Abstract
Normal rat pregnancy is characterized by plasma volume expansion due to renal sodium retention and is associated with a blunted response to natriuretic stimuli, such as atrial natriuretic peptide (ANP). ANP signals via cGMP, and phosphodiesterases (PDE) inactivate cGMP and terminate the natriuretic response. We previously reported that increased medullary PDE-5 activity occurs in rat pregnancy, which may be the mechanism of the blunted natriuretic effect of ANP. Here, we used anesthetized 16-day pregnant and virgin rats to investigate whether intrarenal infusion of a selective PDE-5 inhibitor, sildenafil, would reverse the blunted response to ANP in pregnancy. We measured blood pressure, renal clearances using inulin and p-aminohippuric acid, and electrolyte excretion at baseline and during an ANP infusion. ANP caused a fall in mean arterial pressure in all groups, and sildenafil induced a further reduction. We observed an increase in sodium excretion with ANP in all rats, but this was blunted in the vehicle-infused pregnant rats. This could not be explained by differences in renal hemodynamics and was of tubular origin, as reflected by the reduced rise in fractional excretion of sodium with ANP in the pregnant rat given vehicle (45 ± 11 vs. 204 ± 49%; P < 0.05). However, intrarenal sildenafil increased the natriuretic response and the rise in fractional excretion of sodium to the virgin value (226 ± 23 vs. 245 ± 73%; not significant), whereas the blunting persisted in the contralateral kidney. This demonstrates that increased intrarenal PDE-5 mediates the blunted natriuretic response to ANP during pregnancy and may contribute to the physiological volume expansion.
Keywords: plasma volume, sodium retention, cGMP, hormone signaling, hormone resistance
During Normal Pregnancy, a marked and progressive plasma volume expansion occurs in ~50% of women and in 70–100% of rats (1, 9). The failure of plasma volume to expand during pregnancy is associated with poor outcome and is often a characteristic of preeclampsia (4). The gestational plasma volume expansion is clearly the result of a net sodium retention (1, 9).
The physiological regulation of sodium balance and plasma volume is maintained by a number of counterbalancing systems, including the natriuretic nitric oxide (NO), atrial natriuretic peptide (ANP), pressure-natriuresis system, and the antinatriuretic renin-angiotensin-aldosterone (RAAS) and sympathetic nervous systems. In pregnancy, continual plasma volume expansion and sodium retention suggest that the balance is shifted toward sodium-retaining systems, and yet both NO and ANP levels are maintained or elevated. However, we previously reported that the renal natriuretic actions of ANP are blunted during pregnancy in the rat, as is the pressure natriuretic response (18, 19). cGMP mediates the natriuretic responses to both ANP and increased arterial blood pressure (via NO) (13, 17), and its actions are terminated by phosphodiesterases (PDE), which degrade cGMP to inactive 5′-GMP (3). There are 11 gene families with many variants leading to considerable variability in individual PDE isozymes with regard to location (3). PDE-5 is a cGMP-specific enzyme expressed in many tissues, including renal proximal tubules and medullary collecting ducts, and we have previously reported that inner medullary collecting duct (IMCD) PDE-5 protein expression and activity are increased during pregnancy (23). We have also observed that IMCD PDE-5 abundance and activity increased in animal models of chronic liver disease and nephrotic syndrome, other situations where volume expansion coexists with renal sodium retention and where the natriuretic response to ANP is blunted (22, 28). Taken together, these studies suggest that the increase in renal PDE-5 is responsible for the blunted natriuretic action of ANP. We therefore hypothesized that selective inhibition of PDE-5 in the kidney of the pregnant rat will maintain renal cGMP levels and reinstate the natriuretic response to ANP. We tested this by comparing the natriuretic response to intravenous ANP in virgin and pregnant rats also receiving an intrarenal infusion of a specific PDE-5 inhibitor (sildenafil) or vehicle into the left kidney, with the right kidney serving as control.
METHODS
Female Sprague-Dawley rats aged 3–5 mo were purchased from Harlan Sprague-Dawley (Indianapolis, IN; facility 217). Rats were housed at the University of Florida Animal Care Services Unit at constant temperature and humidity with 12:12-h light-dark cycles and given free access to tap water and standard laboratory chow. Females that were to become pregnant were housed with males; daily vaginal smears were taken, and the presence of sperm was taken as day 1 of pregnancy and confirmed by the presence of fetuses in utero at the time of acute study. All animal studies received approval by the University of Florida Institutional Animals Care and Use Committee.
