Abstract
Background and purpose:
Urocortin is a locally expressed pro-inflammatory peptide. Here we have examined the effects of urocortin on sodium laurate-induced peripheral arterial vasculitis in rats, modelling the mechanisms of thromboangiitis obliterans (TAO).
Experimental approach:
Peripheral vasculitis in rats was induced by sodium laurate and graded by gross appearance on the 12th day after injection. Histological changes in rat femoral arteries were assessed by histopathology and transmission electron microscopy. Blood cell counts, blood rheology, blood coagulation and plasma urocortin, thromboxane B2, prostaglandin E2 and soluble intercellular adhesion molecule-1 levels were measured. Expression of urocortin, corticotrophin-releasing factor (CRF1/2) receptors, cyclooxygenase (COX)-2 and intercellular adhesion molecule-1 (ICAM-1) at both mRNA and protein levels were determined by RT-PCR and Western blot.
Key results:
Rats showed grossly visible signs and symptoms of TAO on the 12th day after sodium laurate injection. In these rats, blood was in a hypercoagulable state; plasma urocortin, prostaglandin E2 and soluble intercellular adhesion molecule-1 levels were elevated; and the expression of urocortin, CRF1 and CRF1α-receptors, COX-2 and ICAM-1 in rat femoral arteries were markedly increased. Exogenous urocortin, given for 12 days after sodium laurate, exacerbated the hypercoagulable state and augmented expression of CRF1α-receptors, COX-2 and ICAM-1. These effects were abolished by a CRF1-receptor antagonist, NBI-27914, or a non-selective CRF-receptor antagonist, astressin, but not by the CRF2-receptor antagonist, antisauvagine-30, given with exogenous urocortin.
Conclusion and implications:
Urocortin exacerbated the hypercoagulable state and vasculitis in a model of TAO induced by sodium laurate in rats, via CRF1-receptors. COX-2 and ICAM-1 might also have contributed to this exacerbation.
Keywords: Urocortin, thromboangiitis obliterans, vasculitis, CRF1-receptor, CRF2- receptor
Introduction
Vasculitis is a pathological process characterized by inflammatory damage to blood vessels, which may be induced by genetic abnormalities, autoimmune or physical damage. Thromboangiitis obliterans (TAO) is a non-atherosclerotic, segmental, inflammatory disease that most commonly affects the small- and medium-sized arteries, veins and nerves of the extremities (Olin, 2000). In the characteristic acute-phase lesion, in association with occlusive cellular thrombosis, the acute inflammation that involves all layers of the vessel wall leads TAO to be classified as a vasculitis (Puéchal and Fiessinger, 2007). However, the exact aetiology for TAO is not yet well defined (Olin, 2000). In sodium laurate-induced arterial occlusive disease of rats, the injected sodium laurate is supposed to cause endothelial cell damage that may lead to the aggregation of platelets in peripheral vascular beds (Ashida et al., 1980). The progression of the disease in this model of vasculitis resembles that reported in the patients with TAO (Nakata et al., 1976; Nielubowicz et al., 1980).
Urocortin, a peptide of the corticotrophin-releasing factor (CRF) family (Vaughan et al., 1995), is expressed in both central and peripheral tissues (Fekete and Zorrilla, 2007). Besides its cardiovascular protective property (Okosi et al., 1998; Oki and Sasano, 2004), urocortin is now considered to be a potent immunomodulatory factor that participates in immune responses (Fekete and Zorrilla, 2007). In contrast to its systemic indirect immunosuppressive effects on the hypothalamic–pituitary adrenal axis, urocortin acts as a locally expressed, autocrine or paracrine, pro-inflammatory factor in a series of inflammatory diseases, such as rheumatoid arthritis and osteoarthritis (Kohno et al., 2001) and ulcerative colitis (Saruta et al., 2004). Moreover, it can stimulate the release of pro-inflammatory mediators under inflammatory conditions (Saruta et al., 2004; Theoharides et al., 2004). Our previous study demonstrated the overexpression of urocortin in lung tissues of rats with allergic asthma and inhalation of aerosolized urocortin increased pulmonary vascular permeability via mast cell infiltration and activation (Wu et al., 2006). This process was mediated by CRF receptors (CRF1 and CRF2) (Singh et al., 1999; nomenclature follows Alexander et al., 2008). We demonstrated for the first time that mast cell degranulation induced by urocortin was mediated by increasing intracellular calcium concentration via CRF1-receptors (Wu et al., 2008). Moreover, our data showed that CRF played a significant role in promoting the development of atherosclerosis (Wu et al., 2009), which also exhibits features of vasculitis (Cipollone and Fazia, 2006). All these results suggested that endogenous urocortin might participate in the pathophysiology of many inflammatory conditions, as a pro-inflammatory mediator.
We therefore hypothesized that urocortin, which is synthesized and secreted by the systemic vasculature, may be a potent, locally expressed, pro-inflammatory factor in TAO, which is classified as a vasculitis with endothelial dysfunction (Joras et al., 2006). In this study, we assessed the role of urocortin in a rat model of TAO induced by the intravascular injection of sodium laurate. We also investigated the type of CRF receptors involved in these effects of urocortin.
Methods
Animals
All animal care and this investigation conform to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996), and were approved by the Ethical Review Board of Nanjing Medical University. Male Wistar rats (200–250 g) were purchased from Shanghai laboratory animal centre. Food and water were given ad libitum unless otherwise noted.
Experimental protocol
Male Wistar rats were randomly divided into seven groups (eight animals per group) as follows: (i) normal group; (ii) sham operated group; (iii) vasculitis (TAO model) group; (iv) urocortin group; (v) urocortin + NBI-27914 group; (vi) urocortin + antisauvagine-30 group; and (vii) urocortin + astressin group. Rats with sodium laurate-induced vasculitis were prepared according to the method described previously (Ashida et al., 1980). Briefly, rats were anaesthetized with 10% (w/v) chloral hydrate (3.5 mL·kg−1, i.p.). The left hind leg was shaved and the femoral artery was exposed by surgical incision and retraction of muscles. Sodium laurate solution (10 mg·mL−1 in normal saline, adjusted to pH 8.0, 0.1 mL per animal) was injected into the left femoral artery while the sham operated group were treated with normal saline as vehicle. All groups were subjected to the surgical procedure except for the normal group. Urocortin (3 nmol·kg−1) and CRF1/2 receptor antagonists (15 nmol·kg−1) (Li et al., 2008) were given s.c., 2 h after sodium laurate injection and once a day for the following 11 days.
The gross appearance of the rat hind legs was checked daily after the operation. The degree of the disease on the 12th day was graded according to the system developed by Murakami et al. (1995), as follows: 0, normal appearance; 1, change in nail colour; 2, change in digit colour; 3, gangrene of digit; 4, loss or mummification of digit.
At the end of the experiment, rats were anaesthetized with 10% chloral hydrate. A blood sample was drawn from the common carotid artery and anti-coagulated with heparin, sodium citrate or EDTA, depending on the variable measured. Plasma was frozen at −80°C for biochemical analysis. Left femoral arteries were collected and divided into two: one was frozen at −80°C until used to provide a homogenate; the other was taken for histological examination.
Assessment of blood cell counts, blood coagulation and blood rheology
Blood cell counts, blood coagulation and blood rheology were measured by the clinical laboratory in the First Affiliated Hospital of Nanjing Medical University.
Plasma urocortin, thromboxane B2, prostaglandin E2 and soluble intercellular adhesion molecule-1 assays
Plasma urocortin, prostaglandin (PG)E2 and soluble intercellular adhesion molecule (sICAM)-1 were determined by ELISA Kits (purchased from Phoenix Pharmaceuticals, USA; R&D Systems, USA; Boster Biological Technology, China respectively). Plasma thromboxane (TX)B2 was measured with an enzyme immunoassay kit (Cayman Chemical, USA) according to the manufacturer's protocol.
Assessment by light and transmission electron microscopy
For light microscopy, samples of rat femoral artery were fixed in 10% neutral formalin for at least 24 h. After being embedded in paraffin, the tissues were cut into 5-µm-thick sections and stained with haematoxylin and eosin before examination.
For electron microscopy, samples of arteries were immediately placed in sodium acetate-buffered 2.5% glutaraldehyde solution and left overnight. The subsequent procedures were kindly performed by the Key Laboratory of Antibody Technique, Ministry of Health, Nanjing Medical University. Artery specimens were then cut into 0.5-µm-thick sections, the sections stained with uranyl acetate and lead citrate and examined in a JEOL 2000FX transmission electron microscope operated at 80 kV.
