Abstract
4-Arylamino and 2- cycloalkyl (including amino substitution) modifications were made in a series of 1H-imidazo-[4,5-c]quinolin-4-amine derivatives as allosteric modulators of the human A3 adenosine receptor (AR). In addition to allosteric modulation of the maximum functional efficacy (in [35S]GTPγS G protein binding assay) of the A3AR agonist Cl-IB-MECA (15), some analogues also weakly inhibited equilibrium radioligand binding at ARs. 4-(3,5-Dichlorophenylamino) (6) or 2-(1-adamantyl) (20) substitution produced allosteric enhancement (twice the maximal agonist efficacy), with minimal inhibition of orthosteric AR binding. 2-(4-Tetrahydropyranyl) substitution abolished allosteric enhancement but preserved inhibition of orthosteric binding. Introduction of nitrogen in the six-membered ring at 2 position, to improve aqueous solubility and provide a derivatization site, greatly reduced the allosteric enhancement. 2-(4-(Benzoylamino)cyclohexyl) analogues 23 and 24 were weak negative A3AR modulators. Thus, consistent with previous findings, the allosteric and orthosteric inhibitory A3AR effects in imidazoquinolines are structurally separable, suggesting the possible design of additional derivatives with enhanced positive or negative allosteric A3AR activity and improved selectivity in comparison to inhibition of orthosteric binding.
Keywords: nucleoside, G protein-coupled receptor, allosterism, adenosine receptor, radioligand binding, imidazoquinolines
Introduction
The adenosine receptors (ARs), of which A1, A2A, A2B, and A3 subtypes have been defined, represent a physiologically important family of G protein-coupled receptors (GPCRs).1,2 ARs are important pharmacological targets in the treatment of a variety of diseases because of their key roles in controlling numerous cellular processes. For example, A3AR agonists are of interest for the treatment of cardiac ischemia, bowel inflammation, protection of skeletal muscle, cancer, and rheumatoid arthritis.3-7 However, therapeutic intervention using a selective AR agonist is subject to side effects related, in part, to the widespread occurrence of the corresponding receptor throughout the body.
Native agonists of a given GPCR bind at a principal (orthosteric) site on the receptor protein to effect its activation. However, an allosteric modulator would bind to a distinct site on the receptor to either enhance (positive modulator) or impede (negative modulator) the action of a native agonist.8-11 The therapeutic application of allosteric modulation has advantages over directly-acting orthosteric GPCR agonists. In a particular disease state, the effect of an endogenous agonist, which may be insufficient to fully compensate for an imbalance, may be magnified in a temporally and/or spatially specific manner by a positive allosteric modulator for therapeutic benefit.12 The allosteric modulator theoretically would have no effect of its own on the unoccupied receptor. An additional advantage of allosteric modulators is that they typically are found to display higher subtype-selectivity than orthosteric ligands of the same receptor. Thus, allosteric action that is dependent on the simultaneous presence of an endogenous ligand ideally can produce a more selective drug action and prevent side effects and possible over-dosage associated with the administration of a conventional orthosteric agonist.
Allosteric modulators of several subtypes of ARs have been reported and their structure-activity relationships (SARs) explored.8,13,14 N-(3,4-Dichlorophenyl)-2-cyclohexyl-1H-imidazo[4,5-c]quinolin-4-amine (LUF6000, 3, Chart 1)15-17 is an allosteric modulator of the human A3AR that increases the maximum efficacy of the agonist 2-chloro-N6-(3-iodobenzyl)adenosine-5′-N-methylcarboxamide (Cl-IB-MECA, 15). A six-membered ring provided the optimal A3AR enhancement in the homologous series of 2-cycloalkyl derivatives 1−4. Compound 3 enhanced the A3AR agonist efficacy in a functional assay and decreased the agonist dissociation rate without influencing agonist potency, because of decreased interaction with the orthosteric binding site on the A3AR. Since the structural requirements for allosteric enhancement at the A3AR are distinct from the requirements to inhibit equilibrium binding, structural manipulation of this family of imidazoquinolines might achieve even greater selectivity.
Chart 1.
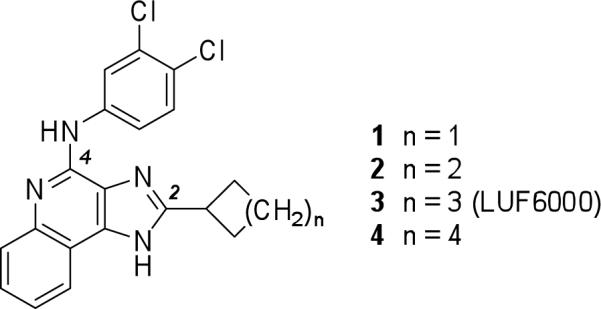
Structures of a series of 2-cycloalkyl imidazoquinoline derivatives previously found to be positive allosteric modulators of the human A3AR.
In the present study we have extended our search for highly effective allosteric enhancer ligands for the A3AR, by modifying the substitutions around the 4-arylamino and 2-cycloalkyl moieties of the imidazoquinoline scaffold. We have also identified weak negative allosteric modulators in the same structural series.
Results and Discussion
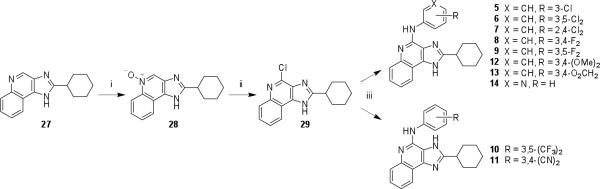
The novel 4-substituted imidazoquinoline derivatives 5−14 (Scheme 1) were prepared from a key 4-chloro-2-cyclohexyl intermediate 29. Oxidation of 2715 with 3-chloroperoxybenzoic acid (m-CPBA) afforded the 5-oxide derivative 28, which was subsequently converted with phosphorus oxychloride into a 4-chloro species 29.15,18 Reaction of 29 with the appropriately substituted aniline provided the desired 4-amino derivatives 5−14 in varying yields (4−100%) where the 3-aminopyridyl derivative 14 being the lowest. Attempts to prepare an ortho-pyridyl derivative (i.e., using 2-aminopyridine) under the same reaction condition with 29 failed.
Scheme 1.
Synthesis of novel 1H-imidazo-[4,5-c]quinolin-4-amine derivatives with structural variation at the 4 position.a
a Reagents: (i) m-CPBA, CHCl3/CH2Cl2/MeOH; (ii) POCl3, toluene/DMF; (iii) R-PhNH2, DMF.
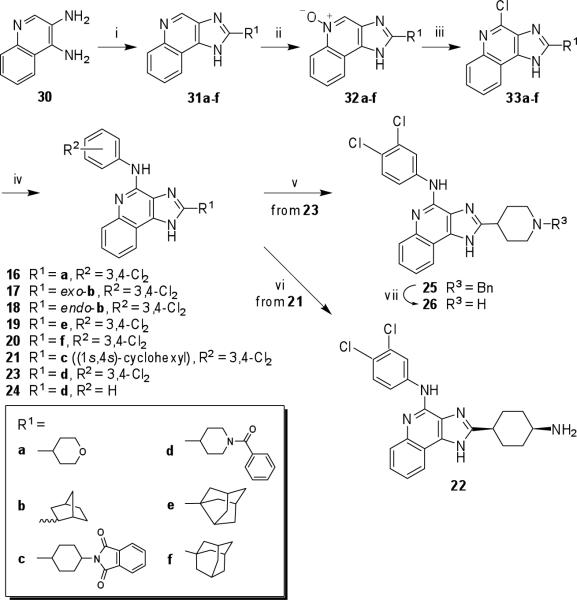
The novel 2-substituted imidazoquinoline derivatives 16−26 (Scheme 2) were prepared similarly. However, here different 2-cycloalkyl groups were appended, each as its carboxylic acid form, to a common intermediate 30 in an earlier synthetic step to give the corresponding imidazoquinoline derivatives 31a-f. In fact, compound 30 also served as a precursor for 27.15 Next, compounds 31a-f were treated with m-CPBA to afford 5-oxide derivatives 32a-f, followed by phosphorus oxychloride to give the respective 4-chloro compounds 33a-f.15,18 Reaction of the 4-chloro-2-cycloalkyl derivatives 33a−f with 3,4-dichloroaniline afforded the desired compounds (16−21, 23). Aniline was used instead in the synthesis of compound 24.
Scheme 2.
Synthesis of novel 1H-imidazo-[4,5-c]quinolin-4-amine derivatives with structural variation at the 2 position.a
a Reagents: (i) polyphosphoric acid, R1CO2H; (ii) m-CPBA, CHCl3/CH2Cl2/MeOH; (iii) POCl3, toluene/DMF; (iv) R2-PhNH2, DMF; (v) LiAlH4, THF; vi) MeNH2, EtOH; (vii) H2/Pd, MeOH.
Several alkylamino derivatives, 22 and 26, were included. In the case of a 2-(4-cis-aminocyclohexyl) analogue 22, it was necessary to remove the amine-protecting group of 21 in order to obtain the final compound. The deprotection was accomplished using methylamine in ethanol. In the case of the piperidine derivative 26, the reduction of the carbonyl group of 23 with lithium aluminum hydride afforded the N-benzyl derivative 25, which was subsequently converted into the desired compound 26 by hydrogenation.
To obtain a general procedure for the aniline substitution reaction, we first attempted the synthesis following the method recently described by Göblyös et al.,15 using a microwave reactor (in ethanol under nitrogen, pre-stirring 60 sec, at 120 °C for 40 min, normal sample absorption, fixed hold time). Although the reaction was performed on a very small scale, we were able to determine preferred conditions to be N,N-dimethylformamide (DMF) as a solvent and using 2−3 equivalents of corresponding aniline with heating at 140 °C overnight under a N2 atmosphere in a tightly sealed Biotage reaction vial. In an earlier attempted reaction at 105 °C under the same conditions, no desired product was obtained.18
The structures of the imidazoquinoline derivatives were analyzed by NMR in dimethyl sulfoxide (DMSO)-d6. In all final compounds except the 2-exo-norbornyl-4-(3,4-dichlorophenyl)amino analogue 18 that was attached in an axial position, the central imidazoquinoline system was clearly attached to the six-membered ring in an equatorial position. This was demonstrated based on the J coupling of the proton at the junction (Jax−ax = ca. 12−11 and Jax−eq = ca. 5−3). We also noted the fact that potentially there are two possible annular tautomers (1H or 3H at the imidazole ring), which may affect the GPCR binding equilibrium depending on the stability of each form in the aqueous media. 2D NOESY experiments in DMSO-d6 (see Supporting Information) suggested the 3H-tautomer as an exclusive form for compounds 10 and 11. Interestingly, for compound 14, two tautomers were observed as correlated by NOE cross-peaks (1H-/3H- ≈ 88:12 by NMR integration in DMSO-d6). Unlike other compounds, detection of two competing tautomers on the NMR time-scale could be due to the formation of a hydrogen bond between the pyridyl nitrogen (acceptor) and the imidazole NH (donor) as a less preferred 3H-tautomer.
All of the imidazoquinoline derivatives were evaluated for interaction with the human A3 receptors and two other ARs as listed in Tables 1 and 2. We first tested the effect of these compounds on the equilibrium binding at A1, A2A, and A3ARs using standard agonist radioligands19-21 [3H]2-chloro-N6-cyclopentyladenosine ([3H]CCPA, 34), [3H]2-[4-(2-carboxylethyl)phenylethylamino]-5′-N-ethylcarboxamidoadenosine ([3H]CGS21680, 35), and [125I]N6-(4-amino-3-iodobenzyl)adenosine-5′-N-methylcarboxamide ([125I]I-AB-MECA, 36), respectively. For compounds 5−14 and 16−26, only the percent inhibition of orthosteric radioligand binding was reported rather than Ki values, because the affinity at all three subtypes is weak and close to the solubility limits of the compounds. It is unknown whether the observed inhibition is of an allosteric or non-allosteric character.
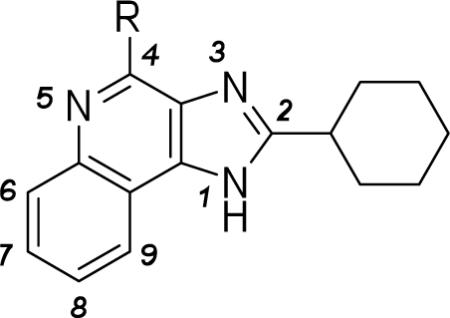
Table 1.
