Abstract
Background and purpose:
We have shown that ginsenoside Rg1 is a novel class of potent phytoestrogen and activates insulin-like growth factor-I receptor (IGF-IR) signalling pathway in human breast cancer MCF-7 cells. The present study tested the hypothesis that the neuroprotective actions of Rg1 involved activation of the IGF-IR signalling pathway in a rat model of Parkinson's disease, induced by 6-hydroxydopamine (6-OHDA).
Experimental approach:
Ovariectomized rats were infused unilaterally with 6-OHDA into the medial forebrain bundle to lesion the nigrostriatal dopamine pathway and treated with Rg1 (1.5 h after 6-OHDA injections) in the absence or presence of the IGF-IR antagonist JB-1 (1 h before Rg1 injections). The rotational behaviour induced by apomorphine and the dopamine content in the striatum were studied. Protein and gene expression of tyrosine hydroxylase, dopamine transporter and Bcl-2 in the substantia nigra were also determined.
Key results:
Rg1 treatment ameliorated the rotational behaviour induced by apomorphine in our model of nigrostriatal injury. This effect was partly blocked by JB-1. 6-OHDA significantly decreased the dopamine content of the striatum and treatment with Rg1 reversed this decrease. Treatment with Rg1 of 6-OHDA-lesioned rats reduced neurotoxicity, as measured by tyrosine hydroxylase, dopamine transporter and Bcl-2 protein and gene level in the substantia nigra. These effects were abolished by JB-1.
Conclusions and implications:
These data provide the first evidence that Rg1 has neuroprotective effects on dopaminergic neurons in the 6-OHDA model of nigrostriatal injury and its actions might involve activation of the IGF-IR signalling pathway.
Keywords: ginsenoside Rg1, 6-hydroxydopamine, insulin-like growth factor-I receptor, dopaminergic system, Parkinson's disease
Introduction
Parkinson's disease (PD) is a neurodegenerative disorder characterized by the progressive loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc) and declining levels of dopamine in the striatum. At the present time, the causes of the neuronal cell death in this disorder is not known but the incidence of PD is less in women than men, suggesting a potential neuroprotective role for female sex steroid hormones (Diamond et al., 1990; Mayeux et al., 1995). The neuroprotective effect of oestrogen (E2) on the dopamine neurons in the SNpc has been widely investigated (Callier et al., 2001; Datla et al., 2003; Murray et al., 2003; Ramirez et al., 2003). Owing to the side effects of hormone replacement therapy, many women turn to phytoestrogens as an alternative to hormone replacement therapy. There is emerging evidence that oestrogen-like compounds may protect against neurotoxicity and, in this context, the phytoestrogen, ginsenoside Rg1, is of particular interest.
Ginsenoside Rg1 is the major pharmacologically active compound of ginseng (Liu and Xiao, 1992). A wide range of neurotrophic and neuroprotective effects of ginsenoside Rg1 both in vivo and in vitro have been reported. In in vitro studies, ginsenoside Rg1 has been reported to protect dopaminergic cells against glutamate (Radad et al., 2004a), 1-methyl-4-phenylpyridinium (MPP+) (Chen et al., 2003; Radad et al., 2004b) and rotenone toxicities (Leung et al., 2007). In vivo studies have demonstrated the protective effect of ginsenoside Rg1 against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced apoptosis in mouse SN neurons (Chen et al., 2002). However, the neuroprotective effect of Rg1 against 6-hydroxydopamine (6-OHDA)-induced nigrostriatal injury in the rat and the possible mechanism is unclear.
Insulin-like growth factor-I receptor (IGF-IR) is a member of the growth factor tyrosine kinase receptor family that signals through the phosphatidylinositol-3-kinase (PI3K) pathway and mitogen-activated protein kinase (MAPK) cascade (Bondy and Cheng, 2004; Laviola et al., 2007). Together with the ligands, insulin-like growth factor-I and II (IGF-I and II) and different binding proteins, they participate in growth and development of the nervous system (Kar et al., 1997). This signalling pathway is also involved in the response of neural tissue to injury and protects neurons against various neurodegenerative stimuli (Guan et al., 2000; Krishnamurthi et al., 2004; Quesada and Micevych, 2004). There is a strong line of evidence supporting the interaction of oestrogen receptor (ER) and IGF-IR signalling pathways in the neuroprotective mechanisms of oestrogen against PD. Pretreatment with oestrogen significantly attenuated the loss of SNpc neurons and the related motor disturbances in the model of nigrostriatal injury induced by 6-OHDA (Quesada and Micevych, 2004). Blockade of IGF-IR by intracerebroventricular JB-1 or of the PI3K/Akt (protein kinase B) pathway by LY294002 attenuated the neuroprotective effects of oestrogen (Quesada and Micevych, 2004; Quesada et al., 2008).
Our previous study demonstrated that ginsenoside Rg1 can stimulate the growth of human breast cancer MCF-7 cells as well as activating the oestrogen response element (ERE)-dependent luciferase activities in HeLa cells, without direct binding to the oestrogen receptor-α (ERα) (Chan et al., 2002). Furthermore, we have demonstrated that ginsenoside Rg1 could activate the IGF-IR-mediated pathway through enhancing tyrosine phosphorylation of insulin receptor substrate-1 (IRS-1) and mitogen-activated protein kinase kinase (MEK), which in turn activate the phosphorylation of the Ser-118 residue in the ERα of MCF-7 cells (Chen et al., 2006; Lau et al., 2008). These results suggest that the action of Rg1 is ER-dependent but it acts via the IGF-IR-mediated pathway to exert its oestrogen-like effects.
In the present study, we hypothesized that the neuroprotective effects of ginsenoside Rg1 against 6-OHDA toxicity in the nigrostriatal system might be mediated through the activation of the IGF-IR-mediated pathway.
Methods
Animals
The Animal Ethic Committee of Qingdao University approved this study. All experimental procedures were in accordance with the National Institute of Health Guide for Care and Use of Laboratory Animals and were performed according to the institutional animal experimental ethical guidelines. Sixty adult female Wistar rats weighing 220–250 g (Experimental Animal Center, Shandong, China) were housed four per cage in stainless steel rust-free cages at 21 ± 2°C and a relative humidity of 60–65% with alternating 12 h periods of light and darkness. Ovariectomy was performed as previously described (Toledo-Aral et al., 2002) to remove the influence of endogenous oestrogen.