Experiments were conducted on day 16 of pregnancy in rats given ANP + vehicle (n = 6) or ANP + sildenafil (n = 7) and in age-matched virgin rats (both n = 6). ANP was obtained from Peninsula Labs (Belmont, CA), and sildenafil was a gift from Pfizer UK. Rats were anesthetized with an intraperitoneal injection of 1.2 mg/100 g body wt of the thiobarbiturate Inactin. The abdomen, left flank, left groin, and neck were shaved, and the rat was placed on a heated table with body temperature maintained at 37 ± 1°C. A tracheostomy was performed, and oxygen was blown across the tracheal tube throughout the experiment. The left femoral vein was cannulated, and an infusion was started at 1 ml · 100 g body wt−1 ·h−1, containing 0.09% NaCl, 2 mg/ml FITC inulin, and 3.6 mg/ml p-aminohippuric acid (PAH). The left femoral artery was cannulated to monitor blood pressure (BP) and to collect blood samples. An abdominal incision was made, and the left kidney was exposed. In pregnant animals, the uterus was covered with parafilm (Pechiney, WI) and kept warm and moist. Both the left and right ureters were cannulated for the collection of urine with PE-10 tubing. For intrarenal drug infusion, PE-10 tubing was inserted into the left ileolumbar artery, passed up the abdominal aorta, and positioned at the junction of the left renal artery without obstructing renal blood flow. Vehicle or drug was infused into the left kidney at 2 μl· 100 g body wt−1 ·min−1 throughout the study, with the right kidney acting as a control.
After a 60-min stabilization period, two × 20-min baseline urine collections and midpoint blood samples (250 μl) were taken. The blood was centrifuged, and 100 μl of plasma were removed to measure inulin, PAH, and sodium concentrations. Red blood cells were resuspended in saline and returned to the animal through the venous catheter. Rat α-ANP was then added to the left femoral vein infusion at 11.6 ng· 100 g body wt−1 ·min−1. In 6 of the 12 virgin and 7 of the 13 pregnant rats, the intrarenal infusion was switched to one containing sildenafil in saline at 0.1 μg·μl−1·min−1, to deliver 0.2 μg· 100 g body wt−1 ·min−1. Two additional 20-min urine collections and blood samples were taken. The uterus and pups were checked to confirm normal perfusion, and the intrarenal cannula was flushed with lissamine green dye to confirm the correct positioning of the intrarenal catheter. The rat was killed with an overdose of anesthetic, and the left kidney was removed and weighed.
Analytical and statistical methods
Inulin concentrations in urine and plasma were measured in a black, clear-bottom, 96-well plate on a Tecan Safire optical system, reading fluorescence at 485-nm excitation and 530-nm emission, with 10 μl of sample or standard + 190 μl of HEPES buffer. PAH concentration was established by colorimetric assay previously described (2), and sodium concentrations were measured on a flame photometer using cesium as the internal standard [1:100 dilution of sample in 1.5 mmol/l CsCl solution (Instrumentation Laboratory)]. All chemicals were obtained from Sigma (St. Louis, MO) unless stated otherwise.
Data are presented as means ± SE. Paired and unpaired t-tests were used to compare between kidneys, drug treatments, and time points. The nonparametric Mann-Whitney U-test was used to compare baseline and post-ANP fractional excretion of sodium.
RESULTS
The 16-day pregnant rats were heavier than the age-matched virgins (291 ± 6 vs. 248 ± 6 g; P < 0.05). As shown in Table 1, the baseline BP was similar in virgin and pregnant rats, and infusion of ANP lowered BP significantly in the vehicle-infused virgin rats and both the sildenafil-infused virgin and pregnant rats (P < 0.05). A nonsignificant fall in BP was seen in the vehicle-infused pregnant group. In both pregnant and virgin rats, the fall in BP with intrarenal PDE-5 inhibition was greater than with vehicle (Table 1). The baseline hematocrit was lower in pregnant than in virgin rats, and PDE-5 inhibition did not change hematocrit levels in any group. There were no differences in plasma sodium concentration between virgin and pregnant rats at baseline or with ANP infusion. Baseline values of glomerular filtration rate, renal vascular resistance, renal plasma flow, and urine flow were similar between all groups, and ANP infusion had inconsistent effects on glomerular filtration rate, renal vascular resistance, and renal plasma flow with a tendency toward renal vasodilation.