Semi-quantitative RT-PCR analysis
Total RNAs were extracted from femoral arteries, using TRIzol (Invitrogen) according to the manufacturer's protocol. For cDNA synthesis, Moloney murine leukemia virus (Invitrogen) was applied as the reverse transcriptase. For PCR reaction, Taq DNA polymerase (Promega) was used in the reaction system. Primers applied in the experiment were synthesized from published sequences (Baigent and Lowry, 2000; Ohnaka et al., 2000; Taal et al., 2000; Brar et al., 2004) (Table 1). The products were analysed by electrophoresis in 2.0% agarose gel containing 0.5 µg·mL−1 ethidium bromide. Specific genes were verified by their predicted sizes. GAPDH was set as the internal control.
Table 1.
Summary of the RT-PCR primer sequences used to amplify GAPDH, urocortin, CRF1-receptors, CRF2-receptors, COX-2 and ICAM-1 from rat tissues
| Sequences | Product size (bp) | Annealing T(C) | ||
|---|---|---|---|---|
| GAPDH | Sense | TCCCAGAGCTGAACGGGAAGCTCACTG | 339 | 68.1 |
| Antisense | TGGAGGCCATGTAGGCCATGAGGTCCA | |||
| Urocortin | Sense | GCTACGCTCCTGGTAGCGTTGCTGCTTCTG | 356 | 68.1 |
| Antisense | GCCGATCACTTGCCCACCGAGTCGAATATG | |||
| CRF1 | Sense | AAGGCGGGATCCAGGCAGTAGAGA | 508 | 60.8 |
| Antisense | TCCCGGTAGCCATTGTTTGTCGTG | |||
| CRF1α | Sense | TCCTACGCAACGCCAC | 147 | 58.8 |
| Antisense | AGCAGCCCTCACCGAAC | |||
| CRF2 | Sense | CTGGTGGCTGCTTTCCTGCTTTTC | 425 | 58.1 |
| Antisense | ATGGGGCCCTGGTAGATGTAGTCC | |||
| COX-2 | Sense | TTCACCAGACAGATTGCTGGC | 530 | 63.5 |
| Antisense | AGTCTGGAGTGGGAGGCACTTG | |||
| ICAM-1 | Sense | AGAAGGACTGCTTGGGGAA | 332 | 58.1 |
| Antisense | CCTCTGGCGGTAATAGGTG | |||
CRF, corticotrophin-releasing factor; COX, cyclooxygenase; ICAM-1, intercellular adhesion molecule-1.
Western blot analysis
The protein samples were separated on a 10% SDS-polyacrylamide gel and electrophoretically transferred to PVDF membranes in Tris-glycine transfer buffer. Then membranes were blocked in 5% (wt/vol) instant non-fat dried milk for 2 h at room temperature, and incubated with primary antibody for CRF1-receptor (1:400), CRF2-receptor (1:400), COX-2 (1:1000), ICAM-1 (1:500) or β-actin (1:200, Boster Biological Technology) at 4°C overnight. After three washes with TBST (50 mmol·L−1 Tris-HCl, pH 7.5, 150 mmol·L−1 NaCl, 0.05% Tween 20), the membranes were incubated with secondary horseradish peroxidase-conjugated IgG for 2 h at room temperature. Immunoreactive proteins were visualized by LumiGLO chemiluminescent reagent and peroxide (Cell Signaling Technology). The light-emitting bands were detected with X-ray films (Kodak, Rochester, NY).
Statistical analysis
Data were expressed as means ± SEM. The significance for the difference among groups was analysed with spss 11.0 by one-way anova with Student–Newman–Keuls multiple comparison methods. The Kruskal–Wallis H test was used to compare the gross appearance of the vasculitis among groups. P < 0.05 was considered statistically significant.
Materials
Rat urocortin, CRF1-receptor antagonist NBI-27914, CRF2-receptor antagonist, antisauvagine-30 and the non-selective CRF-receptor antagonist, astressin were purchased from Sigma (Missouri, USA). Sodium laurate was provided by International Laboratory (California, USA). Polyclonal urocortin and antibodies to CRF1- and CRF2-receptors were obtained from Santa Cruz Biotechnology (California, USA). Polyclonal COX-2 and monoclonal ICAM-1 antibodies were from Abcam (Cambridge, UK). All other reagents used were derived from commercial sources.
Results
Rats showed gross signs and symptoms of TAO and histological changes after sodium laurate injection
The rat model of TAO, induced by sodium laurate, has been widely accepted. In our study, as reported previously (Nakata et al., 1976; Nielubowicz et al., 1980), the whole left hind paw went pale 2 or 3 min after injection of sodium laurate. On the 12th day, in sham operated and normal rats (Figure 1A and B), no ischaemic signs were found; while rats in the vasculitis (TAO model) group showed typical signs and symptoms of TAO (Figure 1C), with four rats displaying obvious signs of gangrene, and two with loss or mummification of paws.
Figure 1.
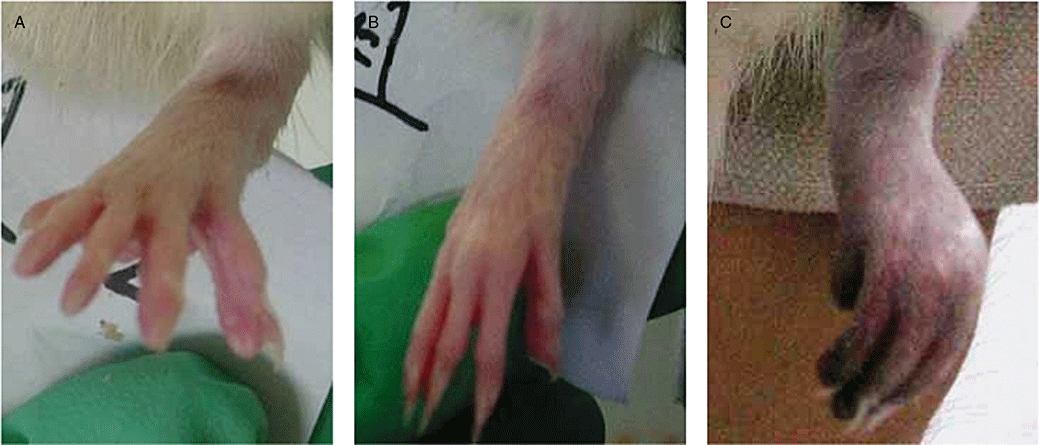
Gross appearance of rat paws in normal, sham operated and thromboangiitis obliterans (TAO) model rats on the 12th day after sodium laurate injection. Paws in normal (A), sham operated (B) and TAO model (C) rats.
Histological changes were also observed in sections of the femoral artery examined by light microscopy (Figure 2A and B). The sections from the TAO model rats (Figure 2B), stained with haematoxylin and eosin, showed obvious thrombi, which resulted in the narrowing or complete occlusion of the vessel lumen, and some showed recanalization of the artery. To investigate ultrastructural changes in the arterial tissue, sections were examined by transmission electron microscopy. The endothelium in sections from normal rats looked intact with regular arrangement of the endothelial cells [Figure 2C (a)]; while in arteries from the TAO model rats, the artery intima was modified with gaps in the endothelial sheet and desquamation in many places, and proliferation and hypertrophy of endothelium were also extensively found [Figure 2C (b)]. Under the intima, smooth muscle cells were in a regular order in normal arteries [Figure 2C (c)]; while in arteries from the TAO group, muscle cells were disordered and most showed multiformity and degeneration [Figure 2C (d)]. These findings were comparable with those found in TAO patients (Cui Pan Cui et al., 2000).
Figure 2.
