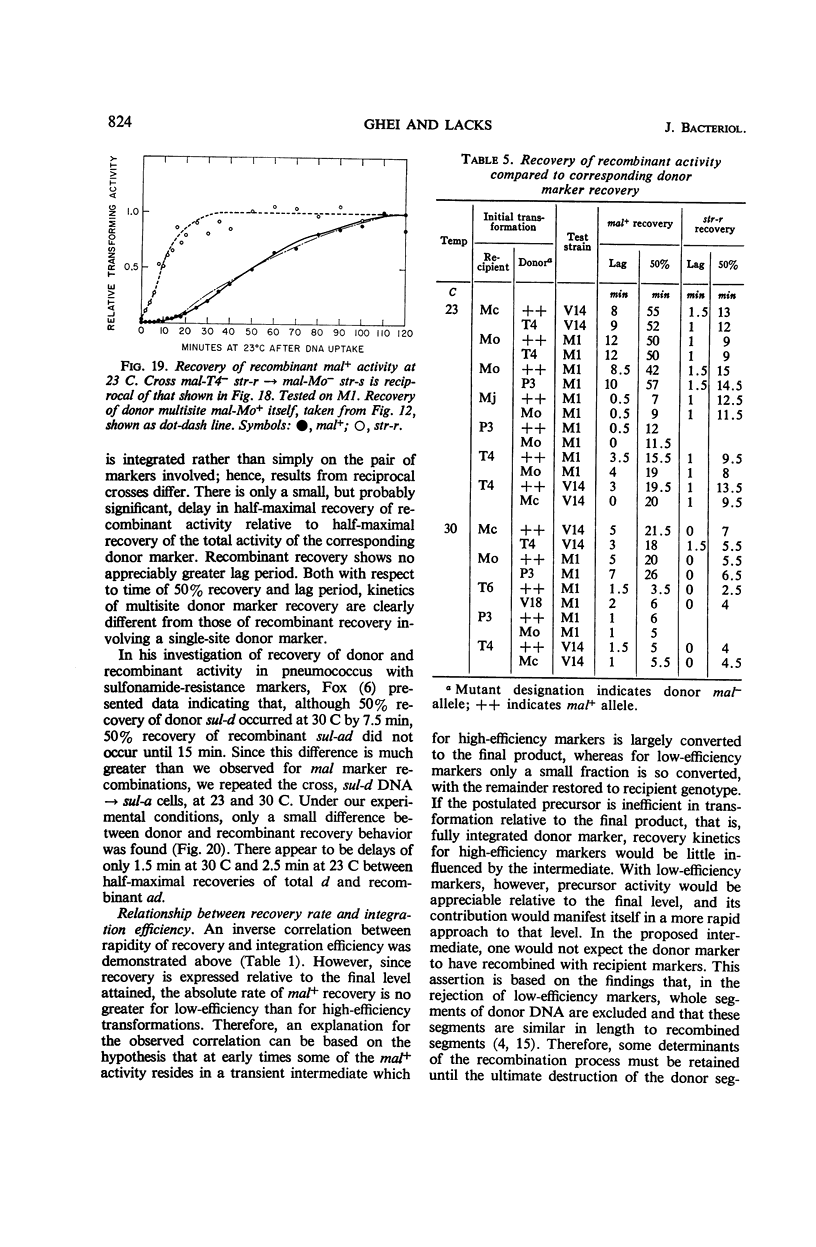
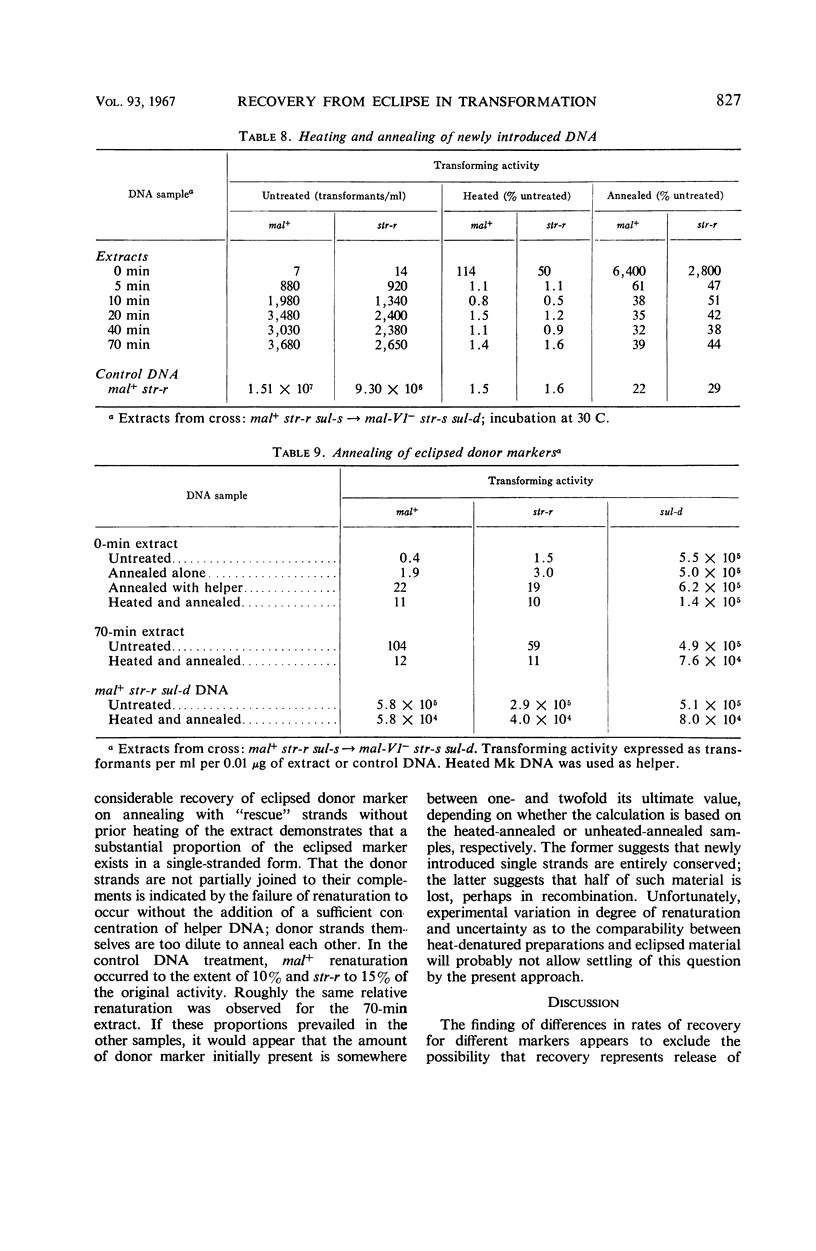
Abstract
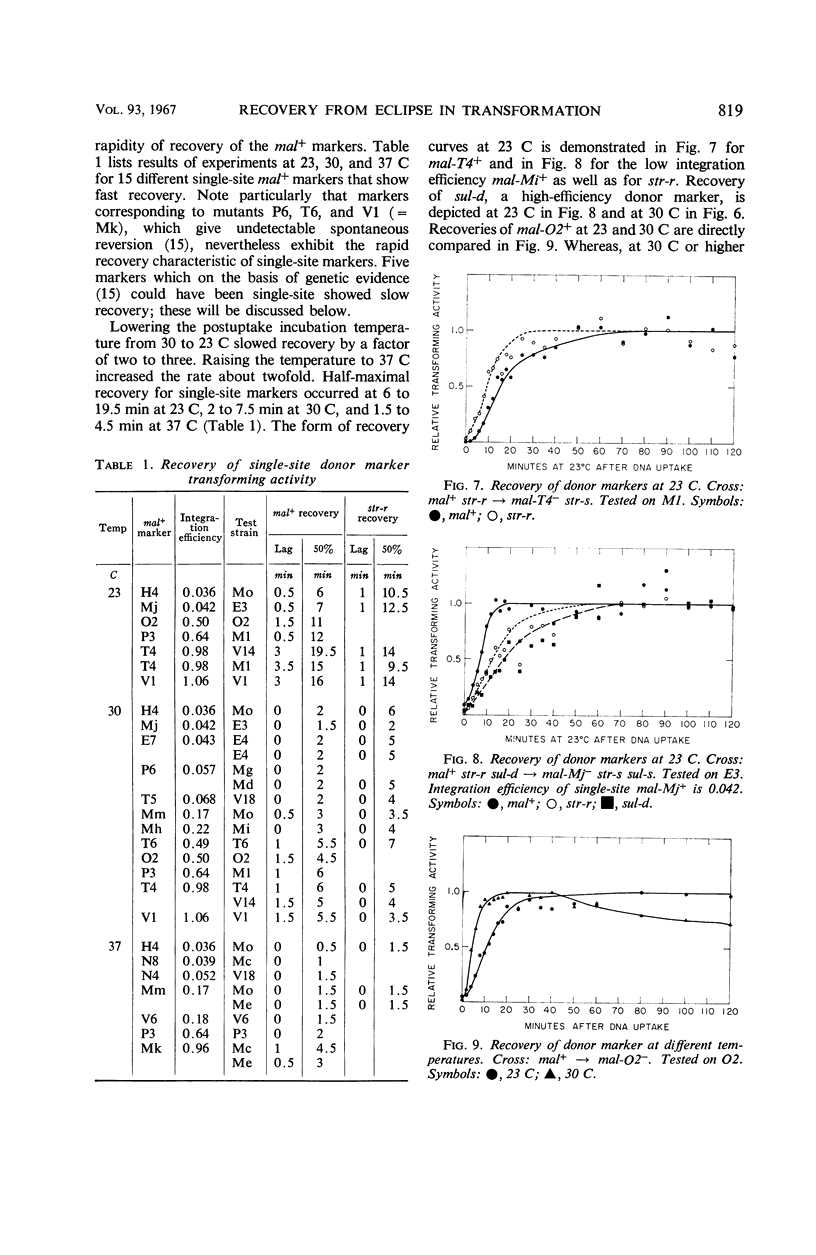
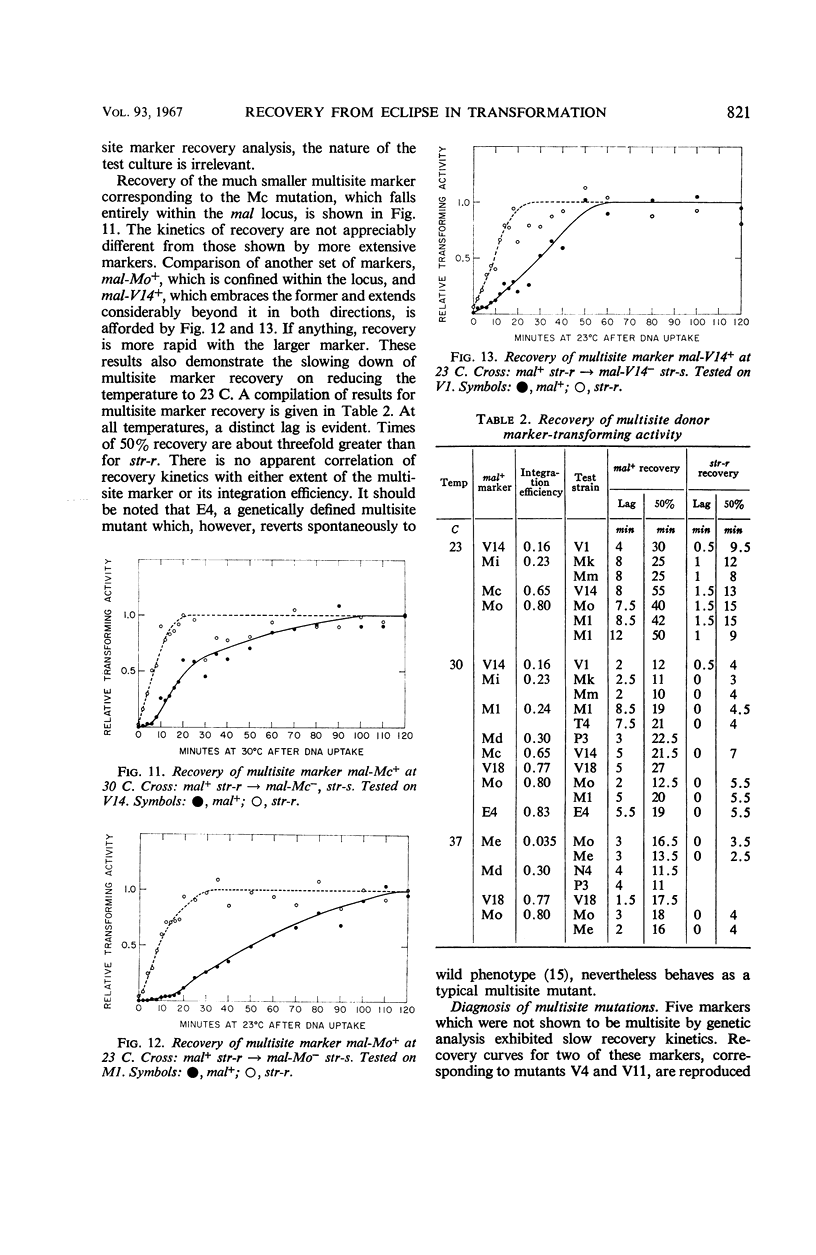
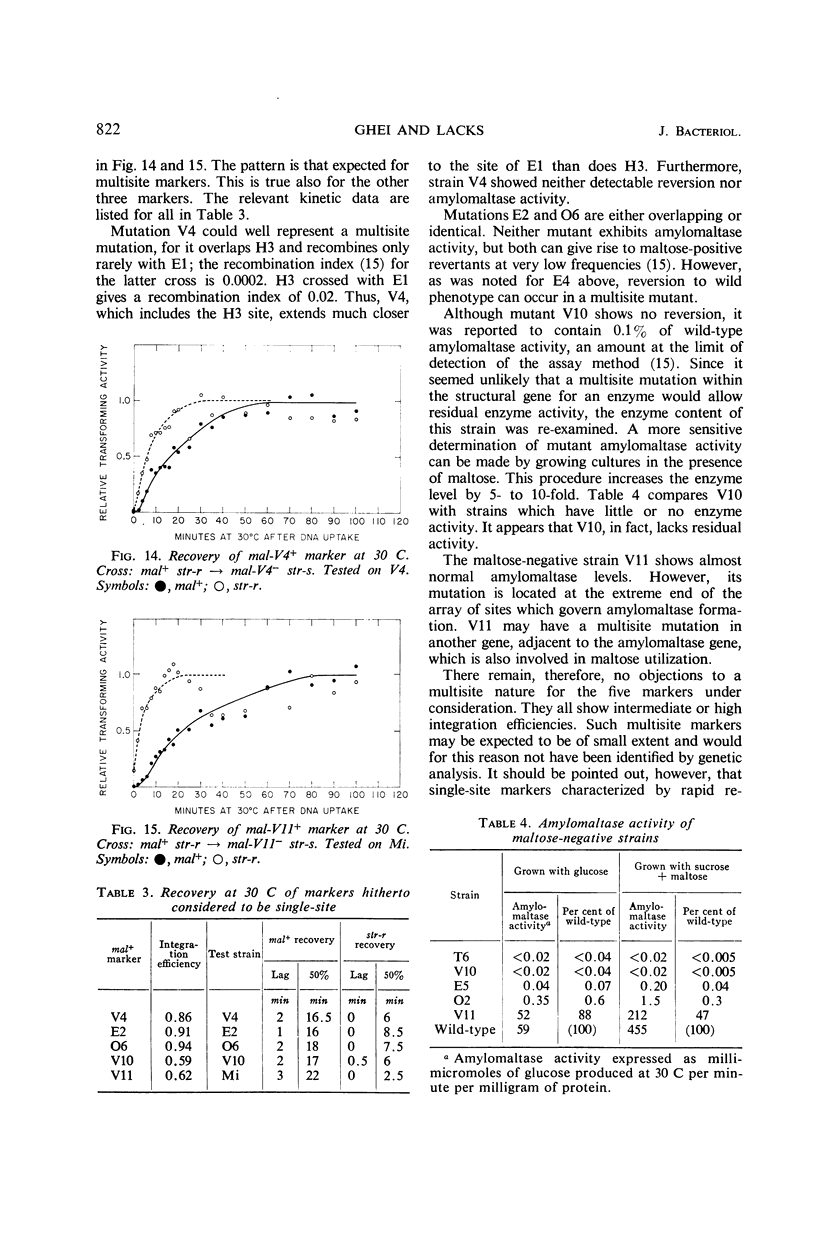
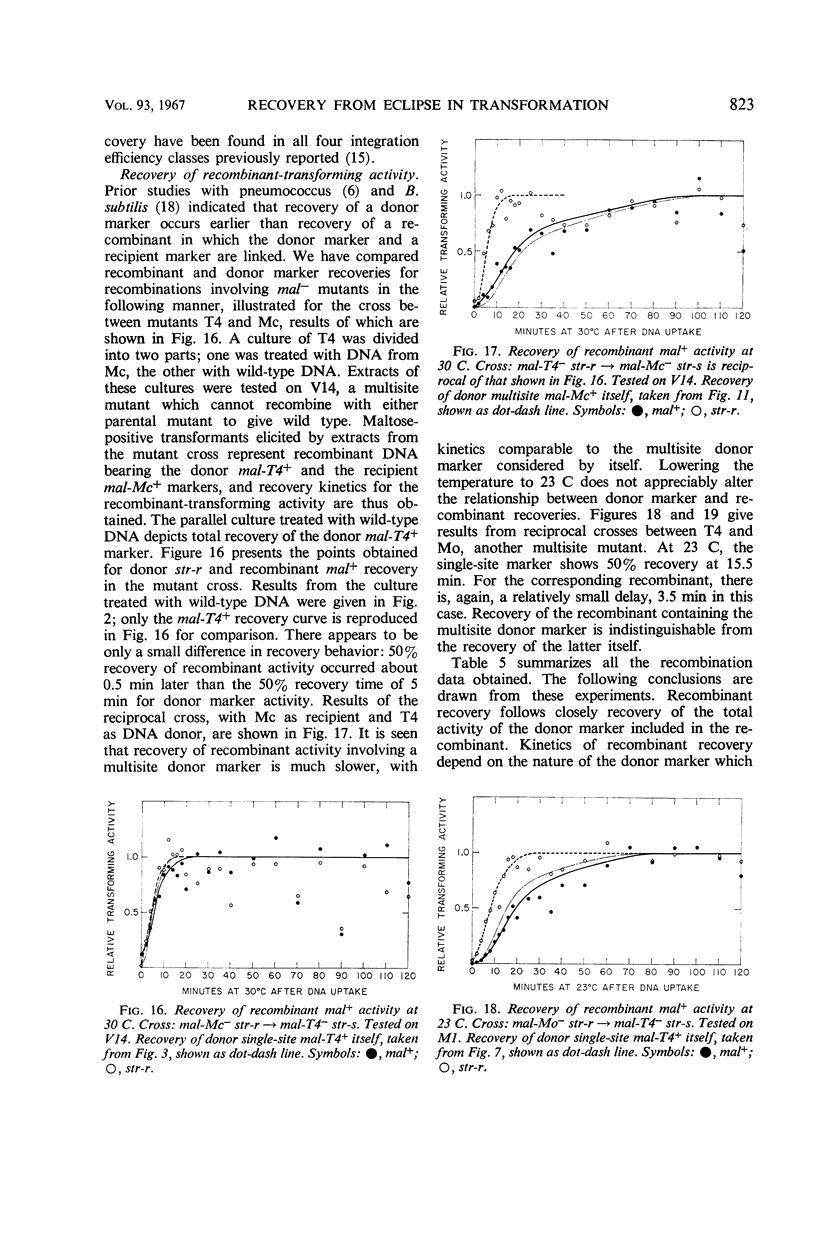
After the uptake of deoxyribonucleic acid (DNA), donor marker-transforming activity is temporarily lost. Restoration of the activity by annealing in vitro supports the idea that donor DNA is single-stranded at this stage. Kinetics of in vivo recovery from eclipse were examined for various markers at three temperatures. Sigmoidal recovery curves at lower temperatures indicate that the process consists of several steps. Rate of recovery was found to depend on the nature of the donor marker. Single-site markers recover much more