Abstract
Trichloroethylene (TCE, CAS 79-01-6) is a widely used industrial chemical, and a common environmental pollutant. TCE is a well-known carcinogen in rodents and is classified as “probably carcinogenic to humans”. Several analytical methods have been proposed for detection of TCE metabolites in biological media utilizing derivatization-free techniques; however, none of them is suitable for simultaneous detection of both oxidative metabolites and glutathione conjugates of TCE in small volume biological samples. Here, we report a new combination of methods for assessment of major TCE metabolites: dichloroacetic acid (DCA), trichloroacetic acid (TCA), S-(1,2-dichlorovinyl)-l-cysteine (DCVC), and S-(1,2-dichlorovinyl) glutathione (DCVG). First, DCA and TCA were extracted with ether. Second, the remaining aqueous fraction underwent solid phase extraction for DCVC and DCVG. Then, DCA and TCA were measured by hydrophilic interaction liquid chromatography ion exchange negative electrospray ionization tandem mass spectrometry, while DCVC and DCVG were measured by reverse phase positive electrospray ionization tandem mass spectrometry. This method was applied successfully to measure all 4 TCE metabolites in as little as 50 μl of serum from mice orally exposed to TCE (2,100 mg/kg, 2 hrs). Serum concentrations (mean±standard deviation) of the TCE metabolites obtained with this method are comparable or equivalent to those previously reported in the literature: DCA, 0.122±0.014 nmol/ml (limit of detection: 0.01 nmol/ml); TCA, 256±30 nmol/ml (0.4 nmol/ml); DCVG, 0.037±0.015 nmol/ml (0.001 nmol/ml); DCVC, 0.0024±0.0009 nmol/ml (0.001 nmol/ml). This method opens new opportunities to increase throughput and decrease number of animals required for mechanistic studies on TCE in rodents.
Keywords: trichloroethylene metabolites; HPLC-ESI-MS/MS; dichloroacetic acid; trichloroacetic acid; S-(1,2-dichlorovinyl)-l-cysteine; S-(1,2-dichlorovinyl)glutathione; HILIC
1. Introduction
Trichloroethylene (TCE) is a widely used cleaning solvent and chemical ingredient. Due to its high production volume and use in a variety of manufacturing processes, TCE is a ubiquitous soil and groundwater contaminant present in as many as 60% of the hazardous waste sites on the U.S. EPA National Priority List (Agency for Toxic Substances and Disease Registry, 1997; Wu and Schaum, 2000). Acute exposure to TCE results primarily in central nervous system effects, while long-term exposure can result in hepatotoxicity (Bull, 2000), nephrotoxicity (Lash et al., 2000b), and neurotoxicity (Barton and Clewell, III, 2000). Adverse reproductive and developmental effects of TCE have also been reported, although the data are considered controversial (Pastino et al., 2000). TCE is classified as reasonably anticipated to be a human carcinogen by the US National Toxicology Program (National Toxicology Program, 2004) and as Group 2A: probably carcinogenic in humans by the International Agency for Research on Cancer (International Agency for Research on Cancer, 1995) based on limited evidence of carcinogenicity from studies in humans and sufficient evidence of carcinogenicity from studies in experimental animals. Despite the existence of a large database of information on TCE effects, significant gaps in knowledge remain. Not surprisingly, the regulatory agencies and a broad scientific community show continuing interest in research on TCE toxicity (National Academy of Science, 2006).
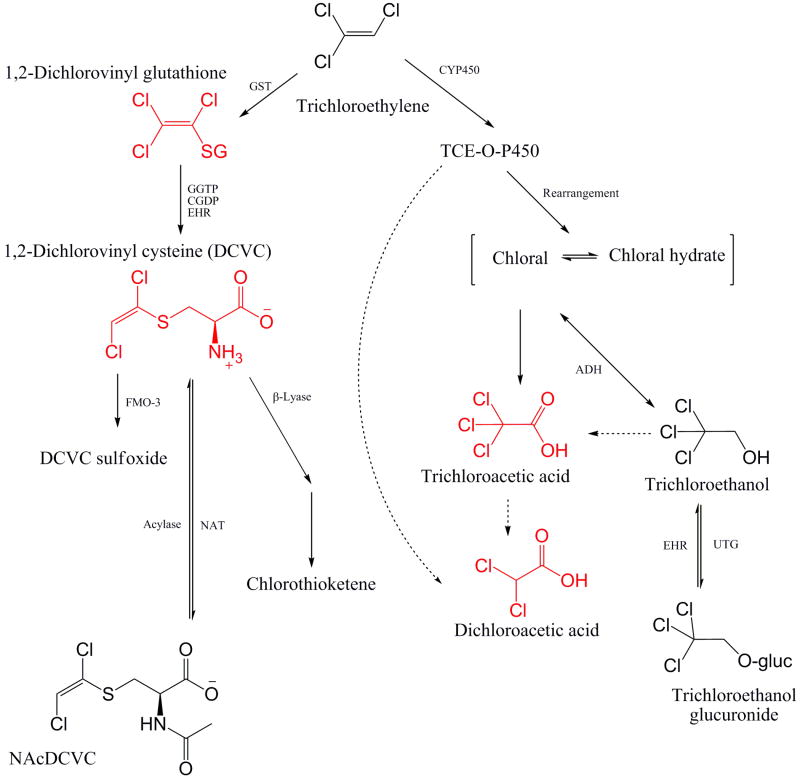
The metabolic transformations of TCE are complex and involve formation of many metabolites that vary in toxicity and organ-specific effects (Lash et al., 2000a). Two primary pathways, cytochrome P450-dependent oxidation and glutathione conjugation, are responsible for biotransformation of TCE (Fig. 1). Liver and kidney are primary target organs, but TCE can be metabolized in other tissues as well. Of the various metabolites, dichloroacetic acid (DCA), trichloroacetic acid (TCA), and chloral hydrate (CH) have been suggested to contribute to liver carcinogenesis in mice, while the reactive thiols from S-(1,2-dichlorovinyl)-l-cysteine (DCVC) are thought to be responsible for kidney toxicity (Bull, 2000; Bruning and Bolt, 2000; Lash et al., 2000a; Chiu et al., 2006a). The inherent complexity of TCE metabolism and apparent multiple potential modes of action make this chemical equally challenging for toxicology research, risk assessment, and policy making.