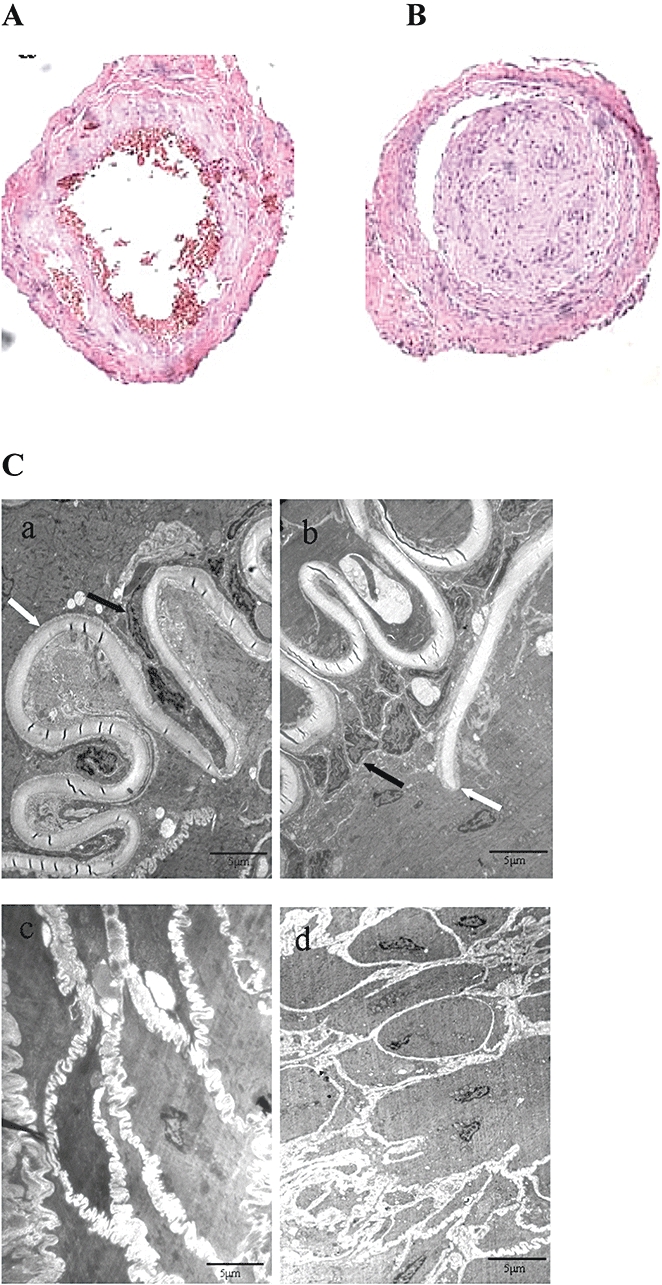
Histological examination of femoral arteries in normal (A) and thromboangiitis obliterans (TAO) model (B) rats (haematoxylin and eosin staining, magnification: 100×). In (C), ultrastructure of femoral arteries in normal (a and c) and TAO model (b and d) rats. a and b, artery intima; c and d, medial membrane. Black arrows show endothelial cells and white arrows show inner elastic layer. In picture b, black arrow indicates endothelial cell proliferation, and the white arrow indicates discontinuity of the internal elastic lamina (transmission electron microscopy; magnification: 4000×).
Urocortin and CRF1-receptors were increased in TAO model rats
As shown in Figure 3, plasma urocortin was increased markedly in the TAO model group, compared with the levels in the sham operated or normal groups (P < 0.05). There was no difference between normal and sham operated group.
Figure 3.
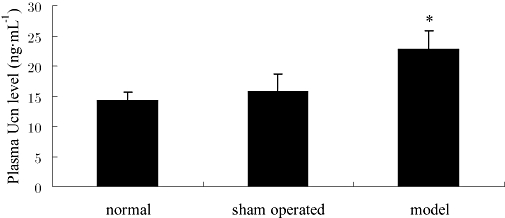
Plasma urocortin (Ucn) levels in normal, sham operated and thromboangiitis obliterans model groups. Data are presented as means ± SEM (n= 8). *P < 0.05, versus sham operated group.
RT-PCR analysis (Figure 4A and B) showed that the mRNA for urocortin and CRF1-receptors were increased in femoral arteries from the TAO group relative to the levels found in the normal and sham operated rat femoral arteries. We also showed that mRNA for the CRF1α-receptor was increased in the model rats (Figure 4A and B). The levels of mRNA for the CRF2-receptor were not significantly altered in samples from the TAO group.
Figure 4.
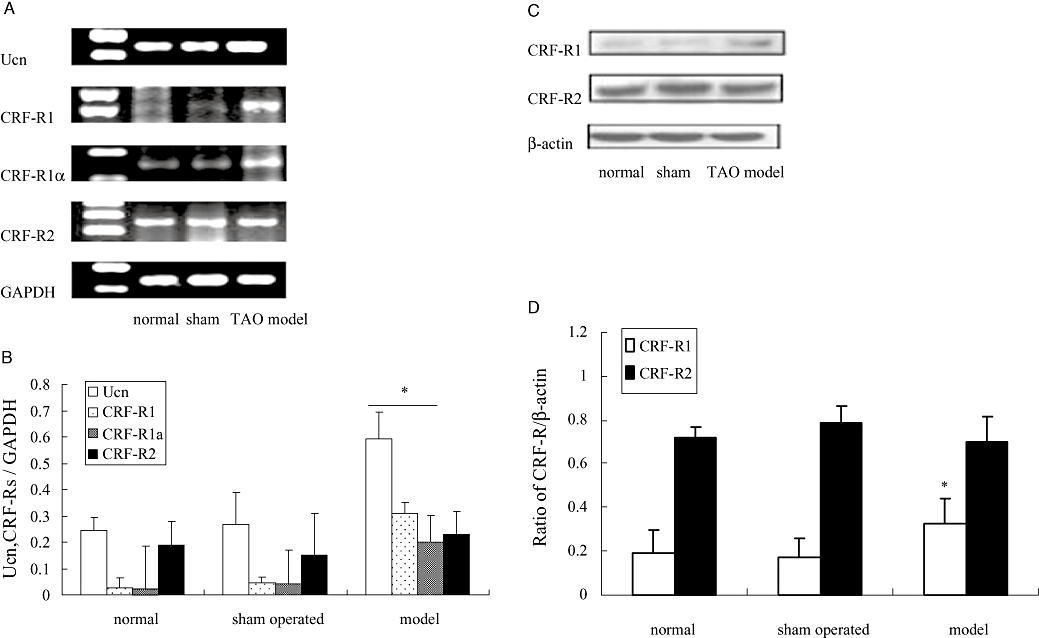
Expression of mRNA and Western blot analysis for urocortin and corticotrophin-releasing factor (CRF)1/2 receptors in femoral arteries from normal, sham operated and thromboangiitis obliterans (TAO) model rats. In (A), a representative record of the expression of mRNA is shown (Ucn, urocortin; CRF-R1, CRF1-receptor; CRF-R1α, CRF1α-receptor; CRF-R2, CRF2-receptor) with GAPDH as an internal control. Summary data are shown in (B); data are presented as means ± SEM (n= 8). *P < 0.05, versus sham operated group. In (C), a representative Western blot for CRF1-receptors and CRF2-receptors is shown (β-actin as control) with summary data in (D); data presented as means ± SEM (n= 8). *P < 0.05, versus sham operated group.
Subsequently, we also measured protein levels of CRF1- and CRF2-receptors in rat femoral arteries by Western blot analysis. As shown in Figure 4C and D, compared with sham operated group, the expression of CRF1-receptor protein in the TAO model group was significantly increased (P < 0.05), while the expression of CRF2-receptor protein remained constant.
Effects of exogenous urocortin and CRF1/2 receptor antagonists on TAO model rats
In order to ascertain whether urocortin contributed to the TAO model, exogenous urocortin and CRF1/2 receptor antagonists were added to rats treated with sodium laurate. As shown in Figure 5A, adding exogenous urocortin for 12 days to the TAO model rats exacerbated the gross effects of the ischaemia, with three rats displaying obvious signs of gangrene and three with loss or mummification of paws. These signs of intensified ischaemia were markedly decreased in the groups given the CRF1-receptor antagonist, NBI-27914 (Figure 5B) or the non-selective CRF-receptor antagonist, astressin (Figure 5D) along with the exogenous urocortin. However, the CRF2-receptor antagonist, antisauvagine-30 (Figure 5C), did not modify the gross effects of exogenous urocortin.
Figure 5.
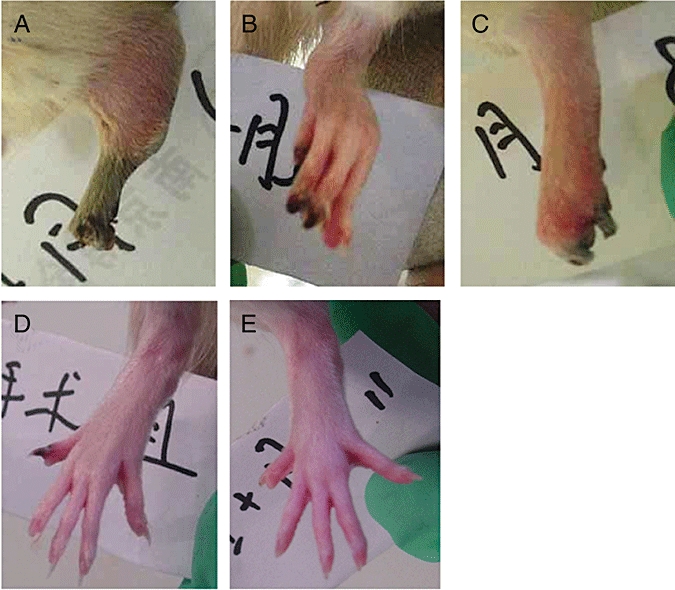
Gross appearance of rat paws after urocortin and corticotrophin-releasing factor type 1 and 2 receptor antagonists (12th day). A, urocortin group; B, urocortin + NBI-27914 group; C, urocortin + anti-sauvagine-30 group; D, urocortin + astressin group; E, sham operated group.