Stereotactic 6-OHDA lesions
Following a 2-week post-surgical recovery after ovariectomy, unilateral infusion of 6-OHDA into the medial forebrain bundle (MFB) was conducted as described previously (Earl et al., 1996). Briefly, the rats were anaesthetized with chloral hydrate (400 mg·kg−1, i.p.) and placed in a stereotaxic apparatus. Then, 2.5 and 3 µL 6-OHDA (3.6 µg·µL−1 in 0.9% saline containing 0.02% w·v−1 ascorbic acid) or 2.5 and 3 µL vehicle (control group) were injected unilaterally into the two sites of the left MFB according to the coordinates: (i) TB: −2.3 mm; AP: −4.4 mm; ML: 1.2 mm; V: 7.8 mm; and (ii) TB: +3.4 mm; AP: −4.0 mm; ML: 0.8 mm; V: 8.0 mm (Paxinos et al., 1985). The sham-operated rats received an identical volume of the ascorbic acid vehicle. All injections were made at a rate of 1 µL·min−1, and the needle remained in position for a further 5 min to prevent reflux along the injection tract. After the injections of 6-OHDA, a cannula made of stainless steel (outer diameter: 0.4 mm; inner diameter: 0.3 mm) was planted into the lateral ventricle according to the coordinates: AP: −0.8 mm; ML: 1.4 mm; V: 3.7 mm. The cannula was cemented in place with dental cement using skull screws as anchors. Stainless steel stylets were used to keep the cannula sealed. After the surgery, the 60 rats were randomly divided into five groups: (i) control group: Sham rats were subjected to microinjection with 2 µL normal saline containing 0.1% ethanol into the lateral cerebral ventricle, followed by injection with saline (1 mL·kg−1) intraperitoneally 1 h after microinjection. The treatment lasted 14 consecutive days; (ii) 6-OHDA group: 6-OHDA-treated rat were subjected to treatments as described for control group; (iii) Rg1 group: similar treatment as in 6-OHDA group except that Rg1 (10 mg·kg−1, 10 mg·mL−1) instead of saline was injected intraperitoneally 1 h after microinjection. The dosages were chosen based on a previous reported study (Chen et al., 2005); (iv) Rg1 + JB-1 group: similar treatment as in Rg1 group except 2 µL JB-1 (0.5µg, 0.25 µg·µL−1) instead of normal saline containing 0.1% ethanol was used in microinjection; and (v) JB-1 group: similar treatment as in the 6-OHDA group except that 2 µL JB-1 instead of normal saline containing 0.1% ethanol was used in microinjection. On day 15, animals from each group were randomly divided into two groups. One group was used for immunocytochemistry studies (see below). The other group was killed, and tissues of stratum and SN were rapidly dissected and stored at −80°C until being used for HPLC analysis and reverse transcriptase-polymerase chain reaction (RT-PCR) assay. In total, 30 rats were used for immunohistochemistry studies, and 30 rats were used for the determination of the levels of dopamine and metabolites in striatum and the gene expressions in the SN.
Apomorphine-induced rotation behaviour
Rotational behaviour contralateral to the lesioned side was induced by i.p. injection of 0.05 mg·kg−1 apomorphine on day 15 after 6-OHDA infusion. Rats were placed in individual hemispherical stainless steel bowls (diameter 32 cm), with a harness around their chests. The harness was connected to an automatic four-channel rotameter (Qingdao University, Qingdao, China). Rotational behaviour was counted for 30 min to evaluate the effects of different treatments on the rotational behaviour in rats. All rats were allowed to adapt to the bowls for about 30 min before the apomorphine injection.
Determination of dopamine content
The striatum on both sides was weighed and then stored in liquid nitrogen. Samples were prepared as described previously (Jiang et al., 2006). Briefly, the samples were homogenized in 0.3 mL liquid A (0.4 M perchloric acid). After centrifugation at 13 500×g for 20 min (4–8°C), 80 µL of the supernatant was transferred to Eppendorf tubes to which 40 µL liquid B (20 mM citromalic acid-potassium, 300 mM dipotassium phosphate, 2 mM EDTA-2Na) was added. The supernatant was used to determine the content of dopamine by means of HPLC with electrochemical detection. Separation was achieved on a PE C18 reversed-phase column. The mobile phase consisted 20 mM citrate-phosphate buffer, pH 3.5, 50 mM sodium acetic acid, 3.75 mM sodium octane sulphonic acid and 0.134 mM EDTA-2Na and 5% (v·v−1) methanol. The flow rate was maintained at 1.0 mL·min−1. A 2465 electrochemical detector (Waters Corp., Milford, MA, USA) was operated in screen mode. Dopamine and its metabolites were quantified by peak height comparison with standards that were run on the day of analysis. Results were expressed as ng·mg−1 wet weight of the brain tissue.
Immunohistochemistry
Rats were anaesthetized with chloral hydrate (400 mg·kg−1, i.p.) and perfused transcardially with 0.9% saline followed by 4% paraformaldehyde in phosphate buffer (0.1 mM, pH 7.4). Brains were removed and post-fixed in 4% paraformaldehyde for 6 h and then transferred into 20% sucrose and stored at 4°C for 48 h. Serial coronal sections were cut through the SNpc (located approximately 5.0–6.0 mm from bregma) at 16 µm on a freezing cryostat (Leica, Nussloch, Germany) and divided into three consecutive series. Sections were placed in 99.7% methanol containing 0.3% hydrogen peroxide for 30 min to eliminate endogenous peroxidase. After washing with phosphate-buffered saline, the sections were pre-incubated in blocking buffer (phosphate-buffered saline containing 10% goat serum) to reduce non-specific binding. The sections were incubated overnight at 4°C in a humidified chamber with a polyclonal rabbit anti-rat tyrosine hydroxylase (TH; 1:3 000),rat monoclonal antibody to the dopamine transporter (DAT; 1:200) or a polyclonal rabbit anti-rat Bcl-2 antibody (1:200). The sections were followed by incubation in biotinylated goat anti-rabbit IgG for 30 min at 37°C and subsequently in streptavidin peroxidase for 30 min at 37°C. TH, DAT and Bcl-2-immunoreactivity (Bcl-2-IR) was visualized by diaminobenzidine (DAB). Magnification used to count TH-immunoreactive (TH-IR) cells on the ipsilateral (lesioned) and the contralateral (unlesioned) side of each section was 400× (40× objective) on an Olympus microscope. Three sections of each rat were used for counting and matched for level as close as possible from animal to animal. The average number of positive cells in four random fields per slide was used to represent the neuron density of one section. The mean for three brain sections was taken from each rat, and there were six rats in each group. Negative control study was performed by the omission of the primary antibody. Counting was always performed by two individuals unaware of the treatments. For DAT-immunoreactivity (DAT-IR) and Bcl-2-IR, the three brain sections were scanned and the optical density (OD) quantitated using the SimplePCI image analysis software. The OD background in the area around the cells of substantia nigra pars reticulata (SNR) was subtracted from the OD values of the SNpc. The mean for three brain sections was taken from each rat, and there were six rats in each group. Micrographs were taken with a camera mounted on a phototube using Kodak film (200×).
Semi-quantitative RT-PCR for TH, DAT, Bcl-2 and GAPDH expression
Total RNA was isolated from the SN of rat by using Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to the standard protocol. Total RNA (3 µg) was used to generate cDNA in each sample using SuperScript II reverse transcriptase with oligo(dT) 12–18 primers (Promega, Madison, WI, USA). The evaluation of TH, DAT and Bcl-2 expression was performed by semi-quantitative RT-PCR as previously described (Chen et al., 2006). PCR amplification was performed on a PTC 200 thermal cycler (MJ Research, Minneapolis, MN, USA). Cycle-response for each PCR product was determined to derive the optimal cycle for each gene candidate. For TH, DAT, Bcl-2 and GAPDH, the primers used were 5′-ACACAGCGGAAGAGATTGCT -3′ (TH forward) and 5′-CCCAGAGATGCAAGTCCAAT-3′(TH reverse), 5′-AAGATCTGCCCTGTCCTGAAAG-3′ (DAT forward) and 5′-CATCGATCCACACAGATGCCTC-3′ (DAT reverse), 5′-GTGGGATACTGGAGATGAAG-3′ (Bcl-2 forward) and 5′-GTGGGATACTGGAGATGAAG-3′ (Bcl-2 reverse), and 5′-GACCAC AGTCCATGCCATCACTGC-3′ (GAPDH forward) and 5′-GCTGTTGAAGTCGCAGGAGACAAC-3′ (GAPDH reverse) to yield products of 400, 540, 453 and 340 bp, with 34, 40, 33 and 25 PCR cycles respectively. The PCR products were analysed using agarose gel electrophoresis. Optical densities of ethidium bromide-stained DNA bands were quantified using an UV-Illuminated Imager (Shanghai Tanon Corp., Shanghai, China), and the mRNA expression levels were normalized to the expression of GAPDH.
