SUMMARY
An effective immune response against infectious agents involves massive expansion of CD8+ T cells. Once the infection is cleared, the majority of these effector cells die through unknown mechanisms, leaving behind only memory precursor cells. How is expansion controlled to maximize pathogen clearance and minimize immunopathology? We found, following Listeria infection, plasma TGF-β levels increased concomitant with the expansion of effector CD8+ T cells. Blocking TGF-β signaling did not affect effector function of CD8+ T cells. However, TGF-β controlled effector cell number by lowering Bcl-2 levels and selectively promoting the apoptosis of short-lived effector cells. TGF-β-mediated apoptosis of this effector sub-population occurred during clonal expansion and contraction, while IL-15 promoted their survival only during contraction. We demonstrate that the number of effector CD8+ T cells is tightly controlled by multiple extrinsic signals throughout effector differentiation; this plasticity should be exploited during vaccine design and immunotherapy against tumors and autoimmune diseases.
Keywords: TGF-beta, IL-15, CD8+ effector, short-lived effector, apoptosis, survival, clonal expansion, Bcl-2
INTRODUCTION
A functional immune system mounts an effective response to foreign antigens, maintains homeostasis, and avoids autoimmunity. In the immune system, effector CD8+ T cells play a central role in the host response to viral infections and cancers, and are major contributors to immunological memory (Klebanoff et al., 2006; Williams and Bevan, 2007). In addition to designing better vaccines against infectious disease, a central goal for immunologists is to also explore the possibility of using vaccines against tumors and certain autoimmune diseases (Klebanoff et al., 2006; Waldmann, 2006). A very important aspect of designing such therapy is to first understand the combination of signals that promote activation, proliferation, differentiation, and survival of different subsets of effector T cells (Joshi and Kaech, 2008).
There are several stages that CD8+ T cells pass through once they have come in contact with their cognate antigen on the surface of an activated antigen presenting cell (APC). First, encounter with antigen induces naïve T cells to expand massively, a process called clonal expansion, and differentiate into effector T cells. These effector CD8+ T cells acquire lytic and cytotoxic activity by producing perforin and granzymes and by secreting inflammatory cytokines such as IFN-γ and TNFα (Kaech and Wherry, 2007; Williams and Bevan, 2007). The high proliferative capacity and cytotoxic activity of antigen-activated CD8+ T cells requires an effective control of their life span in order to maintain lymphocyte homeostasis and to avoid immunopathology. A contraction period then follows 1-2 weeks after the initial antigenic encounter, during which the majority of effector cells die rapidly by apoptosis (Hand and Kaech, 2008; Marrack and Kappler, 2004). A small proportion of effector cells survive and further differentiates into long-lived memory cells (Kaech and Wherry, 2007). Given the long lifespan of memory cells and their enhanced ability to provide protection against recurrent infections, increasing the quantity and quality of memory CD8+ T cells is crucial to improving the efficacy of most vaccines (Seder et al., 2008). Finally, another important focus in the vaccine field is to generate enhanced expansion of effector cells due to the direct relationship between the number of effector cells at the peak of expansion and the final number of memory cells that are formed (Hou et al., 1994; Murali-Krishna et al., 1998). However, very little is known about the signals that influence the pool size of the effector cells.
Extensive cellular differentiation occurs as newly activated T cells become potent effector T cells and ultimately memory T cells (Joshi and Kaech, 2008; Kaech and Wherry, 2007). Functional and phenotypic characterization of different subsets of effector cells may be more informative than the mere numbers of total cells (Seder et al., 2008). Thus, defining the subsets of T cells that will provide the most robust protective immunity for the specific pathogen or tumor of interest, and identifying the extrinsic and intrinsic signals that lead to the development of these subsets of T cells, is the current goal for immunotherapies (Klebanoff et al., 2006). Recent elegant work has described multiple subpopulations of effector CD8+ T cells that exist during clonal expansion in response to some viral and bacterial infections (Joshi et al., 2007; Sarkar et al., 2008). The subset of effector CD8+ T cells that survive and become memory cells are sometimes referred to as memory precursor effector cells (MPECs); whereas, the population that dies during contraction is referred to as short-lived effector cells (SLECs) (Joshi et al., 2007; Joshi and Kaech, 2008). Both of these populations have been shown to have very similar functional ability at the peak of the immune response; however, they greatly differ in their memory potential and survivability (Joshi et al., 2007; Sarkar et al., 2008). A major challenge that remains is to identify extrinsic and intrinsic signals that make SLECs susceptible to apoptosis and the signals that promote the survival and maintenance of the MPECs (Hand and Kaech, 2008).
The common gamma chain cytokines IL-2, IL-7, and IL-15 are critical regulators of activated T cell proliferation, survival, and memory T cell formation (Boyman et al., 2007; Ma et al., 2006; Malek, 2008). IL-2 is a secreted cytokine that binds pre-formed heterotrimeric receptors made up of the high affinity IL-2Rα (CD25), IL-2/15Rβ (CD122), and the common γ-chain (cγc) receptor on the surface of target cells (Malek, 2008). IL-2 levels peak early during infection, but decay as the infection is cleared, which mirrors the pattern of CD25 expression on antigen stimulated T cells (Malek, 2008). IL-2 plays an important role in the differentiation and early expansion of a functional effector population and is required for secondary expansion of memory CD8+ T cells, but does not seem to cause the contraction of effector T cells (Blattman et al., 2003; D’Souza and Lefrancois, 2003; Williams et al., 2006). IL-7 is also a secreted cytokine that binds to the dimeric IL-7Rα (CD127) and the cγc receptor on the surface of target cells (Ma et al., 2006). Effector T cells expressing high levels of the IL-7Rα during acute infections are considered memory precursor cells (Joshi and Kaech, 2008; Kaech and Wherry, 2007). However, transgenic over-expression of IL-7Rα had no effect on effector or memory T cell survival, demonstrating that the IL-7Rα expression does not instruct the formation of memory T cells by allowing them to better compete for IL-7 (Hand et al., 2007; Haring et al., 2008). Thus, the down-regulation of IL-7Rα appears to be an indicator of reduced memory CD8+ T cell potential, but is not the major cause of effector T cell death after acute infections such as LCMV and Listeria (Hand and Kaech, 2008). IL-15 is a membrane bound cytokine that is presented in trans on the surface of monocytes or dendritic cells by the IL-15Rα (Ma et al., 2006). Cells that express CD122 and the cγc receptor can respond to trans-presented IL-15 and transduce IL-15 signaling through this complex (Burkett et al., 2003; Dubois et al., 2002). It has been proposed that aside from its role in supporting long-term maintenance of memory cells, IL-15 can also regulate CD8+ T cell contraction (Kennedy et al., 2000; Rubinstein et al., 2008; Yajima et al., 2006). IL-15 is induced by inflammation and interferons, therefore it has been hypothesized that as infection clears, a reduction in IL-15 contributes to the contraction of the IL-7Rlo effector cells (Dubois et al., 2002; Waldmann, 2006; Zhang et al., 1998). More recent work indicates that in response to some acute infections, the survival of MPECs depends on both IL-7 and IL-15, whereas the survival of SLECs depends on IL-15 as these cells lack IL-7Rα (Joshi et al., 2007; Rubinstein et al., 2008; Yajima et al., 2006). In addition, exogenous administration of IL-2 or IL-15 greatly improves the survival of SLECs during contraction without inducing their proliferation (Rubinstein et al., 2008). In line with these observations, it is thought that SLECs die for reasons other than sole deprivation from these cytokines, although the extrinsic signals that further promote the apoptosis of SLECs have not yet been identified (Joshi and Kaech, 2008).
Transforming Growth Factor β (TGF-β) is a pleiotropic cytokine with potent regulatory activity (Li and Flavell, 2008a, b; Li et al., 2006b). In mammals, three members of the TGF-β family (TGF-β1, TGF-β2, and TGF-β3) have been identified, with TGF-β1 being the predominant form expressed in the immune system (Li et al., 2006b). Monomeric TGF-β binds to TGF-β receptor II (TGF-βRII), triggering the kinase activity of the cytoplasmic domain that in turn activates TGF-βRI. The activated receptor complex leads to nuclear translocation of Smad molecules, and transcription of target genes (Li et al., 2006b). To understand the role of TGF-β signaling in T cell biology, we developed a mouse model where the expression of a Dominant-Negative form of TGFβRII (DNR), lacking the kinase domain, inhibits TGF-β signaling in both CD4+ and CD8+ T cells (Gorelik and Flavell, 2000). T cells in this model expand at a higher rate, acquire an activated phenotype, produce effector cytokines, and the mice eventually succumb to autoimmunity (Gorelik and Flavell, 2000). Remarkably, these DNR mice also mount a strong antitumor response, associated with the expansion and enhanced activities of tumor-specific CTLs (Gorelik and Flavell, 2001). We have also generated T-cell specific TGF-βRII-deficient mice by crossing a CD4-Cre transgenic line with a strain of floxed TGF-βRII mice (Li et al 2006b). These mice have a more severe phenotype compared to DNR mice and develop a lethal inflammatory phenotype. Using these mouse models, the pivotal function of TGF-β under steady state conditions has been identified to maintain tolerance through the regulation of lymphocyte proliferation, differentiation, and survival (Gorelik and Flavell, 2000; Li and Flavell, 2008a, b). It is known that the regulatory activity of TGF-β is modulated based on the cell type, its differentiation state, expression of costimulatory molecules, and the presence of other cytokines (Li and Flavell, 2008a, b; Li et al., 2006b). However, the role of TGF-β in controlling CD8+ T cell activation, proliferation, differentiation, and survival has not been addressed under infectious conditions, where T cells must become activated and their basal homeostasis is temporarily interrupted.
To understand the role of TGF-β signaling during effector CD8+ T cell differentiation and function, we crossed OTI transgenic mice, expressing OVA257-264/Kb-specific TCR, to our TGFβ-DNR mice, and generated OVA-specific naïve CD8+ T cells that are blocked in TGF-β signaling (OTI-DNR). Recombinant Listeria monocytogenes expressing OVA (LM-OVA) was used to activate the OTI-DNR and wild type OTI T cells after adoptive co-transfer into naïve hosts. Unlike the circumstance when TGF-β signaling is blocked under steady state conditions (Li et al., 2006a; Marie et al., 2006), we show here that blockade of TGF-β signaling during effector T cell differentiation does not alter ex vivo cytokine production and in vivo CTL activity of CD8+ effector T cells. Surprisingly, we found that clonally expanding CD8+ T cells undergo massive apoptosis even before reaching the “contraction” phase. However, blockade of TGF-β signaling on effector CD8+ T cells caused a 3-fold increase in their total number, which was a result of reduced apoptosis of short-lived effector cells during clonal expansion. We further demonstrate that TGF-β provides an extrinsic signal to further down-regulate Bcl-2 levels in effector cells and selectively promotes the apoptosis of SLECs. Finally, we present evidence for a model in which TGF-β and IL-15 provide opposing extrinsic signals to control the number of SLECs during clonal expansion and contraction. These findings identify a unique role for TGF-β in controlling the number of clonally expanding effector CD8+ T cells under acute inflammatory conditions. The relevance and implications of these findings for vaccine design and potential immunotherapy against tumors and certain autoimmune diseases are further discussed.
RESULTS
Peak of TGF-β expression during Listeria infection corresponds to peak of effector CD8+ T cell clonal expansion
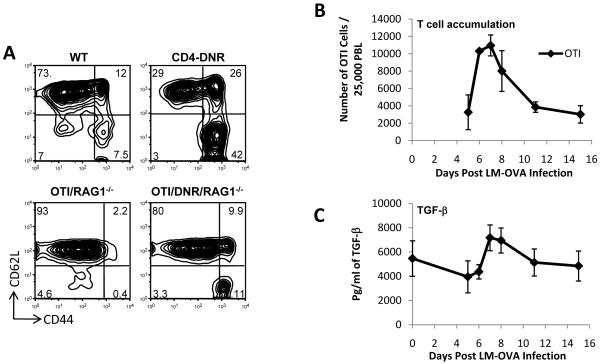
Over-expression of a dominant negative form of TGF-β receptor II specifically in T cells under the control of the CD4 promoter (CD4-DNR), lacking the CD8 silencer, results in the accumulation of CD44hi effector/memory T cells (Figure 1A, top) (Gorelik and Flavell, 2000). These observations, and numerous other studies, underline the importance of TGF-β in maintaining peripheral tolerance under steady state conditions (Li and Flavell, 2008a, b; Li et al., 2006b). However, the biological role of TGF-β during an acute infection is still poorly understood. To assess whether TGF-β plays a role in CD8+ T cell biology during an acute infection, we adoptively transferred 5×104 naïve OTI T cells into wild type mice and infected them 1 day later with LM-OVA. OTI T cell expansion was measured amongst the peripheral blood lymphocytes (Figure 1B), while total TGF-β levels were measured from blood plasma (Figure 1C). The observation that plasma TGF-β levels rise during an acute infection with similar kinetics to the accumulation of CD8+ T cells, prompted us to further investigate the role of TGF-β in effector T cell development and function.