Potency of 4-anilino substituted 1H-imidazo-[4,5-c]quinolin-4-amine derivatives in binding assays at the human A1 and A3ARs expressed in CHO cells and at the human A2A in HEK-293 cells and the allosteric effects at the human A3AR.a
 | ||||||
|---|---|---|---|---|---|---|
| No. | R = | A1AR, %displ. at 10 μM | A2AAR, %displ. at 10 μM | A3AR, %displ. at 10 μM | A3AR ag. dissociation,b % at 10 μM | [35S]GTPγS binding in A3AR cells,c % at 10 μM |
| 3 | 3,4-Cl2-PhNH | 37±9 | −11.2±2.8 | 40.5±13.4 | 192±7 | 208±14 |
| 5 | 3-Cl-PhNH | 53.3±3.8 | 42.8±3.7 | 79.2±1.3 | 194±3 | 200±13 |
| 6 | 3,5-Cl2-PhNH | 16.7±2.9 | −11.4±10.9 | 32.7±4.8 | 184±5 | 209±22 |
| 7 | 2,4-Cl2-PhNH | 48.0±1.1 | 67.2±2.4 | 43.9±0.1 | 174±5 | 187±20 |
| 8 | 3,4-F2-PhNH | 14.6±4.8 | 26.5±7.3 | 68.8±1.2 | 187±1 | 197±17 |
| 9 | 3,5-F2-PhNH | 36.6±5.3 | 17.2±2.1 | 70.1±7.9 | 188±3 | 171±7 |
| 10 | 3,5-(CF3)2-PhNH | 7.2±2.3 | 15.6±0.3 | 21.8±1.4 | 137±5 | 143±3 |
| 11 | 3,4-(CN)2-PhNH | 33.1±4.1 | 37.2±1.5 | 42.2±2.9 | 129±7 | 138±7 |
| 12 | 3,4-(OMe)2-PhNH | 75.6±2.1 | 93.8±1.6 | 98.4±0.1 | 173±1 | 182±14 |
| 13 | 3,4-O2CH2-PhNH | 65.4±3.3 | 49.0±0.7 | 83.9±1.1 | 185±6 | 166±11 |
| 14 | 3-pyridyl-NH | 73.1±2.5 | 78.1±1.8 | 64.8±6.9 | 114±5 | 119±1 |
All experiments were performed using adherent mammalian cells stably transfected with cDNA encoding the human ARs. Binding at human A1, A2A, and A3ARs in this study was carried out as described in the Experimental Procedures using [3H]34, [3H]35, or [125I]36 as a radioligand.19-21 Values from the present study are expressed as mean ± s.e.m., n = 3−5. Percentage inhibition at A1, A2A, or A3 receptors is expressed as the mean value from 2−4 separate experiments with similar results performed in duplicate.
Dissociation: % decrease of [125I]36 dissociation at 60 min (control = 100%).
Increase of efficacy in the stimulation of the binding of [35S]GTPγS: compared to maximal effect induced by 10 μM 15 alone (set to 100%). It should be noted that the Emax of 15 in this functional assay was recently demonstrated to be about 50% of that of 37.17 In the adenylate cyclase assay, 15 and 37 were both full agonists.15
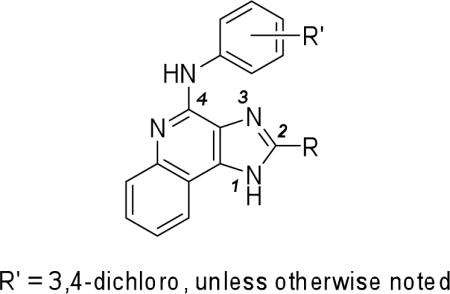
Table 2.
Potency of 2-cycloalkyl substituted 1H-imidazo-[4,5-c]quinolin-4-amine derivatives in binding assays at the human A1 and A3ARs expressed in CHO cells and at the human A2A in HEK-293 cells and the allosteric effects at the human A3AR.a
 | ||||||
|---|---|---|---|---|---|---|
| No. | R = | A1AR, %displ. at 10 μM | A2AAR, %displ. at 10 μM | A3AR, %displ. at 10 μM | A3AR ag. dissociation,c % at 10 μM | [35S]GTPγS binding in A3AR cells,d % at 10 μM |
| 1b | 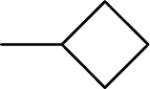 |
−5.2 | 0.4 | 52 | 116±3 | 126±3b |
| 2b | 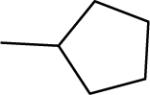 |
15 | 0 | 67e | 144±9 | 141±5b |
| 3 | 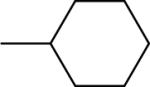 |
37±9 | −11.2±2.8 | 40.5±13.4 | 192±7 | 208±14 |
| 4b | 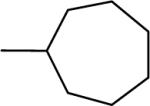 |
−4.2 | −2.2 | 68 | 130±2 | 115±7b |
| 16 | 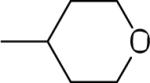 |
37.2±8.6 | −11.9±2.8 | 69.6±1.4 | 115±12 | 114±7 |
| 17 | 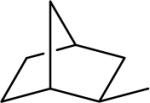 |
77.9±12.2 | 5.8±1.0 | 61.2±1.4 | 187±16 | 201±26 |
| 18 | 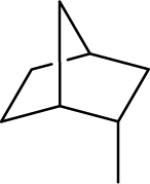 |
44.8±10.5 | 39.4±1.9 | 50.5±5.5 | 168±8 | 168±13 |
| 19 | 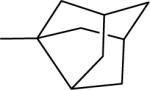 |
14.5±2.7 | −17.3±6.9 | 3.3±4.1 | 139±10 | 156±2 |
| 20 | 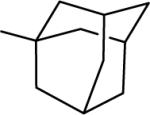 |
13.8±6.9 | −4.4±12.5 | 17.2±6.7 | 196±13 | 210±12 |
| 21 | 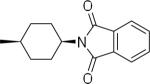 |
21.4±4.3 | 56.8±1.7 | 5.6±4.9 | 102±5 | 96±5 |
| 22 | 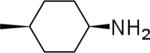 |
31.7±16.2 | 15.8±1.2 | 4.2±6.1 | 107±11 | 111±6 |
| 23 | 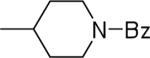 |
20.2±10.1 | 8.8±1.9 | 29.8±0.6 | 91±9 | 93±7 |
| 24 | 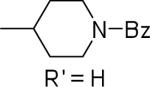 |
12.4±1.1 | 43.4±5.5 | 35.3±1.5 | 92±5 | 93±1 |
| 25 | 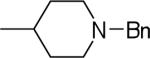 |
17.8±3.6 | −3.1±1.6 | 46.3±0.8 | 93±6 | 97±5 |
| 26 | 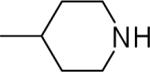 |
10.9±8.6 | 7.0±0.7 | 52.8±1.1 | 101±7 | 99±3 |
All experiments were performed using adherent mammalian cells stably transfected with cDNA encoding the human ARs. Binding at human A1, A2A and A3ARs in this study was carried out as described in the Experimental Procedures using [3H]34, [3H]35, or [125I]36 as a radioligand.19-21 Values from the present study are expressed as mean ± s.e.m., n = 3−5. Percentage inhibition at A1, A2A, or A3 receptors is expressed as the mean value from 2−4 separate experiments with similar results performed in duplicate.
Values from Göblyös et al,15 the functional enhancement of which was measured with the cyclic AMP assay.26
Dissociation: % decrease of [125I]36 dissociation at 60 min (control = 100%).
Increase of efficacy in the stimulation of the binding of [35S]GTPγS: compared to maximal effect induced by 10 μM 15 alone (set to 100%). It should be noted that the Emax of 15 in this functional assay was recently demonstrated to be about 50% of that of 37.17 In the adenylate cyclase assay, 15 and 37 were both full agonists.15
Ki value in a binding assay15 = 4690±970 nM.
Ability to allosterically modulate the A3AR was determined using two methods: effects of the imidazoquinoline on the dissociation rate of 36 ([125I]I-AB-MECA) and on the binding to the G protein of the stable GTP analogue [35S]guanosine-5′-(γ-thiotriphosphate) ([35S]GTPγS).22 Depending on the functional assay used, 15 (Cl-IB-MECA) may appear to be either a full15 or partial agonist17,23-25 at the A3AR. The earlier series of imidazoquinoline derivatives displayed dual and apparently opposite actions as positive allosteric modulators of agonist action and as inhibitors of radioligand binding.15 In the previous study, compound 3 induced a substantial functional enhancement of the effects of the A3AR agonist 15 as determined using an agonist radioligand dissociation kinetic assay and a cyclic AMP assay,15,26 while there was only a weak inhibition of equilibrium radioligand binding at ARs at the concentration used.
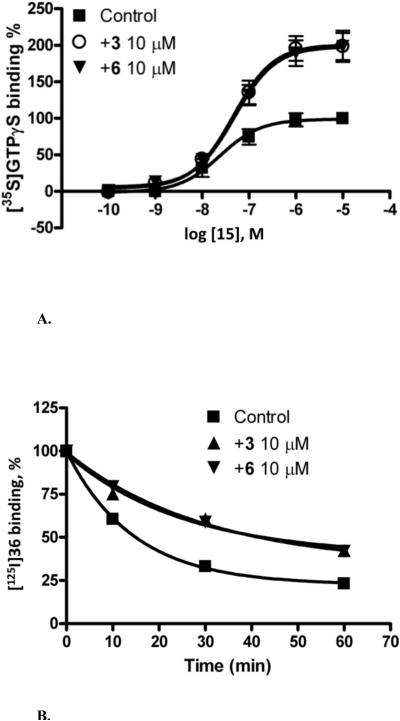
The potentiation of the maximum efficacy of the agonist 15 by arylhalo derivatives 5−9 (Table 1) was high and similar to that observed with the lead compound 3. The percent of maximal functional effect ranged from 171% for 9 to 209% for 6. Among these halo analogues, 6 had the least inhibition of orthosteric binding to ARs. The degree of enhancement of [35S]GTPγS binding by 6 over a range of concentrations of 15 up to 10 μM was indistinguishable from the effect of the lead compound 3 (Figure 1A). Similarly, the marked decrease in dissociation rate of the A3AR agonist radioligand [125I]36 produced by 6 was indistinguishable from the effect of compound 3 (Figure 1B). A 3,5-di-(trifluoromethyl) analogue 10 and another aniline derivative 11 that was disubstituted with electron withdrawing groups displayed an intermediate degree of allosteric enhancement. Other 3,4-disubstituted anilines 12 and 13 that contained electron-donating groups displayed a high degree of allosteric enhancement, but also substantial inhibition of orthosteric binding to the A3AR and other ARs. The 3-pyridinylamine 14 had a low degree of allosteric enhancement.
Figure 1.
Allosteric modulation of the human A3AR by compound 6. A) Functional assay of the human A3AR. The % stimulation of binding of [35S]GTPγS by increasing concentrations of 15 under control conditions or in the presence of 10 μM of compound 3 or 6. B) Radioligand binding studies on the human A3AR. Study of the dissociation kinetics of the agonist radioligand [125I]36 under control conditions and in the presence of 10 μM of compound 3 or 6.
