Abstract
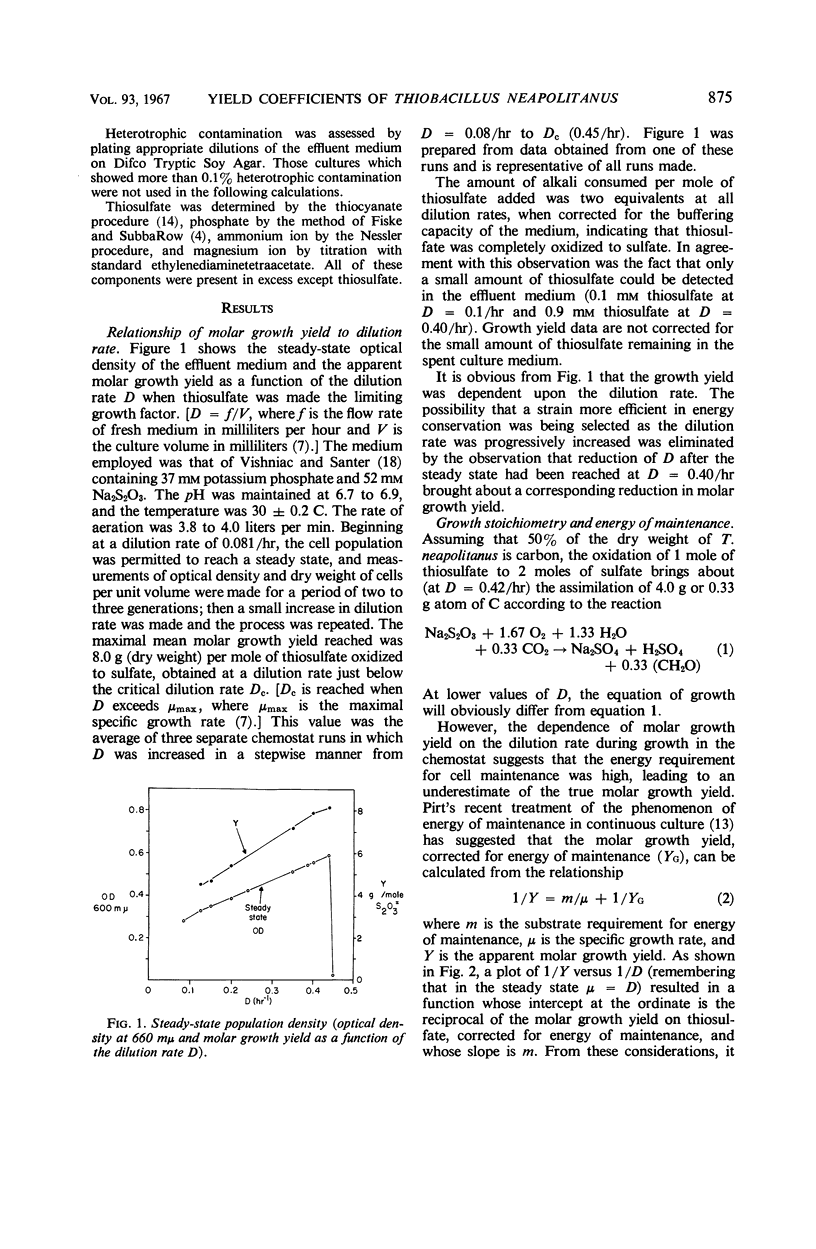
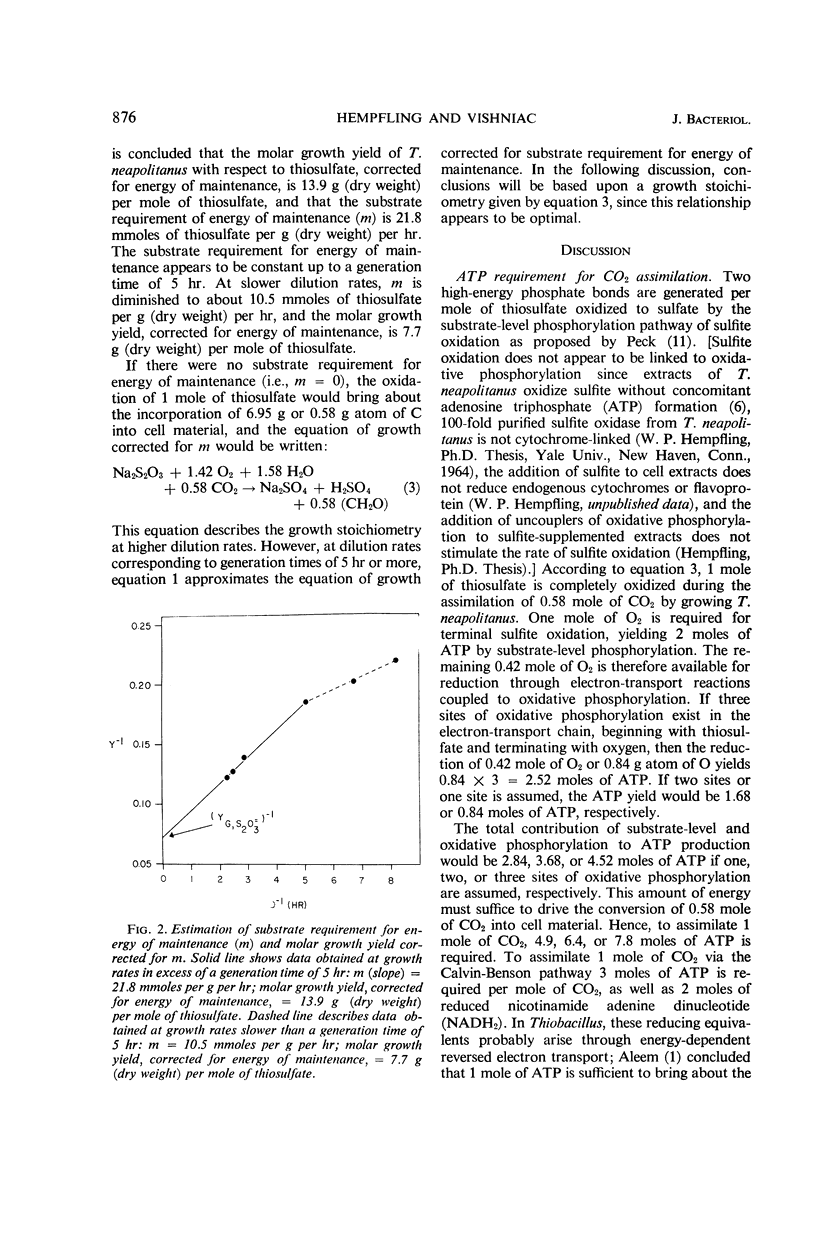
Thiobacillus neapolitanus, when grown in continuous culture with thiosulfate limiting growth, possessed an apparent maximal molar growth yield of 8.0 g (dry weight) per mole of thiosulfate. The substrate requirement for energy of maintenance was the highest yet reported, amounting to 21.8 mmoles of thiosulfate per g per hr. The molar growth yield, corrected for this maintenance energy requirement, was 13.9 g (dry weight) per mole of thiosulfate. It was concluded that substrate-level phosphorylation during sulfite oxidation accounted for about 45% of the adenosine triphosphate (ATP) requirement for CO2 assimilation and maintenance during growth on limiting thiosulfate, that three sites of energy conservation exist in the electron-transport chain terminating in oxygen, and that 7.8 moles of ATP are required to fix and assimilate 1 mole of CO2 into cell material.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aleem M. I. Generation of reducing power in chemosynthesis. 3. Energy-linked reduction of pyridine nucleotides in Thiobacillus novellus. J Bacteriol. 1966 Feb;91(2):729–736. doi: 10.1128/jb.91.2.729-736.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAUCHOP T., ELSDEN S. R. The growth of micro-organisms in relation to their energy supply. J Gen Microbiol. 1960 Dec;23:457–469. doi: 10.1099/00221287-23-3-457. [DOI] [PubMed] [Google Scholar]
- HEINZ E., PATLAK C. S. Energy expenditure by active transport mechanisms. Biochim Biophys Acta. 1960 Nov 4;44:324–334. doi: 10.1016/0006-3002(60)91568-7. [DOI] [PubMed] [Google Scholar]
- HERBERT D., ELSWORTH R., TELLING R. C. The continuous culture of bacteria; a theoretical and experimental study. J Gen Microbiol. 1956 Jul;14(3):601–622. doi: 10.1099/00221287-14-3-601. [DOI] [PubMed] [Google Scholar]
- Hempfling W. P., Vishniac W. Oxidative phosphorylation in extracts of thiobacillus X. Biochem Z. 1965 Aug 6;342(3):272–287. [PubMed] [Google Scholar]