Statistical analysis
Data are reported as the mean ± SEM. Significance of differences between group means was determined by one-way analysis of variance (anova) followed by Tukey's multiple comparison test. A value of P < 0.05 was considered statistically significant.
Materials
Ginsenoside Rg1 was purchased from Norman Bethune University (Changchun, China). The purity of Rg1 was determined by HPLC and found to be more than 99% pure. JB-1 was purchased from Bachem (Bubendorf, Switzerland). Unless otherwise specified, the reagents used were obtained from Sigma-Aldrich. The reagents for RT-PCR were purchased from Life Technologies Inc. (Carlsbad, CA, USA). Monoclonal anti-rat DAT was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Polyclonal anti-rat Bcl-2 was purchased from Biosynthesis Biotechnology Co. Ltd. (Beijing, China).
Results
Apomorphine-induced rotations were reduced following Rg1 treatment and the IGF-IR antagonist JB-1 blocks this effect of Rg1
Unilateral 6-OHDA lesions of the nigrostriatal dopaminergic pathway produce an imbalance of dopamine between the lesioned and the unlesioned striatum, followed by postsynaptic dopamine receptor supersensitivity in the lesioned side. Treatment with a dopamine agonist such as apomorphine leads to contralateral rotational behaviour in denervated animals (Schwarting and Huston, 1996). Assessment of rotational behaviour in 6-OHDA-lesioned rats was consistent with damage to dopaminergic neurons in the SN and the decrease of dopamine content in the striatum (Toledo-Aral et al., 2002; Jiang et al., 2006). Figure 1 shows that the 6-OHDA-induced dopaminergic denervation of the striatum exhibited 230 contralateral turns per 30 min following an administration of apomorphine. Rats lesioned with 6-OHDA and treated with ginsenoside Rg1 showed a significant improvement – less turns after apomorphine. Co-treatment with the IGF-IR antagonist JB-1 partly blocked the effect of Rg1 on the apomorphine-induced rotational behaviour. The turning rate in the JB-1 + 6-OHDA group was also significantly decreased compared with the 6-OHDA group (Figure 1). Sham-treated animals showed no contralateral rotational behaviour (data not shown).
Figure 1.
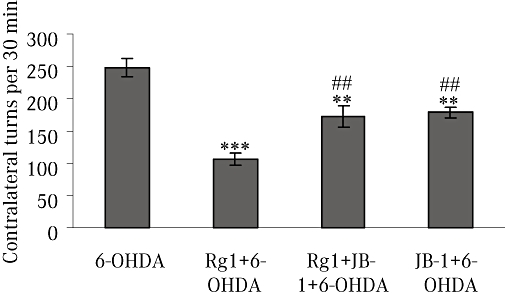
Apomorphine-induced rotation behaviour. Rotational behaviour was counted for 30 min after i.p. injection of 0.05 mg·kg−1 apomorphine in the 6-hydroxydopamine (6-OHDA) group, Rg1 + 6-OHDA group, Rg1 + JB-1 + 6-OHDA group and JB-1 + 6-OHDA group. All values are expressed as mean ± SEM. n= 12. **P < 0.01, ***P < 0.001 versus the 6-OHDA group, ##P < 0.01 versus the Rg1 + 6-OHDA group.
The IGF-IR antagonist JB-1 blocks Rg1 improvement of striatal dopamine after 6-OHDA lesions
Our results are consistent with reports that microinjection of 6-OHDA into the MFB caused a decrease in striatal dopamine (Toledo-Aral et al., 2002). In our model, after 15 days of 6-OHDA lesions, the dopamine level in the striatum was significantly decreased on the lesioned side in the 6-OHDA treatment group, compared with the unlesioned side and that of control animals. However, treatment of lesioned rats with ginsenoside Rg1 (10 mg·kg−1) reduced neurotoxicity, with the loss of dopamine being less than that in the lesioned side of the striatum in rats given 6-OHDA alone (Figure 2). Pretreatment with the IGF-IR antagonist JB-1 abolished Rg1-mediated attenuation of 6-OHDA-induced depletion of striatal dopamine. The dopamine levels were not changed on the lesioned side of the striatum between JB-1 + 6-OHDA treatment group and the 6-OHDA treatment group. No changes in dopamine content were found in the unlesioned side of the striatum in all groups (Figure 2).
Figure 2.
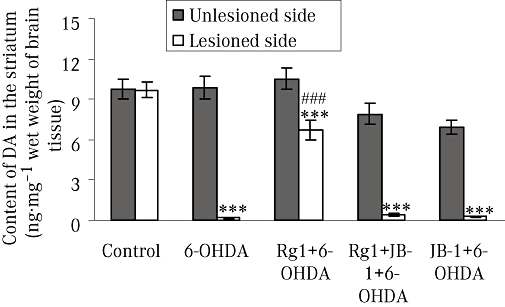
Effect of Rg1 on the dopamine (DA) content of the striatum of rats with 6-hydroxydopamine (6-OHDA) induced Parkinson's disease and the blocking effect of JB-1 (ng·mg−1 wet weight of brain tissue). The unlesioned and lesioned side of striatum were used to determine the content of dopamine by HPLC. All values are expressed as mean ± SEM. n= 6. ***P < 0.001 versus the control group, ###P < 0.001 versus the lesioned side of 6-OHDA group.
Rg1 attenuates 6-OHDA-induced loss of TH-IR and DAT-IR neurons in the SNpc and JB-1 blocks Rg1 protection against these 6-OHDA lesions
Both TH and DAT are expressed in dopaminergic neurons, and they may be the best marker for dopaminergic nerve cells. All animals that had unilateral 6-OHDA injection into the MFB showed severe nigrastriatal lesions with a loss of more than 85% of TH-IR neurons in the lesioned side of SNpc. In contrast, about 4.2 times as many TH-IR nigral neurons in the Rg1-treated animals survived the treatment with 6-OHDA. The number of TH-IR neurons in the lesioned side of the SNpc after the blockade of IGF-IRs was similar to that in rats receiving 6-OHDA only (Figure 3).
Figure 3.
