white matter. J. Neurosci. Res. 2005;79:74–80. doi: 10.1002/jnr.20335. [DOI] [PubMed] [Google Scholar]
- 114.Cruz NF, Ball KK, Dienel GA. Functional imaging of focal brain activation in conscious rats: impact of [(14)C]glucose metabolite spreading and release. J. Neurosci. Res. 2007;85:3254–3266. doi: 10.1002/jnr.21193. [DOI] [PubMed] [Google Scholar]
- 115.Hyder F, Patel AB, Gjedde A, Rothman DL, Behar KL, Shulman RG. Neuronal-glial glucose oxidation and glutamatergic-GABAergic function. J. Cereb. Blood Flow Metab. 2006;26:865–877. doi: 10.1038/sj.jcbfm.9600263. [DOI] [PubMed] [Google Scholar]
- 116.Simpson IA, Carruthers A, Vannucci SJ. Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J. Cereb. Blood Flow Metab. 2007;27:1766–1791. doi: 10.1038/sj.jcbfm.9600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hertz L, Dienel GA. Lactate transport and transporters: general principles and functional roles in brain cells. J. Neurosci. Res. 2005;79:11–18. doi: 10.1002/jnr.20294. [DOI] [PubMed] [Google Scholar]
- 118.Pierre K, Pellerin L. Monocarboxylate transporters in the central nervous system: distribution, regulation and function. J. Neurochem. 2005;94:1–14. doi: 10.1111/j.1471-4159.2005.03168.x. [DOI] [PubMed] [Google Scholar]
- 119.Pellerin L, Bouzier-Sore AK, Aubert A, Serres S, Merle M, Costalat R, Magistretti PJ. Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia. 2007;55:1251–1262. doi: 10.1002/glia.20528. [DOI] [PubMed] [Google Scholar]
- 120.Wender R, Brown AM, Fern R, Swanson RA, Farrell K, Ransom BR. Astrocytic glycogen influences axon function and survival during glucose deprivation in central white matter. J. Neurosci. 2000;20:6804–6810. doi: 10.1523/JNEUROSCI.20-18-06804.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dringen R, Schmoll D, Cesar M, Hamprecht B. Incorporation of radioactivity from [14C]lactate into the glycogen of cultured mouse astroglial cells. Evidence for gluconeogenesis in brain cells. Biol. Chem. Hoppe Seyler. 1993;374:343–347. doi: 10.1515/bchm3.1993.374.1-6.343. [DOI] [PubMed] [Google Scholar]
- 122.Loffler T, Al-Robaiy S, Bigl M, Eschrich K, Schliebs R. Expression of fructose-1,6-bisphosphatase mRNA isoforms in normal and basal forebrain cholinergic lesioned rat brain. Int. J. Dev. Neurosci. 2001;19:279–285. doi: 10.1016/s0736-5748(01)00011-9. [DOI] [PubMed] [Google Scholar]
- 123.Mazzio E, Soliman KF. The role of glycolysis and gluconeogenesis in the cytoprotection of neuroblastoma cells against 1-methyl 4-phenylpyridinium ion toxicity. Neurotoxicology. 2003;24:137–147. doi: 10.1016/s0161-813x(02)00110-9. [DOI] [PubMed] [Google Scholar]
- 124.Hevor TK. Some aspects of carbohydrate metabolism in the brain. Biochimie. 1994;76:111–120. doi: 10.1016/0300-9084(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 125.Hevor TK, Delorme P, Beauvillain JC. Glycogen synthesis and immunocytochemical study of fructose-1,6- biphosphatase in methionine sulfoximine epileptogenic rodent brain. J. Cereb. Blood Flow Metab. 1986;6:292–297. doi: 10.1038/jcbfm.1986.51. [DOI] [PubMed] [Google Scholar]
- 126.Hevor TK, Delorme P, Gayet J. Glycogen content and fructose-1, 6-biphosphatase activity in methionine sulfoximine epileptogenic mouse brain and liver after protein synthesis inhibition. Neuropathol. Appl. Neurobiol. 1985;11:129–139. doi: 10.1111/j.1365-2990.1985.tb00010.x. [DOI] [PubMed] [Google Scholar]
- 127.Cataldo AM, Broadwell RD. Cytochemical identification of cerebral glycogen and glucose-6-phosphatase activity under normal and experimental conditions. II. Choroid plexus and ependymal epithelia, endothelia and pericytes. J. Neurocytol. 1986;15:511–524. doi: 10.1007/BF01611733. [DOI] [PubMed] [Google Scholar]