Fig. 1.
Schematic representation of the metabolic pathways of trichloroethylene. GST, glutathione S-transferase; GGTP, γ-glutamyltransferase; CGDP, cysteinyl-glycine dipeptidase; EHR, enterohepatic recirculation; FMO-3, flavin monooxigenase-3; NAT, N-acetyltransferase; TCE-O-P450, oxygenated TCE-cytochrome (CYP) P450 transition state complex; ADH, alcohol dehydrogenase; ALDH, aldehyde dehydrogenase; UTG, uridine diphosphate-glucuronosyltransferase. Dotted lines represent putative pathways [Adaped from (McKinney et al., 1959; National Academy of Science, 2006; Chiu et al., 2006b)].
Many measurement methods for TCE metabolites have been developed and used for pharmacokinetic studies (Delinsky et al., 2005a; Chiu et al., 2006b). Most of these are based on gas chromatography-electron capture detector (GC-ECD) or gas chromatography-mass spectrometry (GC-MS). While these techniques achieve good limit of detection (LOD), their application to measurements of DCA in blood samples has been criticized as artifact-prone (Ketcha et al., 1996; Merdink et al., 1998). Direct injection or liquid chromatography followed by tandem mass spectrometry methods have been also proposed for TCA or DCA (Brashear et al., 1997; Kuklenyik et al., 2002; Delinsky et al., 2005b). For DCVC and DCVG, few methods have been reported (Commandeur and Vermeulen, 1990; Lash et al., 1995; Finkelstein et al., 1995; Bloemen et al., 2001); however, all of these require sample derivatization and have high LOD, limiting their utility for small biological samples. To address current limitations in the analytical studies of TCE metabolism in small volume biological samples, we developed a derivatization-free method with good sensitivity for measurements of key metabolites that have been implicated in liver and kidney toxicity and carcinogenicity of TCE (National Academy of Science, 2006). This technique uses sequential extraction of DCA, TCA, DCVG and DCVC from as little as 50 μl serum followed by analyte detection with high performance liquid chromatography-electrospray ionization tandem mass spectrometry.
2. Material and methods
2.1. Chemicals and reagents
HPLC-grade acetonitrile (ACN), HPLC-grade water, HPLC-grade methanol, spectrophotometric grade diethyl ether, ammonium hydroxide (29%), trichloroacetic acid (TCA, 100%), ACS grade sulfuric acid (98%), ACS grade acetic acid (100%) and 2-(2-methoxyethoxy)ethanol (2-MEE, 99%) were purchased from Fisher Scientific (Pittsburgh, PA). Dichloroacetic acid (DCA, 99%), difluoroacetic acid (DFA, 98%), trifluoroacetic acid (TFA, 99.5%), and ammonium formate salt (99.9%) were purchased from Sigma (St. Louis, MO). DCVC, [13C5,15N]DCVC, DCVG and [13C4,15N]DCVG were synthesized by modification of a published method (McKinney et al., 1959), details are available from Dr. Avram Gold (golda@email.unc.edu).
2.2. Animal treatments
Male mice (B6C3F1, aged 14-16 weeks) were obtained from the Jackson Laboratory (Bar Harbor, ME) and housed in polycarbonate cages on Sani-Chips irradiated hardwood bedding (P.J. Murphy Forest Products Corp., Montville, NJ). Animals were fed NTP-2000 wafer diet (Zeigler Brothers, Inc., Gardners, PA) and water ad libitum, and maintained on a 12 h light-dark cycle. Mice were administered a single dose (2.1 g/kg) of TCE diluted with corn oil (10 ml/kg) by gavage. Animals were sacrificed 2 hrs after dosing under nembutal (100 mg/kg, i.p., Abbott Laboratories, Chicago, IL) anesthesia and blood was collected from posterior vena cava. Serum was separated by centrifugation with using 1.1-ml Z-gel tubes (Sarstedt AG & Co., Germany) at 16,000×g for 15 min, and stored at -80°C until assayed. The animal studies were conducted under a protocol approved by the Institutional Animal Care and Use Committee at the University of North Carolina at Chapel Hill.
2.3. LC/MS Analysis
Levels of DCA, TCA, DCVG and DCVC in serum were determined as follows. An aqueous mixture of internal standards (5 μl; DFA and TFA, 500 nmol/ml each; [13C5,15N]DCVC and [13C4,15N]DCVG, 2.5 nmol/ml each) was spiked to diluted serum specimen (50 μl) with water (100 μl). Then, serum protein was removed by filter centrifugation (Microcon YM-10, Danvers, MA) at 14,000 × g for 30 min at 25 °C. Subsequently, 2 ml of diethyl ether was added to extract haloacetic acids after acidifying the media with 100 μl of 3% (v/v) sulfuric acid. The ether layer was transferred to another vial and reduced in volume under N2, and finally transferred to 300 μl glass vial insert containing 5 μl of water for solvent transfer before dryness. The residue was reconstituted in 100 μl of mobile phase: 68.6% ACN, 29.4% 40 mM ammonium formate (pH 9.1) and 2% 2-MEE. The aqueous fraction left after the ether extraction above was neutralized with 5 μl of 28% NH4OH prior to extraction of DCVG and DCVC through a solid phase extraction (SPE) cartridge (StrataTM X-AW, 30 mg 96-well plate; Phenomenex, CA). After conditioning with 300 μl of methanol, followed by equilibration with 300 μl of water, the samples (∼ 300 μl) were loaded, washing with 300 μl water, and finally eluted with 250 μl of basic methanol (pH adjusted at 10.8 by 29% NH4OH). Light vacuum (up to 50 mmHg) was applied to expedite washing and elution. The final eluant was collected into 300 μl glass vial inserts and dried in a Speed Vac Concentrator (Thermo-Fisher, CA) before reconstitution with 20 μl of 80:20 water/methanol containing 0.1% acetic acid.