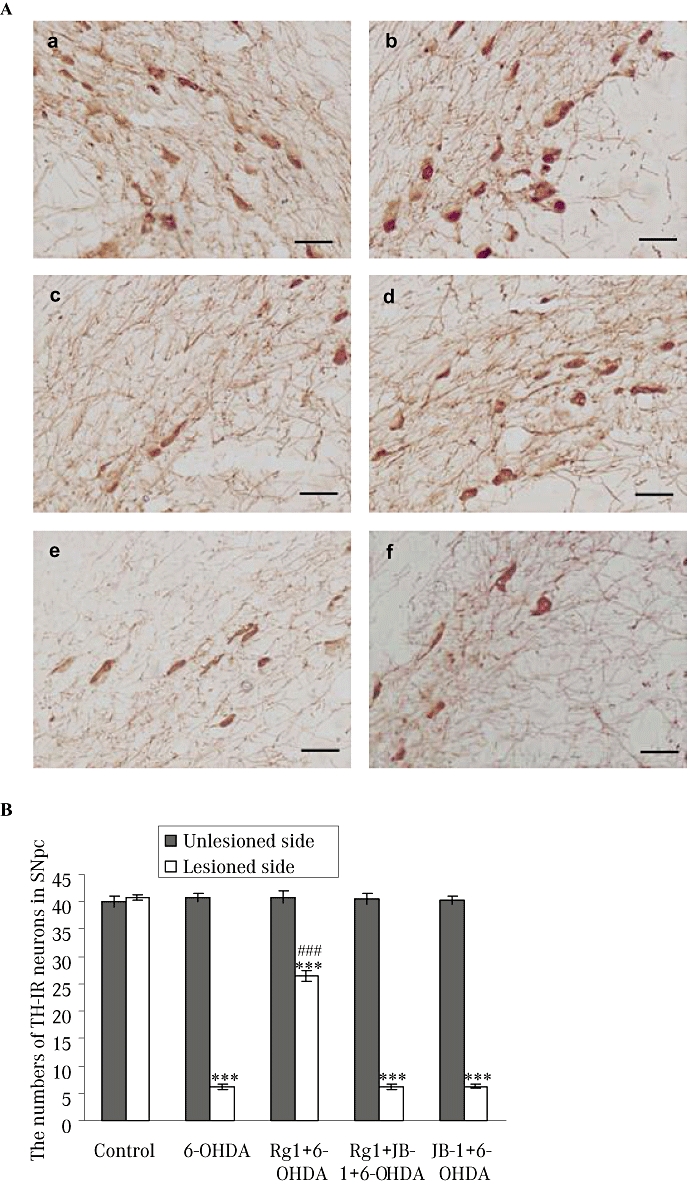
Effect of Rg1 on the tyrosine hydroxylase-immunoreactive (TH-IR) neurons in the substantia nigra pars compacta (SNpc) of the rat model of 6-hydroxydopamine (6-OHDA) induced Parkinson's disease and the blocking effect of JB-1. (A) Representative microphotographs of coronal sections immunostained for TH-IR neurons in the unlesioned side of the SNpc in the control (sham) group (a) and the lesioned side of the SNpc in the control (sham) group (b), 6-OHDA group (c), Rg1 + 6-OHDA group (d), Rg1 + JB-1 + 6-OHDA group (e) and JB-1 + 6-OHDA group (f). Bar = 30 µm. (B) Quantitative analysis of TH-IR neurons in the unlesioned and lesioned side of the SNpc. All values are expressed as mean ± SEM. n= 6. ***P < 0.001 versus the control group, ###P < 0.001 versus the lesioned side of 6-OHDA group.
The OD value (shown as grey level in Figure 4B) of DAT-IR neurons in the lesioned side of the SNpc was also significantly decreased in the 6-OHDA treatment group. Rg1 treatment resulted in an approximately 1.32-fold increase in the OD value of DAT-IR neurons compared with the 6-OHDA lesion only group, and this effect could be completely blocked by JB-1 (Figure 4). No significant changes were observed in the unlesioned side of the SNpc in any group.
Figure 4.
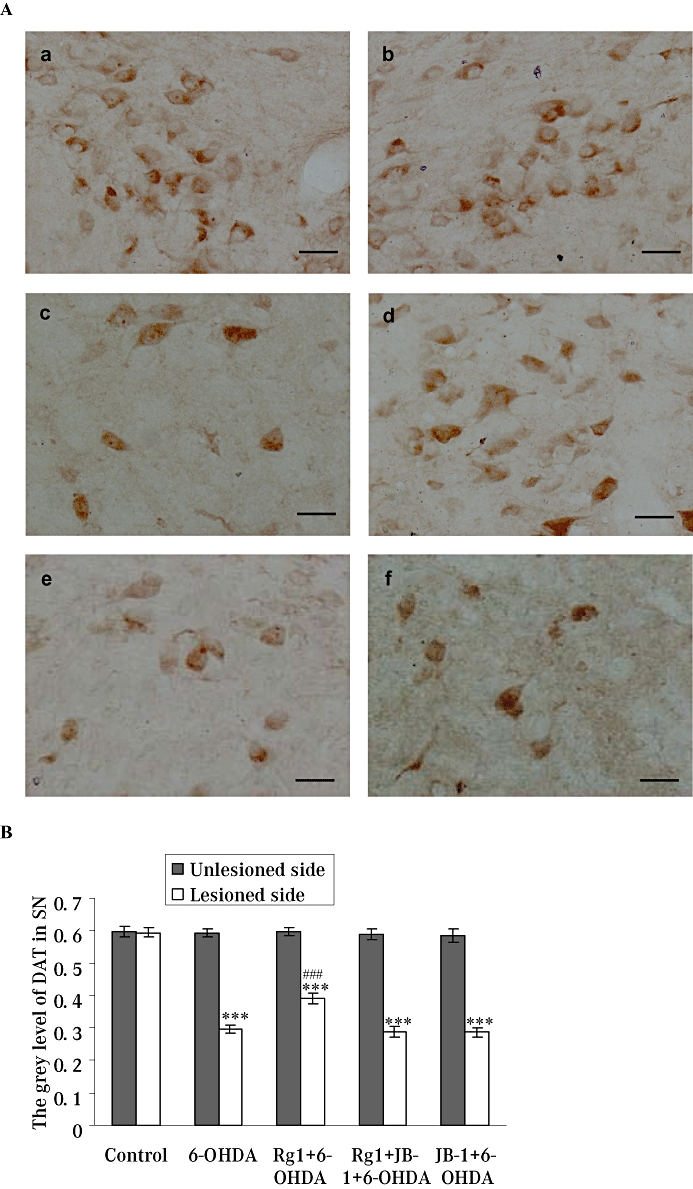
Effect of Rg1 on the dopamine transporter-immunoreactivity (DAT-IR) neurons in the substantia nigra pars compacta (SNpc) of the rat model of Parkinson's disease induced by 6-hydroxydopamine (6-OHDA) and the blocking effect of JB-1. (A) Representative microphotographs of coronal sections immunostained for DAT-IR neurons the unlesioned side of the SNpc in the control (sham) group (a) and the lesioned side of the SNpc in the control (sham) group (b), 6-OHDA group (c), Rg1 + 6-OHDA group (d), Rg1 + JB-1 + 6-OHDA group (e) and JB-1 + 6-OHDA group (f). Bar = 30 µm. (B) Quantitative analysis of DAT-IR neurons in the unlesioned and lesioned side of the SNpc. All values are expressed as mean ± SEM. n= 6. ***P < 0.001 versus the control group, ###P < 0.001 versus the lesioned side of 6-OHDA group.