Figure 1. TGF-β signaling may be important for controlling the number and function of effector CD8+ T cells.
(A) Splenocytes from 6 week old mice under steady state conditions were gated on CD8+ T cells. Data shown is representative of at least 10 different sets of mice with similar profiles.
(B) 5×104 naïve OTI/RAG1-/- cells were adoptively transferred into wild type B6 hosts and OTI cell expansion in response to LM-OVA infection was measured in a longitudinal experiment. Data is plotted as the fraction of OTI cells among 25,000 other peripheral blood lymphocytes (PBLs). Average and s.d. of 4 mice is shown.
(C) Total TGF-β levels detected with ELISA after acidification of plasma collected from infected mice from part (B).
Blockade of TGF-β signaling in CD8+ T cells results in enhanced accumulation of effector cells at the peak of clonal expansion
To obtain large numbers of antigen-specific naïve CD8+ T cells that express the TGF-βRII DNR, we crossed the CD4-DNR mice onto OTI transgenic mice and further crossed them onto a RAG1-/- and CD45.1 congenic marker (Figure 1A, bottom). Unlike the original CD4-DNR mice (Gorelik and Flavell, 2000) (Figure 1A, top), the OTI/DNR/RAG1-/- (OTI-DNR) mice do not develop autoimmunity, and large numbers of antigen specific naïve (CD62Lhi-CD44lo) T cells can be isolated from young mice (Figure 1A, bottom). To address the role of TGF-β signaling during CD8+ T cell differentiation and effector generation in response to an acute infection, we wanted to perform competitive adoptive co-transfer of OTI and OTI-DNR naïve cells into the same host followed by in vivo activation using LM-OVA. Further, we employed low numbers (around 25,000 CD8+ T cells of each type) to avoid the artifacts that can accompany transfers of too many cells (Marzo et al., 2005).
DNR-bearing T cells cannot be adoptively transferred into C57Bl/6 mice, because they are immunologically rejected since they contain human rather than mouse DNR sequence (Supplementary Figure 1, please also see the attached manuscript for more details). Instead, we used our CD11c-DNR mice as recipients of adoptive transfers (Laouar et al., 2005). These mice express the same human TGF-βRII DNR transgene in dendritic cells and natural killer cells under the control of the CD11c promoter, and therefore are immunologically tolerant to the human DNR sequence. These mice have been well characterized and no observable defect has been detected in any of the T cell compartments (Laouar et al., 2005). Nevertheless, to control for any untoward effects of the CD11c-DNR transgene on the data, all of the experiments involving adoptive transfers were performed side by side with transfers of wild type OTI cells into both CD11c-DNR transgene-positive and -negative mice. For the remainder of this study, the main figures contain the comparison of OTI and OTI-DNR cells transferred into the CD11c-DNR hosts (labeled as “host”), whereas the accompanying supplementary figures contain the same analysis of the OTI cells transferred into transgene-negative wild type littermates. It is noteworthy that no significant difference was observed in our studies between the results obtained when OTI cells were transferred into either host.
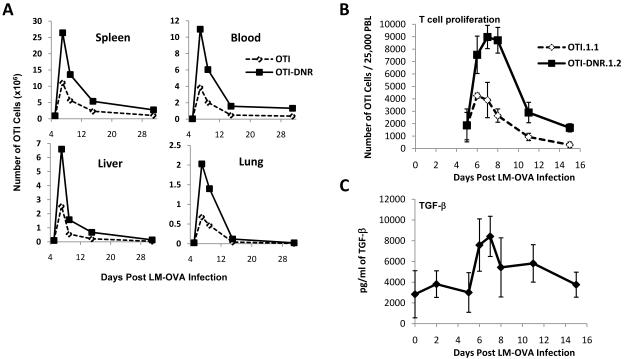
When OTI and OTI-DNR cells were co-transferred and activated with LM-OVA, a massive (3 fold) increase in the number of OTI-DNR effector cells was observed in blood and both lymphoid and non-lymphoid organs (Figure 2A-B). To assure this difference was not somehow related to a significant reduction in the amount of antigen present during priming in the CD11c-DNR mice, we measured bacterial burden 3 days post infection. We detected similar numbers of bacteria in the spleen and liver of wild type and CD11c-DNR mice that had no transgenic T cells or had been adoptively transferred with either 5×104 OTI or OTI-DNR cells (Supplementary Figure 2). When spleens were analyzed for the presence of transgenic T cells on day 3 post infection, no transgenic T cells could be detected among 1×106 live total lymphocytes that were analyzed (data not shown), emphasizing the physiological numbers of OTI and OTI-DNR naïve precursor cells in our adoptive transfer system.
Figure 2. A 3-fold increase in the number of OTI-DNR compared to OTI cells at the peak of CD8+ T cell clonal expansion in response to LM-OVA.
(A) 2.5×104 each of naïve OTI/RAG1-/- CD45.1.1 and OTI/DNR/RAG1-/-CD45.1.2 cells were adoptively co-transferred into naïve hosts, followed by infection with LM-OVA one day later. The absolute number of each OTI and OTI-DNR population from each organ is plotted over time. For the blood measurements, the number of OTI cells per 1 mL of blood is plotted. Data is representative of two independent experiments with similar results.
(B-C) Co-transfer and infection was performed as in part A, except the same group of mice were bled for a longitudinal experiment to compare the kinetics of OTI and OTI-DNR T cell accumulation among PBLs while measuring plasma TGF-β levels in the same infected mice. Average and s.d. is shown. Data is representative of two independent experiments with similar results and a total of 10-15 mice in each experiment.
Notably, the difference between OTI and OTI-DNR cell numbers became apparent only after day 5 post infection (p.i.) (Figure 2A). This also suggests that the difference in OTI-DNR accumulation is not related to a difference in priming and activation of the transgenic T cells. To further confirm that the impact of blocking TGF-β signaling occurs during clonal expansion and is not related to differential priming/activation of the T cells, we performed similar adoptive co-transfer experiments using TGFβRIIflox/flox or TGFβRII flx/+ OTI cells crossed to estrogen receptor cre (ER-Cre) transgenic mice. In this system, cre activation is induced by the addition of tamoxifen, allowing temporally controlled deletion of floxed genes (Hayashi and McMahon, 2002). Since non-toxic dose of tamoxifen treatment was not sufficient to delete TGFβRII in every cell, we further crossed these mice to a cre-reporter line where expression of the Yellow Fluorescent Protein (YFP) indicates the presence of nuclear cre and reports the deletion of all floxed DNA (Srinivas et al., 2001). Thus, in cells where tamoxifen treatment has lead to cre activation, both the floxed region of the TGFβRII and the STOP fragment upstream of the YFP gene will be deleted, generating yellow cells. Using this system, we induced the deletion of the receptor on day 4 p.i. (Supplementary Figure 3). When we compared the YFP+ fraction in the TGFβRIIflx/flx and TGFβRII+/flx cells, we observed the same 3 fold increase in the frequency of OTI/TGF-βRII-/- compared to OTI/TGF-βRII-/+ cells at the peak of clonal expansion as we saw when we compared OTI-DNR and OTI cells (Supplementary Figure 3B and Figure 2B). This comparison further validated our observations in the CD11c-DNR mice and confirmed a role for TGF-β in controlling the number of effector CD8+ T cells during the clonal expansion phase. However, due to the complexity of this system and the small fraction of T cells that could be induced by tamoxifen to delete the TGF-βRII, we continued our investigations using the OTI and OTI-DNR adoptive co-transfer into the CD11c-DNR mice, as described above.
Similar to infection in wild type hosts (Figure 1C), a 3-fold increase in plasma TGF-β levels was also detected in the CD11c-DNR recipients during the peak of T cell proliferation (Figure 2C). Remarkably, the time at which the difference in the expansion between OTI and OTI-DNR cells first becomes apparent (around day 6 post infection) directly correlated with the time at which plasma TGF-β levels also peaked during the course of infection, suggesting a direct effect of TGF-β on the number of effector CD8+ T cells. TGF-β levels dropped by the middle of the contraction phase and returned to baseline by two weeks post infection. Collectively, these data suggest that TGF-β does not play a major role during the early phases of CD8+ T cell priming and activation, but rather, that it plays a key role in determining the number of effector T cells at the peak of clonal expansion.
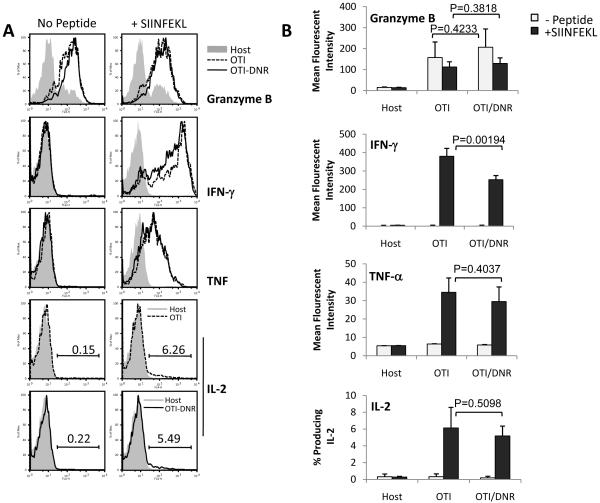
Blockade of TGF-β signaling does not alter ex vivo cytokine production or in vivo cytolytic activity of effector CD8+ T cells
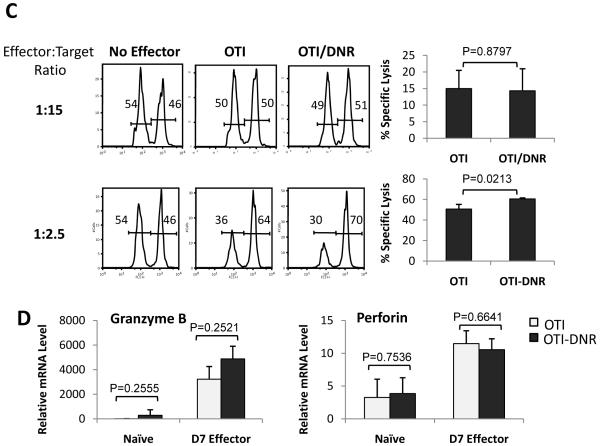
Many studies have suggested that under steady state conditions or in the presence of tumors, TGF-β inhibits cytokine production and CTL activity of CD8+ T cells (Ahmadzadeh and Rosenberg, 2005; Li et al., 2006a; Marie et al., 2006; Thomas and Massague, 2005; Trapani, 2005). To address whether TGF-β plays a similar role during effector T cell differentiation in response to an acute infection, we compared ex vivo cytokine production and in vivo CTL activity of effector OTI and OTI-DNR cells that were generated in the same host (Figure 3). No significant difference in granzyme B, TNF-α, or IL-2 production was observed between OTI and OTI-DNR day 7 effector T cells when re-stimulated ex vivo with SIINFEKL peptide (Figure 3A-B and Supplementary Figure 4A). OTI-DNR cells contained a larger population of the low-IFN-γ producing effector cells, which resulted in slightly lower average mean fluorescent intensity of the whole population (Figure 3A-B, compare histogram to bar graphs). To compare CTL activity of OTI and OTI-DNR effector T cells, day 7 effectors were isolated by cell sorting and the same number of each effector population was retransferred into naïve hosts along with different ratios of target cells, and specific lysis was measured in vivo (Figure 3C). Once again, no difference was observed in the cytolytic activity of OTI and OTI-DNR effector cells (Figure 3C and Supplementary Figure 4B). These findings were consistent with similar mRNA levels of both granzyme B and perforin in the OTI and OTI-DNR effector cells (Figure 3D). Collectively, these data suggest that TGF-β does not play a major role in inhibiting the effector function of CD8+ T cells under acute Listeria infection conditions.
Figure 3. Blockade of TGF-β signaling does not alter cytokine production and CTL activity of effector CD8+ T cells.
(A) Adoptive co-transfer and LM-OVA infection was performed as described in Fig. 2A. Intracellular cytokine staining of spleen cells isolated 7 days p.i. Host (CD45.2.2), OTI (CD45.1.1), and OTI-DNR (CD45.1.2) cells were identified based on staining with CD8 and the corresponding congenic markers. Splenocytes were stimulated with or without SIINFEKL peptide. 1 Representative histogram is shown.
(B) Average and s.d. of 4 different co-transfers. Data is representative of 3 independent experiments with similar results.
(C) In vivo CTL assay performed with effector OTI and OTI-DNR cells isolated from the spleen of infected mice 7 days p.i. Representative raw data is shown on the left. Summary of two independent experiments is shown on the right, with average and s.d. of 4 recipients of each population.
(D) Relative mRNA levels of cytolytic molecules from day 7 effector cells. Samples were first normalized to HPRT. Relative fold increase over naïve OTI cells is shown. Data is average and s.d. of 3 independent experiments.