HPLC-ESI-MS/MS with a Finnigan Surveyor autosampler and pump coupled to a Finnigan TSQ Quantum triple-quadrupole mass spectrometer was used for determination of DCA and TCA. The source was operated in the negative ion mode. The mass analyzer was operated in the multiple reaction monitoring (MRM) mode: DCA (m/z 127 → 83), DFA (m/z 95 → 51), TCA m/z 161 → 117), and TFA (m/z 113 → 69). Sample injection volume was 5-15 μl. A Luna amino column (150 × 2.0 mm, 3 μm; Phenomenex, CA) was used in an isocratic mode (200 μl/min) with a mobile phase as described above. During the first three minutes the effluent flow was diverted to waste. MS settings were as follows: electrospray voltage (3 kV), capillary temperature (275 °C), sheath and auxiliary gas pressures (nitrogen; 40 and 10 arbitrary units), collision energy (12 V), tube lens offset (SRM table reference), Q2 collision gas pressure (0.5 mTorr) for each analytes. Quantification was based on peak areas relative to the fluoride analogues.
HPLC-ESI-MS/MS with an Aquity UPLC® system (Waters, Milford, MA) coupled to a TSQ Quantum Ultra triple quadrupole mass analyzer (Thermo Finnigan, San Jose, CA) using a heat-assisted electrospray ionization (HESI) source in positive ion mode was used for determination of DCVC and DCVG. Sample injection volume was 15 μl. A YMC ODS-AQ analytic column (150 × 2 mm, 3 μm; Waters, Milford, MA) was operated with a linear gradient of 20% methanol 0.1% acetic acid for 0.5 min, then to 52% methanol 0.1% acetic acid in 7.5 min, and subsequently to 20% methanol 0.1% acetic acid at a flow rate of 200 μl/min. During the 15 min run, the effluent for the first 2.5 min was diverted to waste to prevent salts from entering the MS. Analytes were detected in MRM mode, monitoring the transition of the m/z 216 → 127 and 199 for DCVC, m/z 222 → 129 for [13C5,15N]DCVC, m/z 402 → 273 for DCVG and m/z 407 → 278 for [13C4,15N]DCVG. The transition of m/z 216 → 127 was used to confirm DCVC and combined with the other transition of m/z 216 → 199 by Xcalibur® (Ver. 2.0, ThermoFinnigan, San Jose, CA). MS settings were as follows: electrospray voltage (3.5 kV), capillary temperature (250 °C), sheath and auxiliary gas pressures (nitrogen; 30 and 35 arbitrary units), collision energy (14-20 V), tube lens offset (SRM table reference), Q2 collision gas pressure (1.0 mTorr). The MS and electrospay ionization parameters were optimized by direct infusion of standards using Xcalibur®.
2.5. Method Validation
Samples for calibration curves and quality control were prepared with commercially available mouse serum (C57BL/6J strain, Innovative Research, Novi, MI) and compared to the results from serum obtained from B6C3F1 mice to determine whether mouse strain had an effect on the measurements. Standards were prepared with two replicates by spiking serum with chemical standards at 0.02, 0.1, 0.5, 1, 2 and 10 nmol/ml for DCA; 0.4, 2, 4, 8, 40, 400 and 800 nmol/ml for TCA; 0.001, 0.005, 0.025, 0.05, 0.1, 0.5 for DCVG and DCVC. Quantification was based on peak areas relative to the stable isotope-labeled internal standards.
Quality assurance was done at 0.02 and 0.1 nmol/ml (DCA); 40, 400, and 800 nmol/ml (TCA); 0.001, 0.025 and 0.1 nmol/ml (DCVG); 0.005, 0.05 and 0.1 nmol/ml (DCVC) by preparing samples in the same way as the calibration samples. Limits of detection were determined by a signal-to-noise ratio of 3:1 based on the 15 μl injection volume. The lower limit of quantitation (LLOQ) was defined as the lowest concentration to be detectable at about 20% of precision (expressed in CV, % coefficient of variation) and 20% of accuracy. Absence of the artifactual transformation of TCA into DCA during sample preparation was confirmed by spiking TCA standards with naive mouse serum (TCA concentrations used: 500, 1000 or 1500 nmol/ml), or whole mouse blood (TCA concentrations used: 100, 500 or 1000 nmol/ml).
Absolute and relative recovery, as well as matrix effects, were estimated as detailed elsewhere (Delinsky et al., 2005b) using “low,” “medium” and “high” concentration samples (n=5 for each analyte/concentration) for TCA (80, 400 and 4000 nmol/ml), DCA (20, 100 and 1000 nmol/ml), DCVC and DCVG (both at 1, 5, and 50 nmol/ml).
Chemical standards for haloacetic acids were found to be stable in stock solution at -20°C for up to three months; however, diluted standards and biological samples following extraction should not be subjected to repeat freezing and thawing (data not shown). In contrast, both stock solutions of the chemical standards for DCVG and DCVC, as well as extracted samples, showed stable peak shapes even after six months at -20°C storage and repeat freeze-thaw cycles (data not shown). To estimate sample stability during mass spectrometry analysis, since the autosampler was operated at room temperature over a 24-hr period, a quality control sample of the chemical standard diluted with the mobile phase was included in each batch as the first and the last sample. No sample degradation was observed (data not shown).
2.5. Statistical analysis
All statistical analyses were performed using SAS software for Windows v. 9.13 (SAS Institute, Cary, NC). The overall assay precision for each analyte was estimated as CV from three sets of calibration curve samples and positive controls which were measured on different time points. The precision (CV) was estimated as , where is the estimated error variance obtained from a mixed-effects model, where log-transformed levels of each analytes were regressed on a fixed effect (nominal levels of QC samples) and three random effects (batch, assay date, residual error) by implementing SAS PROC MIXED (Kim et al., 2006; Rappaport and Kupper, 2008). Inter-day and intra-day variability were estimated by covariance parameters in the mixed-effect model. Classified precision and accuracy were estimated by simple statistics of mean and standard deviation of the estimated concentration of the analytes. Construction of calibration curves were also estimated using SAS PROC REG after combining to average several batches of calibration samples by each nominal concentration.
