Abstract
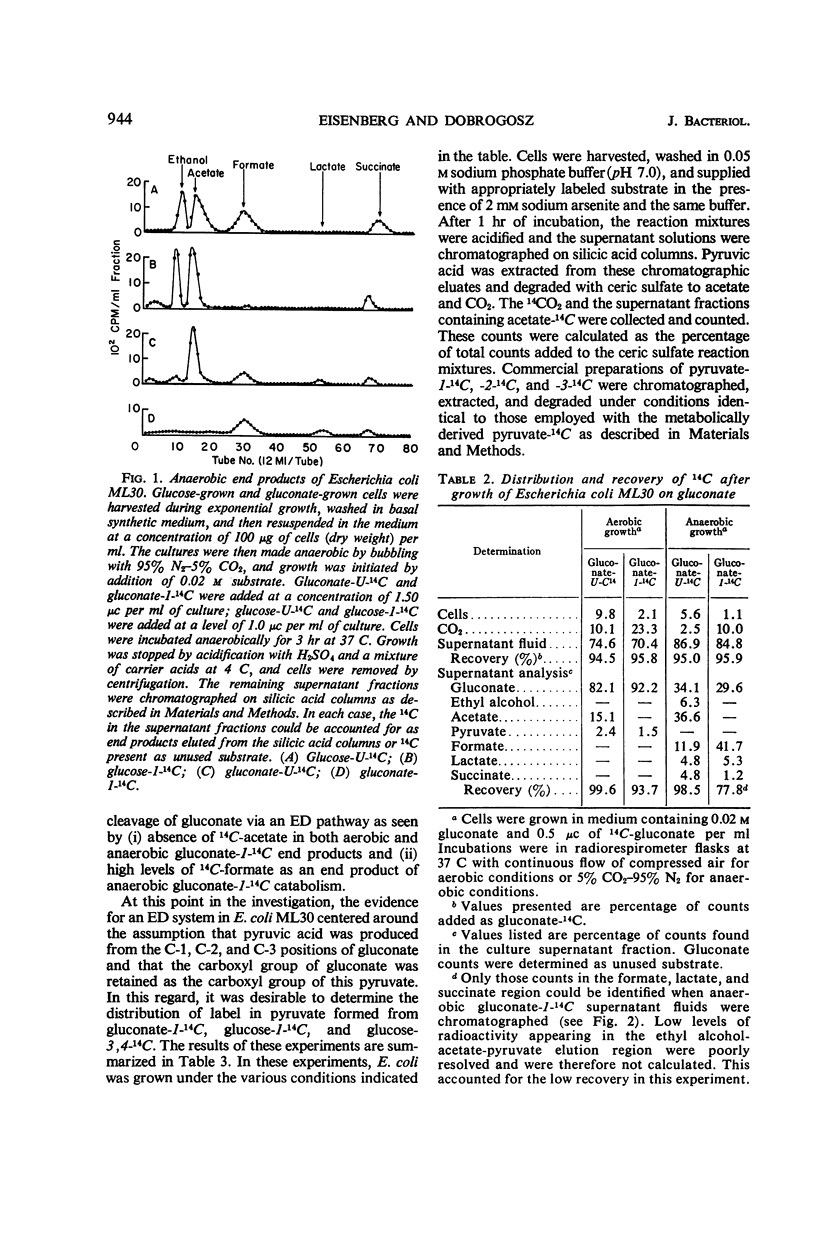
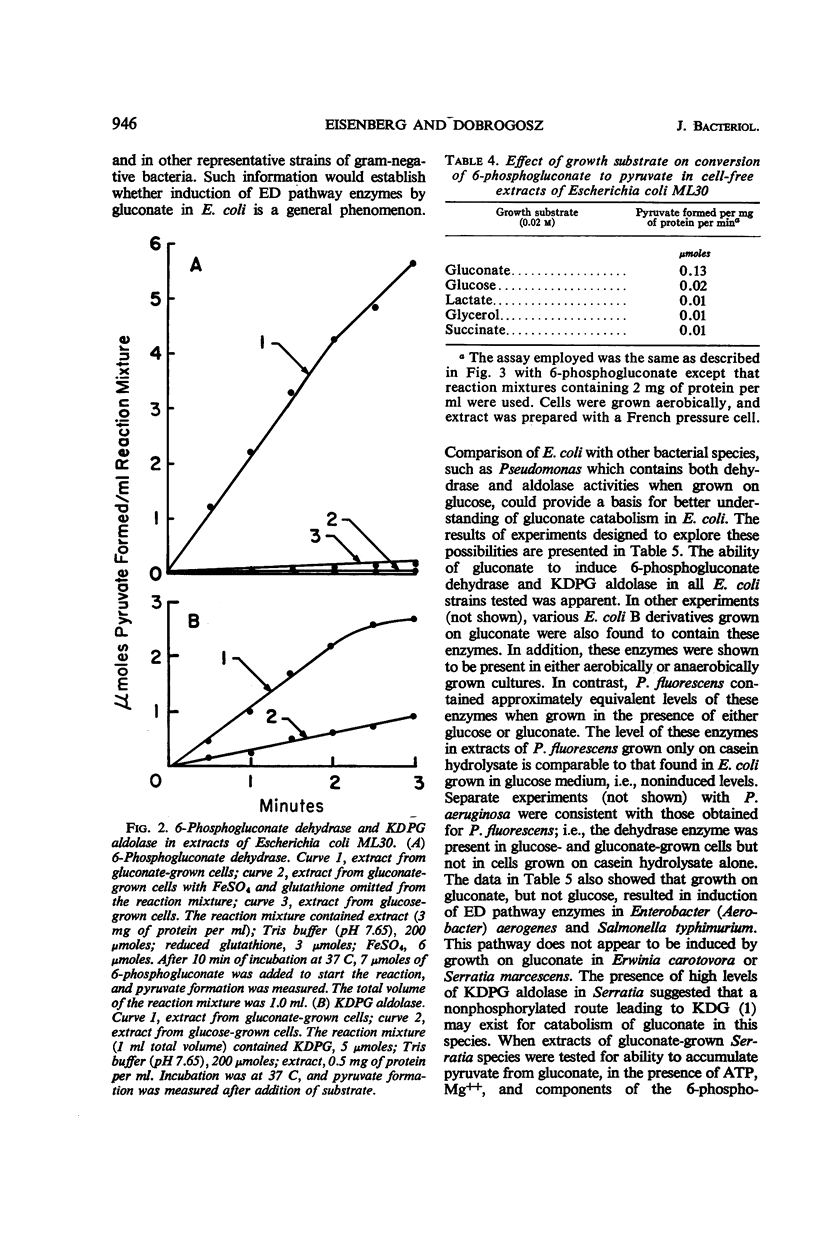
On the basis of information available in the literature, gluconate dissimilation in Escherichia coli is thought to occur via the hexose monophosphate pathway. Evidence is presented in this study that gluconate is catabolized in this organism via an inducible Entner-Doudoroff pathway. This evidence is based on chromatographic examination of end products produced from 14C-labeled gluconate or glucose, distribution of 14C in the carbon atoms of pyruvate formed from specifically labeled 14C-glucose and 14C-gluconate, and the ability of cell-free extracts to produce pyruvate from 6-phosphogluconate. Degradation of gluconate by an Entner-Doudoroff pathway occurred simultaneously with a glycolytic cleavage of glucose. A relationship between gluconate-induced, Entner-Doudoroff pathway activity and catabolism of glucose in Escherichia coli and other bacterial species is discussed.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASHWELL G., WAHBA A. J., HICKMAN J. Uronic acid metabolism in bacteria. I. Purification and properties of uronic acid isomerase in Escherichia coli. J Biol Chem. 1960 Jun;235:1559–1565. [PubMed] [Google Scholar]
- BLACKWOOD A. C., LEDINGHAM G. A., NEISH A. C. Dissimilation of glucose at controlled pH values by pigmented and non-pigmented strains of Escherichia coli. J Bacteriol. 1956 Oct;72(4):497–499. doi: 10.1128/jb.72.4.497-499.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN S. S., SCOTT D. B. M. Gluconokinase and the oxidative path for glucose-6-phosphate utilization. Nature. 1950 Nov 4;166(4227):781–782. doi: 10.1038/166781b0. [DOI] [PubMed] [Google Scholar]
- COHEN S. S. Utilization of gluconate and glucose in growing and virus-infected Escherichia coli. Nature. 1951 Oct 27;168(4278):746–747. doi: 10.1038/168746a0. [DOI] [PubMed] [Google Scholar]