rapidly than multisite markers corresponding to recipient deletions. Single-site markers vary somewhat in recovery rate, with rapidity of recovery inversely related to integration efficiency. Appearance of a recombinant-transforming activity lags only slightly behind recovery of its constituent donor marker.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BODMER W. F., GANESAN A. T. BIOCHEMICAL AND GENETIC STUDIES OF INTEGRATION AND RECOMBINATION IN BACILLUS SUBTILIS TRANSFORMATION. Genetics. 1964 Oct;50:717–738. doi: 10.1093/genetics/50.4.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPHRUSSI-TAYLOR H. [The status of the transforming DNA during the 1st phases of bacterial transformation]. C R Seances Soc Biol Fil. 1960;154:1951–1955. [PubMed] [Google Scholar]
- Ephrussi-Taylor H., Gray T. C. Genetic studies of recombining DNA in pneumococcal transformation. J Gen Physiol. 1966 Jul;49(6):211–231. doi: 10.1085/jgp.49.6.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ephrussi-Taylor H., Sicard A. M., Kamen R. Genetic Recombination in DNA-Induced Transformation of Pneumococcus. I. the Problem of Relative Efficiency of Transforming Factors. Genetics. 1965 Mar;51(3):455–475. doi: 10.1093/genetics/51.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOX M. S., ALLEN M. K. ON THE MECHANISM OF DEOXYRIBONUCLEATE INTEGRATION IN PNEUMOCOCCAL TRANSFORMATION. Proc Natl Acad Sci U S A. 1964 Aug;52:412–419. doi: 10.1073/pnas.52.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOX M. S., HOTCHKISS R. D. Fate of transforming deoxyribonucleate following fixation by transformable bacteria. Nature. 