3. Results and discussion
The objective of this work was the development of a sensitive and quantitative analytical method for simultaneous detection of both oxidative and conjugated metabolites of TCE in small-volume biological samples. We started with a recent method for DCA only (Delinsky et al., 2005b), and extended the method for TCA as well. Also, we were able to adapt the method to the analysis of DCVG and DCVC in the concurrent sample.
3.1. Product ion transitions
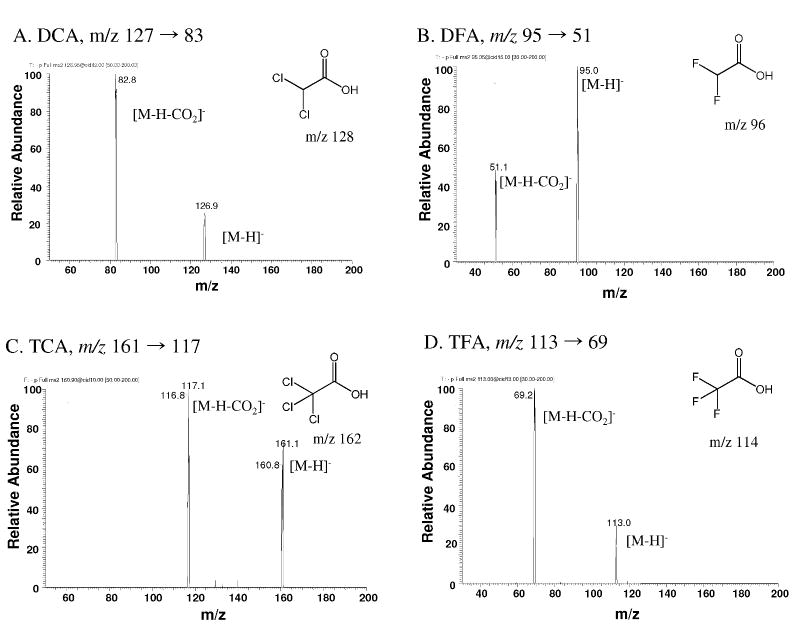
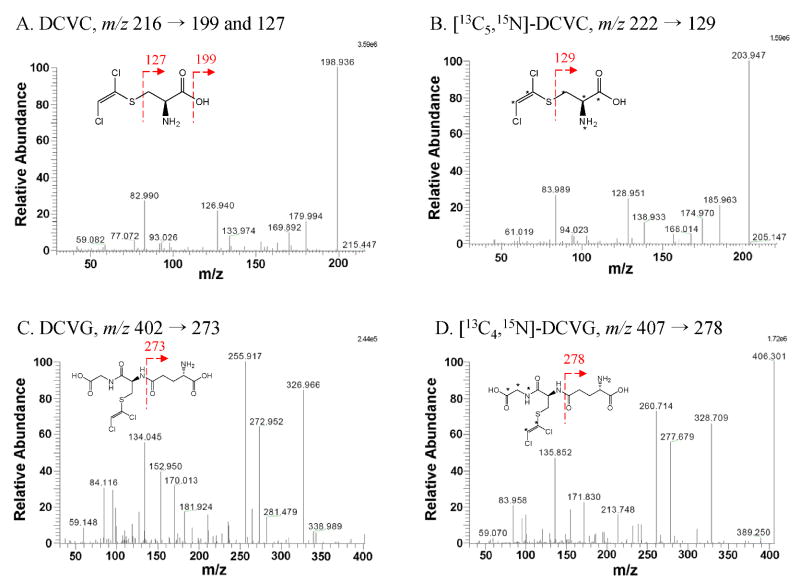
The MS/MS product ion spectra of each analyte were obtained by direct infusion of each chemical standard (Fig. 2-3). DCA and TCA each fragmented into two major product ions, while DCVC and DCVG produced more complex breakdown patterns depending on the MS conditions such as collision energy and collision gas pressure. Transition reactions (Fig. 3) were monitored by a collision energy giving optimal signal-to-noise ratio for the major transition reaction of the haloacetic acid analytes in the negative ion mode and the DCVC and DCVG in the positive ion mode.
Fig. 2.
MS/MS product ion spectra of [M-H]- ions of chemical standards. (A) dichloroacetic acid, (B) difluoroacetic acid, (C) trichloroacetic acid, and (D) trifluoroacetic acid.
Fig. 3.
MS/MS product ion spectra of [M+H]+ ions of chemical standards. (A) S-(1,2-dichlorovinyl)-L-cysteine, (B) [13C5,15N] S-(1,2-dichlorovinyl)cysteine, (C) S-(1,2-dichlorovinyl)glutathione, and (D) [13C4,15N] S-(1,2-dichlorovinyl)glutathione. Asterisks indicate isotope-labeled position.
3.2. Effects of the mobile phase pH and 2-MEE on DCA and TCA measurements
We tested the effects of mobile phase composition and pH on analyte retention times. As reported previously (Olsen, 2001; Delinsky et al., 2005b), increased aqueous phase volume facilitates elution of the analytes. Ammonium formate buffer was necessary to prevent unwanted retention of the analytes on the column. Also by increasing pH of the mobile phase [specifically for 40 mM ammonium formate fraction] from 6.15 (without pH-adjustment) to 9.15 (adjusted with NH4OH), retention times were reduced as follows: 16.0 → 5.5 min for DCA; 17.8 → 5.7 min for DFA; 10.0 → 4.3 min for TCA; and 9.2 → 4.2 min for TFA. A decrease in retention time with increased pH is likely a result of deprotonation of the amino groups of the stationary phase and consequent decrease in affinity for the stationary phase for the carboxylate analytes.