The IGF-IR antagonist JB-1 blocks the effects of Rg1 on gene expressions of TH and DAT after 6-OHDA lesions
The distribution of TH-IR and DAT-IR neurons roughly paralleled the distribution of TH and DAT mRNA in the SNpc (Cerruti et al., 1994). Gene expression of TH and DAT were also detected in the present study. 6-OHDA treatment decreased the gene expression of TH and DAT in the lesioned side of the SN. However, Rg1 treatment significantly antagonized this 6-OHDA-induced decrease in TH and DAT gene expression in the 6-OHDA-lesioned rat. The TH and DAT gene expression after the blockade of IGF-IR were similar to that in the 6-OHDA lesion only group. No significant changes were observed in the unlesioned side of the SN in all groups (Figures 5 & 6).
Figure 5.
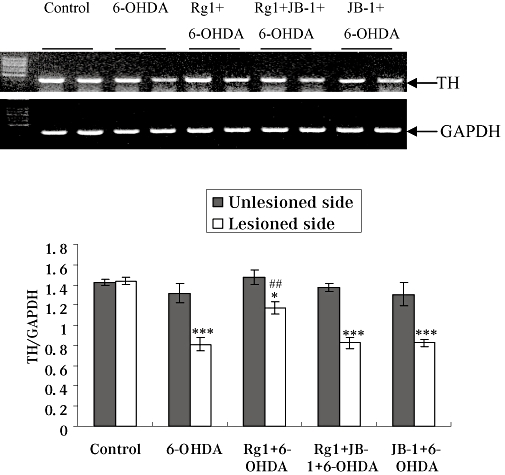
Effect of Rg1 on tyrosine hydroxylase (TH) gene expression in the substantia nigra (SN) of the rat model of Parkinson's disease induced by 6-hydroxydopamine (6-OHDA) and the blocking effect of JB-1. Total RNA was isolated from the unlesioned and lesioned side of SN and subjected to semi-quantitative RT-PCR analysis of TH and GAPDH mRNA expression. The TH mRNA expression level was expressed as a ratio of the expression of GAPDH. Summary results shown are expressed as mean ± SEM. n= 6. *P < 0.05, ***P < 0.001 versus the control group, ##P < 0.01 versus the lesioned side of 6-OHDA group.
Figure 6.
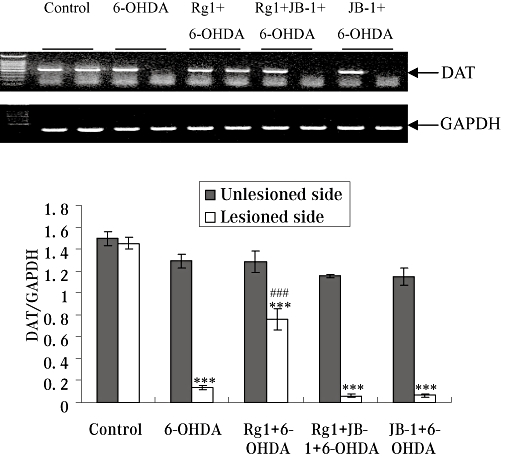
Effect of Rg1 on dopamine transporter (DAT) gene expression in the substantia nigra (SN) of the rat model of Parkinson's disease induced by 6-hydroxydopamine (6-OHDA) and the blocking effect of JB-1. Total RNA was isolated from the unlesioned and lesioned side of the SN and subjected to semi-quantitative RT-PCR analysis of DAT and GAPDH mRNA expression. The DAT mRNA expression level was expressed as a ratio of the expression of GAPDH. Summary results shown are expressed as mean ± SEM. n= 6. ***P < 0.01 versus the control group, ###P < 0.01 versus the lesioned side of 6-OHDA group.
Rg1 attenuates 6-OHDA-induced decrease on protein and gene expression of Bcl-2 and JB-1 blocks these effects of Rg1
Bcl-2 plays its anti-apoptotic role by maintaining mitochondrial integrity, thereby blocking cytochrome c release and the serial intrinsic cascade. The Bcl-2 protein and gene expression in the lesioned side of the SN was markedly decreased by 6-OHDA treatment. On the contrary, after treatment with Rg1, protein and gene expression of Bcl-2 in the lesioned side were significantly higher than the vehicle-treated 6-OHDA group. The protein and gene expressions of Bcl-2 after the blockade of IGF-IR were similar to that in the 6-OHDA-lesioned only group. No significant changes were observed in the unlesioned side of the SN in all groups (Figures 7 & 8).
Figure 7.
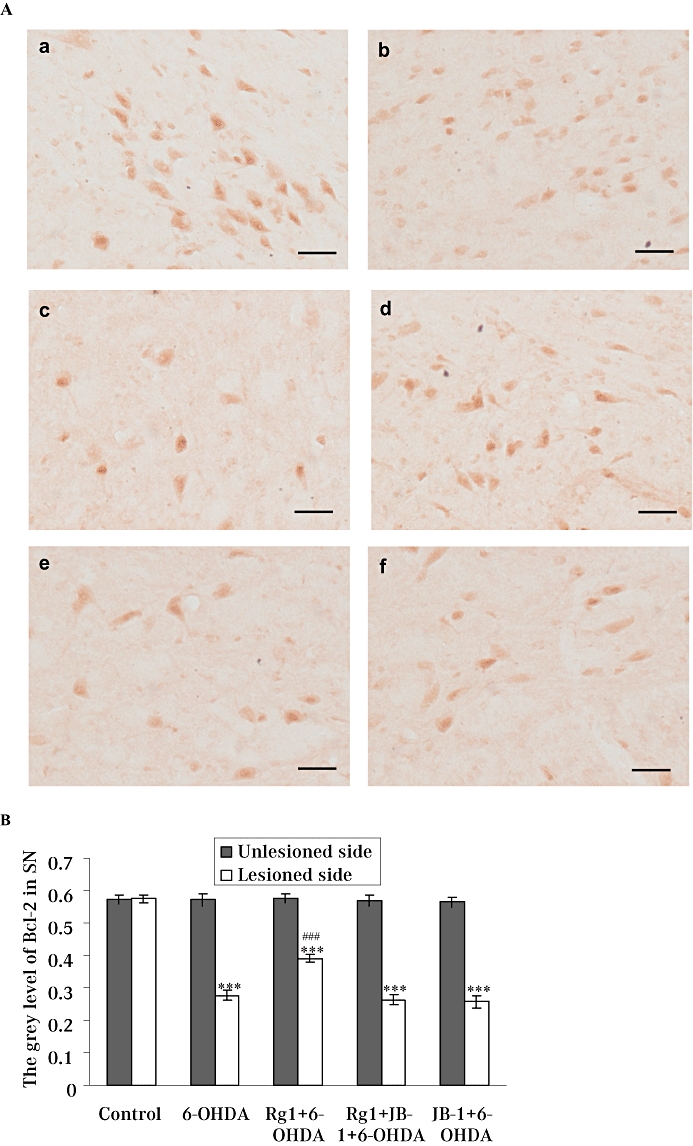
Effect of Rg1 on the Bcl-2 protein expression in the substantia nigra pars compacta (SNpc) of the rat model of Parkinson's disease induced by 6-hydroxydopamine (6-OHDA) and the blocking effect of JB-1. (A) Representative microphotographs of coronal sections immunostained for Bcl-2 protein expression in the unlesioned side of the SNpc in the control (sham) group (a) and the lesioned side of the SNpc in the control (sham) group (b), 6-OHDA group (c), Rg1 + 6-OHDA group (d), Rg1 + JB-1 + 6-OHDA group (e) and JB-1 + 6-OHDA group (f). Bar = 30µm. (B) Quantitative analysis of Bcl-2 protein expression in the unlesioned and lesioned side of the SNpc. All values are expressed as mean ± SEM. n= 6. ***P < 0.001 versus the control group, ###P < 0.001 versus the lesioned side of 6-OHDA group.
