Abstract
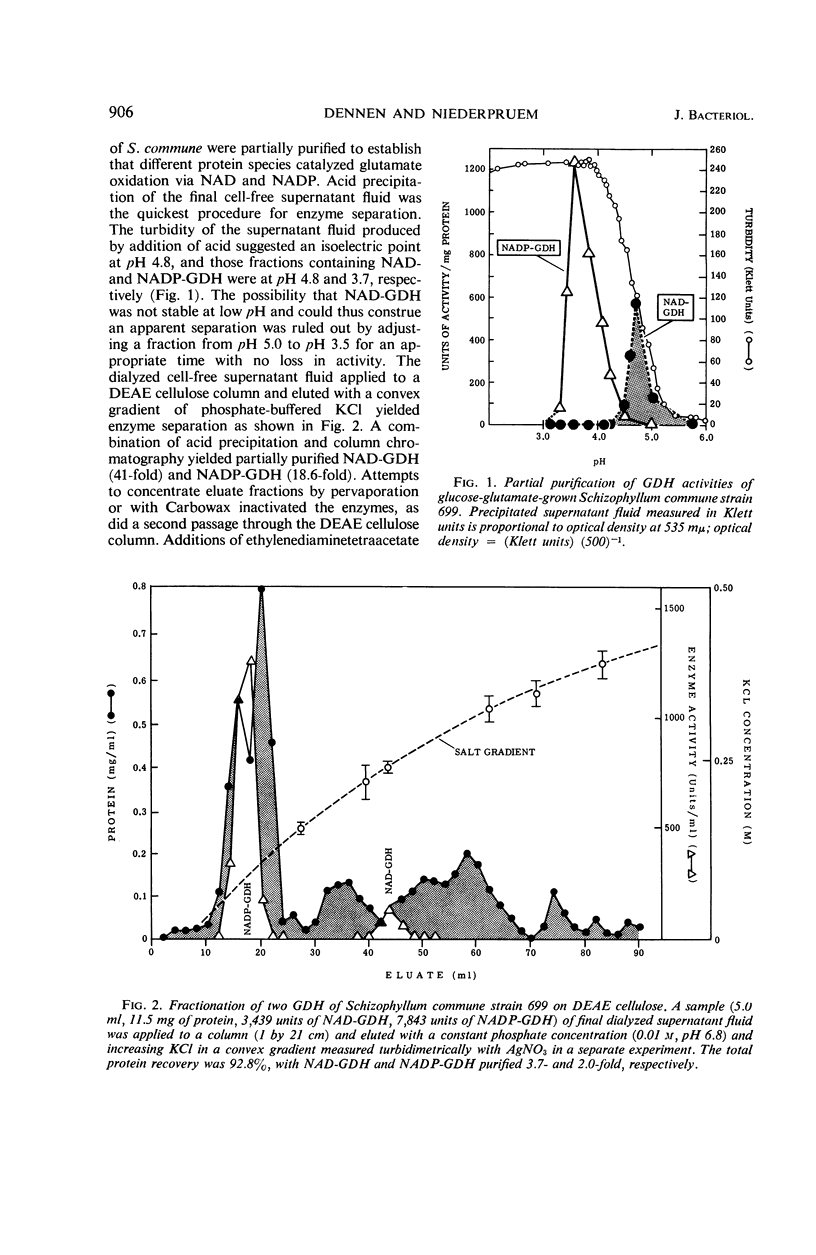
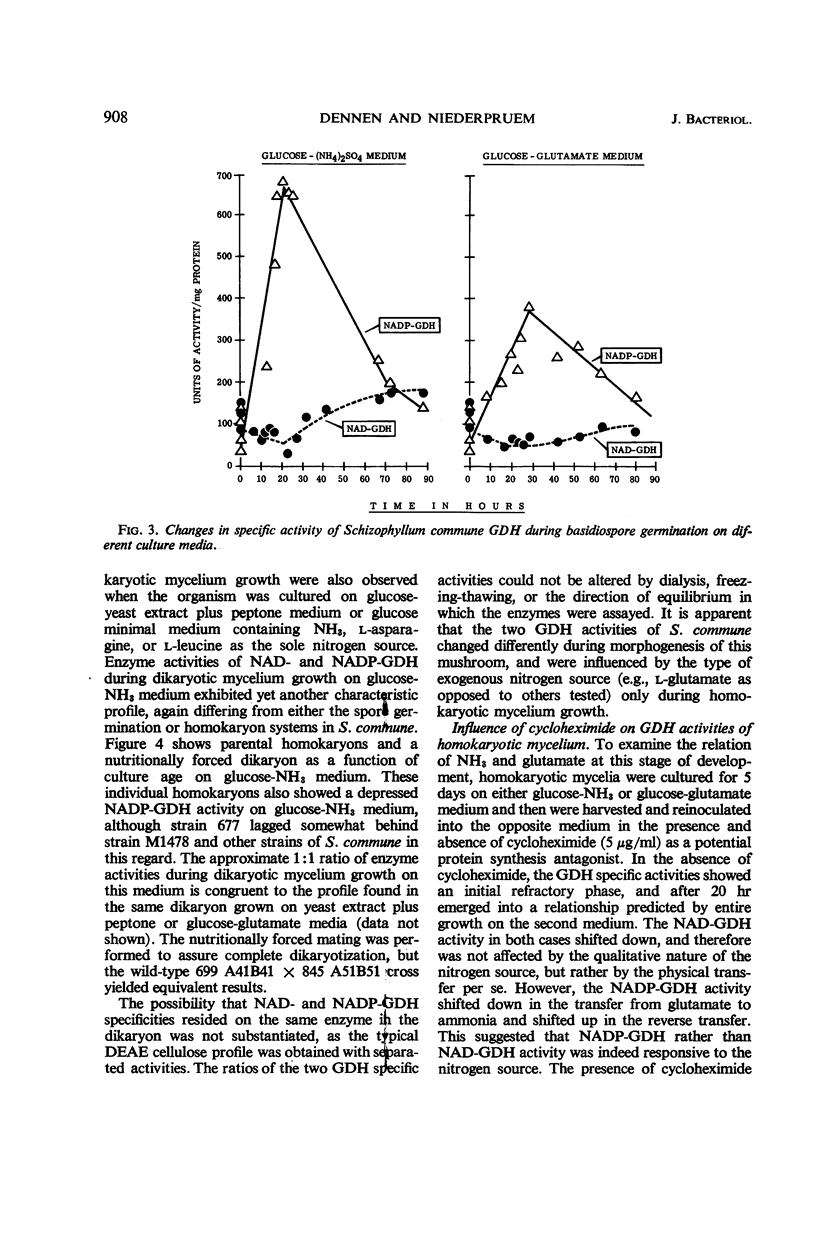
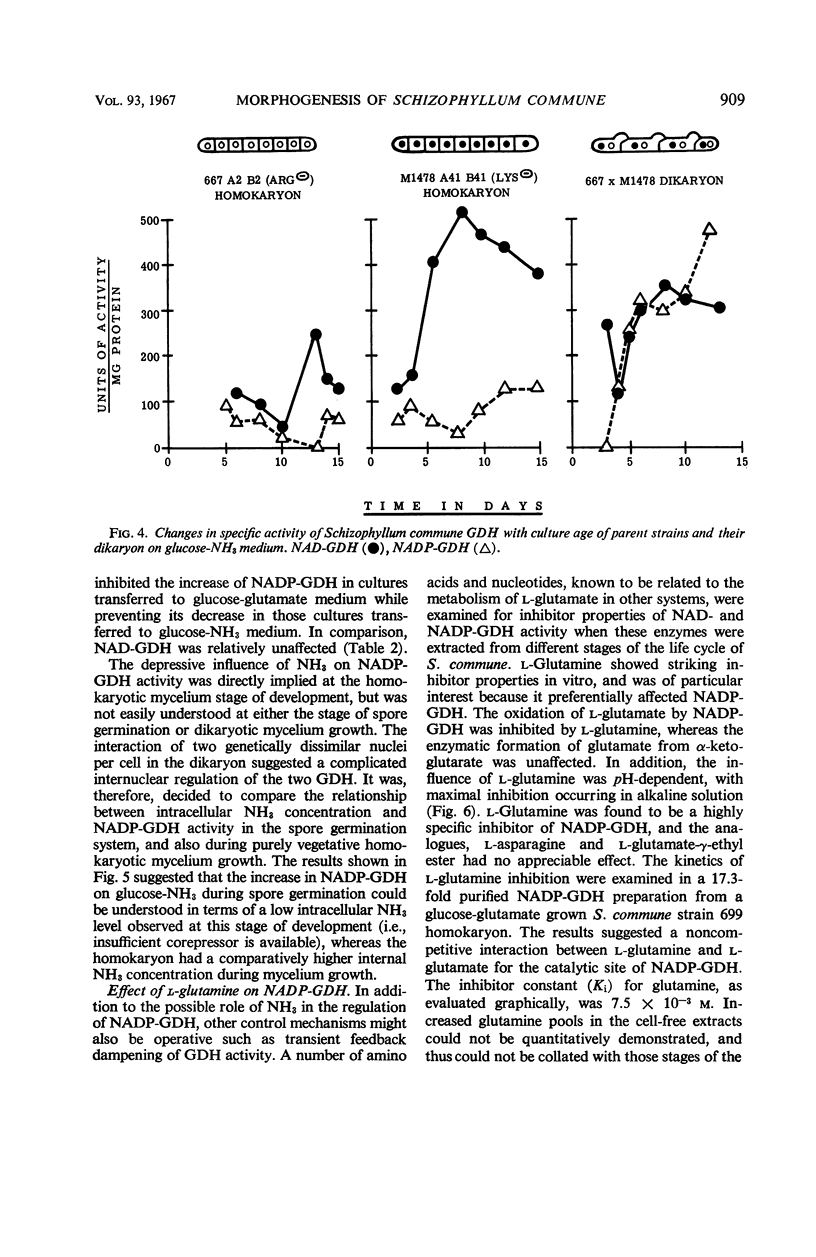
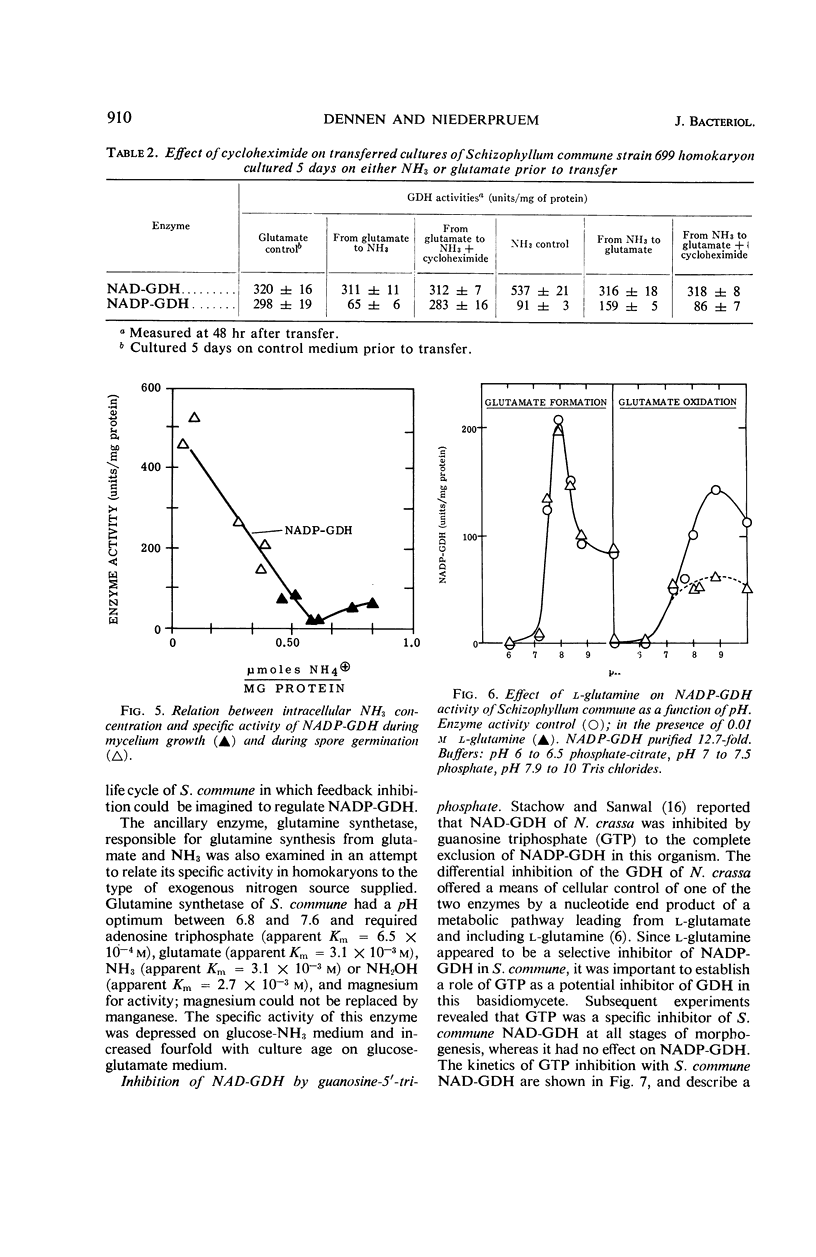
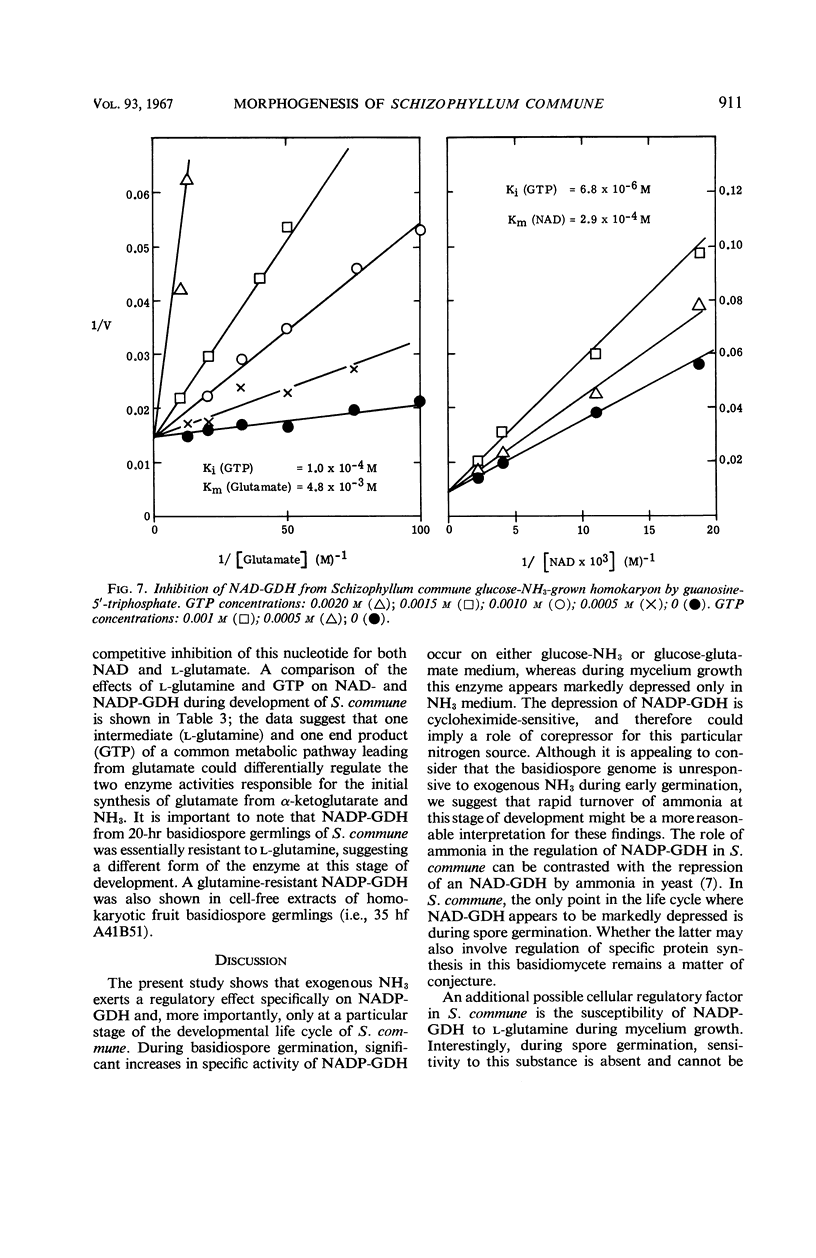
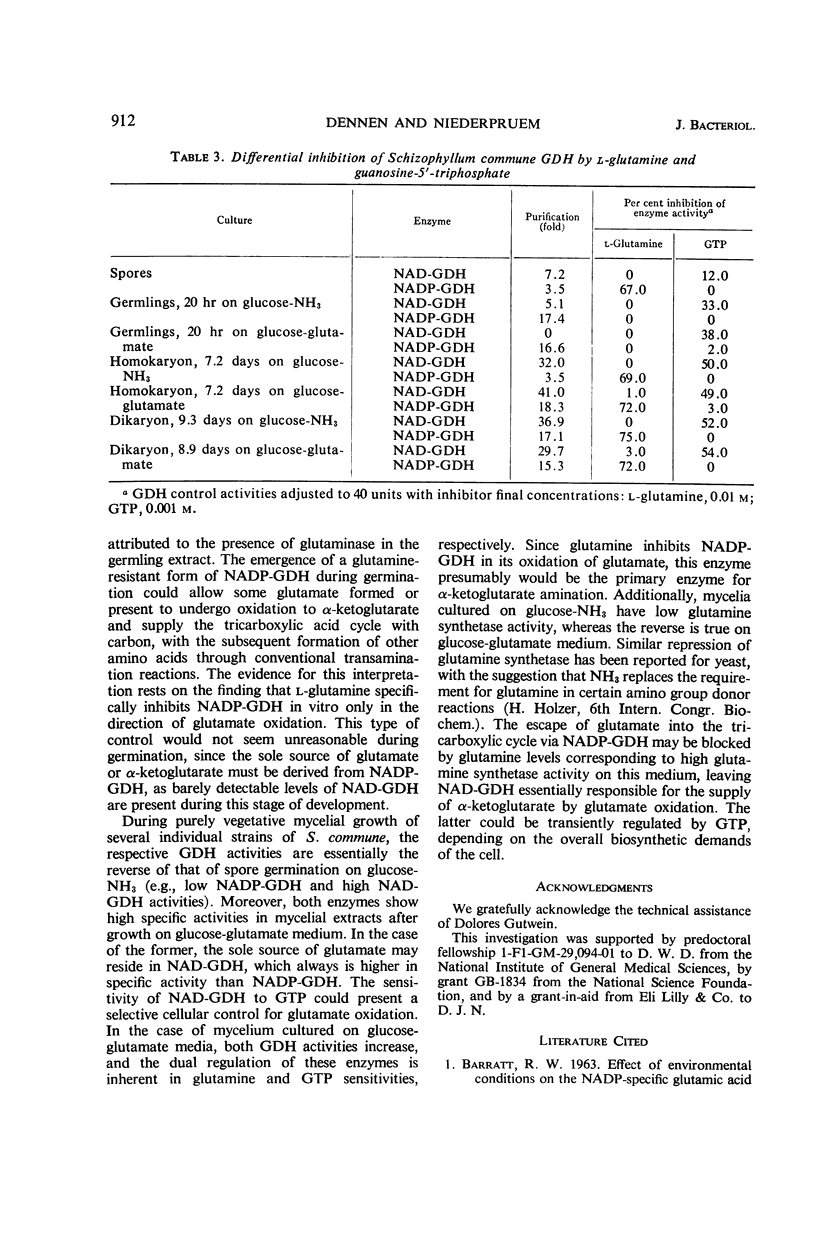
The specific activities of two glutamate dehydrogenases (GDH), one requiring nicotinamide adenine dinucleotide (NAD) and the other specific for nicotinamide adenine dinucleotide phosphate (NADP), varied during growth of Schizophyllum commune as a function of the stage of the life cycle and the exogenous nitrogen source. During basidiospore