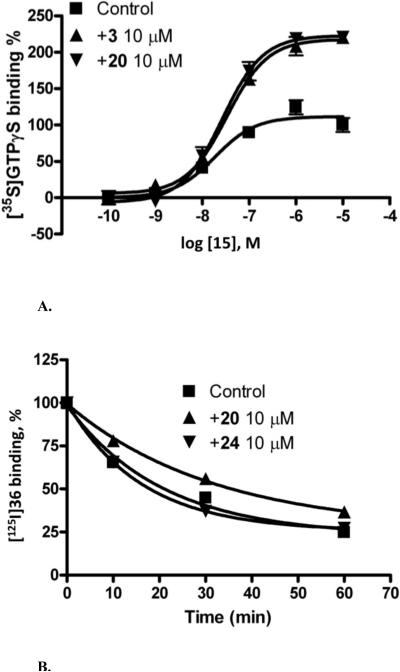
Highly variable biological activities were observed for the 2 position derivatives 16−26 (Table 2). Replacement of the distal methylene group of the 2-cyclohexyl ring of 3 with an ether oxygen in the tetrahydropyranyl derivative 16 abolished allosteric modulation of the A3AR, but retained a similar degree of orthosteric inhibition. Inclusion of a methylene bridge across the 2-cyclohexyl ring of 3 in 17 and 18 resulted in considerable allosteric potentiation. Multiply bridged cycloalkyl substitution in the 2-(1-adamantyl)-4-(3,4-dichlorophenyl)amino analogue 20 resulted in high allosteric potentiation with minimal effects on the binding of orthosteric ligands at A1, A2A, and A3ARs. The 2-exo-noradamantyl-4-(3,4-dichlorophenyl)amino analogue 19 displayed only moderate allosteric potentiation. The (decreasing) order of allosteric enhancement by these bridged cycloalkyl derivatives was: 20, 17 > 18 > 19. The adamantyl derivative 20 increased [35S]GTPγS binding over a range of concentrations of 15 up to 10 μM in a manner indistinguishable from the effect of 3 (Figure 2A).
Figure 2.
Allosteric modulation of the human A3AR by compound 20 or 24. A) Functional assay of the human A3AR. The % stimulation of binding of [35S]GTPγS by increasing concentrations of 15 under control conditions or in the presence of 10 μM of compound 3 or 20. B) Radioligand binding studies on the human A3AR. Study of the dissociation kinetics of the agonist radioligand [125I]36 under control conditions and in the presence of 10 μM of compound 20 or 24.
Introduction of a nitrogen atom in (or as substituted at) the six-membered ring in 21−26 in an attempt to improve aqueous solubility and to provide a site for further derivatization greatly reduced the allosteric enhancement of this series. Variable degrees of inhibition of orthosteric binding at the AR were observed in this group with the most pronounced inhibition found for 21 and 24 at the A2AAR and for 25 and 26 at the A3AR. The slightly decreased agonist efficacy (91−93% of control in both functional assays, Table 2) of 2-(N-benzoyl-4-piperidinyl) analogues 23 and 24 was suggestive of possible negative allosteric modulation of the A3AR. The effect of compound 24 on the dissociation kinetics of the agonist radioligand was only slightly different from control (Figure 2B).
Conclusions
In this study, structural modification of the 1H-imidazo-[4,5-c]quinolin-4-amine 3 has demonstrated that a limited set of substituents at the 2 and 4 positions are tolerated to preserve the allosteric enhancement of agonist action at the A3AR. Notably, the haloanilino derivatives 5, 6, and 8 and the bridged 2-cycloalkyl analogues 17 and 20 approximately doubled the maximum efficacy of the agonist 15 in the [35S]GTPγS assay. The highest potentiation of the maximum efficacy of the agonist 15, without increased inhibition of orthosteric binding, was observed for the 2-(1-adamantyl)-4-(3,4-dichlorophenyl)amino analogue 20, with 210% activity of control in the [35S]GTPγS binding assay. Compounds 6 and 20 were preferred as selective allosteric enhancers of the A3AR due to the minimal effect on binding at the orthosteric sites of the three ARs examined. Substitution of a 4-tetrahydropyran moiety at the 2 position completely abolished allosteric enhancement but preserved inhibition of othosteric binding. Thus, as extension of previous findings, the allosteric and orthosteric inhibitory effects at the A3AR in this series of imidazoquinolines are structurally separable. These biological results suggest that it will be possible to design additional derivatives that would display enhanced positive or negative allosteric activity at this receptor and improved selectivity in comparison to inhibition of orthosteric binding.
Experimental Procedures
General
Glassware was oven-dried and cooled in a desiccator before use. All reactions were carried out under a dry nitrogen atmosphere. Solvents were purchased as anhydrous grade and used without further purification. Suppliers of some commercial compounds are listed as follows: 3,4-diaminoquinoline (30) was purchased from Tyger Scientific, Inc.; 3,5-bis(trifluoromethyl)aniline and 4-aminophthalonitrile were purchased from Acros Organics; polyphosphoric acid, m-CPBA, phosphorus oxychloride (POCl3), chloroform (CHCl3), methylene chloride (CH2Cl2), DMF, toluene, diisopropyl ether, methanol (MeOH), tetrahydrofuran (THF), and most of other reagents and solvents were purchased from Sigma-Aldrich; dimethyl sulfoxide (DMSO)-d6, chloroform-d (CDCl3), and CD3OD were purchased from Cambridge Isotope Laboratories. All reagents were of commercial grade and were used without further purification unless otherwise noted.
NMR spectra were recorded on either a Varian Inova/Gemini 300 or a Bruker DRX-600 spectrometer at 25.0 °C under an optimized parameter setting for each sample, unless otherwise mentioned. For compounds 5−14, 28, and 29, 1H NMR chemical shifts were measured relative to the residual solvent peak at 2.50 ppm in DMSO-d6 and at 3.31 ppm in CD3OD or in a mixture of CD3OD/CDCl3. For compounds 16−26, 31a−f, 32a−f, and 33a−f, 1H NMR chemical shifts were measured relative to tetramethylsilane at 0.00 ppm in CDCl3 and the residual water peak at 3.30 ppm in CD3OD. 13C NMR chemical shifts were measured relative to the residual solvent peak at 49.15 ppm in CD3OD or in a mixture of CD3OD/CDCl3. Suggested NMR peak assignments of some target compounds are shown in Supporting Information based on 2D COSY and NOESY experiments.
Analytical or preparative thin layer chromatography (TLC) was performed on either 0.2 mm silica coated sheets with F254 indicator (Sigma-Aldrich) or 0.2 mm reversed-phase C18 silica coated sheets (pore size: 60 Å, Whatman Inc.). Visualization of the products on the TLC plate was aided by the use of UV light, ninhydrin, or potassium permanganate. Column chromatography was performed on 230−400 mesh silica gel (pore size: 60 Å, Sigma-Aldrich). The tested imidazoquinoline derivatives were confirmed by HPLC to possess a ≥ 96% purity.
The electrospray ionization (ESI) MS experiments were performed on a Micromass/Waters LCT Premier Electrospray time-of-flight (TOF) mass spectrometer coupled with a Waters HPLC system at the Mass Spectrometry Facility, NIDDK, NIH.
General procedure for 2-substituted 1H-imidazo[4,5-c]quinoline (31a−f)
Polyphosphoric acid (1.3 mL/mmol) was added to 3,4-diaminoquinoline (30) (100 mg, 0.63 mmol) and the appropriate carboxylic acid (1.2 equiv). The mixture was stirred at 100 °C for 5 h. Then the reaction was cooled to 0 °C and to it was slowly added NH4OH till pH = 8−9. The mixture was extracted with ethyl acetate (3 × 15 mL), the combined organic extracts were washed with water, brine, and again with water, and then dried over MgSO4. The solution was filtered, the solvent was evaporated, and the residue was dried in vacuo. The residue obtained was subjected to preparative silica gel column chromatography (100:1 to 8:1 CHCl3/MeOH).
2-(Tetrahydro-2H-pyran-4-yl)-1H-imidazo[4,5-c]quinoline (31a)
Yield: 107.6 mg (67%). 1H NMR (300 MHz, CD3OD/CDCl3) δ 13.40 (s, 1H), 9.13 (s, 1H), 8.35 (s, 1H), 8.08 (m, 1H), 7.65 (m, 2H), 3.98 (m, 2H), 3.35 (dd, 2H, J = 11.4, 10.2 Hz), 3.28 (t, 1H, J = 11.3 Hz), 2.05 (m, 2H), 1.95 (dd, 1H, J = 11.6, 4.2 Hz), 1.93 (dd, 1H, J = 11.6, 4.2 Hz); 13C NMR (75 MHz, CD3OD/CDCl3) δ 143.4, 129.5, 126.3, 121.6, 66.7, 31.07; HRMS (ESI) calcd for C15H16N3O+ (M + H+): 254.1288, found: 254.1293.
2-(2-Norbornanyl)-1H-imidazo[4,5-c]quinoline (31b)
Yield: 86.43 mg (52%). 1H NMR (300 MHz, CD3OD/CDCl3) δ 13.33 (bs, 1H), 9.11 (s, 1H), 8.32 (s, 1H), 8.08 (m, 1H), 7.64 (m, 2H), 3.45 (m, 1H), 3.36 (s, 2H), 3.17 (s, 2H), 3.06 (s, 1H), 2.72 (m, 1H), 2.58 (m, 1H), 2.50 (s, 1H), 2.38 (m, 1H), 1.44 (m, 7H); 13C NMR (75 MHz, CD3OD/CDCl3) δ 143.85, 129.57, 125.98, 121.43, 48.59, 42.59, 36.61, 35.78, 29.10, 28.59, 23.60; HRMS (ESI) calcd for C17H18N3+ (M + H+): 264.1495, found: 264.0561.
2-(4-(1,3-Dioxoisoindolin-2-yl)cyclohexyl)-1H-imidazo[4,5-c]quinoline (31c)
Yield: 88.0 mg (35%). 1H NMR (300 MHz, CD3OD/CDCl3) δ 13.35 (bs, 1H), 9.13 (s, 1H), 8.35 (s, 1H), 8.07 (m, 1H), 7.69 (m, 6H), 4.16 (m, 1H), 3.06 (t, 1H, J = 12.2 Hz), 2.36 (m, 4H), 1.88 (m, 4H); 13C NMR (75 MHz, CD3OD/CDCl3) δ 167.9, 167.7, 143.4, 134.3, 129.5, 126.1, 122.9, 121.7, 49.5, 36.9, 30.7, 28.8; HRMS (ESI) calcd for C24H21N4O2+ (M + H+): 397.1659, found: 397.1234.
2-(1-Benzoylpiperidin-4-yl)-1H-imidazo[4,5-c]quinoline (31d)
Yield: 142 mg (67%). 1H NMR (300 MHz, CD3OD/CDCl3) δ 13.38 (s, 1H), 9.13 (s, 1H), 8.35 (s, 1H), 8.09 (s, 1H), 7.66 (s, 1H), 7.46 (s, 1H), 4.56 (s, 1H), 3.71 (s, 1H), 3.13 (s, 1H), 2.17 (s, 1H), 1.91 (s, 1H); 13C NMR (75 MHz, CD3OD/CDCl3) δ 169.1, 143.4, 136.3, 129.4, 128.5, 126.6, 121.4, 59.8, 46.8, 41.2, 35.8, 30.4; HRMS (ESI) calcd for C22H21N4O+ (M + H+): 357.1710, found: 357.1732.
2-(3-Noradamantanyl)-1H-imidazo[4,5-c]quinoline (31e)
Yield: 118.7 mg (68%). 1H NMR (300 MHz, CD3OD/CDCl3) δ 8.98 (s, 1H), 8.36 (s, 1H), 8.03 (d, 1H, J = 7.8 Hz), 7.52 (m, 2H), 2.77 (t, 1H, J = 6.9 Hz), 2.33 (s, 2H), 2.20 (m, 2H), 2.06 (m, 3H), 1.99 (m, 2H), 1.68 (m, 4H); 13C NMR (75 MHz, CD3OD/CDCl3) δ 162.7, 143.2, 128.3, 121.5, 45.6, 43.6, 38.1, 37.8, 37.5, 37.3, 34.3, 30.4; HRMS (ESI) calcd for C19H20N3+ (M + H+): 290.1652, found: 290.1670.
2-(1-Adamantanyl)-1H-imidazo[4,5-c]quinoline (31f)
Yield: 75.8 mg (39%). 1H NMR (300 MHz, CD3OD/CDCl3) δ 9.11 (s, 1H), 8.30 (s, 1H), 8.15 (d, 1H, J = 8.4 Hz), 7.53 (t, 1H, J = 7.2 Hz), 7.43 (d, 1H, J = 7.2 Hz), 2.21 (s, 6H), 2.05 (s, 4H), 1.72 (m, 6H); 13C NMR (75 MHz, CD3OD/CDCl3) δ 143.6, 129.1, 127.4, 126.6, 121.9, 41.6, 36.5, 36.0, 228.3, 1.2; HRMS (ESI) calcd for C20H22N3+ (M + H+): 304.1808, found: 304.1823.