To quantify the gross effects of ischaemia in each group, these effects were graded into five levels, as described in the Methods and shown in Table 2. Rats from each group were graded on the 12th day and the group grades compared, using the Kruskal–Wallis H test. Compared with the values from the sham operated group, those from the TAO model and TAO + urocortin groups were significantly higher, that is, more intense signs of ischaemia. In agreement with the gross observations, the grades in the groups given NBI-27914 or astressin were lower and not different from those in the sham operated group. No such attenuation of ischaemic signs was present in the group given antisauvagine-30 and no statistical significance was found between normal and sham operated groups (Table 2). These data indicate that antagonism of CRF1-receptors, but not of CRF2-receptors, could alleviate the gross signs of the vasculitis.
Table 2.
Grades of disease on the 12th day after sodium laurate injection
|
Grade of disease |
Significance | |||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||
| Normal | 8 | 0 | 0 | 0 | 0 | |
| Sham operated | 8 | 0 | 0 | 0 | 0 | |
| TAO model | 2 | 0 | 0 | 4 | 2 | ** |
| Ucn | 1 | 1 | 0 | 3 | 3 | ** |
| Ucn + NBI-27914 | 5 | 0 | 0 | 2 | 1 | † |
| Ucn + antisauvagine-30 | 2 | 0 | 2 | 1 | 3 | ** |
| Ucn + astressin | 5 | 1 | 1 | 1 | 0 | †# |
On the 12th day after sodium laurate injection, rats from each group were graded according to the gross appearance of the affected paw (see Methods). Kruskal–Wallis H test was carried out to compare the disease condition among groups.
P < 0.01, versus sham operated group;
P < 0.05, versus TAO model group;
P < 0.05, versus Ucn group.
TAO, thromboangiitis obliterans; TAO model, single injection of sodium laurate only; Ucn, TAO model + daily urocortin for 12 days; Ucn + NBI 27914 or + antisauvagine-3 or + astressin, Ucn + daily injections of each antagonist for 12 days.
In further analysis of the effects of exogenous urocortin and CRF1/2 receptor antagonists in the TAO model rats, blood cell counts, blood coagulation and blood rheology of each group were investigated. Blood platelet count was markedly elevated (P < 0.05) in TAO model group (Table 3A). As expected, blood coagulation parameters (Table 3B) in the TAO model rats were very different from those in the sham operated group: prothrombin time, thrombin time and activated partial thromboplastin time were all significantly shortened while fibrinogen and D-dimer values were increased. Compared with the TAO model group, adding exogenous urocortin further shortened prothrombin time, thrombin time, activated partial thromboplastin time and increased blood platelet count, fibrinogen and D-dimer levels. Treatment with NBI-27914 and astressin reversed those parameters, to almost normal levels. However, it seems that urocortin had no influence on other parameters of rat blood cells and on blood rheology (Table 3C). Compared with the TAO model or urocortin groups, treatment with antisauvagine-30 had no significant effect on any of the parameters measured.
Table 3A.
Effects of urocortin and CRF1/2 receptor antagonists on rat blood cell counts
| Blood platelet count (108 mL−1) | Red blood cell count (109 mL−1) | Haemoglobin (g·L−1) | Leucocyte count (106 mL−1) | Neutrophil (106 mL−1) | |
|---|---|---|---|---|---|
| Normal | 7.12 ± 0.31 | 6.51 ± 0.31 | 131.25 ± 6.17 | 4.93 ± 0.21 | 1.08 ± 0.20 |
| Sham operated | 7.35 ± 0.51 | 6.21 ± 0.49 | 128.22 ± 4.73 | 5.01 ± 0.40 | 1.16 ± 0.11 |
| TAO model | 9.30 ± 0.49* | 6.11 ± 0.63 | 129.98 ± 7.44 | 5.11 ± 0.61 | 1.19 ± 0.28 |
| Ucn | 11.71 ± 0.72*# | 6.23 ± 0.14 | 130.05 ± 5.10 | 5.08 ± 0.58 | 1.09 ± 0.09 |
| Ucn + NBI-27914 | 7.87 ± 0.62#† | 6.83 ± 0.72 | 135.12 ± 9.39 | 4.95 ± 0.13 | 1.13 ± 0.19 |
| Ucn + antisauvagine-30 | 11.07 ± 0.82 | 6.61 ± 0.42 | 132.26 ± 8.17 | 5.00 ± 0.46 | 1.14 ± 0.24 |
| Ucn + astressin | 7.61 ± 0.71#† | 6.47 ± 0.55 | 128.78 ± 6.85 | 4.97 ± 0.71 | 1.15 ± 0.17 |
Values are presented as means ± SEM (n= 8).
P < 0.05, versus sham operated group;
P < 0.05, versus model group;
P < 0.05, versus urocortin group.
CRF1/2, corticotrophin-releasing factor type 1 and 2 receptors; TAO, thromboangiitis obliterans; TAO model, single injection of sodium laurate only; Ucn, TAO model + daily urocortin for 12 days; Ucn + NBI 27914 or + antisauvagine-3 or + astressin, Ucn + daily injections of each antagonist for 12 days.
Table 3B.
Effects of urocortin and CRF1/2 receptor antagonists on rat blood coagulation
| Prothrombin time (s) | Thrombin time (s) | Activated partial thromboplastin time(s) | Fibrinogen level (g·L−1) | D-dimer (ng·mL−1) | |
|---|---|---|---|---|---|
| Normal | 37.93 ± 1.61 | 25.27 ± 2.12 | 38.91 ± 3.10 | 2.98 ± 0.11 | 0.60 ± 0.02 |
| Sham operated | 38.20 ± 0.98 | 24.00 ± 1.91 | 35.90 ± 2.74 | 3.12 ± 0.20 | 0.57 ± 0.06 |
| TAO model | 26.50 ± 1.23* | 10.33 ± 1.69* | 20.03 ± 2.18* | 5.94 ± 0.47* | 1.34 ± 0.10* |
| Ucn | 20.20 ± 1,50*# | 8.07 ± 1.78*# | 15.11 ± 3.53*# | 6.90 ± 0.35*# | 1.66 ± 0.19*# |
| Ucn + NBI-27914 | 35.38 ± 0.72#† | 22.65 ± 3.12#† | 36.80 ± 4.61#† | 3.53 ± 0.29#† | 0.78 ± 0.09#† |
| Ucn + antisauvagine-30 | 21.01 ± 1.11 | 11.20 ± 2.19 | 18.31 ± 3.50 | 6.28 ± 0.61 | 1.58 ± 0.21 |
| Ucn + astressin | 36.83 ± 1.31#† | 23.79 ± 1.70#† | 38.77 ± 5.07#† | 3.30 ± 0.42#† | 0.59 ± 0.13#† |
Values are presented as means ± SEM (n= 8).
P < 0.05, versus sham operated group;
P < 0.05, versus model group;
P < 0.05, versus urocortin group.
CRF1/2, corticotrophin-releasing factor type 1 and 2 receptors; TAO, thromboangiitis obliterans; TAO model, single injection of sodium laurate only; Ucn, TAO model + daily urocortin for 12 days; Ucn + NBI 27914 or + antisauvagine-3 or + astressin, Ucn + daily injections of each antagonist for 12 days
Table 3C.