Table 1.
Measurements of rat groups at baseline and after ANP infusion
| MAP, mmHg | Hct, ml/100ml | PNa, meq/l | GFR, ml/min |
RVR, mmHg·ml−1·min |
RPF, ml/min |
V, μl/min |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Left kidney | Right kidney | Left kidney | Right kidney | Left kidney | Right kidney | Left kidney | Right kidney | ||||
| Virgin vehicle | |||||||||||
| Baseline | 120±2 | 45.6±0.4 | 143±1 | 0.96±0.1 | 0.88±0.1 | 28.7±3.5 | 30.9±4.5 | 2.2±0.3 | 2.3±0.4 | 2.4±0.3 | 2.3±0.2 |
| ANP infusion | 114±2† | 46.3±0.6 | 147±5 | 1.10±0.1† | 0.79±0.2 | 20.4±3.7† | 21.6±5.8† | 3.4±0.5† | 3.2±0.5 | 4.5±0.3† | 4.0±0.6† |
| Virgin sildenafil | |||||||||||
| Baseline | 123±6 | 45.6±0.5 | 144±3 | 1.09±0.2 | 1.10±0.1 | 32.9±4.5 | 25.6±2.9 | 2.1±0.2 | 2.2±0.2 | 2.4±0.1 | 2.2±0.2 |
| ANP infusion | 105±4†‡ | 46.3±0.6 | 142±4 | 1.10±0.1 | 1.13±0.2 | 24.3±2.3† | 20.3±1.8† | 2.7±0.4 | 2.8±0.4 | 4.4±0.3† | 4.0±0.3† |
| Pregnant vehicle | |||||||||||
| Baseline | 118±2 | 41.2±0.2* | 141±1 | 0.94±0.1 | 0.88±0.1 | 25.6±3.0 | 33.0±4.5 | 2.8±0.3 | 2.3±0.4 | 2.6±0.2 | 2.5±0.1 |
| ANP infusion | 111±4 | 41.6±0.2* | 142±1 | 1.13±0.1† | 1.06±0.1† | 20.4±1.9† | 24.3±2.3† | 3.3±0.4 | 2.7±0.3† | 3.1±0.3*† | 2.9±0.3*† |
| Pregnant sildenafil | |||||||||||
| Baseline | 120±2 | 41.5±1.1* | 141±2 | 0.76±0.1 | 0.86±0.1 | 27.7±3.3 | 28.6±2.7 | 2.6±0.3 | 2.6±0.3 | 2.8±0.2 | 2.9±0.5 |
| ANP infusion | 102±4†‡ | 42.1±0.9* | 143±1 | 0.95±0.1 | 1.01±0.1 | 16.6±1.3*† | 21.9±1.9 | 3.6±0.3 | 2.7±0.3 | 4.6±0.3† | 3.2±0.3* |
Values are means ± SE. MAP, mean arterial pressure; Hct, hemotocrit; PNa, plasma sodium concentration; GFR, glomerular filtration rate; RVR, renal vascular resistance; RPF, renal plasma flow; V, urine flow.