General procedure for 2-substituted 1H-imidazo[4,5-c]quinoline-5-oxide (28, 32a−f)
The appropriate 2-substituted 1H-imidazo[4,5-c]quinoline derivatives (27, 31a−f) in a mixture of CHCl3 (2.5 mL/mmol), CH2Cl2 (2.5 mL/mmol) and MeOH (0.25 mL/mmol) was heated for dissolution. To this mixture was added m-CPBA (2.5 equiv), which was then refluxed for 30 min. The reaction was cooled to rt, Na2CO3 (0.04 g/mmol) was added in one portion as a solid, and then the mixture was refluxed for 1 h. The solvent was removed in vacuo, and the crude product was chromatographed on silica gel (20:1 to 7:1 CH2Cl2/MeOH for 28; 100:1 to 8:1 CHCl3/MeOH for 32a−f) to give the desired compound.
2-Cyclohexyl-1H-imidazo[4,5-c]quinoline 5-oxide (28).15
Scale: 0.612 mmol. Yield: 150 mg (91%). Rf: 0.43 [silica gel, 10:1 CH2Cl2/MeOH]; 1H NMR (300 MHz, CD3OD) δ 9.03 (s, 1H), 8.78−8.72 (m, 1H), 8.47 (m, 1H), 7.89−7.83 (m, 2H), 3.06 (tt, 1H, J = 11.8, 3.5 Hz), 2.20−2.14 (m, 2H), 1.98−1.91 (m, 2H), 1.86−1.69 (m, 3H), 1.60−1.32 (m, 3H); 13C NMR (75 MHz, CD3OD) δ 130.9, 130.6, 130.4, 129.0, 123.5 121.2, 40.2, 32.9, 27.2, 27.0; HRMS (ESI) calcd for C16H18N3O (M + H+): 268.1450, found: 268.1451.
2-(Tetrahydro-2H-pyran-4-yl)-1H-imidazo[4,5-c]quinoline-5-oxide (32a)
Scale: 0.42 mmol. Yield: 65 mg (57%). 1H NMR (300 MHz, CD3OD/CDCl3) δ 9.18 (s, 1H), 8.80 (m, 1H), 8.53 (m, 1H), 7.85 (m, 2H), 4.17 (m, 2H), 3.68 (m, 2H), 3.36 (m, 1H), 2.15 (m, 4H); HRMS (ESI) calcd for C15H16N3O2+ (M + H+): 270.1237, found: 270.1032.
2-(2-Norbornanyl)-1H-imidazo[4,5-c]quinoline-5-oxide (32b)
Scale: 0.25 mmol. Yield: 44.4 mg (60%). 1H NMR (300 MHz, CD3OD/CDCl3) δ 8.77 (s, 1H), 8.05 (s, 1H), 7.95 (s, 1H), 7.81 (m, 2H), 3.13 (mm, 1H), 2.81 (m, 1H), 2.68 (s, 1H), 2.52 (s, 1H), 2.14 (m, 1H), 1.93 (m, 1H), 1.58 (m, 7H); HRMS (ESI) calcd for C17H18N3O+ (M + H+): 280.1444, found: 280.1443.
2-(4-(1,3-Dioxoisoindolin-2-yl)cyclohexyl)-1H-imidazo[4,5-c]quinoline-5-oxide (32c)
Scale: 0.40 mmol. Yield: 65 mg (44%). 1H NMR (300 MHz, CD3OD/CDCl3) δ 8.96 (s, 1H), 8.63 (m, 1H), 8.32 (m, 1H), 7.75 (m, 6H), 4.18 (m, 1H), 3.04 (t, 1H, J = 11.6 Hz), 2.24 (m, 4H), 1.84 (m, 4H); HRMS (ESI) calculated for C24H21N4O2+ (M + H+): 413.1608, found: 413.1463.
2-(1-Benzoylpiperidin-4-yl)-1H-imidazo[4,5-c]quinoline-5-oxide (32d)
Scale: 0.30 mmol. Yield: 74 mg (67%). 1H NMR (300 MHz, CD3OD/CDCl3) δ 8.79 (s, 1H), 8.57 (m, 1H), 8.18 (bs, 1H), 7.76 (s, 1H), 7.68 (m, 1H), 7.54 (m, 5H), 4.59 (m, 1H), 3.76 (m, 1H), 2.93 (m, 2H), 2.02 (m, 2H), 1.85 (m, 4H); HRMS (ESI) calcd for C22H20N4O2+ (M + H+): 372.1586, found: 373.1665.
2-(3-Noradamantanyl)-1H-imidazo[4,5-c]quinoline-5-oxide (32e)
Scale: 0.41 mmol. Yield: 89 mg (71%). 1H NMR (300 MHz, CD3OD/CDCl3) δ 8.77 (s, 1H), 8.52 (m, 1H), 8.29 (s, 1H), 7.71 (m, 1H), 7.53 (m, 2H), 2.67 (t, 1H, J = 6.7 Hz), 2.27 (s, 2H), 2.09 (d, 2H, J = 11.4 Hz), 1.95 (d, 1H, J = 10.8 Hz), 1.87 (m, 3H), 1.58 (m, 4H); HRMS (ESI) calcd for C19H20N3O+ (M + H+): 306.1601, found: 306.1606.
2-(1-Adamantanyl)-1H-imidazo[4,5-c]quinoline-5-oxide (32f)
Scale: 0.25 mmol. Yield: 63 mg (78%). 1H NMR (300 MHz, CD3OD/CDCl3) δ 8.91 (m, 1H), 8.60 (m, 1H), 8.31 (m, 1H), 7.90 (s, 1H), 7.80 (d, 1H, J = 7.5 Hz), 7.54 (m, 1H), 2.08 (s, 10H), 1.76 (s, 6H); HRMS (ESI) calcd for C20H22N3O+ (M + H+): 320.1757, found: 320.1753.
4-Chloro-2-cyclohexyl-1H-imidazo[4,5-c]quinoline (29).15
Compound 28 (150 mg, 560 μmol) was suspended in a mixture of toluene (0.30 mL) and DMF (0.60 mL), and then was treated with 140 μL (1.50 mmol) of POCl3 at 0 °C with stirring. The ice bath was removed, and the reaction was heated at 100 °C for 1.5 h. The mixture was cooled to rt, and a few small pieces of ice were added with stirring. Subsequently, the pH was adjusted to 6−7 with solid NaHCO3. The mixture was sonicated and filtered, rinsing with water and diisopropyl ether, and the collected solid was dried in vacuo to give 97.1 mg (340 μmol, 61%) of 29 as a beige solid. Rf: 0.56 [silica gel, 10:1 CH2Cl2/MeOH]; 1H NMR (600 MHz, DMSO-d6) δ 8.38−8.35 (m, 1H), 8.02−7.99 (m, 1H), 7.71−7.62 (m, 2H), 3.07 (tt, 1H, J = 12.0, 3.5 Hz), 2.16−2.10 (m, 2H), 1.97−1.91 (m, 2H), 1.88−1.75 (m, 3H), 1.59−1.35 (m, 3H); 13C NMR (75 MHz, CD3OD) δ 129.5, 129.3, 128.2, 122.8, 40.4, 33.0, 27.4, 27.0; HRMS (ESI) calcd for C16H17ClN3 (M + H+): 286.1111, found: 286.1106.
General procedure for 2-substituted 4-chloro-1H-imidazo[4,5-c]quinolines (33a−f)
A mixture of toluene (0.45 mL/mmol) and DMF (0.90 mL/mmol) was cooled in an ice bath, and phosphorus oxychloride (2.6 eq.) was added. After 10 min, the appropriate 1H-imidazo[4,5-c]quinolin-5-oxide was added, and the solution was stirred at rt for 10 min. Subsequently, the solution was heated to 100 °C for 30 min. Upon cooling, the solvent was evaporated, and the resulting syrup was poured on chipped ice while stirring. The mixture was then warmed to rt and carefully adjusted to pH 6−7 with solid NaHCO3. After 2 h, the formed solid was filtered off, washed with water and diisopropyl ether and subsequently dried. The residue obtained was subjected to preparative silica gel column chromatography (100:1 to 8:1 CHCl3/MeOH).
4-Chloro-2-(tetrahydro-2H-pyran-4-yl)-1H-imidazo[4,5-c]quinoline (33a)
Scale: 0.27 mmol. Yield: 41 mg (53%). 1H NMR (300 MHz, CD3OD/CDCl3) δ 13.66 (br s, 1H), 8.35 (m, 1H), 8.02 (m, 1H), 7.70 (m, 2H), 4.04 (m, 2H), 3.52 (dd, 2H, J = 11.1, 10.8 Hz), 3.25 (m, 1H), 1.95 (m, 4H); 13C NMR (75 MHz, CD3OD/CDCl3) δ 143.2, 128.1, 126.8, 121.2, 67.6, 36.1, 31.1; HRMS (ESI) calcd for C15H15ClN3O+ (M + H+): 288.0898, found: 288.0713.
4-Chloro-2-(2-norbornanyl)-1H-imidazo[4,5-c]quinoline (33b)
Scale: 0.25 mmol. Yield: 44.4 mg (60%). 1H NMR (300 MHz, CD3OD/CDCl3) δ 8.29 (m, 1H), 7.96 (m, 2H), 7.56 (m, 2H), 3.31 (m, 1H), 3.05 (s, 2H), 2.91 (s, 2H), 2.83 (m, 2H), 2.41 (m, 1H), 2.06 (m, 1H), 1.62 (m, 7H); 13C NMR (75 MHz, CD3OD/CDCl3) δ 143.0, 127.8, 126.5, 121.4, 42.8, 42.0, 36.3, 36.1, 29.6, 28.6; HRMS (ESI) calcd for C17H17ClN3+ (M + H+): 298.1106, found: 298.1104.
4-Chloro-2-(4-(1,3-dioxoisoindolin-2-yl)-1H-imidazo[4,5-c]quinoline (33c)
Scale: 0.27 mmol. Yield: 87 mg (74%). 1H NMR (300 MHz, CD3OD/CDCl3) δ 8.56 (m, 1H), 8.22 (d, 1H, J = 7.9 Hz), 8.09 (d, 1H, J = 8.5 Hz), 7.70 (m, 6H), 4.25 (m, 1H), 3.35 (m, 1H), 2.42 (m, 4H), 1.85 (m, 41H); 13C NMR (75 MHz, CD3OD/CDCl3) δ 168.5, 158.7, 144.2, 143.6, 134.1, 132.5, 132.3, 132.2, 129.0, 128.8, 123.3, 50.0, 37.8, 31.3, 29.3; HRMS (ESI) calcd for C24H20ClN4O2+ (M + H+): 431.1269, found: 431.1260.
4-Chloro-2-(1-benzoylpiperidin-4-yl)-1H-imidazo[4,5-c]quinoline (33d)
Scale: 0.20 mmol. Yield: 42.4 mg (54%). 1H NMR (300 MHz, CD3OD/CDCl3) δ 7.99 (s, 1H), 7.86 (d, 1H, J = 7.5 Hz), 7.48 (m, 3H), 7.26 (m, 5H), 4.63 (m, 1H), 3.76 (m, 1H), 3.18 (m, 1H), 2.87 (m, 1H), 2.05 (m, 2H), 1.92 (m, 4H); 13C NMR (75 MHz, CD3OD/CDCl3) δ 135.9, 129.9, 128.6, 128.3, 128.0, 127.0, 126.7, 121.4, 53.6, 36.8, 30.6; HRMS (ESI) calcd for C22H20ClN4O+ (M + H+): 391.1320, found: 391.1317.
4-Chloro-2-(3-noradamantanyl)-1H-imidazo[4,5-c]quinoline (33e)
Scale: 0.23 mmol. Yield: 60 mg (79%). 1H NMR (300 MHz, CD3OD/CDCl3) δ 8.37 (s, 1H), 8.10 (d, 1H, J = 7.8 Hz), 7.78 (m, 1H), 7.38 (m, 2H), 2.67 (m, 1H), 2.21 (s, 2H), 2.08 (m, 2H), 1.90 (m, 5H), 1.54 (s, 4H); 13C NMR (75 MHz, CD3OD/CDCl3) δ 162.9, 142.9, 128.2, 127.7, 121.4, 45.5, 43.5, 38.1, 37.7, 37.4, 37.2, 36.4, 34.3, 31.2; HRMS (ESI) calcd for C19H19ClN3+ (M + H+): 324.1262, found: 324.1268.