- Dobrogosz W. J. Altered end-product patterns and catabolite repression in Escherichia coli. J Bacteriol. 1966 Jun;91(6):2263–2269. doi: 10.1128/jb.91.6.2263-2269.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGON R. G., WANG C. H. Dissimilation of glucose and gluconic acid by Pseudomonas natriegens. J Bacteriol. 1962 Apr;83:879–886. doi: 10.1128/jb.83.4.879-886.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRAENKEL D. G., HORECKER B. L. PATHWAYS OF D-GLUCOSE METABOLISM IN SALMONELLA TYPHINMURIUM. A STUDY OF A MUTANT LACKING PHOSPHOGLUCOSE ISOMERASE. J Biol Chem. 1964 Sep;239:2765–2771. [PubMed] [Google Scholar]
- FRAMPTON E. W., WOOD W. A. Carbohydrate oxidation by Pseudomonas fluorescens VI. Conversion of 2-keto-6-phosphogluconate to pyruvate. J Biol Chem. 1961 Oct;236:2571–2577. [PubMed] [Google Scholar]
- HORECKER B. L. PATHWAYS OF CARBOHYDRATE METABOLISM AND THEIR PHYSIOLOGICAL SIGNIFICANCE. J Chem Educ. 1965 May;42:244–253. doi: 10.1021/ed042p244. [DOI] [PubMed] [Google Scholar]
- KITOS P. A., WANG C. H., MOHLER B. A., KING T. E., CHELDELIN V. H. Glucose and gluconate dissimilation in Acetobacter suboxydans. J Biol Chem. 1958 Dec;233(6):1295–1298. [PubMed] [Google Scholar]
- KOVACHEVICH R., WOOD W. A. Carbohydrate metabolism by Pseudomonas fluorescens. III. Purification and properties of a 6-phosphogluconate dehydrase. J Biol Chem. 1955 Apr;213(2):745–756. [PubMed] [Google Scholar]
- KOVACHEVICH R., WOOD W. A. Carbohydrate metabolism by Pseudomonas fluorescens. IV. Purification and properties of 2-keto-3-deoxy-6-phosphogluconate aldolase. J Biol Chem. 1955 Apr;213(2):757–767. [PubMed] [Google Scholar]