Effects of urocortin and CRF1/2 receptor antagonists on rat blood rheology
| Erythrocyte sedimentation rate (mm·h−1) | Haematocrit (%) |
Blood viscosity |
Plasma viscosity (mPa s) | ||
|---|---|---|---|---|---|
| 200 S−1(mPa s) | 5 S−1 (mPa s) | ||||
| Normal | 1.08 ± 0.23 | 40.88 ± 1.34 | 5.68 ± 0.03 | 11.34 ± 1.39 | 1.39 ± 0.03 |
| Sham operated | 1.15 ± 0.09 | 40.03 ± 0.31 | 5.77 ± 0.28 | 12.01 ± 2.80 | 1.34 ± 0.05 |
| TAO model | 1.11 ± 0.05 | 39.60 ± 0.18 | 5.81 ± 0.41 | 11.73 ± 0.47 | 1.36 ± 0.02 |
| Ucn | 1.09 ± 0.17 | 39.21 ± 0.99 | 5.69 ± 0.18 | 11.58 ± 1.19 | 1.39 ± 0.04 |
| Ucn + NBI-27914 | 1.13 ± 0.20 | 39.67 ± 2.12 | 5.71 ± 0.33 | 11.21 ± 1.30 | 1.34 ± 0.04 |
| Ucn + antisauvagine-30 | 1.16 ± 0.09 | 40.30 ± 2.08 | 5.61 ± 0.01 | 11.94 ± 1.71 | 1.31 ± 0.01 |
| Ucn + astressin | 1.01 ± 0.17 | 39.63 ± 1.62 | 5.73 ± 0.17 | 11.84 ± 1.55 | 1.33 ± 0.02 |
CRF1/2, corticotrophin-releasing factor type 1 and 2 receptors; TAO, thromboangiitis obliterans; TAO model, single injection of sodium laurate only; Ucn, TAO model + daily urocortin for 12 days; Ucn + NBI 27914 or + antisauvagine-3 or + astressin, Ucn + daily injections of each antagonist for 12 days Values are presented as means ± SEM (n= 8).
TXA2, a potent inducer of platelet aggregation and microvascular contraction (Silver et al., 1973; 1974;), was measured as its metabolite, TXB2. As shown in Figure 6, plasma TXB2 was markedly raised in the TAO model group, over the sham or normal values (P < 0.05), and was further elevated on addition of exogenous urocortin. Treatment with the CRF receptor antagonists, NBI-27914 or astressin but not antisauvagine-30, dramatically reduced plasma TXB2 levels, relative to the TAO model group (P < 0.05).
Figure 6.
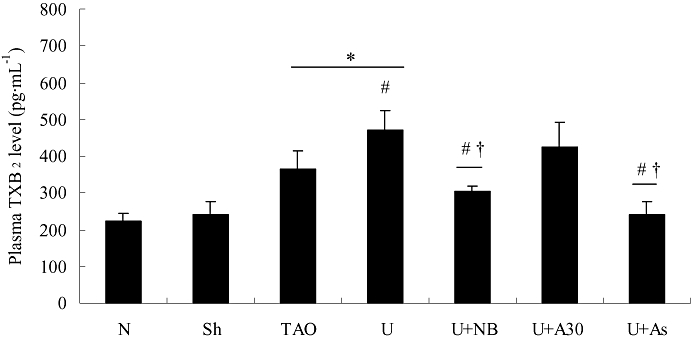
Plasma thromboxane B2 (TXB2) levels in experimental groups. TXB2 levels were higher (than sham) in the thromboangiitis obliterans (TAO) model rats and further increased on addition of exogenous urocortin (Ucn; see Methods). These effects of urocortin were prevented by treatment with NBI-27914 or astressin, but not by antisauvagine-30. Values are presented as means ± SEM (n= 8). *P < 0.05, versus sham operated group; #P < 0.05, versus TAO model group; †P < 0.05, versus urocortin group. N, normal; Sh, sham; TAO, TAO model; U, urocortin; U + NB, U + NBI-27914; U + A30, urocortin + antisauvagine-30; U + As, urocortin + astressin.
Effects of urocortin and CRF1/2 receptor antagonists on expression of COX-2 and ICAM-1
COX-2 and ICAM-1 are two important components of many inflammatory diseases (Dubois et al., 1998; Vane et al., 1998; Witkowska, 2005). Clinical reports indicate that ICAM-1 expression is elevated in TAO patients (Halacheva et al., 2002; Joras et al., 2006) and treatment with COX-2 inhibitors can relieve the disease condition (Nizankowski et al., 1985; Fiessinger and Schäfer, 1990). In the present study, RT-PCR analysis (Figure 7) showed that mRNA for COX-2 and ICAM-1 extracted from femoral arteries were increased in the TAO model and urocortin groups, compared with the sham operated group (P < 0.05) and that treatment with NBI-27914 or astressin but not antisauvagine-30 attenuated the increase due to urocortin group (P < 0.05). Western blot assays to determine protein levels of COX-2 and ICAM-1 (Figure 8) gave results largely in accordance with the RT-PCR data. Again, both NBI-27914 and astressin but not antisauvagine-30 blocked the effect of exogenous urocortin (P < 0.05) on expression of COX-2 and ICAM-1.
Figure 7.
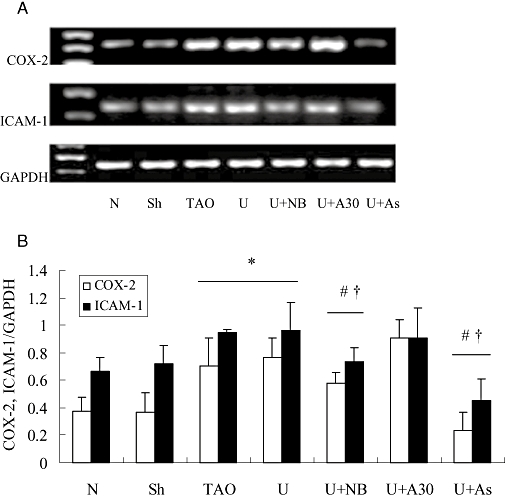
Expression of mRNA for cyclooxygenase (COX)-2 and intercellular adhesion molecule-1 (ICAM-1) in rat femoral arteries from experimental groups. In (A), a representative record of the expression of mRNA is shown, with GAPDH as an internal control. Summary data are shown in (B); data are presented as means ± SEM (n= 8), *P < 0.05, versus sham operated group; #P < 0.05, versus TAO model group; †P < 0.05, versus urocortin group. N, normal; Sh, sham; TAO, TAO model; U, urocortin; U + NB, U + NBI-27914; U + A30, urocortin + antisauvagine-30; U + As, urocortin + astressin.
Figure 8.
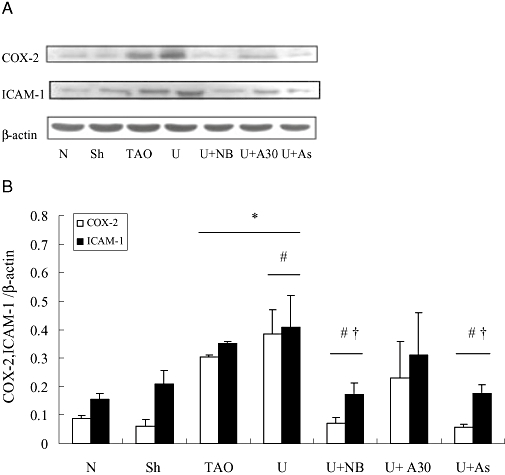
Western blot analysis of cyclooxygenase (COX)-2 and intercellular adhesion molecule-1 (ICAM-1) expression in rat femoral arteries from experimental groups. In (A), a representative blot is shown with β-actin as an internal control. Summary data are shown in (B); data are presented as means ± SEM (n= 8). *P < 0.05, versus sham operated group; #P < 0.05 versus TAO model group; †P < 0.05, versus urocortin group. N, normal; Sh, sham; TAO, TAO model; U, urocortin; U + NB, U + NBI-27914; U + A30, urocortin + antisauvagine-30; U + As, urocortin + astressin.
We also measured plasma PGE2, the main metabolite of COX-2 and sICAM-1 in the experimental groups of rats. As shown in Figure 9, in accordance with the expression of COX-2 and ICAM-1 in rat femoral arteries, plasma PGE2 and sICAM-1 were enhanced in the TAO model group, compared with the sham operated group (P < 0.05) and were further increased on addition of exogenous urocortin (P < 0.05 vs. TAO model group). As observed earlier, the CRF-receptor antagonists NBI-27914 and astressin but not antisauvagine-30 abolished these effects of urocortin (P < 0.05).
Figure 9.
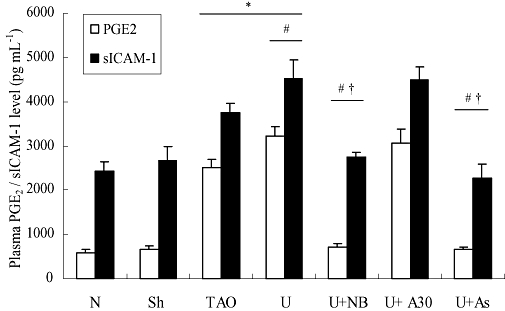
Plasma prostaglandin (PG)E2 and soluble intercellular adhesion molecule (sICAM)-1 levels from experimental groups. Values are presented as means ± SEM (n= 8). *P < 0.05, versus sham operated group; #P < 0.05. versus TAO model group; †P < 0.05, versus urocortin group. N, normal; Sh, sham; TAO, TAO model; U, urocortin; U + NB, U + NBI-27914; U + A30, urocortin + antisauvagine-30; U + As, urocortin + astressin.