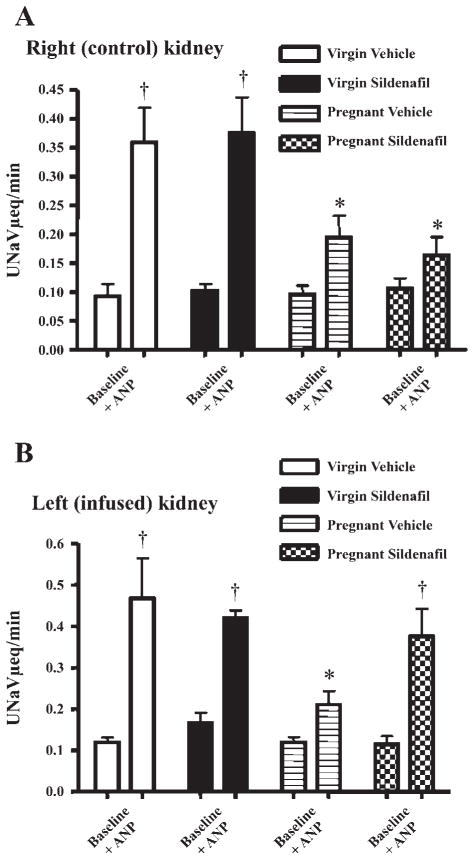
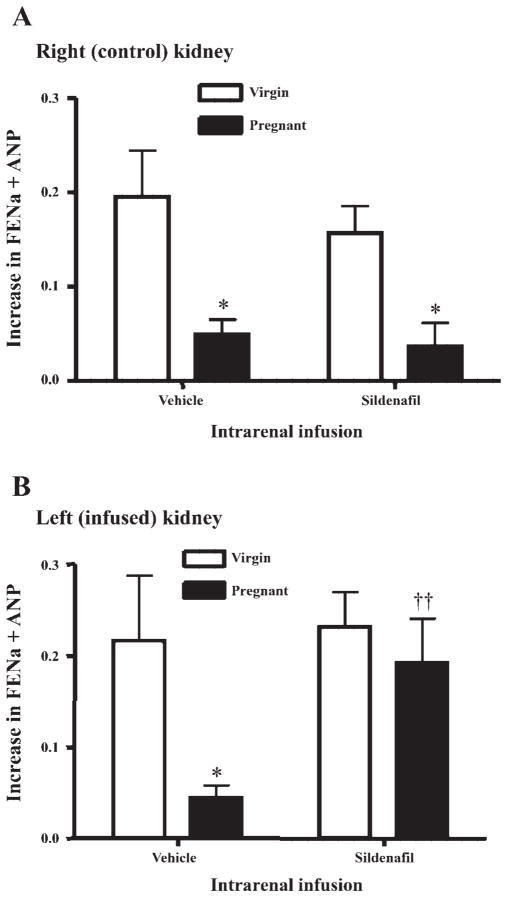
As shown in Fig. 1, the baseline values for urinary sodium excretion (UNaV) were similar in right and left kidneys of virgin and pregnant rats. ANP infusion produced a significant rise in absolute UNaV in all groups; however, the natriuretic effect of ANP was blunted in both kidneys of the vehicle-infused pregnant rats (P < 0.05 vs. virgin). This blunting persisted in the right (noninfused) kidney in the sildenafil-treated pregnant rats but was reversed in the left kidney of the pregnant rats receiving intrarenal sildenafil. The data are most simply expressed in Fig. 2, which presents the change in fractional sodium excretion in response to ANP infusion in all groups. Figure 2A presents data from the right kidneys, where both pregnant groups displayed a blunted natriuretic response to ANP, i.e., reduced increase in fractional sodium excretion vs. similarly treated virgin rats (P < 0.05). Figure 2B displays the data for the change in fractional sodium excretion observed in the left (infused) kidneys. There was a blunted increase in fractional sodium excretion in response to ANP in vehicle-treated pregnant vs. virgin animals (P < 0.05), whereas sildenafil infusion in the pregnant rat restored the full increase in fractional sodium excretion but had no additional effect in the virgin rat.
Fig. 1.
Summary of urinary sodium excretion (UNaV) in the baseline state and during intravenous infusion of atrial natriuretic peptide (ANP; 11.6 ng · 100 g body wt−1 ·min−1) in virgin and pregnant rats receiving an infusion into the left kidney of either 0.9% NaCl (vehicle; 2 μl/min) or sildenafil (0.2 μg · 100 g body wt−1 ·min−1). A: data for the right (control) kidney. B: data for the left (infused) kidney. †P < 0.001; *P < 0.05.
Fig. 2.
Summary of the increase in fractional excretion of sodium (FENa) during ANP infusion in virgin and pregnant rats receiving an infusion into the left kidney of either 0.9% NaCl (vehicle) or sildenafil. A: data for the right (control) kidney. B: data for the left (infused) kidney. *P < 0.001, comparing virgin and pregnant; ††P < 0.001, comparing intrarenal infusion.
Generally similar findings were also observed with the diuretic response (Table 1). ANP infusion produced a greater diuresis in both kidneys of vehicle-treated virgins vs. vehicle-infused pregnant rats (P < 0.05). There was no diuresis in the right kidney of the sildenafil-infused pregnant rats, whereas a robust diuresis was seen in the left kidney due to intrarenal sildenafil.
DISCUSSION
The main novel finding of this study is that the tubular refractoriness to ANP seen in the normal pregnant rat can be reversed by selective intrarenal PDE-5 inhibition. Our earlier observations suggested that IMCD PDE-5 protein abundance and activity are elevated in normal pregnancy, and, in vitro, increased PDE-5 activity causes blunted ANP-induced cGMP release in isolated IMCD from pregnant vs. virgin rats (23). The present finding reinforces these observations with in vivo studies demonstrating that intrarenal infusion of a PDE-5-selective inhibitor restores the natriuretic response to ANP in pregnant rats.