- PACKER L., VISHNIAC W. Chemosynthetic fixation of carbon dioxide and characteristics of hydrogenase in resting cell suspensions of Hydrogenomonas ruhlandii nov. spec. J Bacteriol. 1955 Aug;70(2):216–223. doi: 10.1128/jb.70.2.216-223.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARKER C. D., PRISK J. The oxidation of inorganic compounds of sulphur by various sulphur bacteria. J Gen Microbiol. 1953 Jun;8(3):344–364. doi: 10.1099/00221287-8-3-344. [DOI] [PubMed] [Google Scholar]
- PECK H. D., Jr Symposium on metabolism of inorganic compounds. V. Comparative metabolism of inorganic sulfur compounds in microorganisms. Bacteriol Rev. 1962 Mar;26:67–94. doi: 10.1128/br.26.1.67-94.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck H. D. ADENOSINE 5'-PHOSPHOSULFATE AS AN INTERMEDIATE IN THE OXIDATION OF THIOSULFATE BY THIOBACILLUS THIOPARUS. Proc Natl Acad Sci U S A. 1960 Aug;46(8):1053–1057. doi: 10.1073/pnas.46.8.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirt S. J. The maintenance energy of bacteria in growing cultures. Proc R Soc Lond B Biol Sci. 1965 Oct 12;163(991):224–231. doi: 10.1098/rspb.1965.0069. [DOI] [PubMed] [Google Scholar]
- SORBO B. A colorimetric method for the determination of thiosulfate. Biochim Biophys Acta. 1957 Feb;23(2):412–416. doi: 10.1016/0006-3002(57)90346-3. [DOI] [PubMed] [Google Scholar]
- TRUDINGER P. A. Fixation of carbon dioxide by extracts of the strict autotroph Thiobacillus denitrificans. Biochem J. 1956 Oct;64(2):274–286. doi: 10.1042/bj0640274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VISHNIAC W., SANTER M. The thiobacilli. Bacteriol Rev. 1957 Sep;21(3):195–213. doi: 10.1128/br.21.3.195-213.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VISHNIAC W., TRUDINGER P. A. Symposium on autotrophy. V. Carbon dioxide fixation and substrate oxidation in the chemosynthetic sulfur and hydrogen bacteria. Bacteriol Rev. 1962 Jun;26:168–175. [PMC free article] [PubMed] [Google Scholar]