TGF-β promotes the apoptosis of effector CD8+ T cells
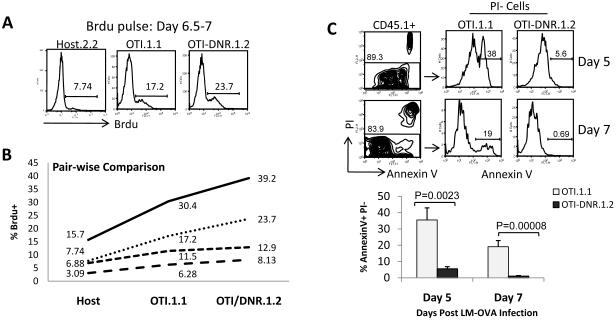
When TGF-β signaling is blocked in CD8+ effector T cells, a 3-fold higher number of effector T cells accumulated at the peak of clonal expansion in vivo (Figure 2). T cell accumulation is a result of the combination of T cell death and proliferation, and it has been suggested that TGF-β can inhibit T cell proliferation and induce apoptosis of activated T cells under a variety of in vitro conditions (Marrack and Kappler, 2004; Sillett et al., 2001; Wolfraim et al., 2004). Therefore, we asked whether blockade of TGF-β signaling in vivo would enhance the proliferative ability of effector T cells, promote their better survival, or both. The proliferation of OTI versus OTI-DNR cells was measured by the incorporation of BrdU near the peak of clonal expansion in adoptive co-transfer experiments (Figure 4A and Supplementary Figure 5A). Although the range in BrdU incorporation differed in each individual mouse, we observed a consistent increase in BrdU incorporation in OTI-DNR compared to OTI cells (Figure 4B). It is generally thought that T cells proliferate during clonal expansion, and die only during the contraction phase (Marrack and Kappler, 2004; Williams and Bevan, 2007). Contrary to this, we observed massive apoptosis of OTI cells on day 5 post infection (Figure 4C and Supplementary Figure 5B), at the time when T cells are still clonally expanding. However, the OTI-DNR effector cells, in the same host, were mostly protected from the apoptosis that occured during (day 5) and at the peak (day 7) of clonal expansion (Figure 4C). These data suggest that blockade of TGF-β signaling allows better proliferation of effector CD8+ T cells, but most importantly, it protects OTI-DNR cells from the massive apoptosis that occurs during clonal expansion.
Figure 4. TGF-β promotes apoptosis of effector CD8+ T cells during clonal expansion.
(A) Histograms showing BrdU incorporation of clonally expanding OTI and OTI-DNR cells from co-transfer experiments. Splenocytes were first gated on CD8+ T cells and host, OTI, and OTI-DNR subpopulations were separated based on congenic markers.
(B) Pair-wise comparison of each subpopulation in 4 different animals with varying degrees of BrdU incorporation. Data are representative of 3 independent experiments with similar results.
(C) Dead versus apoptotic cells from day 5 (PBL) and day 7 (spleen) of infected mice were identified by AnnexinV and PI staining. OTI and OTI-DNR cells were then separated based on congenic markers amongst the PI-population. Average and s.d. of 3 (day 5) and 4 (day 7) mice per group is shown.
Selective accumulation of SLECs at the peak of clonal expansion in the absence of TGF-β signaling
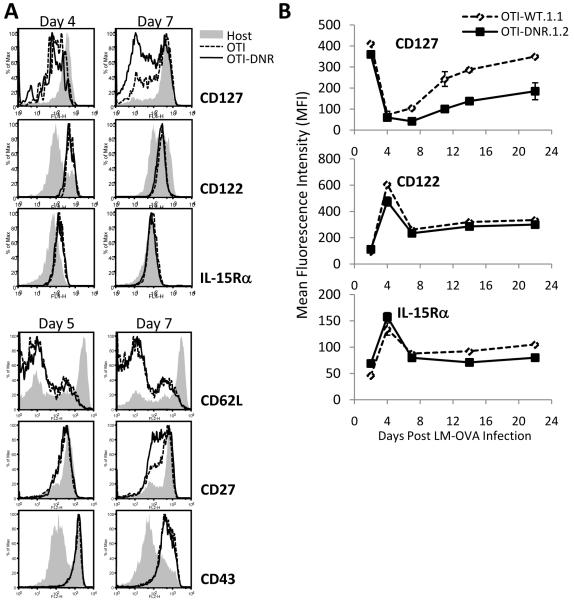
Survival of effector CD8+ T cells is highly dependent on many cell intrinsic and extrinsic factors including signaling from the receptors of the cγc cytokines IL-2, IL-7, and IL-15. As a result, significant changes occur in the surface expression of the receptors for these cytokines during effector CD8+ T cell differentiation (Boyman et al., 2007; Kaech and Wherry, 2007; Masopust et al., 2004; Williams and Bevan, 2007). Since we have shown previously that TGF-β can modulate the expression of CD122, IL-2/15Rβ, via regulating T-bet in CD4+ Th1 cells (Li Immunity 2006), we wanted to see whether the receptor expression for the cγc cytokines was altered on effector CD8+ OTI-DNR compared to OTI cells, resulting in their better survival. Similar to the expansion phenotype, we did not find any difference in surface expression of any of the molecules we analyzed on days 4 or 5 post infection (Figure 5A and Supplementary Figure 6A), including complete down-regulation of CD25 on both OTI and OTI-DNR cells by day 5 post infection (data not shown). However, a difference in the up-regulation of CD127, the IL-7Rα chain, was observed starting at day 7 post infection that also persisted into the early memory phase (Figure 5B). In contrast, no significant difference was detected in the expression of CD122 and IL-15Rα between OTI and OTI-DNR cells throughout effector T cell differentiation (Figure 5B). Similar to CD127, we also observed a difference in the up-regulation of CD27 starting at day 7 post infection, whereas the expression of CD62L and CD43 remained the same between the OTI and OTI-DNR cells (Figure 5A bottom, and Supplementary Figure 6A).
Figure 5. Selective apoptosis of SLECs by TGF-β.
(A) Phenotypic comparisons between OTI and OTI-DNR effector CD8+ T cells. Histograms comparing the expression of each surface molecule on host CD8+ (solid gray), OTI (dashed line), and OTI-DNR (black line) T cells from co-transfer experiments. Data are representative of more than 4 independent experiments with similar results.
(B) Expression pattern of indicated surface receptors was analyzed on OTI and OTI-DNR CD8+ T cells isolated from peripheral blood of LM-OVA infected mice at the indicated time points. Data is average and s.d. of 3-5 individual co-transfers and represents 1 out of 3 independent experiments with similar results.
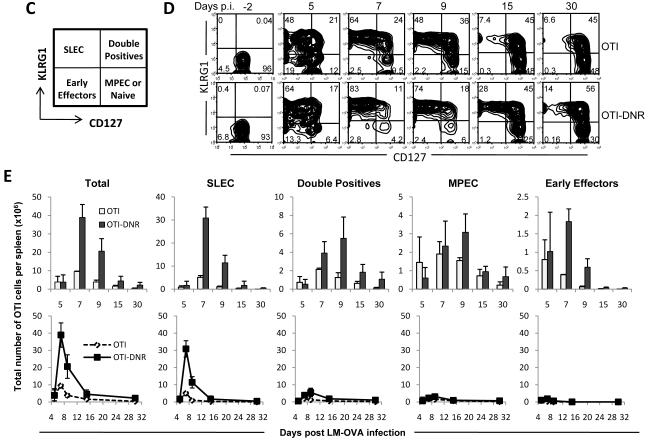
(C) Representative diagram of each effector sub-population separated based on CD127 and KLRG1 expression and the terminology used to describe each subset.
(D) Representation of the difference seen in the percentages of OTI and OTI-DNR SLECs and MPECs isolated from the spleen of infected mice at the indicated time points. Time point -2 days p.i. refers to naïve cells prior to adoptive transfer.
(E) Bar graphs (top) comparing the absolute number of total and each OTI and OTI-DNR sub-population from the spleen of infected mice at each indicated time point. Longitudinal graphs (bottom) comparing the contribution of each sub-population, on the same scale, to the overall increase in the number of OTI-DNR effector cells. Data is average and s.d. of 2-3 mice per time point and represents 1 out of 2 independent experiments with similar results.
It has recently been shown that CD127 and killer cell lectin-like receptor subfamily G1 (KLRG1) can be used to differentiate between two types or subsets of differentiating effector CD8+ T cells. Memory precursor effector cells (MPECs) are enriched in the KLRG1lo-CD127hi population, and short-lived effector cells (SLECs) are enriched in the KLRG1hi-CD127lo and CD27lo population (Joshi et al., 2007; Sarkar et al., 2008). Since we observed a larger fraction of CD127lo and CD27lo cells among the OTI-DNR cells at the peak of clonal expansion (Figure 5A-B), we hypothesized that blockade of TGF-β signaling may be altering the differentiation and/or the survival of MPECs and SLECs. To test this hypothesis, we determined the absolute numbers of each OTI and OTI-DNR effector sub-population, based on CD127 and KLRG1 expression, throughout effector differentiation (Figure 5C-E and Supplementary Figure 6B-C). OTI-DNR cells had a higher percentage of SLECs (KLRG1hi-CD127lo) and a lower percentage of MPECs (KLRG1lo-CD127hi) compared to OTI cells (Figure 5D). However, when these percentages were converted to absolute numbers for each sub-population in the spleen of infected mice, it became evident that the number of MPECs remained similar between OTI and OTI-DNR cells, whereas there were almost 6 times more OTI-DNR SLECs compared to OTI SLECs at the peak of clonal expansion (Figure 5E, top). The significant contribution of the OTI-DNR SLEC sub-population to the overall increase of the total number of OTI-DNR cells is most evident when the sub-populations are plotted on the same scale (Figure 5E, bottom). These data suggest that TGF-β signaling on effector CD8+ T cells selectively promotes the apoptosis of SLECs.
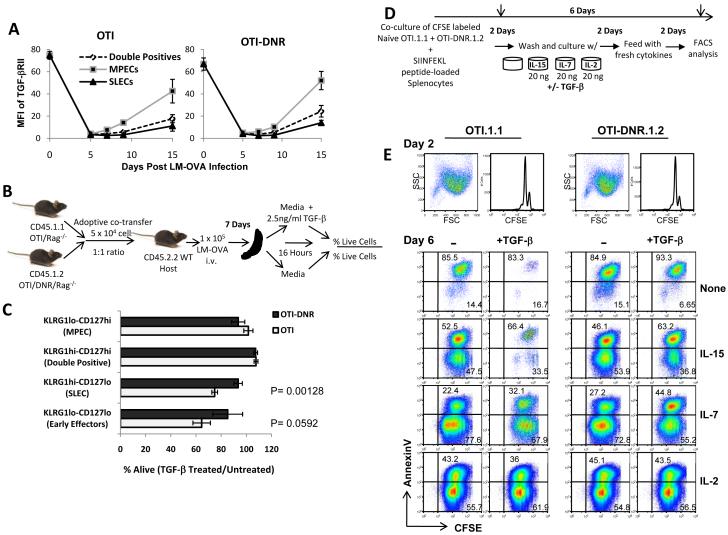
TGF-β promotes the apoptosis of SLECs ex vivo, despite low levels of TGF-βRII expression on their surface
We next wanted to explore the mechanism by which TGF-β exerts its apoptotic effects selectively on SLECs and not on MPECs. One obvious explanation for this selective mechanism may lie within differential expression of TGF-β receptor on the different subpopulations. Therefore, we measured and compared TGFβRII surface expression on naïve and effector sub-populations of OTI and OTI-DNR cells and found very high expression of TGF-βRII on naïve compared to effector T cells (Figure 6A and Supplementary Figure 7A). In fact, on day 5 post infection, the expression of TGF-βRII was almost indistinguishable from the isotype control. However, by about day 7 p.i., the effector cells began up-regulating the surface expression of TGF-βRII, and by day 15 post infection, a clear difference was observed between the level of TGF-βRII expression on the different sub-populations. The MPECs expressed the highest level followed by the Double Positive and SLEC subpopulations (Figure 6A and Supplementary Figure 7A). Importantly, the expression pattern of TGF-βRII did not provide a clear explanation for the selective apoptotic effect of TGF-β on SLECs during clonal expansion; however, it raised the question as to whether the SLECs are even capable of responding to TGF-β around day 7 post infection. To address this, we generated in vivo day 7 effector cells (in co-transfer experiments), recovered these cells and treated ex vivo half the recovered splenocytes with recombinant TGF-β, while the other half was just incubated in media without TGF-β for 16 hours (Figure 6B and Supplementary Figure 7B). Apoptotic effect of TGF-β on each effector sub-population was determined by measuring the percent of live cells in TGF-β treated compared to untreated cells. Interestingly, TGF-β had its greatest apoptotic impact on the OTI CD127lo sub-populations while it did not significantly impact the OTI CD127hi sub-populations or the OTI-DNR cells (Figure 6C and Supplementary Figure 7C). These data suggest that SLECs can indeed respond to TGF-β despite the low levels of TGF-βRII on their surface, and that TGF-β has the same selective apoptotic effect on the OTI SLEC sub-population ex vivo as it does in vivo.
Figure 6. TGF-β promotes the apoptosis of effector CD8+ T cells both ex vivo and in vitro.