Volume homeostasis involves a balance between sodium-retaining systems (sympathetic nervous system and RAAS) and the natriuretic NO and ANP systems. In the normal nonpregnant state, sodium excretion is exquisitely regulated to maintain plasma and extracellular fluid volume at a constant value. In normal pregnancy, this balance is perturbed, and plasma volume expansion develops with persistent renal sodium retention (1). This must mean that the antinatriuretic stimuli predominate, and there is marked upregulation of the antinatriuretic RAAS in normal pregnancy. However, there are also significant increases in renal NO production during normal pregnancy, at least in rat (6). The natriuretic ANP increases moderately in late pregnant women (16), although there is little change in the cardiac content or plasma concentration of ANP in pregnant rats (15, 20).
Thus both natriuretic and antinatriuretic systems increase in pregnancy, which seems counterintuitive given the known net sodium retention that occurs. According to Kristensen et al. (15), the natriuretic response to administered ANP was similar in late pregnant and virgin rats when studied under volume-depleted conditions. In women, a low dose of ANP produced a minimal natriuresis in late pregnancy and no impact on sodium excretion when they were studied 4 mo postpartum (12). However, other evidence suggests that a refractoriness develops to ANP that would allow the antinatriuretic RAAS to predominate. For example, the natriuretic response to infused ANP is blunted in pregnant goats (24). We have previously reported that the conscious, chronically catheterized pregnant rat becomes refractory to the tubular natriuretic (but not hemodynamic) actions of ANP (18). Similar observations have also been made in both anesthetized and conscious acutely prepared pregnant rats (5), and we observed that this ANP refractoriness persists in vitro (23). This loss of response to ANP can be viewed as an adaptation facilitating continued volume expansion in pregnancy, resembling that seen in nephrotic syndrome and cirrhosis. The pressure natriuresis is another important natriuretic response, “central to the long-term regulation of sodium balance, extracellular fluid and plasma volume, and blood pressure” (11). When the acute pressure natriuresis system is operating normally, it should not be possible to develop positive sodium balance and cumulative PVE. However, in normal pregnancy, the pressure natriuresis relationship is blunted (19); in addition, because tubular responsiveness to increased BP requires the renal actions of NO (17), this suggests that the tubular responsiveness to NO is also blunted in pregnancy.
The second messenger cGMP is common to the natriuretic responses that are blunted in normal pregnancy. ANP stimulates the particulate guanylyl cyclase, whereas NO stimulates the soluble guanylyl cyclase (13, 14), and stimulation of either results in an increase in intracellular cGMP, which mediates direct tubular natriuretic actions. Thus, if the renal tubular actions of cGMP are somehow impaired in pregnancy, this could account for a generalized loss of natriuretic responsiveness. This scenario resembles the pathophysiology of inappropriate renal sodium retention seen in nephrotic syndrome, cirrhosis, and heart failure. In these pathological conditions characterized by excessive renal sodium retention and volume expansion, the tubular responsiveness to ANP is also lost (21, 22, 27, 28). Binding of ANP to IMCD cells was not different in normal pregnancy or in nephrotic or cirrhotic rats compared with normal rats (21, 23, 28), reinforcing the notion that this is a postreceptor alteration.