- 128.Hertz L, Peng L, Dienel GA. Energy metabolism in astrocytes: high rate of oxidative metabolism and spatiotemporal dependence on glycolysis/glycogenolysis. J. Cereb. Blood Flow Metab. 2007;27:219–249. doi: 10.1038/sj.jcbfm.9600343. [DOI] [PubMed] [Google Scholar]
- 129.Hertz L. The astrocyte-neuron lactate shuttle: a challenge of a challenge. J. Cereb. Blood Flow Metab. 2004;24:1241–1248. doi: 10.1097/00004647-200411000-00008. [DOI] [PubMed] [Google Scholar]
- 130.Gruetter R. Glycogen: the forgotten cerebral energy store. J. Neurosci. Res. 2003;74:179–183. doi: 10.1002/jnr.10785. [DOI] [PubMed] [Google Scholar]
- 131.Gruetter R, Adriany G, Choi IY, Henry PG, Lei H, Oz G. Localized in vivo 13C NMR spectroscopy of the brain. NMR Biomed. 2003;16:313–338. doi: 10.1002/nbm.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Choi IY, Gruetter R. In vivo 13C NMR assessment of brain glycogen concentration and turnover in the awake rat. Neurochem. Int. 2003;43:317–322. doi: 10.1016/s0197-0186(03)00018-4. [DOI] [PubMed] [Google Scholar]
- 133.Choi IY, Seaquist ER, Gruetter R. Effect of hypoglycemia on brain glycogen metabolism in vivo. J. Neurosci. Res. 2003;72:25–32. doi: 10.1002/jnr.10574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hertz L, Dringen R, Schousboe A, Robinson SR. Astrocytes: glutamate producers for neurons. J. Neurosci. Res. 1999;57:417–428. [PubMed] [Google Scholar]
- 135.Tekkok SB, Brown AM, Westenbroek R, Pellerin L, Ransom BR. Transfer of glycogen-derived lactate from astrocytes to axons via specific monocarboxylate transporters supports mouse optic nerve activity. J. Neurosci. Res. 2005;81:644–652. doi: 10.1002/jnr.20573. [DOI] [PubMed] [Google Scholar]
- 136.Shulman RG, Hyder F, Rothman DL. Cerebral energetics and the glycogen shunt: neurochemical basis of functional imaging. Proc. Natl. Acad. Sci. USA. 2001;98:6417–6422. doi: 10.1073/pnas.101129298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Salm AK, McCarthy KD. The evidence for astrocytes as a target for central noradrenergic activity: expression of adrenergic receptors. Brain Res. Bull. 1992;29:265–275. doi: 10.1016/0361-9230(92)90056-4. [DOI] [PubMed] [Google Scholar]
- 138.Masmoudi-Kouki O, Gandolfo P, Castel H, Leprince J, Fournier A, Dejda A, Vaudry H, Tonon MC. Role of PACAP and VIP in astroglial functions. Peptides. 2007;28:1753–1760. doi: 10.1016/j.peptides.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 139.Magistretti PJ, Cardinaux JR, Martin JL. VIP and PACAP in the CNS: regulators of glial energy metabolism and modulators of glutamatergic signaling. Ann. N. Y. Acad. Sci. 1998;865:213–225. doi: 10.1111/j.1749-6632.1998.tb11181.x. [DOI] [PubMed] [Google Scholar]
- 140.Waschek JA. Vasoactive intestinal peptide: an important trophic factor and developmental regulator? Dev. Neurosci. 1995;17:1–7. doi: 10.1159/000111268. [DOI] [PubMed] [Google Scholar]
- 141.Laming PR, Kimelberg H, Robinson S, Salm A, Hawrylak N, Muller C, Roots B, Ng K. Neuronal-glial interactions and behaviour. Neurosci. Biobehav. Rev. 2000;24:295–340. doi: 10.1016/s0149-7634(99)00080-9. [DOI] [PubMed] [Google Scholar]
- 142.Sorg O, Magistretti PJ. Characterization of the glycogenolysis elicited by vasoactive intestinal peptide, noradrenaline and adenosine in primary cultures of mouse cerebral cortical astrocytes. Brain Res. 1991;563:227–233. doi: 10.1016/0006-8993(91)91538-c. [DOI] [PubMed] [Google Scholar]