germination on either glucose-NH3 or glucose-glutamate medium, NADP-GDH increased six- to eightfold in specific activity, whereas NAD-GDH was depressed. During dikaryotic mycelial growth on either nitrogen source, the two GDH increased in a 1:1 ratio, whereas, during homokaryotic mycelial growth on glucose-NH3, NADP-GDH activity was depressed and NAD-GDH increased six- to eightfold. Homokaryotic mycelium cultured on glucose-glutamate medium yielded high NADP-GDH activities and normal NAD-GDH activities. Intracellular NH3 concentration and NADP-GDH activities were inversely related during spore germination and homokaryotic mycelium growth, whereas guanosine-5′-triphosphate (GTP) and l-glutamine specifically inhibited NAD- and NADP-GDH respectively in vitro. GTP inhibition was shown in extracts from cells at all stages of the life cycle. Basidiospore germling extracts contained an NADP-GDH essentially resistant to l-glutamine inhibition.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DENNEN D. W., NIEDERPRUEM D. J. CONTROL OF GLUTAMATE DEHYDROGENASE IN THE BASIDIOMYCETE SCHIZOPHYLLUM COMMUNE. Life Sci. 1965 Jan;4:93–98. doi: 10.1016/0024-3205(65)90039-1. [DOI] [PubMed] [Google Scholar]