(A) TGF-βRII expression on naïve and effector OTI and OTI-DNR sub-populations. After adoptive co-transfer and LM-OVA infection, OTI and OTI-DNR cells isolated from the spleen of infected mice were stained with CD127 and KLRG1 and either TGFβRII or the isotype control antibodies. MFI of TGF-βRII was determined by subtracting the MFI of background staining observed with isotype control, which varied from 3-6, from the MFI of TGF-βRII staining on the same sub-population of effector cells.
(B) Experimental details of ex vivo treatment of OTI and OTI-DNR effector cells with TGF-β.
(C) Upon experimental procedure described in part (B), OTI and OTI-DNR effector sub-populations were identified by the congenic marker and CD127/KLRG1 staining. The percent of live cells in each sub-population was determined from the forward and side scatter plots. The bar graph represents the fraction of live cells in TGF-β treated versus non-treated samples. Average and s.d. of 4 experimental wells is shown. Data is representative of 1 out of 2 independent experiments.
(D) Experimental details of in vitro treatment of OTI and OTI-DNR effector cells with TGF-β.
(E) Forward and side scatter plots and CFSE dilution of OTI and OTI-DNR cells after being stimulated for 48 hours with SIINFEKL-loaded APCs (top). Cells were washed and cultured in the absence or presence of 5ng/ml of TGF-β and 20ng/ml of IL-15, IL-7, or IL-2 for another 4 days. FACS plots represent total cell recovery and show all the events collected within 1 minute. AnnexinV+ (dead or apoptotic) and AnnexinV- (live) and the CFSE dilution of each fraction is shown. Data is representative of 1 out of 3 independent experiments with similar results.
TGF-β antagonizes the survival effects of IL-15 on clonally expanding T cells in vitro
As inflammation subsides around the peak of clonal expansion and both IL-2 levels and CD25 expression on effector T cells becomes limiting, the MPECs continue to receive survival signals through both IL-7 and IL-15, whereas the survival of SLECs becomes mostly dependent on IL-15, since this population lacks the expression of IL-7Rα (D’Cruz et al., 2009; Hand et al., 2007; Joshi et al., 2007). Therefore, we hypothesized that OTI-DNR SLECs survive better than OTI SLECs, because they can respond better to IL-15, implying that TGF-β may either somehow modulate or override the survival signals provided by IL-15. To test this hypothesis, we wanted to compare the combinatorial effect of TGF-β and various different cγc cytokines on the survival of effector CD8+ T cells; however, treatment of in vivo generated effector cells with these cytokines results in modulation of the perspective receptors (i.e. IL-7 down-regulates IL-7Rα) compromising the analysis on the different sub-populations based on CD127 and KLRG1 expression. Instead, we set up an in vitro culture assay system to specifically address the extrinsic effects of TGF-β alone and in combination with the various cγc cytokines on clonally expanding CD8+ T cells (Figure 6D). Naïve CFSE labeled OTI and OTI-DNR cells were first co-cultured with SIINFEKL peptide-loaded antigen presenting cells for 48 hours, at which point OTI and OTI-DNR cells showed similar growth and cell division (Figure 6E, top). The cells were washed and re-cultured with either TGF-β alone or with TGF-β in combination with IL-15, IL-7, or IL-2 for another 4 days (Figure 6E, bottom). When clonally expanding effector cells were cultured in the presence of TGF-β, most of the cells in the culture underwent apoptosis, and only about 40% of the cells were recovered compared to the No TGF-β control (Figure 6A, row labeled None). As expected, addition of the cγc cytokines greatly enhanced the recovery of all the cells, and the inclusion of TGF-β with the OTI-DNR cells only had a modest effect on all conditions (Figure 6E, right panel). Most notably, inclusion of TGF-β with wild type OTI cells that were cultured in IL-15, compared to those that were cultured in IL-15 alone, resulted in a similar low magnitude of cell recovery (about 40%) as OTI cells cultured in TGF-β alone compared to no TGF-β. However, when cells were cultured in the presence of TGF-β and IL-7, or TGF-β and IL-2, about 80% and 100% of the cells, respectively, could still be recovered compared to the controls (Figure 6E). Thus, TGF-β has a potent apoptotic effect on clonally expanding T cells; however, the presence of IL-2 and IL-7 can fully and partially mask the effects of TGF-β, respectively. Remarkably, under similar conditions, IL-15 is not able to overcome the apoptotic effects of TGF-β. Thus, the magnitude of cell death was similar when cells were cultured with TGF-β alone or with the combination of IL-15 and TGF-β, whereas IL-7 was able to protect the clonally expanding T cells from the apoptotic effects of TGF-β Thus, the magnitude of cell death was similar when cells were cultured with TGF-β alone or with the combination of IL-15 and TGF-β, whereas IL-7 was able to protect the clonally expanding T cells from the apoptotic effects of TGF-β. These observations provide evidence for our hypothesis that the mechanism by which TGF-β selectively promotes apoptosis of SLECs is because these cells respond to IL-15 and not IL-7, whereas MPECs can respond to both IL-15 and IL-7. (Also see DISCUSSION).
TGF-β and IL-15 exert opposing effects on SLECs during an immune response to Listeria infection
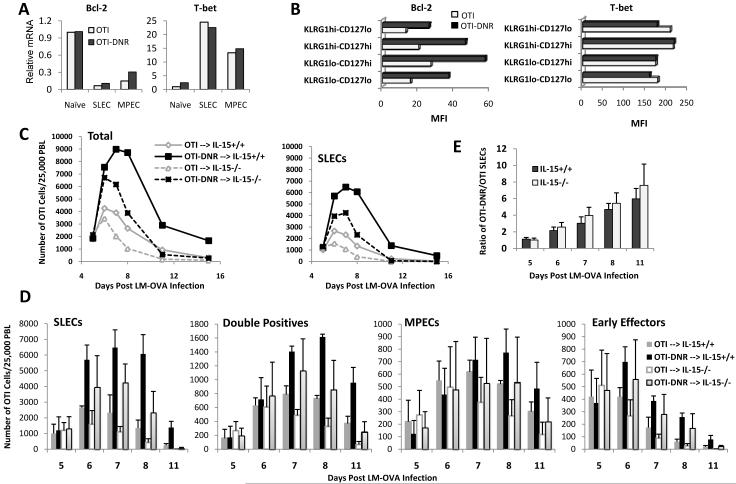
The cγc cytokines IL-2, IL-7, and IL-15 mediate life and death signals by activating the Jak/Stat and PI3K survival pathways. One of the most important outcomes of these signaling pathways is regulation of factors such as Bcl-2 and Bim, which determine survival or death of effector cells (D’Cruz et al., 2009). The observation that TGF-β can override survival signals provided by IL-15 but not those provided by IL-7 or IL-2 (Figure 6E) prompted us to determine whether TGF-β acted on IL-15 signaling, or whether it promoted the apoptosis of SLECs independent of this cytokine. To begin addressing this, we first asked whether OTI-DNR cells biologically respond better to IL-15. In the absence of exogenous TGF-β, both OTI and OTI-DNR cells responded similarly to a titration of doses of IL-15 (Supplementary Figure 8), suggesting that blockade of TGF-β signaling does not change the sensitivity of their responsiveness to IL-15. In support of this, we also did not see a difference in the kinetics of phosphorylation and dephosphorylation of Stat5 upon stimulation with different concentrations of IL-15 and in the presence and absence of TGF-β (Supplementary Figure 9 and data not shown). Subsequently, we also measured the extent of Akt phosphorylation in response to IL-15 in OTI and OTI-DNR cells in the presence or absence of TGF-β, and again did not observe any difference between the two cell types (Data not shown). We concluded from these studies that TGF-β most likely was not overriding IL-15 signaling by inhibiting or dampening the downstream signaling pathways. Finally, we asked whether TGF-β had an impact on the final outcome of these signaling events, namely the expression of Bcl-2 and Bim, whose balance determines the life and death of short-lived effector T cells (Hildeman et al., 2007; Marrack and Kappler, 2004; Wojciechowski et al., 2006). To begin, we isolated mRNA from naïve and day 7 effector OTI and OTI-DNR SLECs and compared the level of expression of several genes involved in function, differentiation, and survival of effector CD8+ T cells (Supplementary Figure 10). This analysis further validated the absence of a role for TGF-β in regulating the expression of genes involved in effector function and differentiation of CD8+ T cells under these inflammatory conditions. However, we consistently found a two fold increase in the expression of Bcl-2 in OTI-DNR compared to OTI effector cells, whereas other molecules such as T-bet, remained comparable at both RNA and protein level (Figure 7A-B and Supplementary Figure 11A). This data suggested that TGF-β is negatively influencing the expression of Bcl-2 in effector T cells. Since signaling by the cγc cytokines is the major inducers of Bcl-2 in effector CD8+ T cells, we wanted to better understand the intertwined relationship between the survival of SLECs, IL-15, TGF-β, and Bcl-2 levels.
Figure 7. TGF-β and IL-15 control the number of SLECs by exerting opposing effects on Bcl-2 levels in vivo.
(A) Quantitative RT PCR comparing mRNA levels of Bcl-2 and T-bet in sorted naïve (prior to adoptive transfer) and effector (day 7 p.i.) OTI and OTI-DNR SLECs and MPECs. Samples were first normalized to HPRT. Fold change from naïve OTI cells is shown. Data is representative of 1 out of 3 independent experiments with similar results.
(B) Intracellular staining of Bcl-2 and T-bet protein in day 7 effector OTI and OTI-DNR sub-populations from a co-transfer experiment. Data is representative of 1 out of 3 independent experiments with similar results.
(C) 2.5×104 each OTI and OTI-DNR cells were co-transferred into either IL-15+/+ or IL-15-/- hosts followed by LM-OVA infection. Fraction of total (left) and SLEC sub-population (right) of OTI and OTI-DNR cells were determined among total PBLs and converted to numbers of each cell type per 25,000 PBL.
(D) Bar graphs comparing the calculated number of each OTI and OTI-DNR sub-population among 25,000 PBLs in IL-15+/+ and IL-15-/- hosts. Average and s.d. of 3 co-transfers into IL-15+/+ and 7 co-transfers into IL-15-/- mice is shown.
(E) The average ratio of OTI-DNR/OTI SLECs in IL-15+/+ versus IL-15-/- hosts at each indicated time point post LM-OVA infection.
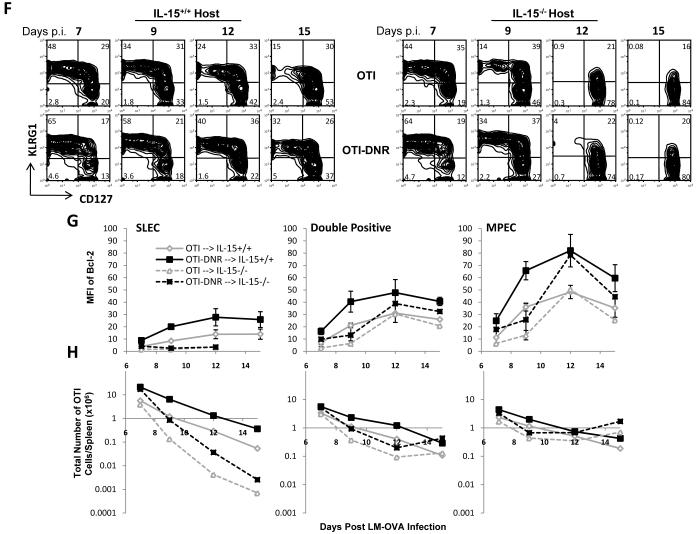
(F) Representation of the difference seen in the percentage of each OTI and OTI-DNR sub-populations from the spleens of infected IL-15+/+ and IL-15-/- hosts at the indicated time points.
(G) Comparison of intracellular Bcl-2 protein levels in each sub-population of OTI and OTI-DNR cells isolated from the spleen of IL-15+/+ or IL-15-/- hosts.
(H) Absolute number of each OTI and OTI-DNR sub-population from the spleens of IL-15+/+ and IL-15-/- hosts. Data is average and s.d. of 2-3 co-transfers for each time point.
To better understand the in vivo dependence of TGF-β on IL-15 for promoting apoptosis of SLECs, we began by performing longitudinal co-transfer experiments comparing the ratio of OTI and OTI-DNR effector cell sub-populations among total PBLs in IL-15+/+ and IL-15-/- hosts (Figure 7C-E and Supplementary Figure 11B-C). Up to day 5 post infection, no significant difference was observed between the ratios of all OTI and OTI-DNR effector cell sub-populations in both IL-15+/+ and IL-15-/- hosts, suggesting that proliferation and survival of early effector CD8+ T cells in response to Listeria infection is independent of both TGF-β and IL-15. This is consistent with a previous report where, using a similar OTI/LM-OVA infection system, the same numbers of CD127hi and CD127lo OTI cells were found in both IL-15+/+ and IL-15-/- hosts at the peak of the immune response (Yajima et al., 2006).