4-Chloro-2-(1-adamantanyl)-1H-imidazo[4,5-c]quinoline (33f)
Scale: 0.20 mmol. Yield: 39 mg (60%). 1H NMR (300 MHz, CD3OD/CDCl3) δ 8.34 (s, 1H), 7.95 (m, 2H), 7.45 (m, 2H), 2.15 (m, 10H), 1.78 (m, 6H); 13C NMR (75 MHz, CD3OD/CDCl3) δ 163.1, 143.0, 132.6, 127.8, 126.5, 121.5, 40.8, 36.4, 36.2, 35.8, 32.2, 28.1; HRMS (ESI) calcd for C20H21ClN3+ (M + H+): 338.1419, found: 338.1414.
General procedure for N-substituted 2-cyclohexyl-1H-imidazo[4,5-c]quinolin-4-amine (5−14)
A solution of compound 29 and an appropriate aniline in DMF was heated at 140 °C overnight under a dry nitrogen atmosphere in a tightly sealed Biotage microwave vial (size: 0.2−0.5 mL or 0.5−2.0 mL) equipped with a magnetic stir bar. The reaction mixture was cooled, the solvent was removed in vacuo, and the crude product was purified by a preparative TLC.
N-(3-Chlorophenyl)-2-cyclohexyl-1H-imidazo[4,5-c]quinolin-4-amine (5)
Compound 29 (6.7 mg, 23 μmol) was reacted with 3-chloroaniline (10 μL, 94 μmol) in DMF (50 μL). The crude product was chromatographed on silica gel (3:1 petroleum ether/EtOAc) to give 5.5 mg (15 μmol, 62%) of 5. Rf: 0.43 [silica gel, 2:1 petroleum ether/EtOAc]; 1H NMR (600 MHz, 4:1 CDCl3/CD3OD) δ 8.30 (s, 1H), 7.94 (d, 1H, J = 7.4 Hz), 7.89 (d, 1H, J = 8.5 Hz), 7.75 (d, 1H, J = 8.0 Hz), 7.48 (t, 1H, J = 7.5 Hz), 7.38 (t, 1H, J = 7.3 Hz), 7.24 (t, 1H, J = 7.9 Hz), 6.94 (d, 1H, J = 8.3 Hz), 2.92 (t, 1H, J = 11.9 Hz), 2.13 (d, 2H, J = 12.6 Hz), 1.90 (d, 2H, J = 13.3 Hz), 1.78 (d, 1H, J = 12.6 Hz), 1.67 (q, 2H, J = 11.7 Hz), 1.44 (q, 2H, J = 12.7 Hz), 1.32 (q, 1H, J = 12.9 Hz); 13C NMR (150 MHz, 4:1 CDCl3/CD3OD) δ 147.1, 143.9, 134.7, 130.1, 127.7, 127.6, 123.3, 121.8, 120.7, 118.8, 117.0, 115.7, 38.8, 32.2, 26.4, 26.1; HRMS (ESI) calcd for C22H22ClN4 (M + H+): 377.1533, found: 377.1528.
2-Cyclohexyl-N-(3,5-dichlorophenyl)-1H-imidazo[4,5-c]quinolin-4-amine (6)
Compound 29 (7.4 mg, 26 μmol) was reacted with 3,5-dichloroaniline (13 mg, 80 μmol) in DMF (50 μL). The crude product was chromatographed on silica gel (3:1 petroleum ether/EtOAc) to give 6.0 mg (15 μmol, 56%) of 6. Rf: 0.50 [silica gel, 2:1 petroleum ether/EtOAc]; 1H NMR (600 MHz, 4:1 CDCl3/CD3OD) δ 8.05 (s, 2H), 7.95 (d, 1H, J = 7.6 Hz), 7.91 (d, 1H, J = 8.3 Hz), 7.49 (t, 1H, J = 7.6 Hz), 7.32 (t, 1H, J = 7.4 Hz), 6.94 (s, 1H), 2.91 (t, 1H, J = 12.0 Hz), 2.13 (d, 2H, J = 11.9 Hz), 1.89 (d, 2H, J = 13.9 Hz), 1.78 (d, 1H, J = 12.2 Hz), 1.66 (q, 2H, J = 12.5 Hz), 1.44 (q, 2H, J = 13.0 Hz), 1.32 (q, 1H, J = 13.1 Hz); 13C NMR (150 MHz, 4:1 CDCl3/CD3OD) δ 158.1, 146.7, 143.7, 143.2, 135.1, 134.8, 127.8, 127.7, 126.7, 123.6, 121.4, 120.8, 116.9, 115.8, 38.8, 32.2, 26.4, 26.1; HRMS (ESI) calcd for C22H21Cl2N4 (M + H+): 411.1143, found: 411.1140.
2-Cyclohexyl-N-(2,4-dichlorophenyl)-1H-imidazo[4,5-c]quinolin-4-amine (7)
Compound 29 (8.2 mg, 29 μmol) was reacted with 2,4-dichloroaniline (14 mg, 87 μmol) in DMF (50 μL). The crude product was chromatographed on silica gel (3:1 hexane/EtOAc) to give 5.0 mg (12 μmol, 42%) of 7. Rf: 0.63 [silica gel, 2:1 petroleum ether/EtOAc]; 1H NMR (600 MHz, 4:1 CDCl3/CD3OD) δ 8.89 (d, 1H, J = 9.4 Hz), 7.98 (d, 1H, J = 7.7 Hz), 7.84 (d, 1H, J = 8.1 Hz), 7.47 (t, 1H, J = 7.6 Hz), 7.39 (d, 1H, J = 2.2 Hz), 7.33 (t, 1H, J = 7.4 Hz), 7.27 (dd, 1H, J = 8.6, 2.3 Hz), 2.96 (tt, 1H, J = 11.9, 3.5 Hz), 2.11 (d, 2H, J = 12.1 Hz), 1.89 (d, 2H, J = 13.7 Hz), 1.77 (d, 1H, J = 12.3 Hz), 1.70 (q, 2H, J = 12.3 Hz), 1.44 (q, 2H, J = 12.8 Hz), 1.32 (q, 1H, J = 12.7 Hz); 13C NMR (150 MHz, 4:1 CDCl3/CD3OD) δ 146.7, 136.1, 135.0, 129.0, 127.7, 127.6 (127.615), 127.6 (127.570), 123.6, 122.1, 120.9, 116.1, 39.1, 32.2, 26.4, 26.1; HRMS (ESI) calcd for C22H21Cl2N4 (M + H+): 411.1143, found: 411.1132.
2-Cyclohexyl-N-(3,4-difluorophenyl)-1H-imidazo[4,5-c]quinolin-4-amine (8)
Compound 29 (7.0 mg, 24 μmol) was reacted with 3,4-difluoroaniline (20 μL, 200 μmol) in DMF (30 μL). The crude product was chromatographed on silica gel (7:4 petroleum ether/EtOAc) to give 7.1 mg (19 μmol, 77%) of 8. Rf: 0.36 [silica gel, 2:1 petroleum ether/EtOAc]; 1H NMR (600 MHz, 4:1 CDCl3/CD3OD) δ 8.31 (m, 1H), 7.93 (d, 1H, J = 7.9 Hz), 7.87 (d, 1H, J = 8.4 Hz), 7.47 (t, 1H, J = 7.7 Hz), 7.44 (d, 1H, J = 8.8 Hz), 7.30 (t, 1H, J = 7.4 Hz), 7.09 (q, 1H, J = 9.3 Hz), 2.91 (t, 1H, J = 11.8 Hz), 2.13 (d, 2H, J = 11.9 Hz), 1.89 (d, 2H, J = 13.2 Hz), 1.78 (d, 1H, J = 12.7 Hz), 1.66 (q, 2H, J = 11.4 Hz), 1.44 (q, 2H, J = 12.8 Hz), 1.32 (q, 1H, J = 13.0 Hz); 13C NMR (150 MHz, 4:1 CDCl3/CD3OD) δ 175.5, 163.8, 158.0, 147.1, 143.9, 134.7, 127.6, 123.2, 120.8, 117.2, 117.1, 115.7, 114.4, 108.3, 108.1, 38.8, 32.2, 26.4, 26.1; HRMS (ESI) calcd for C22H21F2N4 (M + H+): 379.1734, found: 379.1739.
2-Cyclohexyl-N-(3,5-difluorophenyl)-1H-imidazo[4,5-c]quinolin-4-amine (9)
Compound 29 (5.1 mg, 18 μmol) was reacted with 3,5-difluoroaniline (9.5 mg, 72 μmol) in DMF (50 μL). The crude product was chromatographed on silica gel (20:1 CH2Cl2/MeOH, 20:1 CHCl3/MeOH, and then 15:1 CH2Cl2/MeOH) to give 7.4 mg (20 μmol, 100%) of 9. Rf: 0.54 [silica gel, 20:1 CH2Cl2/MeOH]; 1H NMR (600 MHz, DMSO-d6) δ 13.19 (s, 1H), 9.58 (s, 1H), 8.16 (m, 3H), 7.83 (d, 1H, J = 8.2 Hz), 7.53 (t, 1H, J = 7.7 Hz), 7.41 (t, 1H, J = 8.0 Hz), 6.73 (t, 1H, J = 8.8 Hz), 3.00 (tt, 1H, J = 11.7, 3.3 Hz), 2.08 (d, 2H, J = 12.2 Hz), 1.87 (d, 2H, J = 13.1 Hz), 1.77−1.71 (m, 3H), 1.44 (qt, 2H, J = 12.8, 3.0 Hz), 1.32 (qt, 1H, J = 12.5, 3.3 Hz); 13C NMR (150 MHz, 4:1 CDCl3/CD3OD) δ 164.6, 164.4, 162.9, 162.8, 146.8, 143.7, 143.6, 134.8, 127.8, 127.7, 123.5, 120.8, 115.8, 101.6, 101.4, 96.8, 96.6, 96.5, 38.8, 32.2, 26.4, 26.1; HRMS (ESI) calcd for C22H21F2N4 (M + H+): 379.1734, found: 379.1740.
N-(3,5-Bis(trifluoromethyl)phenyl)-2-cyclohexyl-1H-imidazo[4,5-c]quinolin-4-amine (10)
Compound 29 (4.35 mg, 15.2 μmol) was reacted with 3,5-bis(trifluoromethyl)aniline (10.0 μL, 62.7 μmol) in DMF (50 μL). The crude product was chromatographed on silica gel (2:1 hexane/EtOAc, 30:1 CHCl3/MeOH, 80:20:1 hexane/EtOAc/triethylamine, 5:2 hexane/EtOAc, and 35:1 CHCl3/MeOH with a trace amount of citric acid) to give 1.40 mg (2.93 μmol, 19%) of 10. Rf: 0.43 [silica gel, 2:1 hexane/EtOAc]; 1H NMR (600 MHz, DMSO-d6) δ 13.25 (s, 1H), 10.00 (s, 1H), 9.17 (s, 2H), 8.19 (d, 1H, J = 7.7 Hz), 7.76 (d, 1H, J = 8.2 Hz), 7.58 (s, 1H), 7.56 (t, 1H, J = 7.9 Hz), 7.44 (t, 1H, J = 7.6 Hz), 3.02 (tt, 1H, J = 11.8, 3.4 Hz), 2.11 (d, 2H, J = 11.9 Hz), 1.88 (d, 2H, J = 13.5 Hz), 1.78−1.72 (m, 3H), 1.45 (qt, 2H, J = 12.8, 3.3 Hz), 1.32 (qt, 1H, J = 12.6, 3.3 Hz); 13C NMR (150 MHz, 4:1 CDCl3/CD3OD) δ 143.6, 142.9, 135.0, 128.0, 127.8, 123.8, 120.8, 118.2, 115.9, 114.3, 38.8, 32.2, 26.4, 26.1; HRMS (ESI) calcd for C24H21F6N4 (M + H+): 479.1670, found: 479.1680.