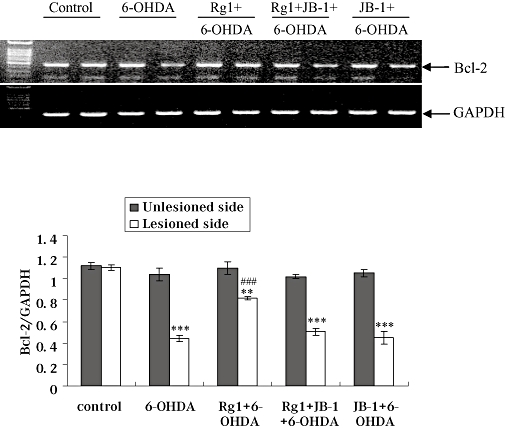
Effect of Rg1 on Bcl-2 gene expression in the substantia nigra (SN) of the rat model of Parkinson's disease induced by 6-hydroxydopamine (6-OHDA) and the blocking effect of JB-1. Total RNA was isolated from the unlesioned and lesioned side of SN and subjected to semi-quantitative RT-PCR analysis of Bcl-2 and GAPDH mRNA expression. The Bcl-2 mRNA expression level was expressed as a ratio of the expression of GAPDH. Summary results shown are expressed as mean ± SEM. n= 6. **P < 0.01, ***P < 0.001 versus the control group, ###P < 0.001 versus the lesioned side of 6-OHDA group.
Discussion
Ginseng products have been used as general tonics and ‘adaptogens’ to enhance physical performance for thousands of years. Ginsenosides are the principal active ingredients in ginseng and are believed to be responsible for most of ginseng's actions. A number of studies have described the beneficial effects of ginsenosides on PD models either in vivo or in vitro (Chen et al., 2002; 2003;Tohda et al., 2002; Radad et al., 2004b). At present, little is known about the neuroprotective roles of Rg1 in the nigrostriatal system in this 6-OHDA model of nigrostriatal injury. The results reported in this paper clearly demonstrate that ginsenoside Rg1 has neuroprotective effects on the nigrostriatal system in this model of PD induced by 6-OHDA. In addition, the neuroprotective effect of ginsenoside Rg1 appears to be mediated by the IGF-IR signalling pathway.
In the present study, we found that apomorphine-induced turning behaviour in the 6-OHDA group was significantly inhibited by Rg1 treatment. We confirmed that ginsenoside Rg1 protected against the 6-OHDA-induced decrease of dopamine content in the lesioned side of the striatum and reversed 6-OHDA-induced decreases in TH-IR and DAT-IR neurons in the lesioned side of the SNpc. The reverse transcription-PCR results also demonstrated that the 6-OHDA-induced decrease in TH and DAT mRNA expression in the lesioned side of the SN could be restored by Rg1 treatment. The IGF-IR antagonist JB-1 suppressed all the Rg1-mediated neuroprotective effects against 6-OHDA-induced neurotoxicity. All of the above values of the unlesioned side of the striatum and the SN were also compared between different groups. No significant changes were observed in the unlesioned side of either the SN or the striatum; although we found JB-1 could induce a slight but non-significant decrease in the content of dopamine in the unlesioned side of stratum compared with 6-OHDA treatment alone group. The changes in dopamine levels were consistent with the changes of the apomorphine-induced rotations between the 6-OHDA treatment group and the JB-1 + 6-OHDA treatment group. The difference of the dopamine level in the striatum between the unlesioned side and lesioned side in the JB-1 + 6-OHDA group was less than that in the 6-OHDA lesion only group, which exhibited a significant decrease in apomorphine-induced rotations compared with the 6-OHDA treatment group.
Although the processes and mechanisms underlying the neuroprotective effects of ginsenoside Rg1 on dopaminergic neurons remain to be elucidated, several studies have shown that ginsenoside Rg1 has protective effects against MPTP-induced apoptosis in the SN (Chen et al., 2002) and against rotenone toxicity through blocking the intrinsic apoptotic pathway and the PI3K/Akt pathway in primary nigral neurons (Leung et al., 2007). These anti-apoptotic effects of Rg1 may be attributed to the enhanced expression of Bcl-2 and Bcl-xl and reduced expression of Bax and NO synthase. Our data also showed that ginsenoside Rg1 partially overcame the decrease of Bcl-2 protein and gene expressions induced by 6-OHDA, therefore positively regulating cell survival. Bcl-2 expression can be modulated by activation of ERE and cAMP response element-binding protein, both transcription factors themselves regulated by ER and Akt (Pugazhenthi et al., 2000; Abraham et al., 2004).
We report here, for the first time, that the neuroprotective effects of Rg1 against 6-OHDA toxicity are mediated, at least partly, through the activation of the IGF-IR signalling pathway. Unlike other well-characterized phytoestrogens such as genistein, the high potency of Rg1 in inducing ERE-luciferase activity (EC50 is 0.05 pM) without binding to ERα (Chan et al., 2002); Chen et al. (2006) suggests that it might be exert oestrogen-like activity via a unique signalling pathway. We had established the relationship between Rg1 and IGF-IR in human breast cancer MCF-7 cells in our previous study (Chen et al., 2006; Lau et al., 2008). Ginsenoside Rg1 can enhance IGF-IR-mediated signalling pathways by increasing tyrosine phosphorylation of IRS-1 and MEK, which in turn activate the phosphorylation of the Ser-118 residue in ERα, in MCF-7 cells. As direct interaction of ginsenosides with specific membrane proteins at the cell surface was previously reported by others (Kimura et al., 1994), the enhancement by Rg1 of the IGF-IR system suggests that Rg1 may mimic growth factors to activate the IGF-IR signalling pathway at the cell surface level. The IGF-IR, a member of the growth factor tyrosine kinase receptor family, plays an important role in regulating neural functions, including the promotion of neuronal differentiation, survival and neuroprotection (Duenas et al., 1996; Garcia-Segura et al., 2000; Cardona-Gomez et al., 2002). There is accumulating evidence for an abundant expression of IGF-IR in neurons and glia in the nigrostriatal system (Quesada et al., 2007). Oestrogen interacts with the IGF-I system to protect nigrostriatal dopamine and maintain motor behaviour in the 6-OHDA model of nigrostriatal injury (Quesada and Micevych, 2004). Blockade of IGF-IRs by intracerebroventricular JB-1 (Quesada and Micevych, 2004) or of the PI3K/Akt pathway by LY294002 attenuated the neuroprotective effects of oestrogen (Quesada et al., 2008). These results suggest that IGF-IR-induced intracellular signalling pathways are involved in the oestrogen mechanism that promotes neuronal survival. It is intriguing to determine the role of the IGF-IR signalling pathways in mediating the neuroprotective effects of Rg1 on dopaminergic neurons. In the present study, the administration of the peptide JB-1, a specific IGF-IR antagonist, in the lateral cerebral ventricle, resulted in the suppression of all the Rg1-mediated neuroprotective effects in the rat model of PD induced by 6-OHDA, indicating that the neuroprotective effects of Rg1 on dopaminergic neurons in SNpc is dependent on IGF-IR. The above results once again confirm that the biological effects of Rg1 are related to the IGF-IR signalling pathway.