Starting at around day 6 post infection, the apoptotic effect of TGF-β was observed in both IL-15+/+ and IL-15-/- hosts, suggesting that TGF-β can promote apoptosis of clonally expanding T cells independent of IL-15 signaling. In fact, the magnitude of the difference between OTI and OTI-DNR SLECs remained the same in both IL-15+/+ and IL-15-/- hosts up until day 11 post infection (Figure 7E). However, the absence of IL-15 had a dramatic effect on the total number of SLECs starting at the peak of clonal expansion and into the contraction phase (around day 7 p.i.) (Figure 7D and 7H and Supplenentary Figure 11C and 11F). In our OTI/LM-OVA system, similar to previous reports, the clonal expansion of effector cells was not altered by the absence of IL-15; however, IL-15 was essential for the survival of effector cells, in particular SLECs, during the contraction phase (D’Cruz et al., 2009; Yajima et al., 2006). Together, these data suggest that in response to Listeria infection, TGF-β promotes the apoptosis of SLECs during CD8+ T cell clonal expansion independently of IL-15 signaling; however, the eventual survival of the remaining SLECs during the contraction phase is dependent on the combination of pro-survival and pro-apoptotic effects of IL-15 and TGF-β, respectively.
TGF-β dampens Bcl-2 expression induced by IL-15 in SLECs
To address the mechanism by which TGF-β selectively promotes the apoptosis of SLECs during the contraction phase, we further explored the combinatorial effect of IL-15 and TGF-β on Bcl-2 levels. We performed adoptive co-transfer experiments in IL-15+/+ and IL-15-/- hosts, and measured intracellular Bcl-2 levels in each sub-population of OTI and OTI-DNR effector cells, focusing on the peak of clonal expansion and contraction, between days 7-15 p.i. (Figure 7F-H and Supplementary Figure 11D-F). Once again, the independence of SLECs on IL-15 up until day 7 p.i. and the dependence of SLECs on IL-15 during the contraction phase is depicted in the similarity between cell numbers in IL-15+/+ and IL-15-/- mice at day 7 p.i. and the dramatic decline of both OTI and OTI-DNR SLECs in IL-15-/- hosts by day 12 p.i., respectively (Figure 7F and 7H and Supplementary Figure 11D and 11F). The rapid contraction of both OTI and OTI-DNR SLECs by day 12 p.i. in IL-15-/- hosts correlated with an absence of Bcl-2 expression in this sub-population in both cell types (Figure 7G and Supplementary Figure 11E). In the presence of IL-15, the Bcl-2 levels continued to slowly rise in SLECs, demonstrating the dependence of this sub-population on IL-15 for the induction of Bcl-2. Importantly, OTI-DNR cells continued to have twice as much Bcl-2 at all time points in the IL-15+/+ hosts, which also correlated with better survival of OTI-DNR SLECs during the contraction phase (Figure 7G-H). Interestingly, IL-15 also significantly contributed to the total Bcl-2 levels in CD127hi sub-populations (Double positives and MPECs) between days 7-12 of the contraction phase. Bcl-2 levels were significantly lower in the CD127hi populations in the IL-15-/- hosts, resulting in lower overall survival of these populations during early contraction (Figure 7F-H and Supplementary Figure 11D-F). However, at later times in contraction between days 12-15, we observed similar overall Bcl-2 levels in the Double positive and MPEC sub-populations in both IL-15+/+ and IL-15-/- hosts. These results suggest that IL-15 plays a critical role in promoting the survival of all effector CD8+ T cells during the early phases of contraction. Where, IL-15 and IL-7 signaling have an additive effect on the induction of Bcl-2 in the MPEC sub-population; whereas, IL-15 signaling alone is responsible for inducing Bcl-2 in the SLEC sub-population. Meanwhile, TGF-β inhibits Bcl-2 expression by about 2-folds in all effector cell populations, dropping in half the already low levels of Bcl-2 in SLECs, making it fall below the survival threshold, and therefore selectively promoting apoptosis of the SLEC sub-population (Supplementary Figure 12).
DISCUSSION
TGF-β has many multi-faceted effects on numerous immune functions, and the outcome of TGF-β signaling is dependent on the cellular and environmental context of the cell (Li and Flavell, 2008a, b). In this study, we addressed the role of TGF-β signaling in effector CD8+ T cells under infectious conditions with Listeria. We genetically blocked TGF-β signaling by using a transgenic mouse model where a dominant negative form of TGF-βRII is expressed in T cells (CD4-DNR)(Gorelik and Flavell, 2000). T cells isolated from these mice cannot be adoptively transferred into wild type C57Bl6 (B6) recipients, because they are immunologically rejected. As a means to prevent the OTI-DNR cells from being rejected, we used CD11c-DNR mice as recipients. We controlled for any environmental differences by co-transferring the OTI-DNR and OTI cells into the same host, and by also performing side by side experiments of OTI cells in CD11c-DNR transgene-negative littermates. Since artifactual results can be obtained by transferring too many transgenic T cells (Marzo et al., 2005) we performed all experiments using low input numbers of co-transferred cells (25,000 of each type of cell per recipient). We also validated our initial findings in a second genetic system using temporal and inducible deletion of the floxed TGF-βRII gene after adoptive transfer into B6 hosts. We did not find any differences in OTI function and cell differentiation in CD11c-DNR versus wild type hosts; however, we cannot formally and completely exclude minor contributions from the CD11c-DNR transgene on our findings. Nevertheless, we describe for the first time that the process of clonal expansion of CD8+ T cells is a combination of cell proliferation and cell death, and we identified a novel role for TGF-β as a key inducer of apoptosis of the short-lived effector CD8+ T cells during an active immune response. Our data suggests that both IL-15 and TGF-β are responsible for controlling the number of short-lived effector CD8+ T cells, where TGF-β promotes their apoptosis during both clonal expansion and contraction and IL-15 promotes their survival during contraction. These findings substantially enhance our basic knowledge of how the number of effector CD8+ T cells is regulated in response to an acute infection, and emphasizes the tightly regulated mechanisms involved in the contraction of effector cells.
The apoptotic effects of TGF-β become apparent around day 6 post Listeria infection. Several mechanisms can explain why the early effector cells are not influenced by TGF-β. Under appropriate inflammatory conditions and upon antigen encounter, TCR signaling supports the very early survival and proliferation of CD8+ T cells. Shortly after, induction of IL-2 promotes the up-regulation of CD25 levels on early effector cells, supporting their further proliferation and differentiation as well as memory recall response (D’Souza and Lefrancois, 2004; Williams et al., 2006). When clonally expanding T cells were cultured in the presence of IL-2 or a combination of IL-2 and TGF-β, no difference was seen in total numbers of cell recovery or CFSE dilution. By contrast, when cells were cultured with IL-15 in combination with TGF-β, cell recovery dropped to 1/3 of the recovery of cells cultured with IL-15 alone, which was mostly due to increased cell death. These data suggest that IL-2 may be able to overcome the inhibitory and apoptotic effects of TGF-β, and may provide an explanation for the mechanism by which early effector cells in vivo are not influenced by TGF-β. However, once the infection is resolved, and IL-2 levels subside, effector CD8+ T cells lose CD25 expression and their ability to respond to IL-2 is decreased. In the periphery, down regulation of CD25 is followed by a peak in CD122 expression around day 4 p.i. Since, in response to LM-OVA, the absence of IL-15 does not seem to influence the survival and the overall numbers of effector cells during clonal expansion, the heightened CD122 expression around day 4 p.i. may serve to make the effector cells more responsive to residual amounts of IL-2, thus further protecting them from apoptotic effects of TGF-β. Interestingly, effector T cells that migrate to non-lymphoid tissues remain more dependent on IL-2 than those circulating in lymphoid organs (D’Souza et al., 2002); IL-2 may be the arsenal protecting these effector cells from the apoptotic effects of TGF-β. Between days 5 and 7, the effector cells become sensitive to apoptotic affects of TGF-β, in particular the SLECs that do not express IL-7Rα. Several events likely contribute to this sensitivity. First, systemic TGF-β levels go up, tripling in amount by day 7 post infection, while at the same time the majority of the effector population becomes KLRG1hi-CD127lo and dependent on IL-15 for survival (Joshi et al., 2007; Yajima et al., 2006). Meanwhile CD122 and IL-15Rα levels that had peaked by day 4 now drop 2-3 fold by day 7, making the SLECs less able to compete for limiting amounts of IL-2 and IL-15. The combination of these events probably shifts the balance between the survival signals that SLECs can receive and the pro-apoptotic signals that they receive via TGF-β, resulting in the death of the majority of SLECs around the peak of proliferation.
The fact that OTI-DNR cells do contract with similar kinetics as wild type OTI cells, suggests that another TGF-β independent mechanism, such as cytokine withdrawal, is responsible for the eventual contraction of all effector CD8+ T cells. Comparing the survival of contracting effector CD8+ T cells in IL-15+/+ and IL-15-/- hosts, or in the presence of exogenously administered recombinant IL-15, has highly suggested that IL-15 may be the factor that determines the ultimate fate of the SLECs, and our data further supports these findings (D’Cruz et al., 2009; Waldmann, 2006; Yajima et al., 2006). The mitochondrial apoptotic pathway that leads to the activation of caspases 3 and 7 has been shown to play an important role in the apoptosis of effector cells during the contraction phase. The activity of the pro-apoptotic molecule Bim is controlled by the relative levels of anti-apoptotic molecules such as Bcl-2, Bcl-XL, and MCL-1 and consequently, Bcl-2 and MCL-1 have been shown to be necessary for the survival of memory T cells (Opferman et al., 2003; Wojciechowski et al., 2007). Bcl-2 levels are down-regulated at the peak of infection in activated T cells, and transgenic expression of Bcl-2 can rescue T cells from contraction (Hildeman et al., 2002; Pellegrini et al., 2003). It is thought that cγc cytokines promote the survival of effector T cells by inducing the expression of Bcl-2 (Nakajima et al., 1997; Vella et al., 1998). We observed much higher levels of Bcl-2 in MPECs compared to SLECs during early contraction, which seemed to be the result of signaling induced by both IL-7 and IL-15. However, toward the latter part of the contraction phase, as Bcl-2 levels in SLECs remained non-existent in IL-15-/- hosts and low in IL-15+/+ hosts, Bcl-2 levels stayed high in MPECs and became equivalent in both IL-15+/+ and IL-15-/- hosts, suggesting that IL-7 eventually becomes the major source of Bcl-2 induction for MPECs. Although, these cells remain responsive to IL-15, because similar to forced expression of Bcl-2, administration of IL-2, IL-7, or IL-15 can prevent the apoptosis of both CD127hi and CD127lo effector T cells during contraction (Rubinstein et al., 2008; Yajima et al., 2006). This indirectly suggests that towards the middle of the contraction phase, IL-15 becomes limiting even in the IL-15+/+ hosts, thereby further limiting the only Bcl-2 inducing signal that SLECs receive. Meanwhile, the 2-fold impact of TGF-β on Bcl-2 levels becomes much more critical for the SLEC population compared to the MPEC population, causing the already low level of Bcl-2 to fall below the survival threshold, which ultimately results in the selective apoptosis of SLECs. This model reconciles our current data and provides an explanation for the selective impact TGF-β has on promoting the apoptosis of SLECs during contraction, while allowing the survival and differentiation of MPECs.
TGF-β is synthesized as an inactive latent form which is unable to bind to its receptor. TGF-β becomes activated by interacting with molecules such as plasmin, matrix metalloproteinase, reactive oxygen species, thrombospondin-1, or integrins αvβ6 or αvβ8 (Li and Flavell, 2008a, b). In vitro acidification mimics these activation steps, and plasma TGF-β was only detected after acidification and re-neutralization of the plasma. This suggests that high levels of TGF-β in plasma, possibly produced by effector T cells, T regulatory cells, or antigen presenting cells, do not necessarily translate to a systemic increase in TGF-β responsiveness. Notably, the cells that can activate TGF-β may also be different than those that produce this potent cytokine, and thus this activation step provides a means for TGF-β to integrate signals from multiple cell types (Li and Flavell, 2008a, b). Remarkably, the inflammatory and T cell phenotypes developed by the dendritic cell-specific αvβ8-deficient mice are very similar to those observed in mice with a T cell-specific deletion of the Tgfb1 gene, suggesting the important role dendritic cells may play in activating T cell produced TGF-β (Li and Flavell, 2008a; Travis et al., 2007). In addition, DC’s have been suggested to be the prominent source of trans-presented IL-15 during acute infection, as they up-regulate the IL-15/IL-15Rα complex in response to inflammatory stimuli (Dubois et al., 2002; Waldmann, 2006). It is therefore plausible to hypothesize that another mechanism whereby TGF-β selectively induces apoptosis of SLECs relates to the means by which these cells come in contact with trans-presented IL-15, which brings them into the vicinity of αvβ8 integrins expressed on the surface of DCs. As a result, the latent form of TGF-β that is abundantly available in plasma during the peak of infection, or made by either the DC or the effector T cell itself, may become locally activated by the αvβ8 integrins. This model would suggest that as the SLECs come in contact with DCs to receive a survival signal through trans-presented IL-15, they also become simultaneously exposed to active form of TGF-β in the same local environment. In contrast, since the MPECs can rely on both trans-presented IL-15 and soluble IL-7 for survival, they can receive survival signals through IL-7 signaling without exposing themselves to the active forms of TGF-β that potentially exists in the local environment near the trans-presented IL-15 on DCs.