In nephrotic and cirrhotic rats, nonselective PDE inhibition corrected the blunted ANP-dependent cGMP accumulation in isolated IMCD cells, and intrarenal infusion of the PDE inhibitor zaprinast corrected the blunted natriuresis in response to acute volume expansion seen in nephrotic and cirrhotic rats in vivo (21, 28). More recently, we found that the PDE-5-selective, competitive antagonist 1,3-dimethyl-6-(2-propoxy-5-methanesulfonylamidophenyl)pyrazol[3,4d]-pyrimidin-4-(5H)-one (DMPPO) reversed ANP resistance in IMCD cells from both cirrhotic and normal pregnant rats in vitro and reversed the blunted natriuretic response to acute volume expansion (putatively due to endogenous ANP release) in vivo (22, 23). The present study expands on these observations by using the highly PDE-5-selective antagonist sildenafil during infusion of exogenous ANP in normal pregnant and virgin rats. ANP infusion produced a robust rise in UNaV in virgin rats, and this was unaffected by sildenafil in either the infused or contralateral control kidney. In the vehicle-infused pregnant rats, however, the response to ANP was blunted and the absolute change in sodium excretion was reduced compared with the virgin rats, as reported previously (5, 18). This blunting persisted in the right (control, uninfused) kidney in the sildenafil group, whereas intrarenal infusion of sildenafil to the left kidney increased the natriuretic response to ANP to equal the nonpregnant value. There was no difference in the filtered load of sodium or in the renal plasma flow between left and right kidneys in the pregnant sildenafil rats; thus the difference in UNaV between left and right must reflect differences in tubular sodium reabsorption. This experiment was conducted under general anesthesia and immediately after acute surgery, which will likely alter renal sodium reabsorption. It is possible that tubular sodium reabsorption is affected differently by surgical stress in the pregnant vs. virgin rat kidney. However, our use of a paired study design allows comparison between right (control) and left (vehicle or PDE-5 inhibited) renal responses to ANP, allowing a clear demonstration of an up-regulated PDE-5-dependent inhibition of the natriuretic response that is specific to pregnancy. The pattern of diuresis generally paralleled the natriuresis with a marked diuretic response observed during ANP in both kidneys of virgin rats, whereas the response was blunted in pregnant rats but reversed in the kidney infused with sildenafil.
Our study design employed intrarenal infusion of a PDE-5 inhibitor to minimize the marked antihypertensive effect of systemic sildenafil administration. Some sildenafil did enter the general circulation, as indicated by the greater decline in BP seen in both virgin and pregnant rats during intrarenal sildenafil infusion. However, this was not enough to influence sodium excretion in the contralateral kidney of the pregnant rat since this remained blunted. Both kidneys of the virgin rats were exposed to the fall in BP with the combination of ANP and sildenafil, but this had no impact on the natriuretic response to virgins compared with vehicle-infused rats. Thus we are confident that any circulating sildenafil did not significantly impact sodium excretion in the control right kidney.
The lack of an effect of PDE-5 inhibition in the virgin rat during ANP-induced natriuresis is interesting and suggests that, although there is significant PDE-5 protein present in the inner medulla of the virgin rat kidney, it is not active. This lack of renal tubular response to PDE-5 inhibition was also seen earlier in the normal male rats used as controls for the cirrhotic rats receiving intrarenal DMPPO, another PDE-5-selective blocker (21). Obviously, there must be some mechanism to degrade cGMP in the normal virgin kidney and the male kidney, and there is abundant renal PDE-1 (another cGMP-selective PDE) as well as PDE-5 (7). Of the more recently discovered PDEs, there is also renal expression of PDE-9, a potent and cGMP-specific PDE (25), and of PDE-10 and PDE-11, both dual substrate PDEs with affinity for both cAMP and cGMP (8, 26). There are thus abundant PDEs capable of cGMP degradation, although the specifics of location and activation of individual PDEs are not currently known.
In the broad context of volume and BP regulation in pregnancy, this selective increase in renal tubular PDE-5 activity makes perfect sense. The primary vasodilator activated in pregnancy, NO, relies largely on cGMP to evoke its vasodilatory response (14). A generalized increase in PDE-5 would prevent the vasodilatory response of pregnancy that is essential to accommodate the plasma volume expansion without leading to a rise in BP. Of note, in nonpregnant states, a diminished pressure-natriuretic response is pathological and causes a volume-dependent hypertension (11) because there is no compensatory peripheral vasodilation. In contrast, in normal pregnancy, a blunted acute pressure natriuresis coexists with a resetting of the BP response, due to overwhelming vasodilatory stimuli, such that volume expansion is permitted without an increase in BP. It is interesting to note that the opposite change occurs with the antinatriuretic RAAS in normal pregnancy, where the renal tubules remain fully responsive to angiotensin and aldosterone but the vasculature becomes refractory to the vasoconstrictor actions of ANG II (10).
In conclusion, this study demonstrates that the increased renal PDE-5 expression in the normal pregnant kidney has a functional consequence in blunting the cGMP-dependent natriuretic response to ANP. This is likely to extend to other natriuretic agents, notably NO, and may play an important role in permitting the plasma volume expansion essential for optimal pregnancy.
Acknowledgments
Present address of S. Knight: Vascular Biology Center, Medical College of Georgia, Augusta, GA 30912.