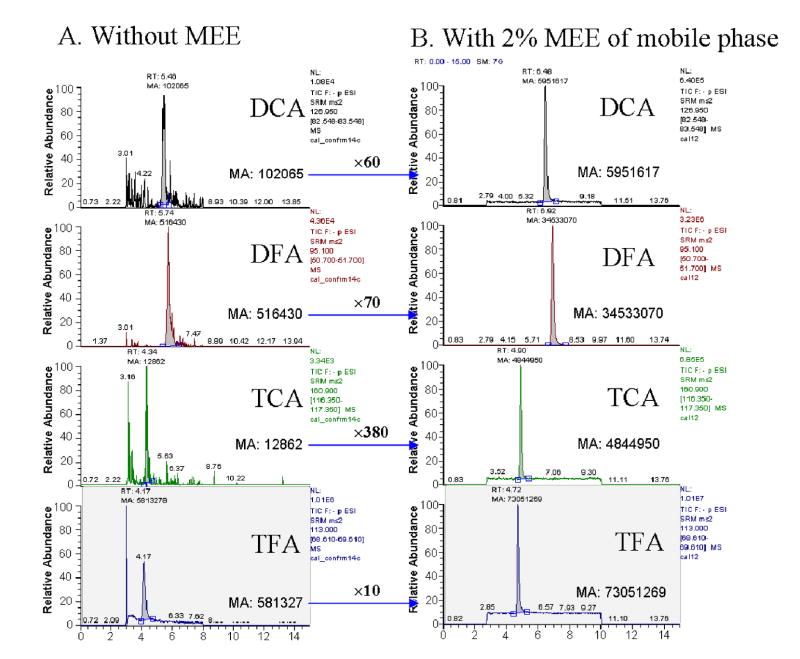
To enhance sensitivity, different mobile phase additives were evaluated: 2-MEE (Yamaguchi et al., 1999; Yamaguchi et al., 2001) and weak acid (e.g. acetic acid) (Apffel et al., 1995; Yamaguchi et al., 2001; Shou and Naidong, 2005). There were some reports in drug analysis using 2-MEE as a post-column additive to improve ionization of the analytes (Yamaguchi et al., 1999; Yamaguchi et al., 2001). Use of 2-MEE resulted in formation of smaller droplets containing a lower percentage of water and buffer due to decreased surface tension and higher boiling point. We found that the postcolumn addition of 2-MEE improved sensitivity of the haloacetic acids remarkably at a flow rate of 15 μl/min, which corresponds to 2% 2-MEE in mobile phase. Premixing of 2-MEE, rather than postcolumn addition to the mobile phase, further improved robustness of the assay and instrumental response, and also eliminated the need for a mixing tee and accessory tubing. The relatively higher boiling point of 2-MEE required raising the temperature of the heated transfer capillary tube to evaporate the analytes and minimize accumulation of impurities. With the capillary temperature set at 275°C we confirmed no thermal decomposition of the analytes. Overall, we were able to achieve a considerable enhancement of signal for each analyte: ∼60-fold for DCA and ∼380-fold for TCA (Fig. 4). To the contrary, addition of acetic acid to mobile phase did not result in signal enhancement but increased the retention time of the analytes.
Fig. 4.
Signal enhancement of haloacetic acids by 2-(2-methoxyethoxy)ethanol. Chromatograms of analytes before (A) and after (B) addition of 2-MEE. Analytes were determined after extraction from control serum spiked with chemical standards: 2 nmol/ml serum for DCA, 8 nmol/ml serum for TCA, and 50 nmol/ml serum for the fluorine analogues as internal standards. MA, manually integrated area of a detected peak.
3.3. Effect of mobile phase additives and MS condition on DCVC and DCVG measurement
Currently available methods for measurement of glutathione conjugates of TCE require sample derivatization and use of GC-ECD, GC-MS, or liquid chromatography-photodiode array detector (LC-UV) instrumentation (Fariss and Reed, 1987; Commandeur and Vermeulen, 1990; Dekant et al., 1990; Cummings and Lash, 2000; Lash et al., 2006; Chiu et al., 2006c). Most of these methods are applicable for the assessment of the mercapturic acid (N-acetyl-S-(1,2-dichlorovinyl)-l-cysteine) and DCVG from biological samples. However, insufficient sensitivity of the existing methods is a serious limitation for studies of kinetics of TCE metabolism through conjugation. Here, we developed a sensitive derivatization-free method applicable to assessment of both DCVC and DCVG from the same sample in which DCA and TCA amounts were measured (see above).
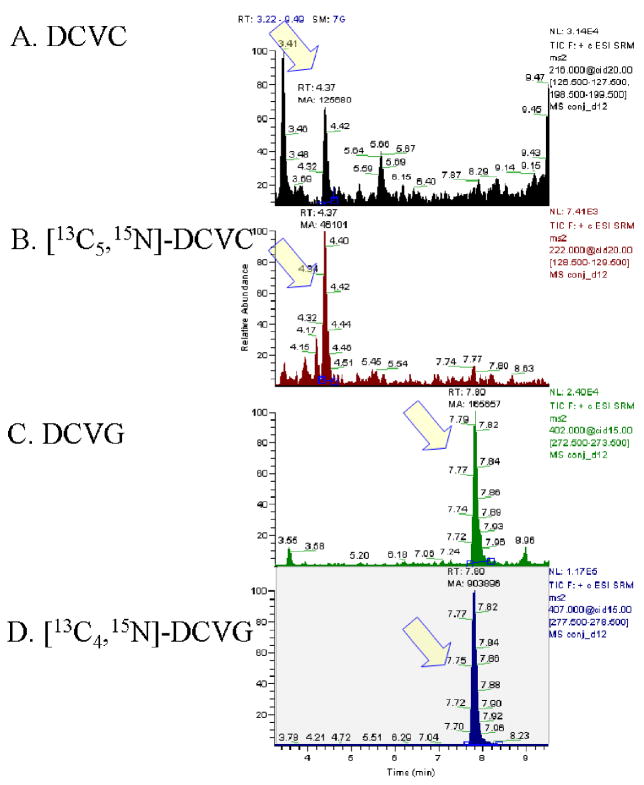
After obtaining the optimized patterns for ion transitions, different mobile phase additives were tested with a goal of improving ionization in the source. Both 0.1% formic acid and 0.1% acetic acid improved the sensitivity for each analyte, but the latter was selected due to improved signal-to-noise ratio for DCVC (by 5-fold) and marginally larger AUC for each analyte. MS spray voltage was set at 3.5kV, which provided 2-fold enhancement of signal relative to the default value (3kV). Although the higher voltage was accompanied by more noise, the addition of acetic acid to buffer was beneficial. After diverting the flow to waste for the initial 2.5 min, DCVC elutes first (retention time, 4.4 min), followed by DCVG (retention time, 7.8 min) as shown in Fig. 5.