Discussion
Urocortin is a peptide of the CRF family. In our model of TAO in rats, urocortin potentiated the effects of the peripheral vasculitis and ischaemia, which could be attributed to exacerbation of the hypercoagulable state of the blood. This exacerbation was mediated by CRF1-receptors, probably the CRF1α-receptor subtype.
Urocortin was originally identified in the CNS in 1995 (Vaughan et al., 1995) and more recently, it was found to be widely expressed in the periphery (Fekete and Zorrilla, 2007). Except for its protective roles in some diseases (Oki and Sasano, 2004), urocortin has been found to play a pro-inflammatory role in some inflammatory diseases, such as ulcerative colitis (Saruta et al., 2004) and rheumatoid arthritis (Kohno et al., 2001; Uzuki et al., 2001). Our previous study demonstrated that CRF accelerated progression of atherosclerosis in LDLr−/− mice via CRF1-receptors (Wu et al., 2009). However, its function in peripheral vasculitis and a potential role in modulating TAO progression are not well analysed.
In the present study, rats with a sodium laurate-induced vasculitis were used to model TAO, which is also defined as a vasculitis. This model has previously been used to study TAO (Ashida et al., 1980) and other groups have found that injection of sodium laurate into the rat femoral artery could mimic the signs and symptoms displayed in patients suffering from TAO (Nakata et al., 1976; Nielubowicz et al., 1980). Here, we observed that most of the model rats showed symptoms typical of ischaemia and vasculitis in the hind limb, and that the femoral artery was structurally changed with thrombi or proliferation of endothelial cells. Our results were consistent with previous reports (Nakata et al., 1976; Nielubowicz et al., 1980; Cui Pan Cui et al., 2000). We also found that expression of mRNA and protein for urocortin, CRF1- and CRF1α-receptors (but not for CRF2-receptors) were elevated in the femoral arteries from our TAO model rats, as was plasma urocortin. The CRF1α-receptor, widely expressed throughout the body, is the main mediator of the actions of CRF and urocortin (Teli et al., 2008). Moreover, the CRF1α-receptor is the most efficient receptor in terms of stimulation of cAMP production, while the CRF1c- and CRF1β-receptors have a decreased CRF binding capacity (Pisarchik and Slominski, 2004). Taken together, these observations suggest that urocortin does contribute to the signs and symptoms of TAO.
Previous reports have observed a hypercoagulable state of blood in TAO model rats (Shirakura et al., 1994). In the present study, such a hypercoagulable state was observed in our rat model of vasculitis. Plasma TXA2, a potent inducer of platelet aggregation and microvascular contraction (Silver et al., 1973; 1974;), was measured as its stable product, TXB2, and was greatly elevated in the TAO model rats. This result could be mainly attributed to the augmentation of platelet aggregation, which is the main producer of TXA2. Previous studies indicated that serum levels of a soluble form of ICAM-1 (sICAM-1) were correlated with possibility of disseminated intravascular coagulation or maximum disseminated intravascular coagulation scores (Gando et al., 2002; Chen et al., 2005). Our data showed that plasma sICAM-1 was increased in the TAO model rats, consistent with clinical findings of increased sICAM-1 in TAO patients (Joras et al., 2006).
To investigate the role of urocortin in TAO, the TAO model rats were treated with exogenous urocortin and CRF1/2 receptor antagonists. Adding urocortin significantly enhanced the grossly visible signs of ischaemia and vasculitis and further raised plasma TXB2 and sICAM-1 levels. Treatment of these rats with the CRF1-receptor antagonist, NBI-27914 or the non-selective CRF-receptor antagonist, astressin, the gross signs of vasculitis were attenuated; plasma TXB2 and sICAM-1 were returned to near normal and signs of the hypercoagulable state were decreased. When the rats were treated with the CRF2-receptor antagonist, antisauvagine-30, no such changes were observed.
From the gross, macroscopic findings (Figure 5 and Table 2), the non-selective CRF-receptor antagonist, astressin, could completely reverse whereas the selective CRF1-receptor antagonist, NBI-27914, could only partially reverse, the effects of urocortin and this might suggest a contribution from CRF2-receptors. However, selective blockade of CRF2-receptors with antisauvagine-30 had no significant effect. Overall, it is likely that urocortin promotes a hypercoagulable state, at least partially, through increasing plasma TXB2 and sICAM-1 and that this promoting effect was modulated via CRF1-receptors, rather than CRF2-receptors.
COX-2, the inducible isoform of the rate-limiting enzyme in the biosynthesis of TXA2 and PGs is well established as an important component of many inflammatory diseases (Dubois et al., 1998; Vane et al. and., 1998). It plays a critical role in atherosclerosis and modulates atherosclerotic plaque stability through PG output (Cipollone and Fazia, 2006). In case–control studies, prostacyclin derivatives have shown to be more effective than placebo for the therapy of TAO patients (Nizankowski et al., 1985; Fiessinger and Schäfer, 1990). Another key component of peripheral inflammatory disease, ICAM-1, is regarded as a marker for vasculitis (Witkowska, 2005), in conditions such as atherosclerosis (Witkowska, 2005) and TAO (Halacheva et al., 2002). In patients with TAO, inflammatory cells infiltrate local lesion sites, and ICAM-1 expression in endothelial cells and inflammatory sites was increased greatly (Halacheva et al., 2002; Joras et al., 2006). Our present study found that urocortin increased COX-2 and ICAM-1 expression in femoral arteries via CRF1-receptors, consistent with previous reports that in rat aortic smooth muscle cells, urocortin induced the expression of COX-2 in a time- and dose-dependent manner (Kageyama et al., 2006). Also, CRF-related peptides (CRF and urocortin) up-regulated COX-2 expression and PG output in cultured human placental trophoblasts via CRF1-receptors (Gao et al., 2008). Furthermore, CRF was biologically active in cultured keratinocytes and enhanced expression of hCAM and ICAM-1, stimulated by interferon-γ (Quevedo et al., 2001).
Our present study showed that both endogenous and exogenous urocortin were involved in our model of TAO in rats. On the one hand, endogenous urocortin was increased in these TAO model rats. On the other hand, exogenous urocortin application exacerbated the condition of the TAO rats. Furthermore, treatment with NBI-27914 or with astressin, CRF-receptor antagonists, reduced the urocortin-enhanced TXB2, COX-2 and ICAM-1 expression to a level lower than those in the TAO model group, indicating antagonism of both endogenous and exogenous urocortin.
The pro-inflammatory role of CRF1-receptors in peripheral inflammatory sites has been demonstrated (Kohno et al., 2001; Saruta et al., 2004). Yokotani et al. (2001) found that activation of brain CRF1-receptors raised brain TXA2, which is involved in the central adrenomedullary outflow. In the synovium of rheumatoid arthritis patients, urocortin and CRF-receptors were overexpressed and were significantly correlated with the degree of inflammation (Kohno et al., 2001). Furthermore, peripheral administration of CRF-receptor antibody (Karalis et al., 1991) or the specific CRF1-receptor non-peptide antagonist, antalarmin (Webster et al., 1996), prior to subcutaneous carrageenan injection, significantly suppressed inflammatory exudate volume and cell infiltration. Although there are reports indicating a protective role of urocortin in the cardiovascular system, this effect is mainly mediated via activating CRF2-receptors (Oki and Sasano, 2004). Recent studies have demonstrated that the CRF2-receptor is a tonic suppressor of vascularization (Bale et al., 2002). Genetic deletion of CRF2-receptors resulted in profound postnatal hypervascularization in mice, which was characterized by both an increase in total vessel number and a dramatic increase in vessel diameter. Furthermore, CRF2-receptor activation can inhibit tumour growth via effects on vascularization and cell proliferation (Hao et al., 2008; Wang et al., 2008). However, in the present study, expression of mRNA or protein of CRF2-receptors were the same in normal, sham operated and TAO model groups. In addition, the selective CRF2-receptor antagonist, antisauvagine-30 had no obvious effect on the vasculitis or the hypercoagulable state in TAO rats.