GRANTS
These studies were funded by National Institute of Child Health and Human Development Grant HD-041571.
References
- 1.Baylis C. Glomerular filtration and volume regulation in gravid animal models. Baillieres Clin Obstet Gynaecol. 1994;8:235–264. doi: 10.1016/s0950-3552(05)80320-7. [DOI] [PubMed] [Google Scholar]
- 2.Baylis C, Brango C, Engels K. Renal effects of moderate hemorrhage in the conscious pregnant rat. Am J Physiol Renal Fluid Electrolyte Physiol. 1990;259:F945–F949. doi: 10.1152/ajprenal.1990.259.6.F945. [DOI] [PubMed] [Google Scholar]
- 3.Beavo JA. Cyclic nucleotide phosphodiesterases: functional implications of multiple isoforms. Physiol Rev. 1995;75:725–748. doi: 10.1152/physrev.1995.75.4.725. [DOI] [PubMed] [Google Scholar]
- 4.Chesley LC, Lindheimer MD. Renal hemodynamics and intravascular volume in normal and hypertensive pregnancy. In: Rubin PC, editor. Hypertension: Hypertension in Pregnancy. Vol. 10. Amsterdam: Elsevier; 1988. p. 38. [Google Scholar]
- 5.Corwin E, Castro L, Brizzee B, Solomon S. Atrial natriuretic factor: effects on blood pressure and renal function during pregnancy. Biogenic Amines. 1993;9:271–280. [Google Scholar]
- 6.Danielson LA, Conrad KP. Acute blockade of nitric oxide synthase inhibits renal vasodilation and hyperfiltration during pregnancy in chronically instrumented conscious rats. J Clin Invest. 1995;96:482–490. doi: 10.1172/JCI118059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dousa TP. Cyclic-3′,5′-nucleotide phosphodiesterase isozymes in cell biology and pathophysiology of the kidney. Kidney Int. 1999;55:29–62. doi: 10.1046/j.1523-1755.1999.00233.x. [DOI] [PubMed] [Google Scholar]
- 8.Fawcett L, Baxendale R, Stacey P, McGrouther C, Harrow I, Soderling S, Hetman J, Beavo JA, Phillips SC. Molecular cloning and characterization of a distinct human phosphodiesterase gene family: PDE11A. Proc Natl Acad Sci USA. 2000;97:3702–3807. doi: 10.1073/pnas.050585197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallery EDM, Hunyor SN, Gyory AZ. Plasma volume concentration: a significant factor in both pregnancy-associated hypertension (preeclampsia) and chronic hypertension in pregnancy. Qu J Med. 1979;48:593–602. [PubMed] [Google Scholar]
- 10.Gant NF, Daley GL, Chand S, Whalley PC, MacDonald PJ. A study of angiotensin II pressor response throughout primigravid pregnancy. J Clin Invest. 1973;52:2682–2689. doi: 10.1172/JCI107462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guyton AC. The surprising kidney-fluid mechanism for pressure control—its infinite gain! Hypertension. 1990;16:725–730. doi: 10.1161/01.hyp.16.6.725. [DOI] [PubMed] [Google Scholar]
- 12.Irons DW, Baylis PH, Davison JM. Effect of atrial natriuretic peptide on renal hemodynamics and sodium excretion during human pregnancy. Am J Physiol Renal Fluid Electrolyte Physiol. 1996;271:F239–F242. doi: 10.1152/ajprenal.1996.271.1.F239. [DOI] [PubMed] [Google Scholar]
- 13.Kishimoto I, Dubois SK, Garbers DL. The heart communicates with the kidney exclusively through the guanylyl cyclase-A receptor: acute handling of sodium and water in response to volume expansion. Proc Natl Acad Sci USA. 1996;93:6215–6219. doi: 10.1073/pnas.93.12.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kone BC, Baylis C. Biosynthesis and homeostatic roles of nitric oxide in the normal kidney. Am J Physiol Renal Physiol. 1997;272:F561–F578. doi: 10.1152/ajprenal.1997.272.5.F561. [DOI] [PubMed] [Google Scholar]