Fig. 5.
Chromatograms of glutathione conjugates of TCE. (A) S-(1,2-dichlorovinyl)-L-cysteine, (B) [13C5,15N] S-(1,2-dichlorovinyl)-L-cysteine, (C) S-(1,2-dichlorovinyl)glutathione, and (D) [13C4,15N] S-(1,2-dichlorovinyl)glutathione. Analytes were determined after extraction from control serum spiked with chemical standards: 1 nmol/ml serum for DCVC and DCVG and 0.25 nmol/ml serum for the internal standards. Arrows indicate the peaks of analytes, and chromatograms were displayed in stick mode.
3.4. Extraction recovery and ion suppression
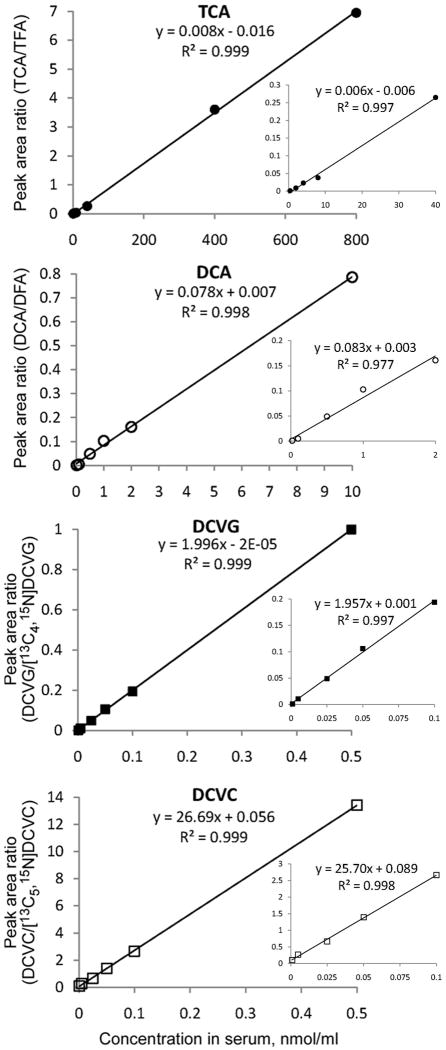
Extraction recovery for TCE metabolites was estimated as detailed elsewhere (Delinsky et al., 2005b). Relative recovery (a range of values depending on the concentration of the standard is shown) of the analytes was as follows: 51.6 - 75.6% for TCA; 64.4 - 80.7% for DCA; 17.9 - 41.2% for DCVG; and 20.7 – 27.3% for DCVC. The absolute recovery was 62.4 – 79.0% for TCA; 51.0 – 100% for DCA; 15.2 – 22.7% for DCVG; and 5.3 – 6.1% for DCVC. It should be noted that a relatively low yield of DCVC is likely due to the remnants of the sulfuric acid after neutralization with NH4OH which may cause low recovery during solid phase extraction. Indeed, we observed a two-fold decrease in recovery of DCVC after washing SPE cartridges with weak acid (pH 4-5) as compared with a regular washing step with alkali (pH>10) methanol (data not shown). It is also possible that DCVC is not a stable moiety under acidic condition during the extraction; however, despite the low yield our method still produces highly linear calibration curves with acceptable precision (Fig. 6).
Fig. 6.
Calibration curves of the analytes trichloroacetic acid (TCA, ●), dichloroacetic acid (DCA, ○), S-(1,2-dichlorovinyl)glutathione (DCVG, ■), and S-(1,2-dichlorovinyl)-L-cysteine (DCVC, □). Full range (large graphs), as well as low concentration range (inset graphs), calibration curves are shown.
Matrix effects were negligible (i.e., ion suppression or ionization enhancement of less than 10%) for TCA, or minor (18.1% ion suppression at low, 7.8 and 39.7% ionization enhancement at medium and high concentrations, respectively) for DCA. However, it should be noted that appreciable ion suppression was observed for DCVG (15.6–44.9%) and DCVC (70.0-77.6%). While the detection of both glutathione conjugates is challenging, the use of the isotope-labeled internal standards, as performed in this study, is necessary to achieve acceptable precision and accuracy (see below).
3.5. Linearity of the calibration curves, limit of detection (LOD) and limit of quantitation (LOQ)
Standard calibration curves (Fig. 6) showed a reliable response at a wide range of concentrations: 0.02 – 10.0 nmol/ml for DCA, 0.4 – 800 nmol/ml for TCA, and 0.0001 – 0.5 nmol/ml for both DCVG and DCVC, with high coefficients of determination. Since no evidence that strain effect impacts the calibration curve was found by comparing the results between serum from B6C3F1 and C57BL/6J mice, we combined data from both strains to construct the calibration curve.
Based on a volume of 50 μl serum, 15 μl of injection volume and a signal-to-noise ratio of 3:1, we could detect as little as 0.01 nmol/ml of DCA, 0.4 nmol/ml of TCA, 0.001 nmol/ml of DCVG and 0.001 nmol/ml of DCVC successfully, while the LLOQ were found to be: 0.02 [0.021 ± 0.004 (means ± SD)] nmol/ml for DCA, 0.4 [0.301 ± 0.041] nmol/ml for TCA, 0.005 [0.0066 ± 0.0005] nmol/ml for DCVG, and 0.005 [0.0049 ± 0.0007] nmol/ml for DCVC after consideration of about 20% CV of precision (standard deviation divided by measurement means, %) and 20% bias (difference between the given concentration and estimated concentration normalized by each nominal concentration, %).