The discovery that the IGF-IR is involved in the neuroprotective action of ginsenoside Rg1 against 6-OHDA-induced neurotoxicity on dopaminergic neurons provides new insights for understanding the pharmacological actions of ginseng. Thus, it is highly likely that the protective effect of Rg1 in the SN is mediated through the activation of the downstream signalling molecules of the IGF-IR signalling pathway, such as PI3K and/or MAPK pathway. The PI3K pathway is known to protect cells from several apoptotic stimuli (D'Astous et al., 2006). The MAPK cascade is essential for cell growth and plays a crucial role in apoptosis inhibition (Yoon and Seger, 2006). Further study will be needed to determine the role of the PI3K and the MAPK pathways in mediating the neuroprotective actions of ginsenoside Rg1 on SNpc dopaminergic neurons in 6-OHDA-lesioned rats.
In conclusion, we have provided evidence that the phytoestrogen, ginsenoside Rg1, has neuroprotective effects on dopaminergic neurons against 6-OHDA-induced neuronal damage in the nigrostriatal system and its actions might involve an activation of the IGF-IR signalling pathway. This newly discovered mechanism of action of Rg1 provides new insights into the pharmacological effects of ginsenoside Rg1 in the CNS
Acknowledgments
This work was supported by the National Natural Science Foundation of China (30570573), the Education Department Foundation of Shandong Province (J05L01) and Natural Science Foundation of Shandong Province (Y2007D49).
Glossary
Abbreviations:
- 6-OHDA
6-hydroxydopamine
- Akt
protein kinase B
- Bcl-2-IR
Bcl-2-immunoreactivity
- DAT
dopamine transporter
- DAT-IR
DAT-immunoreactivity
- E2
17β-oestradiol
- ER
oestrogen receptor
- IGF-I
insulin-like growth factor-I
- IGF-IR
insulin-like growth factor-I receptor
- IRS-1
insulin receptor substrate-1
- MAPK
mitogen-activated protein kinase
- MEK
mitogen-activated protein kinase kinase
- MFB
medial forebrain bundle
- MPP+
1-methyl-4-phenylpyridinium
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- PD
Parkinson's disease
- PI3K
phosphatidylinositol-3-kinase
- SNpc
substantia nigra pars compacta
- TH
tyrosine hydroxylase
- TH-IR
TH-immunoreactive
Conflict of interest
None.
References
- Figure 8.Abraham IM, Todman MG, Korach KS, Herbison AE. Critical in vivo roles for classical estrogen receptors in rapid estrogen actions on intracellular signaling in mouse brain. Endocrinology. 2004;145:3055–3061. doi: 10.1210/en.2003-1676. [DOI] [PubMed] [Google Scholar]
- Bondy CA, Cheng CM. Signaling by insulin-like growth factor 1 in brain. Eur J Pharmacol. 2004;490:25–31. doi: 10.1016/j.ejphar.2004.02.042. [DOI] [PubMed] [Google Scholar]
- Callier S, Morissette M, Grandbois M, Pelaprat D, Di Paolo T. Neuroprotective properties of 17beta-estradiol, progesterone, and raloxifene in MPTP C57Bl/6 mice. Synapse. 2001;41:131–138. doi: 10.1002/syn.1067. [DOI] [PubMed] [Google Scholar]
- Cardona-Gomez GP, Mendez P, DonCarlos LL, Azcoitia I, Garcia-Segura LM. Interactions of estrogen and insulin-like growth factor-I in the brain: molecular mechanisms and functional implications. J Steroid Biochem Mol Biol. 2002;83:211–217. doi: 10.1016/s0960-0760(02)00261-3. [DOI] [PubMed] [Google Scholar]
- Cerruti C, Pilotte NS, Uhl G, Kuhar MJ. Reduction in dopamine transporter mRNA after cessation of repeated cocaine administration. Brain Res Mol Brain Res. 1994;22:132–138. doi: 10.1016/0169-328x(94)90040-x. [DOI] [PubMed] [Google Scholar]
- Chan RY, Chen WF, Dong A, Guo D, Wong MS. Estrogen-like activity of ginsenoside Rg1 derived from Panax notoginseng. J Clin Endocrinol Metab. 2002;87:3691–3695. doi: 10.1210/jcem.87.8.8717. [DOI] [PubMed] [Google Scholar]
- Chen WF, Lau WS, Cheung PY, Guo DA, Wong MS. Activation of insulin-like growth factor I receptor-mediated pathway by ginsenoside Rg1. Br J Pharmacol. 2006;147:542–551. doi: 10.1038/sj.bjp.0706640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XC, Chen Y, Zhu YG, Fang F, Chen LM. Protective effect of ginsenoside Rg1 against MPTP-induced apoptosis in mouse substantia nigra neurons. Acta Pharmacol Sin. 2002;23:829–834. [PubMed] [Google Scholar]
- Chen XC, Fang F, Zhu YG, Chen LM, Zhou YC, Chen Y. Protective effect of ginsenoside Rg1 on MPP+-induced apoptosis in SHSY5Y cells. J Neural Transm. 2003;110:835–845. doi: 10.1007/s00702-003-0005-y. [DOI] [PubMed] [Google Scholar]
- Chen XC, Zhou YC, Chen Y, Zhu YG, Fang F, Chen LM. Ginsenoside Rg1 reduces MPTP-induced substantia nigra neuron loss by suppressing oxidative stress. Acta Pharmacol Sin. 2005;26:56–62. doi: 10.1111/j.1745-7254.2005.00019.x. [DOI] [PubMed] [Google Scholar]
- D'Astous M, Mendez P, Morissette M, Garcia-Segura LM, Di Paolo T. Implication of the phosphatidylinositol-3 kinase/protein kinase B signaling pathway in the neuroprotective effect of estradiol in the striatum of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mice. Mol Pharmacol. 2006;69:1492–1498. doi: 10.1124/mol.105.018671. [DOI] [PubMed] [Google Scholar]
- Datla KP, Murray HE, Pillai AV, Gillies GE, Dexter DT. Differences in dopaminergic neuroprotective effects of estrogen during estrous cycle. Neuroreport. 2003;14:47–50. doi: 10.1097/00001756-200301200-00009. [DOI] [PubMed] [Google Scholar]
- Diamond SG, Markham CH, Hoehn MM, McDowell FH, Muenter MD. An examination of male–female differences in progression and mortality of Parkinson's disease. Neurology. 1990;40:763–766. doi: 10.1212/wnl.40.5.763. [DOI] [PubMed] [Google Scholar]
- Duenas M, Torres-Aleman I, Naftolin F, Garcia-Segura LM. Interaction of insulin-like growth factor-I and estradiol signaling pathways on hypothalamic neuronal differentiation. Neuroscience. 1996;74:531–539. doi: 10.1016/0306-4522(96)00142-x. [DOI] [PubMed] [Google Scholar]
- Earl CD, Reum T, Xie JX, Sautter J, Kupsch A, Oertel WH. Foetal nigral cell suspension grafts influence dopamine release in the non-grafted side in the 6-hydroxydopamine rat model of Parkinson's disease: in vivo voltammetric data. Exp Brain Res. 1996;109:179–184. doi: 10.1007/BF00228642. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Cardona-Gomez GP, Chowen JA, Azcoitia I. Insulin-like growth factor-I receptors and estrogen receptors interact in the promotion of neuronal survival and neuroprotection. J Neurocytol. 2000;29:425–437. doi: 10.1023/a:1007125626308. [DOI] [PubMed] [Google Scholar]