- Krebs H. A., Johnson W. A. Metabolism of ketonic acids in animal tissues. Biochem J. 1937 Apr;31(4):645–660. doi: 10.1042/bj0310645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lockwood L. B., Tabenkin B., Ward G. E. The Production of Gluconic Acid and 2-Keto-Gluconic Acid from Glucose by Species of Pseudomonas and Phytomonas. J Bacteriol. 1941 Jul;42(1):51–61. doi: 10.1128/jb.42.1.51-61.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis W. F., Jr, Magasanik B. Nature of the effector of catabolite repression of beta-galactosidase in Escherichia coli. J Bacteriol. 1966 Jul;92(1):170–177. doi: 10.1128/jb.92.1.170-177.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCNAIR SCOTT D. B. The oxidative pathway of carbohydrate metabolism in Escherichia coli. III. Glucose 6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase in cells grown under different conditions. Biochem J. 1956 Aug;63(4):587–593. doi: 10.1042/bj0630587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NARROD S. A., WOOD W. A. Carbohydrate oxidation by Pseudomonas fluorescens. V. Evidence for gluconokinase and 2-ketogluconokinase. J Biol Chem. 1956 May;220(1):45–55. [PubMed] [Google Scholar]
- Okinaka R. T., Dobrogosz W. J. Enhanced Catabolite Repression in Escherichia coli by Growth on Combined Substrates. J Bacteriol. 1966 Aug;92(2):526–527. doi: 10.1128/jb.92.2.526-527.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOKATCH J. T., GUNSALUS I. C. Aldonic acid metabolism. I. Pathway of carbon in an inducible gluconate fermentation by Streptococcus faecalis. J Bacteriol. 1957 Apr;73(4):452–460. doi: 10.1128/jb.73.4.452-460.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes J. L. FERMENTATION OF GLUCOSE BY SUSPENSIONS OF ESCHERICHIA COLI. J Bacteriol. 1949 Feb;57(2):147–158. doi: 10.1128/jb.57.2.147-158.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG C. H., STERN I., GILMOUR C. M., KLUNGSOYR S., REED D. J., BIALY J. J., CHRISTENSEN B. E., CHELDELIN V. H. Comparative study of glucose catabolism by the radiorespirometric method. J Bacteriol. 1958 Aug;76(2):207–216. doi: 10.1128/jb.76.2.207-216.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]