- HARTMAN S. C., BUCHANAN J. M. Nucleic acids, purines, pyrimidines (nucleotide synthesis). Annu Rev Biochem. 1959;28:365–410. doi: 10.1146/annurev.bi.28.070159.002053. [DOI] [PubMed] [Google Scholar]
- HIERHOLZER G., HOLZER H. REPRESSION DER SYNTHESE VON DPN-ABHAENGIGER GLUTAMINSAEUREDEHYDROGENASE IN SACCHAROMYCES CEREVISIAE DURCH AMMONIUMIONEN. Biochem Z. 1963 Dec 3;339:175–185. [PubMed] [Google Scholar]
- Hackett D. P., Haas D. W., Griffiths S. K., Niederpruem D. J. Studies on Development of Cyanide-resistant Respiration in Potato Tuber Slices. Plant Physiol. 1960 Jan;35(1):8–19. doi: 10.1104/pp.35.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATO K., KOIKE S., YAMADA K., YAMADA H., TANAKA S. Di- and triphosphopyridine nucleotide linked glutamic dehydrogenases of Piricularia oryzae and their behaviors in glutamate media. Arch Biochem Biophys. 1962 Aug;98:346–347. doi: 10.1016/0003-9861(62)90195-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MUFTIC M. K. A NEW PHENOL-HYPOCHLORITE REACTION FOR AMMONIA. Nature. 1964 Feb 8;201:622–623. doi: 10.1038/201622a0. [DOI] [PubMed] [Google Scholar]
- NIEDERPRUEM D. J., HOBBS H., HENRY L. NUTRITIONAL STUDIES OF DEVELOPMENT IN SCHIZOPHYLLIUM COMMUNE. J Bacteriol. 1964 Dec;88:1721–1729. doi: 10.1128/jb.88.6.1721-1729.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANWAL B. D. Diphosphopyridine nucleotide and triphosphopyridine nucleotide linked glutamic dehydrogenases of Fusarium. Arch Biochem Biophys. 1961 May;93:377–386. doi: 10.1016/0003-9861(61)90281-8. [DOI] [PubMed] [Google Scholar]
- SANWAL B. D., LATA M. Concurrent regulation of glutamic acid dehydrogenases of Neurospora. Arch Biochem Biophys. 1962 Jun;97:582–588. doi: 10.1016/0003-9861(62)90127-3. [DOI] [PubMed] [Google Scholar]