- Guan J, Krishnamurthi R, Waldvogel HJ, Faull RL, Clark R, Gluckman P. N-terminal tripeptide of IGF-1 (GPE) prevents the loss of TH positive neurons after 6-OHDA induced nigral lesion in rats. Brain Res. 2000;859:286–292. doi: 10.1016/s0006-8993(00)01988-0. [DOI] [PubMed] [Google Scholar]
- Jiang H, Luan Z, Wang J, Xie J. Neuroprotective effects of iron chelator Desferal on dopaminergic neurons in the substantia nigra of rats with iron-overload. Neurochem Int. 2006;49:605–609. doi: 10.1016/j.neuint.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Kar S, Seto D, Dore S, Hanisch U, Quirion R. Insulin-like growth factors-I and -II differentially regulate endogenous acetylcholine release from the rat hippocampal formation. Proc Natl Acad Sci USA. 1997;94:14054–41059. doi: 10.1073/pnas.94.25.14054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T, Saunders PA, Kim HS, Rheu HM, Oh KW, Ho IK. Interactions of ginsenosides with ligand-bindings of GABA(A) and GABA(B) receptors. Gen Pharmacol. 1994;25:193–199. doi: 10.1016/0306-3623(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Krishnamurthi R, Stott S, Maingay M, Faull RL, McCarthy D, Gluckman P, et al. N-terminal tripeptide of IGF-1 improves functional deficits after 6-OHDA lesion in rats. Neuroreport. 2004;15:1601–1604. doi: 10.1097/01.wnr.0000127461.15985.07. [DOI] [PubMed] [Google Scholar]
- Lau WS, Chan RY, Guo DA, Wong MS. Ginsenoside Rg1 exerts estrogen-like activities via ligand-independent activation of ERalpha pathway. J Steroid Biochem Mol Biol. 2008;108:64–71. doi: 10.1016/j.jsbmb.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Laviola L, Natalicchio A, Giorgino F. The IGF-I signaling pathway. Curr Pharm Des. 2007;13:663–669. doi: 10.2174/138161207780249146. [DOI] [PubMed] [Google Scholar]
- Leung KW, Yung KK, Mak NK, Chan YS, Fan TP, Wong RN. Neuroprotective effects of ginsenoside-Rg1 in primary nigral neurons against rotenone toxicity. Neuropharmacology. 2007;52:827–835. doi: 10.1016/j.neuropharm.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Liu CX, Xiao PG. Recent advances on ginseng research in China. J Ethnopharmacol. 1992;36:27–38. doi: 10.1016/0378-8741(92)90057-x. [DOI] [PubMed] [Google Scholar]
- Mayeux R, Marder K, Cote LJ, Denaro J, Hemenegildo N, Mejia H, et al. The frequency of idiopathic Parkinson's disease by age, ethnic group, and sex in northern Manhattan, 1988–1993. Am J Epidemiol. 1995;142:820–827. doi: 10.1093/oxfordjournals.aje.a117721. [DOI] [PubMed] [Google Scholar]
- Murray HE, Pillai AV, McArthur SR, Razvi N, Datla KP, Dexter DT, et al. Dose- and sex-dependent effects of the neurotoxin 6-hydroxydopamine on the nigrostriatal dopaminergic pathway of adult rats: differential actions of estrogen in males and females. Neuroscience. 2003;116:213–222. doi: 10.1016/s0306-4522(02)00578-x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C, Pennisi M, Topple A. Bregma, lambda and the interaural midpoint in stereotaxic surgery with rats of different sex, strain and weight. J Neurosci Methods. 1985;13:139–143. doi: 10.1016/0165-0270(85)90026-3. [DOI] [PubMed] [Google Scholar]
- Pugazhenthi S, Nesterova A, Sable C, Heidenreich KA, Boxer LM, Heasley LE, et al. Akt/protein kinase B up-regulates Bcl-2 expression through cAMP-response element-binding protein. J Biol Chem. 2000;275:10761–10766. doi: 10.1074/jbc.275.15.10761. [DOI] [PubMed] [Google Scholar]
- Quesada A, Micevych PE. Estrogen interacts with the IGF-1 system to protect nigrostriatal dopamine and maintain motoric behavior after 6-hydroxdopamine lesions. J Neurosci Res. 2004;75:107–116. doi: 10.1002/jnr.10833. [DOI] [PubMed] [Google Scholar]
- Quesada A, Romeo HE, Micevych P. Distribution and localization patterns of estrogen receptor-beta and insulin-like growth factor-1 receptors in neurons and glial cells of the female rat substantia nigra: localization of ERbeta and IGF-IR in substantia nigra. J Comp Neurol. 2007;503:198–208. doi: 10.1002/cne.21358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada A, Lee BY, Micevych PE. PI3 kinase/Akt activation mediates estrogen and IGF-1 nigral DA neuronal neuroprotection against a unilateral rat model of Parkinson's disease. Dev Neurobiol. 2008;68:632–644. doi: 10.1002/dneu.20609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radad K, Gille G, Moldzio R, Saito H, Rausch WD. Ginsenosides Rb1 and Rg1 effects on mesencephalic dopaminergic cells stressed with glutamate. Brain Res. 2004a;1021:41–53. doi: 10.1016/j.brainres.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Radad K, Gille G, Moldzio R, Saito H, Ishige K, Rausch WD. Ginsenosides Rb1 and Rg1 effects on survival and neurite growth of MPP+-affected mesencephalic dopaminergic cells. J Neural Transm. 2004b;111:37–45. doi: 10.1007/s00702-003-0063-1. [DOI] [PubMed] [Google Scholar]
- Ramirez AD, Liu X, Menniti FS. Repeated estradiol treatment prevents MPTP-induced dopamine depletion in male mice. Neuroendocrinology. 2003;77:223–231. doi: 10.1159/000070277. [DOI] [PubMed] [Google Scholar]
- Schwarting RK, Huston JP. The unilateral 6-hydroxydopamine lesion model in behavioral brain research. Analysis of functional deficits, recovery and treatments. Prog Neurobiol. 1996;50:275–331. doi: 10.1016/s0301-0082(96)00040-8. [DOI] [PubMed] [Google Scholar]
- Tohda C, Matsumoto N, Zou K, Meselhy MR, Komatsu K. Axonal and dendritic extension by protopanaxadiol-type saponins from ginseng drugs in SK-N-SH cells. Jpn J Pharmacol. 2002;90:254–262. doi: 10.1254/jjp.90.254. [DOI] [PubMed] [Google Scholar]
- Toledo-Aral JJ, Méndez-Ferrer S, Pardal R, López-Barneo J. Dopaminergic cells of the carotid body: physiological significance and possible therapeutic applications in Parkinson's disease. Brain Res Bull. 2002;57:847–853. doi: 10.1016/s0361-9230(01)00771-7. [DOI] [PubMed] [Google Scholar]
- Yoon S, Seger R. The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors. 2006;24:21–44. doi: 10.1080/02699050500284218. [DOI] [PubMed] [Google Scholar]