4-(2-Cyclohexyl-1H-imidazo[4,5-c]quinolin-4-ylamino)phthalonitrile (11)
Compound 29 (4.50 mg, 15.7 μmol) was reacted with 4-aminophthalonitrile (11.6 mg, 78.8 μmol) in DMF (50 μL). The crude product was chromatographed on reversed-phase C18 silica gel (9:1 MeOH/H2O) and normal-phase silica gel (15:1 CHCl3/MeOH) to give 0.45 mg (1.1 μmol, 7.3%) of 11. Rf: 0.80 [silica gel, 10:1 CHCl3/MeOH]; 1H NMR (600 MHz, DMSO-d6) δ 13.30 (s, 1H), 10.19 (s, 1H), 9.07 (s, 1H), 8.78 (d, 1H, J = 7.6 Hz), 8.21 (d, 1H, J = 7.2 Hz), 8.04 (d, 1H, J = 8.9 Hz), 7.89 (d, 1H, J = 8.2 Hz), 7.58 (t, 1H, J = 7.6 Hz), 7.48 (t, 1H, J = 7.1 Hz), 3.02 (tt, 1H, J = 12.1, 3.4 Hz), 2.09 (d, 2H, J = 10.7 Hz), 1.87 (d, 2H, J = 13.2 Hz), 1.78−1.72 (m, 3H), 1.45 (qt, 2H, J = 12.8, 3.3 Hz), 1.32 (qt, 1H, J = 12.9, 3.5 Hz); 13C NMR (150 MHz, 4:1 CDCl3/CD3OD) δ 158.5, 146.2, 145.7, 143.3, 134.6, 130.0, 128.2, 128.0, 124.4, 122.6, 121.8, 120.9, 116.9, 116.7, 116.5, 116.1, 38.8, 32.2, 26.4, 26.1; HRMS (ESI) calcd for C24H21N6 (M + H+): 393.1828, found: 393.1835.
2-Cyclohexyl-N-(3,4-dimethoxyphenyl)-1H-imidazo[4,5-c]quinolin-4-amine (12)
Compound 29 (7.8 mg, 27 μmol) was reacted with 3,4-dimethoxyaniline (13 mg, 83 μmol) in DMF (50 μL). The crude product was chromatographed on silica gel (2:3 hexane/EtOAc) to give 2.5 mg (6.2 μmol, 23%) of 12. Rf: 0.33 [silica gel, 1:1 petroleum ether/EtOAc]; 1H NMR (600 MHz, 4:1 CDCl3/CD3OD) δ 7.98 (s, 1H), 7.92 (d, 1H, J = 6.6 Hz), 7.80 (d, 1H, J = 8.6 Hz), 7.45 (t, 1H, J = 7.6 Hz), 7.33 (d, 1H, J = 9.7 Hz), 7.27 (t, 1H, J = 7.1 Hz), 6.87 (d, 1H, J = 8.1 Hz), 3.95 (s, 3H), 3.84 (s, 3H), 2.92 (t, 1H, J = 11.9 Hz), 2.13 (d, 2H, J = 11.0 Hz), 1.89 (d, 2H, J = 13.2 Hz), 1.78 (d, 1H, J = 11.6 Hz), 1.67 (q, 2H, J = 12.1 Hz), 1.44 (q, 2H, J = 13.0 Hz), 1.32 (q, 1H, J = 13.0 Hz); 13C NMR (150 MHz, 4:1 CDCl3/CD3OD) δ 149.3, 144.3, 144.1, 135.1, 127.5, 127.3, 122.7, 120.7, 112.4, 111.0, 104.5, 56.6, 56.0, 38.9, 32.2, 26.4, 26.1; HRMS (ESI) calcd for C24H27N4O2 (M + H+): 403.2134, found: 403.2100.
N-(Benzo[d][1,3]dioxol-5-yl)-2-cyclohexyl-1H-imidazo[4,5-c]quinolin-4-amine (13)
Compound 29 (13.7 mg, 47.9 μmol) was reacted with 3,4-(methylenedioxy)aniline (16.3 mg, 115 μmol) in DMF (100 μL). The crude product was chromatographed on silica gel (2:3 and 1:1 hexane/EtOAc) to give 5.4 mg (14 μmol, 29%) of 13. Rf: 0.59 [silica gel, 1:1 hexane/EtOAc]; 1H NMR (600 MHz, 4:1 CDCl3/CD3OD) δ 7.91 (br s, 1H), 7.82 (d, 1H, J = 8.6 Hz), 7.80 (s, 1H), 7.44 (t, 1H, J = 7.7 Hz), 7.26 (t, 1H, J = 6.8 Hz), 7.21 (d, 1H, J = 7.3 Hz), 6.78 (d, 1H, J = 8.5 Hz), 5.91 (s, 2H), 2.91 (t, 1H, J = 11.6 Hz), 2.12 (d, 2H, J = 12.0 Hz), 1.89 (d, 2H, J = 13.5 Hz), 1.77 (d, 1H, J = 13.4 Hz), 1.66 (q, 2H, J = 11.9 Hz), 1.43 (q, 2H, J = 12.7 Hz), 1.31 (q, 1H, J = 12.8 Hz); 13C NMR (150 MHz, 4:1 CDCl3/CD3OD) δ 147.9, 144.0, 135.6, 127.5, 127.2, 122.8, 120.7, 115.6, 112.1, 108.5, 102.2, 101.2, 38.9, 32.2, 26.4, 26.1; HRMS (ESI) calcd for C23H23N4O2 (M + H+): 387.1821, found: 387.1814.
2-Cyclohexyl-N-(pyridin-3-yl)-1H-imidazo[4,5-c]quinolin-4-amine (14)
Compound 29 (6.67 mg, 23.3 μmol) was reacted with 3-aminopyridine (11.0 mg, 116 μmol) in DMF (50 μL). The crude product was chromatographed on normal-phase silica gel (10:1 and 3:1 CHCl3/MeOH, and 90:3:1 CHCl3/MeOH/triethylamine), on reversed-phase C18 silica gel (4:1 MeOH/H2O), and again on normal-phase silica gel (15:1 CHCl3/MeOH) to give 0.35 mg (0.99 μmol, 4.2%) of 14. Rf: 0.57 [silica gel, 200:10:1 CHCl3/MeOH/triethylamine]; 1H NMR (600 MHz, DMSO-d6) [major tautomer] δ 13.16 (s, 1H), 9.36 (d, 1H, J = 2.6 Hz), 9.31 (s, 1H), 8.73 (ddd, 1H, J = 8.2, 2.5, 1.3 Hz), 8.17 (dd, 1H, J = 4.7, 1.5 Hz), 8.14 (dd, 1H, J = 8.2, 1.0 Hz), 7.78 (d, 1H, J = 7.7 Hz), 7.50 (td, 1H, J = 7.7, 1.7 Hz), 7.38 (td, 1H, J = 7.4, 1.1 Hz), 7.35 (dd, 1H, J = 8.3, 4.7 Hz), 3.00 (tt, 1H, J = 12.1, 3.5 Hz), 2.09 (d, 2H, J = 12.7 Hz), 1.87 (dt, 2H, J = 13.2, 3.4 Hz), 1.78−1.72 (m, 3H), 1.45 (qt, 2H, J = 13.0, 3.2 Hz), 1.32 (qt, 1H, J = 12.8, 3.3 Hz); 13C NMR (150 MHz, 2:1 CDCl3/CD3OD) δ 158.4, 142.0, 140.3, 139.0, 137.2, 131.5, 130.4, 130.2, 127.9, 127.8, 126.7, 124.5, 123.7, 121.1, 116.1, 39.1, 32.4, 26.6, 26.3; HRMS (ESI) calcd for C21H22N5 (M + H+): 344.1875, found: 344.1873.
General procedure for N-substituted 1H-imidazo[4,5-c]quinolin-4-amine (16−21, 23)
A solution of the appropriate 4-chloro-1H-imidazo[4,5-c]quinoline and 3,4-dichloroaniline in DMF was heated at 140 °C overnight under a dry nitrogen atmosphere. The reaction mixture was cooled, and the solvent was evaporated. The residue obtained was subjected to preparative silica gel column chromatography (100:1 to 8:1 CHCl3/MeOH).
N-(3,4-Dichlorophenyl)-2-(tetrahydro-2H-pyran-4-yl)-1H-imidazo[4,5-c]quinolin-4-amine (16)
Scale: 0.14 mmol. Yield: 20 mg (35%). 1H NMR (300 MHz, CD3OD/CDCl3) δ 8.44 (s, 1H), 7.89 (d, 2H, J = 9.0 Hz), 7.74 (dd, 1H, J = 9.0, 2.5 Hz), 7.50 (m, 1H), 7.37 (m, 2H), 4.10 (tdt, 2H, J = 11.4, 3.3 Hz), 3.59 (m, 2H), 3.21 (m, 1H), 2.04 (m, 4H); 13C NMR (75 MHz, CD3OD/CDCl3) δ 143.4, 140.4, 132.2, 127.4, 127.2, 124.0, 123.2, 120.4, 119.9, 117.9, 115.3, 67.5, 35.5, 31.1; HRMS (ESI) calcd for C21H19Cl2N3O+ (M + H+): 413.0930, found: 413.0952.
N-(3,4-Dichlorophenyl)-2-(exo-norbornanyl)-1H-imidazo[4,5-c]quinolin-4-amine (17)
Scale: 0.139 mmol. Yield: 6.4 mg (11%). 1H NMR (300 MHz, CD3OD/CDCl3) δ 9.54 (s, 1H), 8.49 (s, 1H), 8.00 (d, 1H, J = 8.1 Hz), 7.83 (t, 1H, J = 9.6 Hz), 7.57 (t, 2H, J = 8.4 Hz), 7.31 (m, 1H), 7.27 (m, 12H), 3.99 (m, 1H), 3.59 (s, 1H), 3.42 (dd, 1H, J = 11.4, 6.0 Hz), 2.71 (s, 1H), 2.47 (s, 1H), 2.09 (m, 2H), 1.47 (m, 5H); 13C NMR (75 MHz, CD3OD/CDCl3) δ 130.4, 127.8, 123.4, 120.6, 120.0, 118.4, 42.3, 41.2, 40.5, 37.3, 33.5, 29.9, 24.2; HRMS (ESI) calcd for C23H21Cl2N4+ (M + H+): 423.1138, found: 423.1140.
N-(3,4-Dichlorophenyl)-2-(endo-norbornanyl)-1H-imidazo[4,5-c]quinolin-4-amine (18)
Scale: 0.139 mmol. Yield: 12.6 mg (21%). 1H NMR (300 MHz, CD3OD/CDCl3) δ 9.81 (s, 1H), 8.44 (s, 1H), 7.98 (d, 1H, J = 8.4 Hz), 7.78 (m, 2H), 7.55 (t, 1H, J = 8.5 Hz), 7.36 (s, 1H), 2.99 (dd, 1H, J = 8.7, 4.9 Hz), 2.60 (s, 1H), 2.48 (s, 1H), 2.23 (m, 1H), 1.82 (m, 1H), 1.66 (m, 2H), 1.30 (m, 5H); 13C NMR (75 MHz, CD3OD/CDCl3) δ 130.2, 127.4, 123.1, 120.1, 118.1, 42.7, 41.9, 36.4, 36.3, 36.1, 29.7, 28.9; HRMS (ESI) calcd for C23H21Cl2N4+ (M + H+): 423.1138, found: 423.1151.
N-(3,4-Dichlorophenyl)-2-(3-noradamantanyl)-1H-imidazo[4,5-c]quinolin-4-amine (19)
Scale: 0.186 mmol. Yield: 58.2 mg (70%). 1H NMR (300 MHz, CD3OD/CDCl3) δ 8.23 (s, 1H), 8.06 (d, 1H, J = 8.4 Hz), 7.80 (d, 1H, J = 8.4 Hz), 7.63 (dd, 1H, J = 8.7, 2.4 Hz), 7.43 (m, 1H), 7.31 (m, 2H), 2.76 (t, 1H, J = 6.3 Hz), 2.38 (s, 2H), 2.20 (d, 2H, J = 10.5 Hz), 2.08 (m, 2H), 2.02 (m, 4H), 1.70 (m, 4H); 13C NMR (75 MHz, CD3OD/CDCl3) δ 160.5, 145.9, 142.0, 139.9, 139.8, 132.4, 132.4, 130.3, 127.6, 125.9, 124.9, 123.5, 120.9, 118.8, 115.5, 45.8, 43.8, 37.6, 34.5; HRMS (ESI) calcd for C25H23Cl2N4+ (M + H+): 449.1294, found: 449.1291.