3.6. Precision, accuracy and artifactual formation of DCA from TCA
The overall precision (CV, %) of the method is described in Table 1. The factors of “batch,” “inter-day variation” and measurement error, shown as “residual” were considered. DCA and TCA were most sensitive to the “batch” effect which is likely due to micro-condition of the experimental settings such as content of 2-MEE in the mobile phase. Even though the use of 2-MEE afforded considerable signal enhancement, it introduced additional variability which may, however, be remedied by establishing the calibration curves for these analytes in every batch. To the contrary, DCVG and DCVC “residual” (i.e., measurement) error accounted for 70% (for DCVG) to 85% (for DCVC) of the total variability. The classified precision and accuracy at nominal range of quality assurance samples are as follows: 3.06 – 19.1% CV and 5.61 – 17.1% bias for DCA; 4.81 – 11.0% CV and 7.20 – 23.7% bias for TCA; 8.7 – 11.1% CV and 3.7 – 23.7% bias for DCVG; 12.2 – 14.9% CV and 3.0 – 18.1% bias for DCVC.
Table 1.
Overall precision and variance component by batch, assay date and residual error.
| Chemical | Factor | CV*, % | Variance component, % |
|---|---|---|---|
| Batch | 18.2 | 79.9 | |
| DCA | Date | 0.1 | 0.0 |
| (n=22) | Residual | 9.1 | 20.1 |
| Total | 20.4 | 100.0 | |
| Batch | 22.3 | 75.3 | |
| TCA | Date | 2.9 | 1.3 |
| (n=22) | Residual | 12.3 | 23.4 |
| Total | 25.8 | 100.0 | |
| Batch | 6.2 | 30.7 | |
| DCVG | Date | 0.0 | 0.0 |
| (n=19) | Residual | 9.3 | 69.3 |
| Total | 11.1 | 100.0 | |
| Batch | 0.0 | 0.0 | |
| DCVC | Date | 6.2 | 15.0 |
| (n=34) | Residual | 14.7 | 85.0 |
| Total | 16.0 | 100.0 | |
Overall coefficient of variation (CV) indicates a comprehensive precision of the assay, estimated by covariance estimates using mixed effects model to show variance components by batch, date and residual measurement error. Since the representative calibration curves were established in every batch, the actual assay error is represented by the “residual” component.
We also monitored whether artifactual transformation of TCA into DCA occurred during sample preparation by using mouse serum spiked with TCA standards at 500, 1000 and 1500 nmol/ml. We found no evidence of DCA formation with increases in spiked TCA (data not shown) and conclude that there is no evidence for artifactual formation of DCA during sample preparation in our method.
3.7. Application of the method to in vivo samples
While there are several currently available methods for assessment of TCA, DCA, as well as some glutathione conjugates of TCE, to our knowledge, there are no published reports of measurements of TCA, DCA, DCVC and DCVG from the same in vivo biological sample. To test the utility of our method on the biological samples, B6C3F1 mice were exposed to TCE (2.1 g/kg, i.g.) and serum samples collected 2 hr after exposure. We determined DCA, TCA, DCVG and DCVC in each of the serum samples. Our data (mean±standard deviation, n=3) show the following amounts: DCA, 0.122±0.014 nmol/ml (limit of detection: 0.01 nmol/ml); TCA, 256±30 nmol/ml (LOD 0.4 nmol/ml); DCVG, 0.037±0.015 nmol/ml (LOD 0.001 nmol/ml); DCVC, 0.0024±0.0009 nmol/ml (LOD 0.001 nmol/ml). We compared the measurements of TCE metabolites in the present study to those published in the literature (Table 2). It should be noted that the use of corn oil may dramatically change the absorption of TCE relative to other types of dosing vehicles that more closely approximate aqueous solutions (Withey et al., 1983). Thus, after the adjustment for the TCE dose between the studies, we conclude that values obtained with our method are comparable to previous studies which used the same mouse strain and corn oil as a vehicle.
Table 2.
TCE metabolitesa reported at 2 hr after exposure to TCE.
| Dose (mg/kg, route) |
DCA (nmol/ml) |
TCA (nmol/ml) |
Matrix | Species | Strain | Assay method | LOD on column (pmole) |
Reference |
|---|---|---|---|---|---|---|---|---|
| TCE (2140, oral*) | 0.122 | 256 | Plasma | Mouse | B6C3F1 | LC-MS/MS | 0.075 (DCA LOD) | Present study |
| 3.00 (TCA LOD) | ||||||||
| 0.0375 (DCVG LOD) | ||||||||
| 0.0375 (DCVC LOD) | ||||||||
| TCE (2000, oral#) | 0.306 | NR | Blood | Rat | SD | LC-MS/MS | 0.72 (DCA LOD) | (Delinsky et al., 2005b) |
| TCE (1000, oral#) | ND | NR | (Merdink et al., 1998) | |||||
| TCE (100, iv) | NR | 110 | Blood | Mouse | B6C3F1 | GC-MS | 2.00 (DCA LOD) | |
| CH (50, iv) | NR | 200 | 0.04 (TCA LOD) | |||||
| TCE (5.8, inh)b | ND | 18 | Plasma | Human | NR | MS/MS | 0.30 (DCA LOD) | (Brashear et al., 1997) |
| TCE (1970, oral*) | NDc | NDc | Blood | Rat | F344 (m) | GC-ECD (TCA, DCA), LC-UV (DCVG, DCVC) | (Lash et al., 2006) | |
| 0.05 (DCA LOD) | ||||||||
| 0.05 (TCA LOD) | ||||||||
| 5.0 (DCVC LOD) | ||||||||
| F344 (f) | 50 (DCVG LOD) | |||||||
| TCE (2000, oral*) | NRd | 260 | (Abbas and Fisher, 1997) | |||||
| TCE (1200, oral*) | 1.7 | 355 | 0.40 (DCA LOD)e | |||||
| TCE (600, oral*) | 1.2 | 171 | Blood | Mouse | B6C3F1 | GC-ECD | 0.30 (TCA LOD)e | |
| TCE (300, oral*) | 1.4 | 263 | ||||||
| TCE (1970, oral#) | 14 | 410 | Blood | Mouse | B6C3F1 | (Larson and Bull, 1992) | ||
| TCE (3020, oral#) | ND | 62 | Blood | Rat | SD | GC-ECD | NRf | |
ND, not detected; NR, not reported; LC-MS/MS, liquid chromatography-tandem mass spectrometry; GC-MS, gas chromatography-mass spectrometry; GC-ECD, gas chromatography-electron capture detector; LC-UV, liquid chromatography-ultraviolet detector; LOD, limit of detection; LOQ, limit of quantitation.