The current findings in this study have several potentially important clinical implications. Autoimmunity and inflammatory diseases can be caused by decreased immune suppression, while cancers and certain infectious diseases are often associated with increased immune suppression. Induction of TGF-β producing regulatory T cells has been associated with better prognosis in several autoimmune diseases. For example, a promising immunotherapy for type 1 diabetes (T1D) involves the administration of a CD3-specific monoclonal antibody (Herold et al., 2002), which functions by eliminating autoreactive T cells and promoting the expansion of TGF-β producing regulatory T cells (Chatenoud and Bluestone, 2007). Our data suggest that a mechanism by which this immunotherapy may function is through a TGF-β dependent induction of apoptosis of self-reactive effector T cells. Such depleting immunotherapy also generates a lymphopenic environment where IL-15 presumably becomes highly abundant. We therefore would predict that neutralization of IL-15 under such lymphopenic conditions may help to further destabilize self-reactive effector T cells whose survival may be dependent on IL-15. Targeting IL-15 as a means of immunotherapy against many autoimmune diseases has been suggested and is currently under trial (Waldmann, 2006). Our findings in this study support and provide further mechanistic insights into the success of a combinatorial therapy of simultaneously inducing TGF-β and blocking IL-15 to achieve the best immunotherapeutic results against T1D.
Elimination of most tumors by the immune system involves functional CD8+ cytotoxic T lymphocytes. A number of mechanisms have been identified that mediate resistance to immunotherapy in animal models, including insufficient expansion of tumor antigen-specific CTLs, and suppression of CTL activity by T-regulatory cells (Tregs) or by cytokines within the tumor microenvironment (Klebanoff et al., 2006). Thus pharmacological increase in trafficking of effector T cells into tumors and/or preventing these T cells from undergoing apoptosis or loss of effector function could be very valuable means of immunotherapy. It is considered that tumors that produce TGF-β or promote TGF-β production by surrounding cells advance their progression and allow tumors to evade immune surveillance, although the exact mechanism by which this occurs is not well understood (Wrzesinski et al., 2007). Studies in our laboratory and by other investigators provide compelling evidence that inhibition of TGF-β signaling may address several key mechanisms of resistance and improve the efficacy of tumor immune therapy (Gorelik and Flavell, 2001; Wrzesinski et al., 2007). In the current study we describe a new mechanism whereby tumors may prevent their immune mediated destruction by producing TGF-β and directly promoting the apoptosis of tumor antigen specific effector CD8+ T cells. In addition, many studies have already demonstrated the efficacy of therapeutic uses of IL-15 for eradicating tumors (Klebanoff et al., 2004; Morris et al., 2006). Our findings strongly support these notions and provide a cellular mechanism by which the combinatorial effects of blocking TGF-β signaling in addition to administering IL-15 may have the most beneficial therapeutic outcomes during vaccination or tumor immunotherapy. Such combinatorial therapy would most likely lead to enhanced numbers of effector CD8+ SLECs as well as CD4+ Th1 cells (Li et al., 2006a).
EXPERIMENTAL PROCEDURES
Mice
CD4-TGFβRII-DNR mice (Gorelik and Flavell, 2000) were bred on to OTI Vα2/Vβ5 TCR transgenic specific for OVA257-264 that recognizes SIINFEKL epitope in the context of MHC class I H-2Kb. These mice were then crossed to RAG1-/- (JAX) and CD45.1 (NCI) congenic markers. CD11c-DNR transgenic mice (Laouar et al., 2005) were maintained on a C57BL/6 background. IL-15-/- mice were purchased from Taconic and bred onto the CD11c-DNR mice.
Adoptive co-transfer and LM-OVA infection
Every co-transfer experiment consisted of 1.5-2.5×104 sorted naïve OTI and OTI-DNR cells each on a different congenic marker mixed at a 1:1 ratio. One to two days after adoptive transfer, mice were infected with 1×105 recombinant Listeria monocytogenes, expressing OVA (LM-OVA), which was a generous gift from Hao Shen (U. Penn).
Isolation of peripheral blood lymphocytes (PBL) and calculating cell populations among PBLs
100-150μl of blood from tail vein of infected mice was added to 30μl of 1X heparin (500 units/ml). 500μl of 1X ACK lysis buffer (Lonza) was added directly to the cells and incubated at room temperature for 2-3 minutes. Cells were centrifuged at 4000 rpm for 5 minutes. The top layer was aspirated and another 500μl of 1X ACK lysis buffer was added followed by centrifugation. Cells were resuspended in 500μl of FACS buffer (PBS + 0.5% FBS) and divided into 2-4 wells of a 96-well V-bottom plate and stained with surface antibodies. The same gate was set on a FACS Calibur machine to collect 25,000 live lymphocytes for every time point. The same gate was also set during analysis of the data, and thus the starting number of PBLs was set to 25,000 for every calculation. Total number of host CD8+ T cells was measured as an internal control, which remained roughly around 4,000/25,000 in early and late time points, but increased to 5,000-6,000 at the peak of T cell proliferation. Based on the frequencies obtained from FACS analysis, we then calculated the number of each sub-population out of 25,000 PBLs.
Isolation of lymphocytes from non-lymphoid organs
Peripheral blood was isolated from tail vein of infected mice as described above. Mice were then euthanized and perfused with 30mL of cold PBS to remove all the blood from the liver and lung. Liver and lung were removed, and cell homogenates were digested for 1 hour at 37°C with Digestion buffer [RPMI + 5% FBS + 100 U/ml DNase I (Sigma) + 0.2mg/ml Collagenase IV (Sigma)]. Liver homogenates were centrifuged at 300 rpm for 3 min to remove hepatocytes, and lung homogenates were run through a 70μm filter mesh. Non-hepatic supernatant and lung lymphocytes were centrifuged at 1500 rpm for 10 min. The cell pellet was resuspended in 1 ml of RPMI + 5% FBS and mixed with 4 ml of 27.5% OptiPrep (Axis-Shield; Oslo, Norway). To make a gradient, 1ml of RPMI was carefully layered on top of the cells, and centrifuged at 2700 rpm for 20 min. Lymphocytes were carefully removed from the interface of the gradient and further analyzed.
Antibodies and reagents
All FACS antibodies used were from BD Pharmingen, except for Biotin or PE-CD127 (eBioscience), APC KLRG1 (eBioscience), APC Granzyme B (CALTAG), PE T-bet (eBioscience), Biotin IL-15Rα (R&D Systems), and PE TGFβRII and isotype control (R&D Systems). Recombinant cytokines mouse IL-15 (R&D Systems), mouse IL-7 (PeroTech Inc.), mouse IL-2 (BD Pharmingen), and human TGF-β1 (R&D Systems) were used.
Measuring plasma TGFβ1
100-200μl of tail-vein blood was collected and immediately mixed with 30μl of 25 mM EDTA, and immediately put on ice. Samples were centrifuged at 3,000 rpm for 5 minutes. Plasma was removed, transferred to a new tube, and spun again at 12,000 rpm for 5 minutes to remove residual RBCs and platelets. Plasma samples were kept at -70°C for future use. TGFβ1 Emax ImmunoAssay System (Promega) was used to measure TGF-β1 . Plasma samples were acid treated to activate total TGF-β per manufacturer’s instructions. Non-acid treated samples were diluted 2 folds in the provided 1X Sample Buffer.
BrdU labeling and AnnexinV + PI Staining
Mice were injected intraperitoneally with a single dose of 1 mg BrdU (5-bromodeoxyuridine; Sigma) on day 6 post infection. Spleens were harvested 14 hours later, and stained first for surface markers. Cells were fixed and permeabilized over night in 100μl of 1% PFA + 0.01% Tween 20 at 4°C. Cells were then treated with DNase I (Sigma) at 37°C for 60 minutes. FITC anti-BrdU Ab (BD) was used to detect cells that had incorporated BrdU. Annexin V-FITC and -APC Apoptosis Detection Kit I (BD Pharmingen) was used per manufacturer’s instructions.
Intracellular Cytokine Staining
Splenocytes from day 7 infected mice were incubated with 20μM of SIINFEKL peptide in the presence of BD GolgiStop for 5 hours at 37°C in CO2 incubator. BD Cytofix/Cytoperm was used to stain for intracellular cytokines, Granzyme B, Bcl-2, and T-bet.
In Vivo CTL Assay
OTI and OTI-DNR day 7 effector cells were purified by cell sorting and mixed at various ratios with CFSE labeled target cells as previously described (Ingulli, 2007). The mixture of effector and target cells were injected i.v. into naïve hosts. 14-16 hours later, spleens from the recipient mice were harvested and the ratio of CFSEhi and CFSElo cells was measured by FACS analysis as previously described (Ingulli, 2007).
In vitro T cell cultures and cell recovery assay
Naïve OTI and OTI-DNR CD8+ T cells were purified by cell sorting. 100×106 splenocytes were incubated with 200nM of SIINFEKL peptide at 37°C for 1 hour followed by 2 washes. 3-5×106 naïve cells were mixed with 30-50×106 peptide-loaded splenocytes and the mixture was labeled with 5μM of CFSE. Cells were cultured for 2 days in Complete media (Clicks, 10% FBS, L-Glutamine, BME, pen/strep) in the presence of peptide-loaded APCs. Cells were then washed and mixed again with naïve live splenocytes in the presence of various cytokines as indicated. Two days later, one half of the media was removed and replenished with fresh media and cytokines. After a total of 6 days (2 days with peptide and 4 days with cytokines), cells were harvested, stained with PE CD45.1, Percp CD8, and APC-AnnexinV. Cells were kept on ice at all times and resuspended in the same final volume before FACS analysis. To determine the total number of recovered cells, each sample was collected on FACS Calibur for exactly 1 minute.
Statistical analysis
Student’s t-test (two-tailed) was used to calculate the statistical significance of data comparison. P value of ≤0.05 was considered to be significant.
Supplementary Material
Supplementary Figure 1. DNR transgene-positive cells are rejected in C57Bl6 mice.
2.5×104 sorted naïve OTI.1.1 and OTI-DNR.1.2 cells were adoptively co-transferred into either (A) wild type C57Bl6 (B6) or (B) CD4-DNR recipients. Mice were infected with LM-OVA two days after transfer. T cell proliferation was monitored in peripheral blood lymphocytes (PBL). Data is average and s.d. of 5 co-transfers.
Supplementary Figure 2. Listeria burden in CD11c-DNR compared to B6 hosts 3 days post LM-OVA infection.
5×104 naïve OTI or OTI-DNR cells were adoptively transferred into either CD11c-DNR transgene-positive or -negative hosts, or did not receive any transgenic T cells (None), as indicated. One day later, the mice were infected with 1×105 LM-OVA. On day 3 p.i., spleens and livers were isolated, homogenized in 0.5% Tween-20 and serially diluted and plated on BHI agar plates containing Erythromycin (50μg/ml). Plates were incubated at 37°C over night, and LM-OVA CFUs were measured from (A) Spleens and (B) Livers of infected mice. Limit of detection (LOD) was at 100 CFU/organ. Data shown is from one out of two independent experiments with similar results.
Supplementary Figure 3. Temporal deletion of TGF-βRII after T cell priming and activation results in a 3-fold incre(base in the frequency of TGF-βRII-/- OTI effector CD8+ T cells.
(A) Details of experimental scheme. TGF-βRII floxed mice were crossed to OTI, ER-Cre, YFP-reporter, and put on a RAG1-/- background.
(B) The same group of mice was bled for a longitudinal experiment to compare the kinetics of accumulation of OTI/RII+/- and OTI/RII-/- cells among PBLs. Lymphocytes were first gated on CD8+ T cells, then separated based on their congenic markers (left). Each population was then separated based on the expression of YFP (right three panels). No YFP+ T cells were detected on day 4 post infection, prior to tamoxifen treatment. YPF positive cells represent the cells that have active nuclear Cre thus CD45.1.2 YFP+ cells are RII-/-, and CD45.2.2 YFP+ cells are RII+/-.
Supplementary Figure 4. Cytokine production and cytolytic activity of OTI cells in B6 host.
(A) 5×104 sorted naïve OTI cells were transferred into CD11c-DNR transgene-negative littermates, followed by LM-OVA infection. Intracellular cytokine staining of day 7 effector cells stimulated with or without SIINFEKL peptide. Data are representative of 1 out of 3 mice.
(B) In vivo CTL assay using day 7 effector cells generated in CD11c-DNR transgene-negative mice. Average and s.d. of 3 transfers is shown.
Supplementary Figure 5. TGF-β promotes the death of effector CD8+ T cells in B6 host.
(A) BrdU staining of splenocytes isolated 7 days post infection. Host (CD11c-DNR transgene-negative littermates) and wild type OTI cells were gated on based on staining with CD8 and CD45.1 congenic marker. Data are representative of 1 out of 3 mice.
(B) Dead versus apoptotic cells from day 5 (PBL) and day 7 (spleen) of transgene-negative infected mice were identified by Annexin V and PI staining. Cells were first stained for CD45.1 congenic marker to identify OTI.1.1 cells. Average and s.d. of 2 (day 5) and 3 (day 7) mice per group are shown.