N-(3,4-Dichlorophenyl)-2-(1-adamantanyl)-1H-imidazo[4,5-c]quinolin-4-amine (20)
Scale: 0.115 mmol. Yield: 38 mg (71%). 1H NMR (300 MHz, CD3OD/CDCl3) δ 8.33 (s, 1H), 8.04 (d, 1H, J = 7.5 Hz), 7.81 (d, 1H, J = 8.4 Hz), 7.66 (m, 1H), 7.42 (m, 1H), 7.31 (m, 3H), 2.09 (s, 6H), 1.78 (s, 4H); 13C NMR (75 MHz, CD3OD/CDCl3) δ 161.3, 146.1, 142.4, 140.1, 132.3, 124.5, 120.6, 41.0, 36.3, 35.5, 28.6, 27.6; HRMS (ESI) calcd for C26H25Cl2N4+ (M + H+): 463.1451, found: 463.1456.
N-(3,4-Dichlorophenyl)-2-((1s,4s)-4-(1,3-dioxoisoindolin-2-yl)cyclohexyl)-1H-imidazo[4,5-c]quinolin-4-amine (21)
Scale: 0.044 mmol. Yield: 40 mg (78%). 1H NMR (300 MHz, CD3OD/CDCl3) δ 8.47 (m, 1H), 8.23 (s, 1H), 7.78 (m, 7H), 7.39 (m, 14H), 4.28 (m, 1H), 3.09 (m, 1H), 2.52 (m, 4H), 1.91 (m, 4H); 13C NMR (75 MHz, CD3OD/CDCl3) δ 168.5, 160.1, 143.3, 140.4, 134.0, 132.0, 131.6, 130.2, 129.9, 127.2, 122.9, 121.3, 120.3, 119.8, 118.9, 117.8, 100.2, 37.0, 30.9, 28.9; HRMS (ESI) calcd for C30H24Cl2N5O2+ (M + H+): 556.1302, found: 556.1290.
N-(3,4-Dichlorophenyl)-2-(1-benzoylpiperidin-4-yl)-1H-imidazo[4,5-c]quinolin-4-amine (23)
Scale: 0.108 mmol. Yield: 25 mg (44%). 1H NMR (300 MHz, CD3OD/CDCl3) δ 8.37 (s, 1H), 7.85 (d. 1H, J = 7.3 Hz), 7.82 (d, 1H, J = 8.4 Hz), 7.66 (dd, 1H, J = 8.7, 2.4 Hz), 7.43 (m, 1H), 7.31 (m, 7H), 4.69 (m, 12H), 3.82 (m, 2H), 3.17 (m, 1H), 3.00 (s.1H), 1.92 (m, 4H); 13C NMR (75 MHz, CD3OD/CDCl3) δ 171.2, 143.5, 140.4, 135.3, 132.2, 130.1, 128.6, 127.5, 127.3, 126.5, 124.0, 123.2, 120.4, 119.9, 117.9, 42.1, 36.4, 31.0, 30.5; HRMS (ESI) calcd for C28H24Cl2N5O+ (M + H+): 516.1352, found: 516.1352.
N-Phenyl-2-(1-benzoylpiperidin-4-yl)-1H-imidazo[4,5-c]quinolin-4-amine (24)
A solution of 33d (61.4 mg, 0.156 mmol) and aniline (43 μL, 0.47 mmol) in DMF (0.8 mL) was heating at 140 °C overnight under N2 atmosphere. The reaction mixture was cooled, and the solvent was evaporated. The residue obtained was subjected to preparative silica gel column chromatography (100:1 to 8:1 CHCl3/MeOH). Yield: 40 mg (57%). 1H NMR (300 MHz, CD3OD/CDCl3) δ 7.92 (d, 1H, J = 7.5 Hz), 7.81 (s, 2H), 7.75 (d. 1H, J = 8.1 Hz), 7.33 (dd, 1H, J = 7.2, 1.5 Hz), 7.21 (t, 1H, J = 8.0 Hz), 7.12 (d, 1H, J = 7.3 Hz), 6.86 (t, 1H, J = 7.5 Hz), 4.62 (m, 12H), 3.60 (m, 2H), 3.07 (d, 1H, J= 11.4 Hz), 1.92 (m, 4H); 13C NMR (75 MHz, CD3OD/CDCl3) δ 170.7, 154.8, 143.6, 140.6, 135.6, 129.8, 128.7, 128.5, 127.2, 127.1, 126.6, 122.5, 121.6, 120.5, 118.7, 53.4, 20.8, 13.9; HRMS (ESI) calcd for C28H26N5O+ (M + H+): 448.2132, found: 448.2137.
2-((1s,4s)-4-Aminocyclohexyl)-N-(3,4-dichlorophenyl)-1H-imidazo[4,5-c]quinolin-4-amine (22)
A 33% solution of methylamine in absolute ethanol (0.5 mL) is added to a stirred solution of 21 (10 mg, 0.017 mmol) in ethanol (0.25 mL). The solution was refluxed for 2 h. The mixture was then cooled to rt and the solvent was evaporated under reduced pressure. The residue was purified by flash column chromatography using a mixture of 10:1 EtOAc/MeOH, by volume. Yield: 5 mg (70%). 1H NMR (300 MHz, CD3OD/CDCl3) δ 8.40 (s, 1H), 8.08 (d, 1H, J = 8.1 Hz), 7.87 (d, 1H, 8.7 Hz), 7.75 (dd, 1H, J = 8.7, 2.5 Hz), 7.47 (t, 2H, J = 6.9 Hz), 7.29 (m, 4H), 3.40 (m, 1H), 3.12 (m, 1H), 2.27 (m, 2H), 1.95 (m, 2H), 1.89 (m, 4H); 13C NMR (75 MHz, CD3OD/CDCl3) δ 143.4, 140.4, 132.1, 130.0, 127.4, 124.0, 123.2, 120.8, 119.9, 117.9, 110.7, 105.7, 31.6, 27.4, 25.6; HRMS (ESI) calcd for C22H22Cl2N5+ (M + H+): 426.1247, found: 426.4203.
2-(1-Benzylpiperidin-4-yl)-N-(3,4-dichlorophenyl)-1H-imidazo[4,5-c]quinolin-4-amine (25)
A solution of compound 23 (18 mg, 0.035 mmol) in dry THF (0.18 mL) was added dropwise to a stirred slurry of lithium aluminum hydride (0.89 mL of 0.2 M in THF, 0.175 mmol). After heating under reflux overnight, decomposition of excess hydride was effected by cautious addition of water. The inorganic solids were removed by filtration, the organic layer dried over anhydrous sodium sulfate and filtered, and the solvent was removed under reduced pressure. The final residue was purified by flash column chromatography using a mixture of 10:1 EtOAc/MeOH, by volume. 1H NMR (300 MHz, CD3OD/CDCl3) δ 11.79 (s, 1H), 8.50 (s, 1H), 8.01 (m, 2H), 7.87 (dd, 1H, J = 9.0, 2.6 Hz), 7.78 (m, 1H), 7.53 (m, 7H), 7.37 (s, 2H), 3.59 (m, 2H), 3.09 (m, 2H), 2.06 (m, 2H), 1.76 (s, 4H); 13C NMR (75 MHz, CD3OD/CDCl3) δ 140.4, 136.4, 129.4, 128.1, 127.8, 123.0, 120.3, 119.8, 117.8, 100.2, 61.6, 52.9, 30.1, 28.8; HRMS (ESI) calcd for C28H26Cl2N5+ (M + H+): 502.1560, found: 502.1541.
N-(3,4-Dichlorophenyl)-2-(piperidin-4-yl)-1H-imidazo[4,5-c]quinolin-4-amine (26)
A solution of 25 (6 mg, 0.011 mmol) in dry MeOH (0.5 mL) was hydrogenated at 1 atm in the presence of palladium black (0.011 mg). After 2 h, the catalyst was removed by filtration, and the solvent was evaporated under reduced pressure. The final residue was purified by flash column chromatography using a mixture of 10:1:0.1 EtOAc/MeOH/NH4OH, by volume. 1H NMR (300 MHz, CD3OD/CDCl3) δ 8.51 (m, 1H), 8.03 (m, 2H), 7.72 (m, 1H), 7.60 (m, 1H), 7.43 (m, 2H), 3.38 (m, 2H), 2.35 (m, 2H), 1.19 (m, 12H), 0.90 (m, 12H); HRMS (ESI) calcd for C21H20Cl2N5+ (M + H+): 412.1090, found: 412.1086.
HPLC analysis of compounds 5−14 and 16−26
Purity of compounds was checked using a Hewlett-Packard 1100 HPLC equipped with a Zorbax SB-Aq 5 μ analytical column (50 × 4.6 mm; Agilent Technologies, Palo Alto, CA). System A: linear gradient solvent system; 5 mM TBAP (tetrabutylammonium dihydrogenphosphate) - CH3CN from 50:50 to 0:100 in 13 min; the flow rate was 0.5 mL/min. System B: linear gradient solvent system; 10 mM TEAA (triethylammonium acetate)-CH3CN from 65:35 to 0:100 in 13 min; the flow rate was 0.5 mL/min. Peaks were detected by UV absorption with a diode array detector. The compounds eluted at the following retention times: 5, 7.0 min (system A), 9.4 min (system B); 6, 8.9 min (system A), 10.44 min (system B); 7, 9.2 min (system A), 10.9 min (system B); 8, 7.5 min (system A), 9.3 min (system B); 9, 7.3 min (system A), 10.1 min (system B); 10, 9.4 min (system A), 11.7 min (system B); 11, 7.1 min (system A), 9.8 min (system B); 12, 3.3 min (system A), 8.0 min (system B); 13, 4.7 min (system A), 8.6 min (system B); 14, 7.2 min (system A), 4.7 min (system B); 16, 5.7 min (system A), 8.6 min (system B); 17, 8.4 min (system A), 10.9 min (system B); 18, 8.7 min (system A), 11.2 min (system B); 19, 9.3 min (system A), 11.3 min (system B); 20, 9.7 min (system A), 12.0 min (system B); 21, 9.2 min (system A), 11.5 min (system B); 22, 2.4 min (system A), 10.3 min (system B); 23, 6.5 min (system A), 9.6 min (system B); 24, 2.9 min (system A), 6.9 min (system B). 25, 7.06 min (system A), 10.8 min (system B); 26, 1.93 min (system A), 10.6 min (system B).
Pharmacological Methods
[3H]35 (47 Ci/mmol) was from GE Healthcare Bio-Sciences Corp. (Piscataway, NJ). [3H]34 (CCPA, 42.6 Ci/mmol), [125I]36 (I-AB-MECA, 2000 Ci/mmol), and [35S]GTPγS (1068 Ci/mmol) were from Perkin Elmer Life Sciences (Waltham, MA).
Cell culture and membrane preparation
CHO (Chinese hamster ovary) cells expressing the recombinant human ARs (HEK-293 cells were used for the human A2AAR) were cultured in DMEM and F12 (1:1) supplemented with 10% fetal bovine serum, 100 units/ml penicillin, 100 μg/ml streptomycin and 2 μ mol/ml glutamine. Cells were harvested by trypsinization. After homogenization and suspension, cells were centrifuged at 1000 × g for 10 min, and the pellet was re-suspended in 50 mM Tris·HCl buffer (pH 7.4) containing 10 mM MgCl2. The suspension was homogenized with an electric homogenizer for 10 sec, and was then re-centrifuged at 20,000 × g for 20 min at 4°C. The resultant pellets were resuspended in buffer in the presence of 3 Units/mL adenosine deaminase, and the suspension was stored at −80°C until the binding experiments. The protein concentration was measured using the Bradford assay.27
Binding to the Human A1AR and the A2AAR
For binding to the human A1AR, [3H]34 (2 nM) was incubated with membranes (40 μg/tube) from CHO cells stably expressing the human A1AR at 25 °C for 60 min in 50 mM Tris·HCl buffer (pH 7.4; MgCl2, 10 mM) in a total assay volume of 200 μL. 19 Nonspecific binding was determined using 10 μ M of N6-cyclopentyladenosine. For human A2AAR binding, membranes (20 μg/tube) from HEK-293 cells stably expressing the human A2AAR were incubated with 15 nM [3H]35 at 25 °C for 60 min in 200 μL of 50 mM Tris·HCl, pH 7.4, containing 10 mM MgCl2.20 5′-N-Ethylcarboxamidoadenosine (NECA, 37, 10 μM) was used to define nonspecific binding. Reaction was terminated by filtration with GF/B filters.