- 143.Allaman I, Pellerin L, Magistretti PJ. Protein targeting to glycogen mRNA expression is stimulated by noradrenaline in mouse cortical astrocytes. Glia. 2000;30:382–391. [PubMed] [Google Scholar]
- 144.Cloix JF, Ardourel MY, Hévor T. Glycogen metabolism and brain pathologies. Central Nervous Syst. Agents Med. Chem. 2008;8:187–197. [Google Scholar]
- 145.Oz G, Henry PG, Seaquist ER, Gruetter R. Direct, noninvasive measurement of brain glycogen metabolism in humans. Neurochem. Int. 2003;43:323–329. doi: 10.1016/s0197-0186(03)00019-6. [DOI] [PubMed] [Google Scholar]
- 146.Oz G, Seaquist ER, Kumar A, Criego AB, Benedict LE, Rao JP, Henry PG, Van De Moortele PF, Gruetter R. Human brain glycogen content and metabolism: implications on its role in brain energy metabolism. Am. J. Physiol. Endocrinol. Metab. 2007;292:E946–951. doi: 10.1152/ajpendo.00424.2006. [DOI] [PubMed] [Google Scholar]
- 147.Lei H, Gruetter R. Effect of chronic hypoglycaemia on glucose concentration and glycogen content in rat brain: A localized 13C NMR study. J. Neurochem. 2006;99:260–268. doi: 10.1111/j.1471-4159.2006.04115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lei H, Morgenthaler F, Yue T, Gruetter R. Direct validation of in vivo localized 13C MRS measurements of brain glycogen. Magn. Reson. Med. 2007;57:243–248. doi: 10.1002/mrm.21128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Choi IY, Tkac I, Ugurbil K, Gruetter R. Noninvasive measurements of [1-(13)C]glycogen concentrations and metabolism in rat brain in vivo. J. Neurochem. 1999;73:1300–1308. doi: 10.1046/j.1471-4159.1999.0731300.x. [DOI] [PubMed] [Google Scholar]
- 150.Gruetter R. In vivo 13C NMR studies of compartmentalized cerebral carbohydrate metabolism. Neurochem. Int. 2002;41:143–154. doi: 10.1016/s0197-0186(02)00034-7. [DOI] [PubMed] [Google Scholar]
- 151.Bernard-Hélary K, Ardourel M, Hévor T, Cloix J-F. In vivo and in vitro glycogenic effects of methionine sulfoximine are different in two inbred strains of mice. Brain Res. 2002;929:147–155. doi: 10.1016/s0006-8993(01)03380-7. [DOI] [PubMed] [Google Scholar]
- 152.Delorme P, Hevor T. Glycogen particles in méthionine sulfoximine epileptogenic rodent brain and liver after the administration of methionine actinomycin D. Neuropathol. Appl. Neurobiol. 1985;11:117–128. doi: 10.1111/j.1365-2990.1985.tb00009.x. [DOI] [PubMed] [Google Scholar]
- 153.Folbergrova J. Glycogen and glycogen phosphorylase in the cerebral cortex of mice under the influence of methionine sulphoximine. J. Neurochem. 1973;20:547–557. doi: 10.1111/j.1471-4159.1973.tb12154.x. [DOI] [PubMed] [Google Scholar]
- 154.Folbergrova J, Passonneau JV, Lowry OH, Schulz DW. Glycogen, ammonia and related metabolites in the brain during seizures evoked by methionine sulphoximine. J. Neurochem. 1969;16:191–203. doi: 10.1111/j.1471-4159.1969.tb05937.x. [DOI] [PubMed] [Google Scholar]
- 155.Hevor TK, Delorme P. Biochemical and ultrastructural study of glycogen in cultured astrocytes submitted to the convulsant methionine sulfoximine. Glia. 1991;4:64–69. doi: 10.1002/glia.440040108. [DOI] [PubMed] [Google Scholar]
- 156.Phelps CH. An ultrastructural study of methionine sulphoximine-induced glycogen accumulation in astrocytes of the mouse cerebral cortex. J. Neurocytol. 1975;4:479–490. doi: 10.1007/BF01261377. [DOI] [PubMed] [Google Scholar]