Urocortin induced degranulation of rat lung mast cells by increasing intracellular calcium via CRF1-receptors (Wu et al., 2008). In the present study, levels of CRF1-receptors were elevated in the TAO model group. This might represent both the infiltration of immune cells bearing CRF1-receptors and an effect of inflammatory mediators on CRF1-receptor expression. On the one hand, the pro-inflammatory action of CRF peptides is partially the result of their effects on immune cells (such as mast cell, macrophages and lymphocytes) (Bamberger Wald Bamberger et al., 1998; Theoharides et al., 1998; Agelaki et al., 2002), which can synthesize CRF peptides and express their receptors, CRF1 and CRF2 (Theoharides et al., 1998; Baigent, 2001). On the other hand, inflammatory mediators could induce expression of CRF1-receptors (Inada et al., 2009). Thus any increase of CRF1-receptors could be due to increased immune cells or to increased inflammatory mediators.
In conclusion, we have shown, in a model of TAO induced by sodium laurate in rats, that urocortin could exacerbate the pathological condition by potentiating the hypercoagulable state and the vasculitis. Blockade of CRF1-receptors attenuated the signs and symptoms of TAO by normalizing the hypercoagulable state. Up-regulation of COX-2 and ICAM-1 might also contribute to the exacerbation induced by urocortin.
Acknowledgments
This work was supported by Key Project from Natural Scientific Foundation of Jiangsu Province (No. BK2006727), Project from New Century Excellent Talents (No. NCET-06-0507) and Project funded by the Summit of the ‘Six Great Talents’ of Jiangsu Province (06-C-017).
Glossary
Abbreviations:
- CRF
corticotrophin-releasing factor
- CRF1 receptor
corticotrophin-releasing factor type 1 receptor
- CRF2 receptor
corticotrophin-releasing factor type 2 receptor
- ICAM-1
intercellular adhesion molecule-1
- PG
prostaglandin
- TAO
thromboangiitis obliterans
- TXA2
thromboxane A2
- TXB2
thromboxane B2
Conflict of interest
The authors have no conflict of interest.
References
- Agelaki S, Tsatsanis C, Gravanis A, Margioris AN. Corticotropin-releasing hormone augments proinflammatory cytokine production from macrophages in vitro and in lipopolysaccharide-induced endotoxin shock in mice. Infect Immun. 2002;70:6068–6074. doi: 10.1128/IAI.70.11.6068-6074.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 3rd edn. Br J Pharmacol. 2008;153(Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashida S, Ishihara M, Ogawa H, Abiko Y. Protective effect of ticlopidine onexperimentally induced peripheral arterial occlusive disease in rats. Thromb Res. 1980;18:55–67. doi: 10.1016/0049-3848(80)90170-x. [DOI] [PubMed] [Google Scholar]
- Baigent SM. Peripheral corticotropin-releasing hormone and urocortin in the control of the immune response. Peptides. 2001;22:809–820. doi: 10.1016/s0196-9781(01)00395-3. [DOI] [PubMed] [Google Scholar]
- Baigent SM, Lowry PJ. mRNA expression profiles for corticotrophin-releasing factor (CRF), urocortin, CRF receptors and CRF-binding protein in peripheral rat tissues. J Mol Endocrinol. 2000;25:43–52. doi: 10.1677/jme.0.0250043. [DOI] [PubMed] [Google Scholar]
- Bale TL, Giordano FJ, Hickey RP, Huang Y, Nath AK, Peterson KL, et al. Corticotropin-releasing factor receptor 2 is a tonic suppressor of vascularization. Proc Natl Acad Sci U S A. 2002;99:7734–7739. doi: 10.1073/pnas.102187099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamberger CM, Wald M, Bamberger AM, Ergün S, Beil FU, Schulte HM. Human lymphocytes produce urocortin, but not corticotropin-releasing hormone. J Clin Endocrinol Metab. 1998;83:708–711. doi: 10.1210/jcem.83.2.4693. [DOI] [PubMed] [Google Scholar]
- Brar BK, Chen A, Perrin MH, Vale W. Specificity and regulation of extracellularly regulated Kinase1/2 phosphorylation through Corticotropin-Releasing Factor (CRF) receptors 1 and 2β by the CRF/Urocortin Family of peptides. Endocrinology. 2004;145:1718–1729. doi: 10.1210/en.2003-1023. [DOI] [PubMed] [Google Scholar]
- Chen DY, Lan JL, Lin FJ, Hsieh TY. Association of intercellular adhesion molecule-1 with clinical manifestations and interleukin-18 in patients with active, untreated adult-onset Still's disease. Arthritis Rheum. 2005;53:320–327. doi: 10.1002/art.21164. [DOI] [PubMed] [Google Scholar]
- Cipollone F, Fazia ML. COX-2 and Atherosclerosis. J Cardiovasc Pharmacol. 2006;47:S26–S36. doi: 10.1097/00005344-200605001-00006. [DOI] [PubMed] [Google Scholar]
- Cui XM, Pan L, Cui L, Qi GC, Li ZB. Ultrastructural diagnosis of thromboangiitis obliterans. J Chin Electron Microsc Soc. 2000;19:811–814. [Google Scholar]
- Dubois RN, Abramson SB, Crofford L, Gupta RA, Simon LS, Van De Putte LB, et al. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063–1073. [PubMed] [Google Scholar]
- Fekete EM, Zorrilla EP. Physiology, pharmacology, and therapeutic relevance of urocortins in mammals: ancient CRF paralogs. Front Neuroendocrinol. 2007;28:1–27. doi: 10.1016/j.yfrne.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiessinger JN, Schäfer M. Trial of iloprost versus aspirin treatment for critical limb ischaemia of thromboangiitis obliterans: the TAO study. Lancet. 1990;335:555–557. doi: 10.1016/0140-6736(90)90346-7. [DOI] [PubMed] [Google Scholar]
- Gando S, Kameue T, Matsuda N, Hayakawa M, Ishitani T, Morimoto Y, et al. Combined activation of coagulation and inflammation has an important role in multiple organ dysfunction and poor outcome after severe trauma. Thromb Haemost. 2002;88:943–949. [PubMed] [Google Scholar]
- Gao L, Lu C, Xu C, Tao Y, Cong B, Ni X. Differential regulation of prostaglandin production mediated by CRH receptor type 1 and type 2 in cultured human placental trophoblasts. Endocrinology. 2008;149:2866–2876. doi: 10.1210/en.2007-1377. [DOI] [PubMed] [Google Scholar]
- Halacheva K, Gulubova MV, Manolova I, Petkov D. Expression of ICAM-1, VCAM-1, E-selectin and TNF-alpha on the endothelium of femoral and iliac arteries in thromboangiitis obliterans. Acta Histochem. 2002;104:177–184. doi: 10.1078/0065-1281-00621. [DOI] [PubMed] [Google Scholar]
- Hao Z, Huang Y, Cleman J, Jovin IS, Vale WW, Bale TL, et al. Urocortin2 inhibits tumor growth via effects on vascularization and cell proliferation. Proc Natl Acad Sci USA. 2008;105:3939–3944. doi: 10.1073/pnas.0712366105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada Y, Ikeda K, Tojo K, Sakamoto M, Takada Y, Tajima N. Possible involvement of corticotropin-releasing factor receptor signaling on vascular inflammation. Peptides. 2009;30:365–372. doi: 10.1016/j.peptides.2008.10.015. [DOI] [PubMed] [Google Scholar]
- Joras M, Poredos P, Fras Z. Endothelial dysfunction in Buerger's disease and its relation to markers of inflammation. Eur J Clin Invest. 2006;36:376–382. doi: 10.1111/j.1365-2362.2006.01646.x. [DOI] [PubMed] [Google Scholar]
- Kageyama K, Hanada K, Nigawara T, Moriyama T, Terui K, Sakihara S, et al. Urocortin induces Interleukin-6 gene expression via Cyclooxygenase-2 activity in Aortic Smooth Muscle Cells. Endocrinology. 2006;147:4454–4462. doi: 10.1210/en.2006-0008. [DOI] [PubMed] [Google Scholar]
- Karalis K, Sano H, Redwine J, Listwak S, Wilder RL, Chrousos GP. Autocrine or paracrine inflammatory actions of corticotropinreleasing hormone in vivo. Science. 1991;254:421–423. doi: 10.1126/science.1925600. [DOI] [PubMed] [Google Scholar]