Binding to the Human A3AR
Each tube in the competitive binding assay contained 100 μL membrane suspension (20 μg protein), 50 μL [125I]36 (0.5 nM),21 and 50 μL of increasing concentrations of the test ligands in Tris·HCl buffer (50 mM, pH 7.4) containing 10 mM MgCl2, 1 mM EDTA. Nonspecific binding was determined using 10 μM of 37 (NECA) in the buffer. The mixtures were incubated at 25°C for 60 min. Dissociation was started by the addition of 10 μM 15 in the absence or presence of 10 μ M of each allosteric modulator. Binding reactions were terminated by filtration through Whatman GF/B filters under reduced pressure using a MT-24 cell harvester (Brandell, Gaithersburgh, MD, USA). Filters were washed three times with 9 mL ice-cold buffer. Radioactivity was determined in a Beckman 5500B γ-counter.
[35S]GTPγS binding assay
The preparation of membranes from CHO cells stably expressing human A3AR was as previously described.22 [35S]GTPγS binding was measured in 200 μL buffer containing 50 mM Tris·HCl (pH 7.4), 1 mM EDTA, 1 mM MgCl2, 1 μM GDP, 1 mM dithiothreitol, 100 mM NaCl, 3 Units/mL adenosine deaminase, 0.2 nM [35S]GTPγS, 0.004% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS), and 0.5% bovine serum albumin. Incubations were started by addition of the membrane suspension (10 μg protein/tube) to the test tubes, and carried out in duplicate for 30 min at 25°C. The reaction was stopped by rapid filtration through Whatman GF/B filters, pre-soaked in 50 mM Tris·HCl, 5 mM MgCl2 (pH 7.4) containing 0.02% CHAPS. The filters were washed twice with 3 mL of the buffer mentioned before, and retained radioactivity was measured using liquid scintillation counting. Non-specific binding of [35S]GTPγS was measured in the presence of 10 μM unlabelled GTPγS.
Statistical analysis
Binding and functional parameters were calculated using Prism 5.0 software (GraphPAD, San Diego, CA, USA). IC50 values obtained from competition curves were converted to Ki values using the Cheng-Prusoff equation.28 Data were expressed as mean ± standard error.
Supplementary Material
Acknowledgements
This research was supported in part by the Intramural Research Program of the NIH, National Institute of Diabetes and Digestive and Kidney Diseases and by the European Union (QLK3-CT-2001−51963). We thank Dr. Anikó Göblyös for preparation of compound 27, Dr. John Lloyd for collecting MS data, and Dr. Herman Yeh and Mr. Wesley White for the helpful advice on the NMR experiments. S. de Castro thanks Ministerio de Educación y Ciencia (Spain) for financial support. Y.K. thanks the Can-Fite Biopharma for financial support.
ABBREVIATIONS
- AR
adenosine receptor
- Cl-IB-MECA
2-chloro-N-6-(3-iodobenzyl)adenosine-5′-N-methylcarboxamide
- CCPA
2-chloro-N6-cyclopentyladenosine
- CHAPS
3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate
- DMEM
Dulbecco's modified Eagle's medium
- DMF
N,N-dimethylformamide
- DMSO
dimethyl sulfoxide
- ESI
electrospray ionization
- GPCR
G protein-coupled receptor
- GTPγS
guanosine-5′-(γ-thiotriphosphate)
- I-AB-MECA
N6-(4-amino-3-iodobenzyl)adenosine-5′-N-methylcarboxamide
- m-CPBA
3-chloroperoxybenzoic acid
- NECA
5′-N-ethylcarboxamidoadenosine
- SAR
structure-activity relationship
- TLC
thin layer chromatography
Footnotes
Supporting Information Available: Selected 1H and 2D COSY and NOESY spectra with peak assignments. This material is available free of charge via the Internet at http://pubs.acs.org/jmc.
References
- 1.Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol. Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- 2.Jacobson KA, Gao Z-G. Adenosine receptors as therapeutic targets. Nature Rev. Drug Discovery. 2006;5:247–264. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wan TC, Ge ZD, Tampo A, Mio Y, Bienengraeber MW, Tracey WR, Gross GJ, Kwok WM, Auchampach JA. The A3 adenosine receptor agonist CP-532,903 [N6-(2,5-dichlorobenzyl)-3'-aminoadenosine-5'-N-methylcarboxamide] protects against myocardial ischemia/reperfusion injury via the sarcolemmal ATP-sensitive potassium channel. J. Pharmacol. Exp. Ther. 2008;324:234–243. doi: 10.1124/jpet.107.127480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guzman J, Yu JG, Suntres Z, Bozarov A, Cooke H, Javed N, Auer H, Palatini J, Hassanain HH, Cardounel AJ, Javed A, Grants I, Wunderlich JE, Christofi FL. ADOA3R as a therapeutic target in experimental colitis: proof by validated high-density oligonucleotide microarray analysis. Inflamm. Bowel Dis. 2006;12:766–789. doi: 10.1097/00054725-200608000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Fishman P, Jacobson KA, Ochaion A, Cohen S, Bar-Yehuda S. The anti-cancer effect of A3 adenosine receptor agonists: A novel, targeted therapy. Immunology Endocrine and Metabolic Agents in Medicinal Chemistry. 2007;7:298–303. doi: 10.2174/187152207781369878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silverman MH, Strand V, Markovits D, Nahir M, Reitblat T, Molad Y, Rosner I, Rozenbaum M, Mader R, Adawi M, Caspi D, Tishler M, Langevitz P, Rubinow A, Friedman J, Green L, Tanay A, Ochaion A, Cohen S, Kerns WD, Cohn I, Fishman-Furman S, Farbstein M, Yehuda SB, Fishman P. Clinical evidence for utilization of the A3 adenosine receptor as a target to treat rheumatoid arthritis: Data from a phase II clinical trial. J. Rheumatol. 2008;35:41–48. [PubMed] [Google Scholar]
- 7.Zheng J, Wang R, Zambraski E, Wu D, Jacobson KA, Liang BT. A novel protective action of adenosine A3 receptors: Attenuation of skeletal muscle ischemia and reperfusion injury. Am. J. Physiol., Heart and Circ. Physiol. 2007;293:3685–3691. doi: 10.1152/ajpheart.00819.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruns RF, Fergus JH. Allosteric enhancement of adenosine A1 receptor binding and function by 2-amino-3-benzoylthiophenes. Mol. Pharmacol. 1990;38:939–949. [PubMed] [Google Scholar]
- 9.Gao Z-G, Kim SK, Gross AE, Chen A, Blaustein JB, Jacobson KA. Identification of essential residues involved in the allosteric modulation of the humanA3 adenosine receptor. Mol. Pharmacol. 2003;63:1021–1031. doi: 10.1124/mol.63.5.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bridges TM, Lindsley CW. G-protein-coupled receptors: from classical modes of modulation to allosteric mechanisms. ACS Chem. Biol. 2008;3:530–541. doi: 10.1021/cb800116f. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz TW, Holst B. Allosteric enhancers, allosteric agonists and ago-allosteric modulators: where do they bind and how do they act? Trends Pharmacol. Sci. 2007;28:366–373. doi: 10.1016/j.tips.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Leach K, Sexton PM, Christopoulos A. Allosteric GPCR modulators: taking advantage of permissive receptor pharmacology. Trends Pharmacol. Sci. 2007;28:382–389. doi: 10.1016/j.tips.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Gao Z-G, Jacobson KA. Allosterism in membrane receptors. Drug Discov. Today. 2006;11:191–202. doi: 10.1016/S1359-6446(05)03689-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao Z-G, van Muijlwijk-Koezen JE, Chen A, Müller CE, IJzerman AP, Jacobson KA. Allosteric modulation of A3 adenosine receptors by a series of 3-(2-pyridinyl)isoquinoline derivatives. Mol. Pharmacol. 2001;60:1057–1063. [PMC free article] [PubMed] [Google Scholar]
- 15.Göblyös ,A, Gao Z-G, Brussee J, Connestari R, Santiago SN, Ye K, IJzerman AP, Jacobson KA. Structure activity relationships of 1H-imidazo[4,5-c]quinolin-4-amine derivatives new as allosteric enhancers of the A3 adenosine receptor. J. Med. Chem. 2006;49:3354–3361. doi: 10.1021/jm060086s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao Z-G, Kim SG, Soltysiak KA, Melman N, IJzerman AP, Jacobson KA. Selective allosteric enhancement of agonist binding and function at human A3 adenosine receptors by a series of imidazoquinoline derivatives. Mol. Pharmacol. 2002;62:81–89. doi: 10.1124/mol.62.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao Z-G, Ye K, Göblyös A, IJzerman AP, Jacobson KA. Flexible modulation of agonist efficacy at the human A3 adenosine receptor by an imidazoquinoline allosteric enhancer LUF6000 and its analogues. BMC Pharmacol. 2008;8:20. doi: 10.1186/1471-2210-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Galen PJM, Nissen P, van Wijngaarden I, IJzerman AP, Soudijn W. 1H-imidazo[4,5-c]quinolin-4-amines: novel non-xanthine adenosine antagonists. J. Med. Chem. 1991;34:1202–1206. doi: 10.1021/jm00107a046. [DOI] [PubMed] [Google Scholar]
- 19.Klotz KN, Lohse MJ, Schwabe U, Cristalli G, Vittori S, Grifantini M. 2-Chloro-N6-[3H]cyclopentyladenosine ([3H]CCPA)--a high affinity agonist radioligand for A1 adenosine receptors. Naunyn Schmiedebergs Arch. Pharmacol. 1989;340:679–683. doi: 10.1007/BF00717744. [DOI] [PubMed] [Google Scholar]
- 20.Jarvis MF, Schutz R, Hutchison AJ, Do E, Sills MA, Williams M. [3H]CGS 21680, an A2 selective adenosine receptor agonist directly labels A2 receptors in rat brain tissue. J. Pharmacol. Exp. Ther. 1989;251:888–893. [PubMed] [Google Scholar]
- 21.Olah ME, Gallo-Rodriguez C, Jacobson KA, Stiles GL. 125I-4-Aminobenzyl-5′-N-methylcarboxamidoadenosine, a high affinity radioligand for the rat A3 adenosine receptor. Mol. Pharmacol. 1994;45:978–982. [PMC free article] [PubMed] [Google Scholar]
- 22.Lorenzen A, Lang H, Schwabe U. Activation of various subtypes of G-protein alpha subunits by partial agonists of the adenosine A1 receptor. Biochem. Pharmacol. 1998;56:1287–1293. doi: 10.1016/s0006-2952(98)00207-x. [DOI] [PubMed] [Google Scholar]
- 23.Wolber C, Fozard JR. The receptor mechanism mediating the contractile response to adenosine on lung parenchymal strips from actively sensitised, allergen-challenged Brown Norway rats. Naunyn Schmiedebergs Arch. Pharmacol. 2005;371:158–168. doi: 10.1007/s00210-004-1012-8. [DOI] [PubMed] [Google Scholar]
- 24.Fossetta J, Jackson J, Deno G, Fan X, Du XK, Bober L, Soude-Bermejo A, de Bouteiller O, Caux C, Lunn C, Lundell D, Palmer RK. Pharmacological analysis of calcium responses mediated by the human A3 adenosine receptor in monocyte-derived dendritic cells and recombinant cells. Mol Pharmacol. 2003;63:342–350. doi: 10.1124/mol.63.2.342. [DOI] [PubMed] [Google Scholar]
- 25.Gao ZG, Jacobson KA. Translocation of arrestin induced by human A3 adenosine receptor ligands in an engineered cell line: Comparison with G protein-dependent pathways. Pharmacol. Res. 2008;57:303–311. doi: 10.1016/j.phrs.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nordstedt C, Fredholm BB. A modification of a protein-binding method for rapid quantification of cAMP in cell-culture supernatants and body fluid. Anal. Biochem. 1990;189:231–234. doi: 10.1016/0003-2697(90)90113-n. [DOI] [PubMed] [Google Scholar]
- 27.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 28.Cheng Y-C, Prusoff WH. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 percent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.