- 157.Campbell PN, Work TS, Mellanby E. The isolation of a toxic substance from agenized wheat flour. Biochem. J. 1951;48:106–113. doi: 10.1042/bj0480106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bentley HR, Mc DE, Whitehead JK. Action of nitrogen trichloride on proteins; a synthesis of the toxic factor from methionine. Nature. 1950;165:735. doi: 10.1038/165735b0. [DOI] [PubMed] [Google Scholar]
- 159.Bentley HR, McDermott EE, Whitehead JK. Toxic factor from agonized proteins; methionine as the essential reactant. Nature. 1950;165:150. doi: 10.1038/165150a0. [DOI] [PubMed] [Google Scholar]
- 160.Gastaut H, Toga M, Naquet R. Clinical, electrographical and anatomical study of epilepsy induced in dogs by ingestion of agenised proteins. In: Baldwin M, Bailey P, editors. Temporal Lobe Epilepsy. Illinois: Thomas. Publisher; 1958. pp. 57–69. [Google Scholar]
- 161.Wolfe LS, Elliot KAC. Chemical studies in regulation to convulsive conditions. In: Elliot KAC, Page IH, Quastel JH, editors. Neurochemistry. 2nd. Springfield, USA: 1962. pp. 694–697. [Google Scholar]
- 162.Murakoshi I, Sekine T, Maeshima K, Ikegami F, Yoshinaga K, Fujii Y, Okonogi S. Absolute configuration of L-methionine sulfoximine as a toxic principle in Cnestis palala (Lour.) Merr. Chem. Pharm. Bull. (Tokyo) 1993;41:388–390. doi: 10.1248/cpb.41.388. [DOI] [PubMed] [Google Scholar]
- 163.Jeannoda VL, Rakoto-Ranoromalala DA, Valisolalao J, Creppy EE, Dirheimer G. Natural occurrence of methionine sulfoximine in the Connaraceae family. J. Ethnopharmacol. 1985;14:11–17. doi: 10.1016/0378-8741(85)90023-6. [DOI] [PubMed] [Google Scholar]
- 164.Bernard-Hélary K, Lapouble E, Ardourel M, Hevor T, Cloix J-F. Correlation between brain glycogen and convulsive state in mice submitted to methionine sulfoximine. Life Sci. 2000;67:1773–1781. doi: 10.1016/s0024-3205(00)00756-6. [DOI] [PubMed] [Google Scholar]
- 165.Peters EL, Tower DB. Glutamic acid and glutamine metabolism in cerebral cortex after seizures induced by methionine sulphoximine. J. Neurochem. 1959;5:80–90. doi: 10.1111/j.1471-4159.1959.tb13336.x. [DOI] [PubMed] [Google Scholar]
- 166.Meister A. Inhibition of glutamine synthase and gamma glutamylcysteine synthetase by methionine sulfoximine and related compounds. In: Seiler N, Jung MJ, Koch-Weser J, editors. Enzyme Activated Irreversible Inhibitiors. Amsterdam, The Netherlands: Elsevier; 1978. pp. 187–210. [Google Scholar]
- 167.Somers DL, Beckstead RM. Chronic methionine sulfoximine administration reduces synaptosomal aspartate and glutamate in rat striatum. Neurosci. Lett. 1990;115:335–340. doi: 10.1016/0304-3940(90)90478-r. [DOI] [PubMed] [Google Scholar]
- 168.Fonnum F. Glutamate: A neurotransmitter in mammalian brain. J. Neurochem. 1984;42:1–11. doi: 10.1111/j.1471-4159.1984.tb09689.x. [DOI] [PubMed] [Google Scholar]
- 169.Fonnum F, Paulsen RE. Comparison of transmitter amino acid levels in rat globus pallidus and neostriatum during hypoglycemia or after treatment with methionine sulfoximine or gamma-vinyl gamma-aminobutyric acid. J. Neurochem. 1990;54:1253–1257. doi: 10.1111/j.1471-4159.1990.tb01956.x. [DOI] [PubMed] [Google Scholar]
- 170.Engelsen B, Fonnum F. The effect of methionine sulfoximine, an inhibitor of glutamine synthetase, on the levels of amino acids in the intact and decorticated rat neostriatum. Brain Res. 1985;338:165–168. doi: 10.1016/0006-8993(85)90261-6. [DOI] [PubMed] [Google Scholar]
- 171.Blin M, Crusio WE, Hevor T, Cloix J-F. Chronic inhibition of glutamine synthetase is not associated with impairment of learning and memory in mice. Brain Res. Bull. 2002;57:11–15. doi: 10.1016/s0361-9230(01)00631-1. [DOI] [PubMed] [Google Scholar]