Supplementary Figure 6. Phenotypic analysis of OTI effector cells in B6 host.
(A) Histograms comparing the expression of each molecule on host (solid gray) and OTI (dashed line) CD8+ T cells on day 5 and 7 p.i.. Data are representative of 1 out of 3-4 mice per time point.
(B) CD127-KLRG1 profiles of OTI cells transferred into CD11c-DNR transgene-negative hosts.
(C) Absolute number of each effector subpopulation shown on the same scale as in Figure 5E. Top row shows the contribution of each population over time on the same scale, and the bottom row is the contribution of each sub-population on Individual scales.
Supplementary Figure 7. TGF-β promotes the apoptosis of effector CD8+ T cells ex vivo.
(A) TGF-βRII expression on naïve and effector OTI sub-populations. After adoptive transfer of 5×104 naïve OTI cells into CD11c-DNR transgene-negative littermates and LM-OVA infection, OTI cells isolated from the spleen of infected mice were stained with CD127 and KLRG1 and either TGFβRII or the isotype control antibodies. MFI of TGF-βRII was determined by subtracting the MFI of background staining observed with isotype control, which varied from 3-6, from the MFI of TGF-βRII staining on the same sub-population of effector cells.
(B) Details of experimental scheme.
(C) Upon experimental procedure described in part (B), OTI effector sub-populations were identified by the congenic marker and CD127/KLRG1 staining. The percent of live cells in each fraction was determined by forward and side scatter plots. The bar graph represents the fraction of live cells in TGF-β treated versus non-treated samples. Average and s.d. of 4 experimental wells is shown. Data is representative of 1 out of 2 independent experiments
Supplementary Figure 8. OTI and OTI-DNR cells respond similarly to different concentrations of IL-15.
(A) Representative FACS plots of CFSE dilution and AnnexinV staining after a similar treatment as described in Figure 6D.
(B) Total number of OTI versus OTI-DNR cells recovered in the presence of varying concentrations of IL-15.
(C) Average number of cell cycles normalized by dividing 1000 (Maximum CFSE MFI) by the CFSE MFI at the end of the experiment under the indicated conditions. Each bar represents one individual well.
Supplementary Figure 9. OTI and OTI-DNR effector cells show similar phosphorylation and dephosphorylation of Stat5 in response to IL-15 and IL-2.
Intracellular FACS analysis of pStat5. Day 2 OTI and OTI-DNR effector cells were generated in vitro as described in Figure 6D. Phosphorylation of Stat5 was measured at different time intervals under the indicated conditions as previously described (Hand et al., 2007). Data is representative of 1 out of 2 independent experiments with similar results.
Supplementary Figure 10. Comparison of mRNA levels in naïve and day 7 SLECs.
Quantitative RT PCR comparing mRNA levels of indicated genes in sorted naïve (CD62Lhi-CD44lo) and day 7 SLEC (CD127lo-KLRG1hi) OTI and OTI-DNR cells. Samples were first normalized to HPRT. Fold change from naïve OTI cells is shown.
Supplementary Figure 11. TGF-β and IL-15 control the number of SLECs by exerting opposing effects on Bcl-2 levels in CD1c-DNR transgene-negative mice.
(A) Intracellular staining of Bcl-2 and T-bet protein in day 7 effector OTI sub-populations from a B6 host. Data is plotted on the same scale as in Figure 7B.
(B) 5×104 naïve OTI cells were transferred into either IL-15+/+ or IL-15-/- B6 hosts followed by LM-OVA infection. Fraction of total (left) and SLEC sub-population (right) of OTI cells were determined among total PBLs and converted to numbers per 25,000 PBL.
(C) Bar graphs comparing the calculated number of each OTI sub-population among 25,000 PBLs in IL-15+/+ and IL-15-/- B6 hosts. Average and s.d. of 3 transfers into each IL-15+/+ and IL-15-/- mice is shown.
(D) Representation of the difference seen in the percentage OTI sub-populations from the spleens of infected IL-15+/+ and IL-15-/- B6 hosts at the indicated time points.
(E) Comparison of intracellular Bcl-2 protein levels in each sub-population of splenic OTI cells isolated from either IL-15+/+ or IL-15-/- B6 hosts.
(F) Absolute number of each OTI sub-population from the spleens of IL-15+/+ and IL-15-/- B6 hosts. Data is average and s.d. of 2-3 mice per time point.
Supplementary Figure 12. Relationship between Listeria infection, cγc cytokines, TGF-β, Bcl-2 and effector T cell contraction.
Early during infection, TCR signal and IL-2 promote the survival of all effector CD8+ T cells. As TGF-β levels rise, the SLECs that seem to be surviving during clonal expansion in the absence of any significant signaling from the cγc cytokines, become very sensitive to the apoptotic effects of TGF-β. In the contraction phase, IL-15 supports the survival of both MPECs and SLECs, while TGF-β continues to oppose the survival signals by lowering the Bcl-2 levels. The 2-fold decrease in Bcl-2 levels by TGF-β does not impact the survival of MPECs that already have high levels of Bcl-2, whereas it makes SLECs much more susceptible to apoptosis as it drops the already low levels of Bcl-2 below the survival threshold. Finally, as IL-15 levels subside, the MPECs continue to proliferate and survive in response to IL-7, whereas the SLECs that cannot proliferate eventually die.
ACKNOWLEDGEMENTS
We thank M. Li, T. Willinger, E. Eynon, and S. Kaech for critically reading the manuscript and for helpful discussion; T. Hand for performing AKT phosphorylation experiment, R. Webber for help with breeding the CD11c-DNR mice; and T. Taylor for cell sorting. R.A.F is an investigator of the Howard Hughes Medical Institute. This work is supported by a post-doctoral fellowship grant from Cancer Research Institute (S.S.), with additional support from NIH grants CA121974 and DK051665 (R.A.F.) and JDRF grant 32-2008-352 (R.A.F.). The authors declare that they have no competing financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ahmadzadeh M, Rosenberg SA. TGF-beta 1 attenuates the acquisition and expression of effector function by tumor antigen-specific human memory CD8 T cells. J Immunol. 2005;174:5215–5223. doi: 10.4049/jimmunol.174.9.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattman JN, Grayson JM, Wherry EJ, Kaech SM, Smith KA, Ahmed R. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nature medicine. 2003;9:540–547. doi: 10.1038/nm866. [DOI] [PubMed] [Google Scholar]
- Boyman O, Purton JF, Surh CD, Sprent J. Cytokines and T-cell homeostasis. Current opinion in immunology. 2007;19:320–326. doi: 10.1016/j.coi.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Burkett PR, Koka R, Chien M, Chai S, Chan F, Ma A, Boone DL. IL-15R alpha expression on CD8+ T cells is dispensable for T cell memory. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:4724–4729. doi: 10.1073/pnas.0737048100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatenoud L, Bluestone JA. CD3-specific antibodies: a portal to the treatment of autoimmunity. Nature reviews. 2007;7:622–632. doi: 10.1038/nri2134. [DOI] [PubMed] [Google Scholar]
- D’Cruz LM, Rubinstein MP, Goldrath AW. Surviving the crash: Transitioning from effector to memory CD8(+) T cell. Seminars in immunology. 2009;21:92–98. doi: 10.1016/j.smim.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza WN, Lefrancois L. IL-2 is not required for the initiation of CD8 T cell cycling but sustains expansion. J Immunol. 2003;171:5727–5735. doi: 10.4049/jimmunol.171.11.5727. [DOI] [PubMed] [Google Scholar]
- D’Souza WN, Lefrancois L. Frontline: An in-depth evaluation of the production of IL-2 by antigen-specific CD8 T cells in vivo. European journal of immunology. 2004;34:2977–2985. doi: 10.1002/eji.200425485. [DOI] [PubMed] [Google Scholar]
- D’Souza WN, Schluns KS, Masopust D, Lefrancois L. Essential role for IL-2 in the regulation of antiviral extralymphoid CD8 T cell responses. J Immunol. 2002;168:5566–5572. doi: 10.4049/jimmunol.168.11.5566. [DOI] [PubMed] [Google Scholar]
- Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity. 2002;17:537–547. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- Gorelik L, Flavell RA. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12:171–181. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- Gorelik L, Flavell RA. Immune-mediated eradication of tumors through the blockade of transforming growth factor-beta signaling in T cells. Nature medicine. 2001;7:1118–1122. doi: 10.1038/nm1001-1118. [DOI] [PubMed] [Google Scholar]
- Hand TW, Kaech SM. Intrinsic and extrinsic control of effector T cell survival and memory T cell development. Immunologic research. 2008 doi: 10.1007/s12026-008-8027-z. [DOI] [PubMed] [Google Scholar]
- Hand TW, Morre M, Kaech SM. Expression of IL-7 receptor alpha is necessary but not sufficient for the formation of memory CD8 T cells during viral infection. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:11730–11735. doi: 10.1073/pnas.0705007104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haring JS, Jing X, Bollenbacher-Reilley J, Xue HH, Leonard WJ, Harty JT. Constitutive expression of IL-7 receptor alpha does not support increased expansion or prevent contraction of antigen-specific CD4 or CD8 T cells following Listeria monocytogenes infection. J Immunol. 2008;180:2855–2862. doi: 10.4049/jimmunol.180.5.2855. [DOI] [PubMed] [Google Scholar]
- Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Developmental biology. 2002;244:305–318. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, Gitelman SE, Harlan DM, Xu D, Zivin RA, Bluestone JA. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. The New England journal of medicine. 2002;346:1692–1698. doi: 10.1056/NEJMoa012864. [DOI] [PubMed] [Google Scholar]
- Hildeman D, Jorgensen T, Kappler J, Marrack P. Apoptosis and the homeostatic control of immune responses. Current opinion in immunology. 2007;19:516–521. doi: 10.1016/j.coi.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildeman DA, Zhu Y, Mitchell TC, Bouillet P, Strasser A, Kappler J, Marrack P. Activated T cell death in vivo mediated by proapoptotic bcl-2 family member bim. Immunity. 2002;16:759–767. doi: 10.1016/s1074-7613(02)00322-9. [DOI] [PubMed] [Google Scholar]
- Hou S, Hyland L, Ryan KW, Portner A, Doherty PC. Virus-specific CD8+ T-cell memory determined by clonal burst size. Nature. 1994;369:652–654. doi: 10.1038/369652a0. [DOI] [PubMed] [Google Scholar]
- Ingulli E. Tracing tolerance and immunity in vivo by CFSE-labeling of administered cells. Methods in molecular biology (Clifton, N.J. 2007;380:365–376. doi: 10.1007/978-1-59745-395-0_23. [DOI] [PubMed] [Google Scholar]
- Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi NS, Kaech SM. Effector CD8 T cell development: a balancing act between memory cell potential and terminal differentiation. J Immunol. 2008;180:1309–1315. doi: 10.4049/jimmunol.180.3.1309. [DOI] [PubMed] [Google Scholar]
- Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, Matsuki N, Charrier K, Sedger L, Willis CR, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. The Journal of experimental medicine. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff CA, Finkelstein SE, Surman DR, Lichtman MK, Gattinoni L, Theoret MR, Grewal N, Spiess PJ, Antony PA, Palmer DC, et al. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:1969–1974. doi: 10.1073/pnas.0307298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff CA, Gattinoni L, Restifo NP. CD8+ T-cell memory in tumor immunology and immunotherapy. Immunological reviews. 2006;211:214–224. doi: 10.1111/j.0105-2896.2006.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laouar Y, Sutterwala FS, Gorelik L, Flavell RA. Transforming growth factor-beta controls T helper type 1 cell development through regulation of natural killer cell interferon-gamma. Nature immunology. 2005;6:600–607. doi: 10.1038/ni1197. [DOI] [PubMed] [Google Scholar]
- Li MO, Flavell RA. Contextual regulation of inflammation: a duet by transforming growth factor-beta and interleukin-10. Immunity. 2008a;28:468–476. doi: 10.1016/j.immuni.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Li MO, Flavell RA. TGF-beta: a master of all T cell trades. Cell. 2008b;134:392–404. doi: 10.1016/j.cell.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MO, Sanjabi S, Flavell RA. Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity. 2006a;25:455–471. doi: 10.1016/j.immuni.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annual review of immunology. 2006b;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annual review of immunology. 2006;24:657–679. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- Malek TR. The biology of interleukin-2. Annual review of immunology. 2008;26:453–479. doi: 10.1146/annurev.immunol.26.021607.090357. [DOI] [PubMed] [Google Scholar]
- Marie JC, Liggitt D, Rudensky AY. Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-beta receptor. Immunity. 2006;25:441–454. doi: 10.1016/j.immuni.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Marrack P, Kappler J. Control of T cell viability. Annual review of immunology. 2004;22:765–787. doi: 10.1146/annurev.immunol.22.012703.104554. [DOI] [PubMed] [Google Scholar]