- 15.Kristensen CG, Nakagawa Y, Coe FL, Lindheimer MD. Effect of atrial natriuretic factor in rat pregnancy. Am J Physiol Regul Integr Comp Physiol. 1986;250:R589–R594. doi: 10.1152/ajpregu.1986.250.4.R589. [DOI] [PubMed] [Google Scholar]
- 16.Lowe SA, Macdonald GJ, Brown MA. Atrial natriuretic peptide in pregnancy: response to oral sodium supplementation. Clin Exp Pharmacol Physiol. 1992;19:607–612. doi: 10.1111/j.1440-1681.1992.tb00512.x. [DOI] [PubMed] [Google Scholar]
- 17.Majid DS, Williams A, Navar LG. Inhibition of nitric oxide synthesis attenuates pressure-induced natriuretic responses in anesthetized dogs. Am J Physiol Renal Fluid Electrolyte Physiol. 1993;264:F79–F87. doi: 10.1152/ajprenal.1993.264.1.F79. [DOI] [PubMed] [Google Scholar]
- 18.Masilamani S, Hobbs GR, Baylis C. Pregnant rats are refractory to the natriuretic actions of atrial natriuretic peptide. Am J Physiol Regul Integr Comp Physiol. 1994;267:R1611–R1616. doi: 10.1152/ajpregu.1994.267.6.R1611. [DOI] [PubMed] [Google Scholar]
- 19.Masilamani S, Hobbs GR, Baylis C. The acute pressure natriuresis response blunted and the blood pressure response reset in the normal pregnant rat. Am J Obstet Gynecol. 1998;179:486–491. doi: 10.1016/s0002-9378(98)70384-9. [DOI] [PubMed] [Google Scholar]
- 20.Nadel AS, Ballermann BJ, Anderson S, Brenner BM. Interrelationships among atrial peptides, renin and blood volume in pregnant rats. Am J Physiol Regul Integr Comp Physiol. 1988;254:R793–R800. doi: 10.1152/ajpregu.1988.254.5.R793. [DOI] [PubMed] [Google Scholar]
- 21.Ni X, Cheng Y, Cao L, Gardner DG, Humphreys MH. Mechanisms contributing to renal resistance to atrial natriuretic peptide in rats with common bile-duct ligation. J Am Soc Nephrol. 1996;7:2110–2118. doi: 10.1681/ASN.V7102110. [DOI] [PubMed] [Google Scholar]
- 22.Ni XP, Safai M, Gardner DG, Humphreys MH. Increased cGMP phosphodiesterase activity mediates renal resistance to ANP in rats with bile duct ligation. Kidney Int. 2001;59:1264–1273. doi: 10.1046/j.1523-1755.2001.0590041264.x. [DOI] [PubMed] [Google Scholar]
- 23.Ni XP, Safai M, Rishi R, Baylis C, Humphreys M. Increased activity of cGMP specific phosphodiaesterase (PDE-5) contributes to resistance to ANP in the pregnant rat. J Am Soc Nephrol. 2004;15:1254–1260. doi: 10.1097/01.asn.0000125613.96927.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olsson K, Karlberg BE, Eriksson L. Atrial natriuretic peptide in pregnant and lactating goats. Acta Endocrinol. 1989;120:519–525. doi: 10.1530/acta.0.1200519. [DOI] [PubMed] [Google Scholar]
- 25.Soderling SH, Bayuga SJ, Beavo JA. Identification and characterization of a novel family of cyclic nucleotide phosphodiesterases. J Biol Chem. 1998;273:15553–15558. doi: 10.1074/jbc.273.25.15553. [DOI] [PubMed] [Google Scholar]
- 26.Soderling SH, Bayuga SJ, Beavo JA. Isolation and characterization of a dual-substrate phosphodiesterase gene family: PDE10A. Proc Natl Acad Sci USA. 1999;96:7071–7076. doi: 10.1073/pnas.96.12.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Supaporn T, Sandberg SM, Borgeson DD, Heublein DM, Luchner A, Wei CM, Dousa TP, Burnett JC., Jr Blunted cGMP response to agonists and enhanced glomerular cyclic 3′,5′-nucleotide phosphodiesterase activities in experimental congestive heart failure. Kidney Int. 1996;50:1718–1725. doi: 10.1038/ki.1996.491. [DOI] [PubMed] [Google Scholar]
- 28.Valentin JP, Qiu CB, Muldowney WP, Ying WZ, Gardner DG, Humphreys MH. Cellular basis for blunted volume expansion natriuresis in experimental nephrotic syndrome. J Clin Invest. 1992;90:1302–1312. doi: 10.1172/JCI115995. [DOI] [PMC free article] [PubMed] [Google Scholar]