- 172.Dalsgaard MK, Madsen FF, Secher NH, Laursen H, Quistorff B. High glycogen levels in the hippocampus of patients with epilepsy. J. Cereb. Blood Flow Metab. 2007;27:1137–1141. doi: 10.1038/sj.jcbfm.9600426. [DOI] [PubMed] [Google Scholar]
- 173.Folbergrova J, Druga R, Haugvicova R, Mares P, Otahal J. Anticonvulsant and neuroprotective effect of (S)-3,4-dicarboxyphenylglycine against seizures induced in immature rats by homocysteic acid. Neuropharmacology. 2008;54:665–675. doi: 10.1016/j.neuropharm.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 174.Folbergrova J. Energy metabolism of mouse cerebral cortex during homocysteine convulsions. Brain Res. 1974;81:443–454. doi: 10.1016/0006-8993(74)90842-7. [DOI] [PubMed] [Google Scholar]
- 175.Sacktor B, Wilson JE, Tiekert CG. Regulation of glycolysis in brain, in situ, during convulsions. J. Biol. Chem. 1966;241:5071–5075. [PubMed] [Google Scholar]
- 176.Nahorski SR, Roberts DJ, Stewart GG. Some neurochemical aspects of pentamethylenetetrazole convulsive activity in rat brain. J. Neurochem. 1970;17:621–631. doi: 10.1111/j.1471-4159.1970.tb00542.x. [DOI] [PubMed] [Google Scholar]
- 177.King LJ, Lowry OH, Passonneau JV, Venson V. Effects of convulsants on energy reserves in the cerebral cortex. J. Neurochem. 1967;14:599–611. doi: 10.1111/j.1471-4159.1967.tb09563.x. [DOI] [PubMed] [Google Scholar]
- 178.Folbergrova J, Ingvar M, Siesjo BK. Metabolic changes in cerebral cortex, hippocampus, and cerebellum during sustained bicuculline-induced seizures. J. Neurochem. 1981;37:1228–1238. doi: 10.1111/j.1471-4159.1981.tb04673.x. [DOI] [PubMed] [Google Scholar]
- 179.Chapman AG, Meldrum BS, Siesjo BK. Cerebral metabolic changes during prolonged epileptic seizures in rats. J. Neurochem. 1977;28:1025–1035. doi: 10.1111/j.1471-4159.1977.tb10665.x. [DOI] [PubMed] [Google Scholar]
- 180.Wang W, Lohi H, Skurat AV, DePaoli-Roach AA, Minassian BA, Roach PJ. Glycogen metabolism in tissues from a mouse model of Lafora disease. Arch. Biochem. Biophys. 2007;457:264–269. doi: 10.1016/j.abb.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Vilchez D, Ros S, Cifuentes D, Pujadas L, Valles J, Garcia-Fojeda B, Criado-Garcia O, Fernandez-Sanchez E, Medrano-Fernandez I, Dominguez J, Garcia-Rocha M, Soriano E, Rodriguez de Cordoba S, Guinovart JJ. Mechanism suppressing glycogen synthesis in neurons and its demise in progressive myoclonus epilepsy. Nat. Neurosci. 2007;10:1407–1413. doi: 10.1038/nn1998. [DOI] [PubMed] [Google Scholar]
- 182.Jobe PC, Laird HE. Neurotransmitter abnormalities as determinants of seizure susceptibility and intensity in the genetic models of epilepsy. Biochem. Pharmacol. 1981;30:3137–3144. doi: 10.1016/0006-2952(81)90510-4. [DOI] [PubMed] [Google Scholar]
- 183.Jobe PC, Laird HE 2nd, Ko KH, Ray T, Dailey JW. Abnormalities in monoamine levels in the central nervous system of the genetically epilepsy-prone rat. Epilepsia. 1982;23:359–366. doi: 10.1111/j.1528-1157.1982.tb05421.x. [DOI] [PubMed] [Google Scholar]
- 184.Laird HE 2nd, Dailey JW, Jobe PC. Neurotransmitter abnormalities in genetically epileptic rodents. Fed. Proc. 1984;43:2505–2509. [PubMed] [Google Scholar]
- 185.Lallement G, Delamanche IS, Pernot-Marino I, Baubichon D, Denoyer M, Carpentier P, Blanchet G. Neuroprotective activity of glutamate receptor antagonists against soman-induced hippocampal damage: quantification with an omega 3 site ligand. Brain Res. 1993;618:227–237. doi: 10.1016/0006-8993(93)91270-3. [DOI] [PubMed] [Google Scholar]