- Kohno M, Kawahito Y, Tsubouchi Y, Hashiramoto A, Yamada R, Inoue KI, et al. Urocortin expression in synovium of patients with rheumatoid arthritis and osteoarthritis: relation to inflammatory activity. J Clin Endocrinol Metab. 2001;86:4344–4352. doi: 10.1210/jcem.86.9.7827. [DOI] [PubMed] [Google Scholar]
- Li X, Hu J, Zhang R, Sun X, Zhang Q, Guan X, et al. Urocortin ameliorates diabetic nephropathy in obese db/db mice. Br J Pharmacol. 2008;154:1025–1034. doi: 10.1038/bjp.2008.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T, Sawada K, Taneda K, Hayashi M, Katsuura Y, Tanabe H, et al. Effect of isocarbacyclin methyl ester incorporated in lipid microspheres on experimental models of peripheral obstructive disease. Arzneimittelforschung. 1995;45:991–994. [PubMed] [Google Scholar]
- Nakata Y, Ban I, Hirai M, Shionoya S. Onset and clinicopathological course in Burger's disease. Angiology. 1976;27:509–517. doi: 10.1177/000331977602700904. [DOI] [PubMed] [Google Scholar]
- Nielubowicz J, Rosnowski A, Pruszynski B, Przetakiewicz Z, Potemkowski A. Natural history of Buerger's disease. J Cardiovasc Surg. 1980;21:529–540. [PubMed] [Google Scholar]
- Nizankowski R, Królikowski W, Bielatowicz J, Szczeklik A. Prostacyclin for ischemic ulcers in peripheral arterial disease. A random assignment, placebo controlled study. Thromb Res. 1985;37:21–28. doi: 10.1016/0049-3848(85)90029-5. [DOI] [PubMed] [Google Scholar]
- Ohnaka K, Numaguchi K, Yamakawa T, Inagami T. Induction of Cyclooxygenase-2 by Angiotensin II in cultured rat vascular smooth muscle cells. Hypertension. 2000;35:68–75. doi: 10.1161/01.hyp.35.1.68. [DOI] [PubMed] [Google Scholar]
- Oki Y, Sasano H. Localization and physiological roles of urocortin. Peptides. 2004;25:1745–1749. doi: 10.1016/j.peptides.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Okosi A, Brar BK, Chan M, D'Souza L, Smith E, Stephanou A, et al. Expression and protective effects of urocortin in cardiac myocytes. Neuropeptides. 1998;32:167–171. doi: 10.1016/s0143-4179(98)90033-6. [DOI] [PubMed] [Google Scholar]
- Olin JW. Thromboangiitis obliterans (Buerger's disease) N Engl J Med. 2000;343:864–869. doi: 10.1056/NEJM200009213431207. [DOI] [PubMed] [Google Scholar]
- Pisarchik A, Slominski A. Molecular and functional characterization of novel CRFR1 isoforms from the skin. Eur J Biochem. 2004;271:2821–2830. doi: 10.1111/j.1432-1033.2004.04216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puéchal X, Fiessinger JN. Thromboangiitis obliterans or Buerger's disease: challenges for the rheumatologist. Rheumatology. 2007;46:192–199. doi: 10.1093/rheumatology/kel388. [DOI] [PubMed] [Google Scholar]
- Quevedo ME, Slominski A, Pinto W, Wei E, Wortsman J. Pleiotropic effects of corticotropin releasing hormone on normal human skin keratinocytes. In Vitro Cell Dev Biol Anim. 2001;37:50–54. doi: 10.1290/1071-2690(2001)037<0050:peocrh>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Saruta M, Takahashi K, Suzuki T, Torii A, Kawakami M, Sasano H. Urocortin 1 in colonic mucosa in patients with ulcerative colitis. J Clin Endocrinol Metab. 2004;89:5352–5361. doi: 10.1210/jc.2004-0195. [DOI] [PubMed] [Google Scholar]
- Shirakura S, Higo K, Takeda M, Karasawa A. Antithrombotic effects of KW-3635, a thromboxane A2-receptor antagonist, in guinea pigs. Jpn J Pharmacol. 1994;65:93–98. doi: 10.1254/jjp.65.93. [DOI] [PubMed] [Google Scholar]
- Silver MJ, Smith JB, Ingerman C, Kocsis JJ. Arachidonic acid induced human platelet aggregation and prostaglandin formation. Prostaglandins. 1973;4:863–875. doi: 10.1016/0090-6980(73)90121-4. [DOI] [PubMed] [Google Scholar]
- Silver MJ, Hoch W, Kocsis JJ, Ingerman CM, Smith JB. Arachidonic acid causes sudden death in rabbits. Science. 1974;183:1085–1087. doi: 10.1126/science.183.4129.1085. [DOI] [PubMed] [Google Scholar]
- Singh LK, Boucher W, Pang X, Letourneau R, Seretakis D, Green M, et al. Potent mast cell degranulation and vascular permeability triggered by urocortin through activation of corticotropin-releasing hormone receptors. J Pharmacol Exp Ther. 1999;288:1349–1356. [PubMed] [Google Scholar]
- Taal MW, Zandi-Nejad K, Weening B, Shahsafaei A, Kato S, Lee KW, et al. Proinflammatory gene expression and macrophage recruitment in the rat remnant kidney. Kidney Int. 2000;58:1664–1676. doi: 10.1111/j.1523-1755.2000.00327.x. [DOI] [PubMed] [Google Scholar]
- Teli T, Markovic D, Hewitt ME, Levine MA, Hillhouse EW, Grammatopoulos DK. Structural domains determining signalling characteristics of the CRH-receptor type 1 variant R1beta and response to PKC phosphorylation. Cell Signal. 2008;20:40–49. doi: 10.1016/j.cellsig.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Singh LK, Boucher W, Pang X, Letourneau R, Webster E, et al. Corticotropin-releasing hormone induces skin mast cell degranulation and increased vascular permeability, a possible explanation for its proinflammatory effects. Endocrinology. 1998;139:403–413. doi: 10.1210/endo.139.1.5660. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Donelan JM, Papadopoulou N, Cao J, Kempuraj D, Conti P. Mast cells as targets of corticotropin-releasing factor and related peptides. Trends Pharmacol Sci. 2004;25:563–568. doi: 10.1016/j.tips.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Uzuki M, Sasano H, Muramatsu Y, Totsune K, Takahashi K, Oki Y, et al. Urocortin in the synovial tissue of patients with rheumatoid arthritis. Clin Sci. 2001;100:577–589. [PubMed] [Google Scholar]
- Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- Vaughan J, Donaldson C, Bittencourt J, Perrin MH, Lewis K, Sutton S, et al. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin- releasing factor. Nature. 1995;378:287–292. doi: 10.1038/378287a0. [DOI] [PubMed] [Google Scholar]
- Wang J, Xu Y, Xu Y, Zhu H, Zhang R, Zhang G, et al. Urocortin's inhibition of tumor growth and angiogenesis in hepatocellular carcinoma via corticotrophin-releasing factor receptor 2. Cancer Invest. 2008;26:359–368. doi: 10.1080/07357900701788106. [DOI] [PubMed] [Google Scholar]
- Webster EL, Lewis DB, Torpy DJ, Zachman EK, Rice KC, Chrousos GP. In vivo and in vitro characterization of antalarmin a nonpeptide corticotropin-releasing hormone (CRH) receptor antagonist: suppression of pituitary ACTH release and peripheral inflammation. Endocrinology. 1996;137:5747–5750. doi: 10.1210/endo.137.12.8940412. [DOI] [PubMed] [Google Scholar]
- Witkowska AM. Soluble ICAM-1: a marker of vascular inflammation and lifestyle. Cytokine. 2005;31:127–134. doi: 10.1016/j.cyto.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Wu Y, Xu Y, Zhou H, Tao J, Li S. Expression of urocortin in rat lung and its effect on pulmonary vascular permeability. J Endocrinol. 2006;189:167–178. doi: 10.1677/joe.1.06607. [DOI] [PubMed] [Google Scholar]
- Wu Y, Hu J, Zhang R, Zhou C, Xu Y, Guan X, et al. Enhanced intracellular calcium induced by urocortin is involved in degranulation of rat lung mast cells. Cell Physiol Biochem. 2008;21:173–182. doi: 10.1159/000113759. [DOI] [PubMed] [Google Scholar]
- Wu Y, Zhang R, Zhou C, Xu Y, Guan X, Hu J, et al. Enhanced expression of vascular cell adhesion molecule-1 by corticotrophinreleasing hormone contributes to progression of atherosclerosis in LDL receptor-deficient mice. Atherosclerosis. 2009;203:360–370. doi: 10.1016/j.atherosclerosis.2008.05.059. [DOI] [PubMed] [Google Scholar]
- Yokotani K, Murakami Y, Okada S, Hirata M. Role of brain arachidonic acid cascade on central CRF1 receptor-mediated activation of sympatho-adrenomedullary outflow in rats. Eur J Pharmacol. 2001;419:183–189. doi: 10.1016/s0014-2999(01)00987-6. [DOI] [PubMed] [Google Scholar]