1960 Sep 17;187:1002–1006. doi: 10.1038/1871002a0. [DOI] [PubMed] [Google Scholar]
- FOX M. S., HOTCHKISS R. D. Fate of transforming deoxyribonucleate following fixation by transformable bacteria. Nature. 1960 Sep 17;187:1002–1006. doi: 10.1038/1871002a0. [DOI] [PubMed] [Google Scholar]
- Hotchkiss R. D. CYCLICAL BEHAVIOR IN PNEUMOCOCCAL GROWTH AND TRANSFORMABILITY OCCASIONED BY ENVIRONMENTAL CHANGES. Proc Natl Acad Sci U S A. 1954 Feb;40(2):49–55. doi: 10.1073/pnas.40.2.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LACKS S. Molecular fate of DNA in genetic transformation of Pneumococcus. J Mol Biol. 1962 Jul;5:119–131. doi: 10.1016/s0022-2836(62)80067-9. [DOI] [PubMed] [Google Scholar]
- Lacks S. Integration efficiency and genetic recombination in pneumococcal transformation. Genetics. 1966 Jan;53(1):207–235. doi: 10.1093/genetics/53.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmur J., Lane D. STRAND SEPARATION AND SPECIFIC RECOMBINATION IN DEOXYRIBONUCLEIC ACIDS: BIOLOGICAL STUDIES. Proc Natl Acad Sci U S A. 1960 Apr;46(4):453–461. doi: 10.1073/pnas.46.4.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notani N., Goodgal S. H. On the nature of recombinants formed during transformation in Hemophilus influenzae. J Gen Physiol. 1966 Jul;49(6):197–209. doi: 10.1085/jgp.49.6.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VENEMA G., PRITCHARD R. H., VENEMA-SCHROEDER T. FATE OF TRANSFORMING DEOXYRIBONUCLEIC ACID IN BACILLUS SUBTILIS. J Bacteriol. 1965 May;89:1250–1255. doi: 10.1128/jb.89.5.1250-1255.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VENEMA G., PRITCHARD R. H., VENEMA-SCHROEDER T. PROPERTIES OF NEWLY INTRODUCED TRANSFORMING DEOXYRIBONUCLEIC ACID IN BACILLUS SUBTILIS. J Bacteriol. 1965 Aug;90:343–346. doi: 10.1128/jb.90.2.343-346.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voll M. J., Goodgal S. H. Loss of activity of transforming deoxyribonucleic acid after uptake by Haemophilus influenzae. J Bacteriol. 1965 Oct;90(4):873–883. doi: 10.1128/jb.90.4.873-883.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voll M. J., Goodgal S. H. The stability of recombinant DNA formed during transformation of Hemophilus influenzae. Biochim Biophys Acta. 1966 Apr 18;119(1):65–74. doi: 10.1016/0005-2787(66)90038-4. [DOI] [PubMed] [Google Scholar]