- Marzo AL, Klonowski KD, Le Bon A, Borrow P, Tough DF, Lefrancois L. Initial T cell frequency dictates memory CD8+ T cell lineage commitment. Nature immunology. 2005;6:793–799. doi: 10.1038/ni1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D, Kaech SM, Wherry EJ, Ahmed R. The role of programming in memory T-cell development. Current opinion in immunology. 2004;16:217–225. doi: 10.1016/j.coi.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Morris JC, Janik JE, White JD, Fleisher TA, Brown M, Tsudo M, Goldman CK, Bryant B, Petrus M, Top L, et al. Preclinical and phase I clinical trial of blockade of IL-15 using Mikbeta1 monoclonal antibody in T cell large granular lymphocyte leukemia. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:401–406. doi: 10.1073/pnas.0509575103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murali-Krishna K, Altman JD, Suresh M, Sourdive DJ, Zajac AJ, Miller JD, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- Nakajima H, Shores EW, Noguchi M, Leonard WJ. The common cytokine receptor gamma chain plays an essential role in regulating lymphoid homeostasis. The Journal of experimental medicine. 1997;185:189–195. doi: 10.1084/jem.185.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature. 2003;426:671–676. doi: 10.1038/nature02067. [DOI] [PubMed] [Google Scholar]
- Pellegrini M, Belz G, Bouillet P, Strasser A. Shutdown of an acute T cell immune response to viral infection is mediated by the proapoptotic Bcl-2 homology 3-only protein Bim. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:14175–14180. doi: 10.1073/pnas.2336198100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein MP, Lind NA, Purton JF, Filippou P, Best JA, McGhee PA, Surh CD, Goldrath AW. IL-7 and IL-15 differentially regulate CD8+ T-cell subsets during contraction of the immune response. Blood. 2008;112:3704–3712. doi: 10.1182/blood-2008-06-160945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Kalia V, Haining WN, Konieczny BT, Subramaniam S, Ahmed R. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. The Journal of experimental medicine. 2008;205:625–640. doi: 10.1084/jem.20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nature reviews. 2008;8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- Sillett HK, Cruickshank SM, Southgate J, Trejdosiewicz LK. Transforming growth factor-beta promotes ‘death by neglect’ in post-activated human T cells. Immunology. 2001;102:310–316. doi: 10.1046/j.1365-2567.2001.01185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC developmental biology. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DA, Massague J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer cell. 2005;8:369–380. doi: 10.1016/j.ccr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Trapani JA. The dual adverse effects of TGF-beta secretion on tumor progression. Cancer cell. 2005;8:349–350. doi: 10.1016/j.ccr.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Travis MA, Reizis B, Melton AC, Masteller E, Tang Q, Proctor JM, Wang Y, Bernstein X, Huang X, Reichardt LF, et al. Loss of integrin alpha(v)beta8 on dendritic cells causes autoimmunity and colitis in mice. Nature. 2007;449:361–365. doi: 10.1038/nature06110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vella AT, Dow S, Potter TA, Kappler J, Marrack P. Cytokine-induced survival of activated T cells in vitro and in vivo. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:3810–3815. doi: 10.1073/pnas.95.7.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nature reviews. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annual review of immunology. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojciechowski S, Jordan MB, Zhu Y, White J, Zajac AJ, Hildeman DA. Bim mediates apoptosis of CD127(lo) effector T cells and limits T cell memory. European journal of immunology. 2006;36:1694–1706. doi: 10.1002/eji.200635897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojciechowski S, Tripathi P, Bourdeau T, Acero L, Grimes HL, Katz JD, Finkelman FD, Hildeman DA. Bim/Bcl-2 balance is critical for maintaining naive and memory T cell homeostasis. The Journal of experimental medicine. 2007;204:1665–1675. doi: 10.1084/jem.20070618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfraim LA, Walz TM, James Z, Fernandez T, Letterio JJ. p21Cip1 and p27Kip1 act in synergy to alter the sensitivity of naive T cells to TGF-beta-mediated G1 arrest through modulation of IL-2 responsiveness. J Immunol. 2004;173:3093–3102. doi: 10.4049/jimmunol.173.5.3093. [DOI] [PubMed] [Google Scholar]
- Wrzesinski SH, Wan YY, Flavell RA. Transforming growth factor-beta and the immune response: implications for anticancer therapy. Clin Cancer Res. 2007;13:5262–5270. doi: 10.1158/1078-0432.CCR-07-1157. [DOI] [PubMed] [Google Scholar]
- Yajima T, Yoshihara K, Nakazato K, Kumabe S, Koyasu S, Sad S, Shen H, Kuwano H, Yoshikai Y. IL-15 regulates CD8+ T cell contraction during primary infection. J Immunol. 2006;176:507–515. doi: 10.4049/jimmunol.176.1.507. [DOI] [PubMed] [Google Scholar]
- Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. DNR transgene-positive cells are rejected in C57Bl6 mice.
2.5×104 sorted naïve OTI.1.1 and OTI-DNR.1.2 cells were adoptively co-transferred into either (A) wild type C57Bl6 (B6) or (B) CD4-DNR recipients. Mice were infected with LM-OVA two days after transfer. T cell proliferation was monitored in peripheral blood lymphocytes (PBL). Data is average and s.d. of 5 co-transfers.
Supplementary Figure 2. Listeria burden in CD11c-DNR compared to B6 hosts 3 days post LM-OVA infection.
5×104 naïve OTI or OTI-DNR cells were adoptively transferred into either CD11c-DNR transgene-positive or -negative hosts, or did not receive any transgenic T cells (None), as indicated. One day later, the mice were infected with 1×105 LM-OVA. On day 3 p.i., spleens and livers were isolated, homogenized in 0.5% Tween-20 and serially diluted and plated on BHI agar plates containing Erythromycin (50μg/ml). Plates were incubated at 37°C over night, and LM-OVA CFUs were measured from (A) Spleens and (B) Livers of infected mice. Limit of detection (LOD) was at 100 CFU/organ. Data shown is from one out of two independent experiments with similar results.
Supplementary Figure 3. Temporal deletion of TGF-βRII after T cell priming and activation results in a 3-fold incre(base in the frequency of TGF-βRII-/- OTI effector CD8+ T cells.
(A) Details of experimental scheme. TGF-βRII floxed mice were crossed to OTI, ER-Cre, YFP-reporter, and put on a RAG1-/- background.
(B) The same group of mice was bled for a longitudinal experiment to compare the kinetics of accumulation of OTI/RII+/- and OTI/RII-/- cells among PBLs. Lymphocytes were first gated on CD8+ T cells, then separated based on their congenic markers (left). Each population was then separated based on the expression of YFP (right three panels). No YFP+ T cells were detected on day 4 post infection, prior to tamoxifen treatment. YPF positive cells represent the cells that have active nuclear Cre thus CD45.1.2 YFP+ cells are RII-/-, and CD45.2.2 YFP+ cells are RII+/-.
Supplementary Figure 4. Cytokine production and cytolytic activity of OTI cells in B6 host.
(A) 5×104 sorted naïve OTI cells were transferred into CD11c-DNR transgene-negative littermates, followed by LM-OVA infection. Intracellular cytokine staining of day 7 effector cells stimulated with or without SIINFEKL peptide. Data are representative of 1 out of 3 mice.
(B) In vivo CTL assay using day 7 effector cells generated in CD11c-DNR transgene-negative mice. Average and s.d. of 3 transfers is shown.
Supplementary Figure 5. TGF-β promotes the death of effector CD8+ T cells in B6 host.
(A) BrdU staining of splenocytes isolated 7 days post infection. Host (CD11c-DNR transgene-negative littermates) and wild type OTI cells were gated on based on staining with CD8 and CD45.1 congenic marker. Data are representative of 1 out of 3 mice.
(B) Dead versus apoptotic cells from day 5 (PBL) and day 7 (spleen) of transgene-negative infected mice were identified by Annexin V and PI staining. Cells were first stained for CD45.1 congenic marker to identify OTI.1.1 cells. Average and s.d. of 2 (day 5) and 3 (day 7) mice per group are shown.
Supplementary Figure 6. Phenotypic analysis of OTI effector cells in B6 host.
(A) Histograms comparing the expression of each molecule on host (solid gray) and OTI (dashed line) CD8+ T cells on day 5 and 7 p.i.. Data are representative of 1 out of 3-4 mice per time point.
(B) CD127-KLRG1 profiles of OTI cells transferred into CD11c-DNR transgene-negative hosts.
(C) Absolute number of each effector subpopulation shown on the same scale as in Figure 5E. Top row shows the contribution of each population over time on the same scale, and the bottom row is the contribution of each sub-population on Individual scales.
Supplementary Figure 7. TGF-β promotes the apoptosis of effector CD8+ T cells ex vivo.
(A) TGF-βRII expression on naïve and effector OTI sub-populations. After adoptive transfer of 5×104 naïve OTI cells into CD11c-DNR transgene-negative littermates and LM-OVA infection, OTI cells isolated from the spleen of infected mice were stained with CD127 and KLRG1 and either TGFβRII or the isotype control antibodies. MFI of TGF-βRII was determined by subtracting the MFI of background staining observed with isotype control, which varied from 3-6, from the MFI of TGF-βRII staining on the same sub-population of effector cells.
(B) Details of experimental scheme.
(C) Upon experimental procedure described in part (B), OTI effector sub-populations were identified by the congenic marker and CD127/KLRG1 staining. The percent of live cells in each fraction was determined by forward and side scatter plots. The bar graph represents the fraction of live cells in TGF-β treated versus non-treated samples. Average and s.d. of 4 experimental wells is shown. Data is representative of 1 out of 2 independent experiments
Supplementary Figure 8. OTI and OTI-DNR cells respond similarly to different concentrations of IL-15.
(A) Representative FACS plots of CFSE dilution and AnnexinV staining after a similar treatment as described in Figure 6D.
(B) Total number of OTI versus OTI-DNR cells recovered in the presence of varying concentrations of IL-15.
(C) Average number of cell cycles normalized by dividing 1000 (Maximum CFSE MFI) by the CFSE MFI at the end of the experiment under the indicated conditions. Each bar represents one individual well.
Supplementary Figure 9. OTI and OTI-DNR effector cells show similar phosphorylation and dephosphorylation of Stat5 in response to IL-15 and IL-2.
Intracellular FACS analysis of pStat5. Day 2 OTI and OTI-DNR effector cells were generated in vitro as described in Figure 6D. Phosphorylation of Stat5 was measured at different time intervals under the indicated conditions as previously described (Hand et al., 2007). Data is representative of 1 out of 2 independent experiments with similar results.
Supplementary Figure 10. Comparison of mRNA levels in naïve and day 7 SLECs.
Quantitative RT PCR comparing mRNA levels of indicated genes in sorted naïve (CD62Lhi-CD44lo) and day 7 SLEC (CD127lo-KLRG1hi) OTI and OTI-DNR cells. Samples were first normalized to HPRT. Fold change from naïve OTI cells is shown.
Supplementary Figure 11. TGF-β and IL-15 control the number of SLECs by exerting opposing effects on Bcl-2 levels in CD1c-DNR transgene-negative mice.
(A) Intracellular staining of Bcl-2 and T-bet protein in day 7 effector OTI sub-populations from a B6 host. Data is plotted on the same scale as in Figure 7B.
(B) 5×104 naïve OTI cells were transferred into either IL-15+/+ or IL-15-/- B6 hosts followed by LM-OVA infection. Fraction of total (left) and SLEC sub-population (right) of OTI cells were determined among total PBLs and converted to numbers per 25,000 PBL.
(C) Bar graphs comparing the calculated number of each OTI sub-population among 25,000 PBLs in IL-15+/+ and IL-15-/- B6 hosts. Average and s.d. of 3 transfers into each IL-15+/+ and IL-15-/- mice is shown.
(D) Representation of the difference seen in the percentage OTI sub-populations from the spleens of infected IL-15+/+ and IL-15-/- B6 hosts at the indicated time points.
(E) Comparison of intracellular Bcl-2 protein levels in each sub-population of splenic OTI cells isolated from either IL-15+/+ or IL-15-/- B6 hosts.
(F) Absolute number of each OTI sub-population from the spleens of IL-15+/+ and IL-15-/- B6 hosts. Data is average and s.d. of 2-3 mice per time point.
Supplementary Figure 12. Relationship between Listeria infection, cγc cytokines, TGF-β, Bcl-2 and effector T cell contraction.
Early during infection, TCR signal and IL-2 promote the survival of all effector CD8+ T cells. As TGF-β levels rise, the SLECs that seem to be surviving during clonal expansion in the absence of any significant signaling from the cγc cytokines, become very sensitive to the apoptotic effects of TGF-β. In the contraction phase, IL-15 supports the survival of both MPECs and SLECs, while TGF-β continues to oppose the survival signals by lowering the Bcl-2 levels. The 2-fold decrease in Bcl-2 levels by TGF-β does not impact the survival of MPECs that already have high levels of Bcl-2, whereas it makes SLECs much more susceptible to apoptosis as it drops the already low levels of Bcl-2 below the survival threshold. Finally, as IL-15 levels subside, the MPECs continue to proliferate and survive in response to IL-7, whereas the SLECs that cannot proliferate eventually die.