- 186.Lallement G, Carpentier P, Pernot-Marino I, Baubichon D, Collet A, Blanchet G. Transient impairment of the gabaergic function during initiation of soman-induced seizures. Brain Res. 1993;629:239–244. doi: 10.1016/0006-8993(93)91326-n. [DOI] [PubMed] [Google Scholar]
- 187.Lallement G, Denoyer M, Collet A, Pernot-Marino I, Baubichon D, Monmaur P, Blanchet G. Changes in hippocampal acetylcholine and glutamate extracellular levels during soman-induced seizures: influence of septal cholinoceptive cells. Neurosci. Lett. 1992;139:104–107. doi: 10.1016/0304-3940(92)90868-8. [DOI] [PubMed] [Google Scholar]
- 188.Hevor T. Methionine sulfoximine has no effect on gamma aminobutyric acid concentration in the brain. Biogenic Amines. 1996;12:445–461. [Google Scholar]
- 189.Hevor T, Richard O, Pichon J, Hélary K, Cloix JF. Acetycholine, catecholamine, indolamine, and GABA levels in the brain of rats submitted to a convulsant. In: Qureshi GA, Parvez H, Caudy P, Parvez S, editors. Neurochemical markers of degenerative nervous diseases and drug addiction, Progress in HPLC-HPCE. Vol. 7. The Netherlands: VSP, Zest; 1998. pp. 383–411. [Google Scholar]
- 190.Hevor TK, Delorme P. Possible involvement of indolamines in the glycogenic effect of the convulsant methionine sulfoximine in rat brain. Experientia. 1990;46:710–713. doi: 10.1007/BF01939942. [DOI] [PubMed] [Google Scholar]
- 191.Hevor TK, Aissi E, Delorme P. Correlation between carbohydrate and catecholamine level impairments in methionine sulfoximine epileptogenic rat brain. Neurochem. Res. 1990;15:861–868. doi: 10.1007/BF00965904. [DOI] [PubMed] [Google Scholar]
- 192.Magistretti PJ, Morrison JH, Shoemaker WJ, Sapin V, Bloom FE. Vasoactive intestinal polypeptide induces glycogenolysis in mouse cortical slices: a possible regulatory mechanism for the local control of energy metabolism. Proc. Natl. Acad. Sci. USA. 1981;78:6535–6539. doi: 10.1073/pnas.78.10.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Pentreath VW, Seal LH, Kai-Kai MA. Incorporation of [3H]2-deoxyglucose into glycogen in nervous tissues. Neuroscience. 1982;7:759–767. doi: 10.1016/0306-4522(82)90081-1. [DOI] [PubMed] [Google Scholar]
- 194.Quach TT, Duchemin AM, Rose C, Schwartz JC. 3H-Glycogen hydrolysis elicited by histamine in mouse brain slices: selective involvement of H1 receptors. Mol. Pharmacol. 1980;17:301–308. [PubMed] [Google Scholar]
- 195.Quach TT, Rose C, Duchemin AM, Schwartz JC. Glycogenolysis induced by serotonin in brain: identification of a new class of receptor. Nature. 1982;298:373–375. doi: 10.1038/298373a0. [DOI] [PubMed] [Google Scholar]
- 196.Quach TT, Rose C, Schwartz JC. [3H]Glycogen hydrolysis in brain slices: responses to neurotransmitters and modulation of noradrenaline receptors. J. Neurochem. 1978;30:1335–1341. doi: 10.1111/j.1471-4159.1978.tb10464.x. [DOI] [PubMed] [Google Scholar]
- 197.Ingvar M, Lindvall O, Folbergrova J, Siesjo BK. Influence of lesions of the noradrenergic locus coeruleus system on the cerebral metabolic response to bicuculline-induced seizures. Brain Res. 1983;264:225–231. doi: 10.1016/0006-8993(83)90820-x. [DOI] [PubMed] [Google Scholar]
- 198.Fabene PF, Weiczner R, Marzola P, Nicolato E, Calderan L, Andrioli A, Farkas E, Sule Z, Mihaly A, Sbarbati A. Structural and functional MRI following 4-aminopyridine-induced seizures: a comparative imaging and anatomical study. Neurobiol. Dis. 2006;21:80–89. doi: 10.1016/j.nbd.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 199.Fabene PF, Sbarbati A. In vivo MRI in different models of experimental epilepsy. Curr. Drug Targets. 2004;5:629–636. doi: 10.2174/1389450043345218. [DOI] [PubMed] [Google Scholar]