DCVG (0.037 nmol/ml) and DCVC (0.0024 nmol/ml) are not shown here due to the sparseness of the measurements while Lash et al. (2006) reported DCVC of 0.009 nmol/ml in blood of rats after TCE treatment by oral gavage at 1970 mg/kg.
Dose in ref. (Lin et al., 2001) was estimated as follows: alveolar ventilation 2.7 L/hr/kg × concentration 0.537 mg/L (100 ppm) × exposure period 4 hr.
This study used three doses: 260 mg/kg, 660 mg/kg and 1970 mg/kg on female (f) and male (m) F344 rats, and assessed multiple time points. Although TCA in blood was below the LOD at any time point for 1970 mg/kg dosage, it was 0.124 nmol/ml (at 24 hr, female) and 9.67 nmol/ml (at 48 hr, female) for 660 mg/kg dosage; 15.8-18.2 nmol/ml (at 2-8 hr, female). The inconsistent dose-response relationship was also observed for DCVG in blood. Rats produced more DCVG between 2 and 48 hr for 263 mg/kg dosage than for 657 mg/kg; however, DCVG was detected about 0.01 to 0.08 nmol/ml between 4 and 48 hr for higher dosages, where females produce higher amounts.
Not reported at 2 hr (next available observation, 15 nmol/ml at 4 hr).
LOD on column imputed by an original method(Ketcha et al., 1996) due to lack of information.
LOD on column not reported. Limit of detection on sample-base were 4 nmol/ml of DCA and 15 nmol/ml of TCA.
Administered in corn oil as vehicle
Administered in an aqueous emulsion as vehicle.
Since toxic effects of TCE arise from its metabolites, better understanding of TCE metabolism is needed to address gender- or species-susceptibility and target organ specificity (National Academy of Science, 2006). It requires the exploration of tissue-dosimetry and shape of dose-response relationship, which requires sensitive methodology for quantitation of TCE metabolites in vivo. For instance, a panel of the National Academy of Science pointed out the need “to determine the metabolic pathway and yield for forming DCA from TCA whether via TCA or via other pathway(s)”(National Academy of Science, 2006); however, DCA has seldom been determined in vivo after exposure to TCE except for artifactual overestimations (Ketcha et al., 1996; Brashear et al., 1997; Merdink et al., 1998; Delinsky et al., 2005b). Thus, the present method can be used to address many issues relating to measurement challenges and facilitate risk assessment of TCE exposures through means for sensitive determination of DCA and TCA with concurrent quantitation of DCVG and DCVC.
In conclusion, we developed a novel combination of methods for measurements of major TCE metabolites DCA, TCA, DCVG and DCVC from small volume biological samples by using HPLC-ESI-MS/MS. The present method allows considerably robust and sensitive detection of multiple metabolites from the same sample and can applied successfully to in vivo experiments aimed at understanding of TCE metabolism.
Acknowledgments
This research was supported by the National Institute of Environmental Health Sciences grants P42 ES005948 and P30 ES010126.
Footnotes
Conflict of interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbas R, Fisher JW. A physiologically based pharmacokinetic model for trichloroethylene and its metabolites, chloral hydrate, trichloroacetate, dichloroacetate, trichloroethanol, and trichloroethanol glucuronide in B6C3F1 mice. Toxicol Appl Pharmacol. 1997;147:15–30. doi: 10.1006/taap.1997.8190. [DOI] [PubMed] [Google Scholar]
- Agency for Toxic Substances and Disease Registry. Toxicological profile for trichloroethylene. U.S. Department of Health and Human Services; Washington, DC: 1997. [PubMed] [Google Scholar]
- Apffel A, Fischer S, Goldberg G, Goodley PC, Kuhlmann FE. Enhanced sensitivity for peptide mapping with electrospray liquid chromatography-mass spectrometry in the presence of signal suppression due to trifluoroacetic acid-containing mobile phases. J Chromatogr A. 1995;712:177–190. doi: 10.1016/0021-9673(95)00175-m. [DOI] [PubMed] [Google Scholar]
- Barton HA, Clewell HJ., III Evaluating noncancer effects of trichloroethylene: dosimetry, mode of action, and risk assessment. Environ Health Perspect. 2000;108 2:323–334. doi: 10.1289/ehp.00108s2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloemen LJ, Monster AC, Kezic S, Commandeur JN, Veulemans H, Vermeulen NP, Wilmer JW. Study on the cytochrome P-450- and glutathione-dependent biotransformation of trichloroethylene in humans. Int Arch Occup Environ Health. 2001;74:102–108. doi: 10.1007/s004200000198. [DOI] [PubMed] [Google Scholar]
- Brashear WT, Bishop CT, Abbas R. Electrospray analysis of biological samples for trace amounts of trichloroacetic acid, dichloroacetic acid, and monochloroacetic acid. J Anal Toxicol. 1997;21:330–334. doi: 10.1093/jat/21.5.330. [DOI] [PubMed] [Google Scholar]
- Bruning T, Bolt HM. Renal toxicity and carcinogenicity of trichloroethylene: key results, mechanisms, and controversies. Crit Rev Toxicol. 2000;30:253–285. doi: 10.1080/10408440091159202. [DOI] [PubMed] [Google Scholar]
- Bull RJ. Mode of action of liver tumor induction by trichloroethylene and its metabolites, trichloroacetate and dichloroacetate. Environ Health Perspect. 2000;108 2:241–259. doi: 10.1289/ehp.00108s2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu WA, Caldwell JC, Keshava N, Scott CS. Key scientific issues in the health risk assessment of trichloroethylene. Environ Health Perspect. 2006a;114:1445–1449. doi: 10.1289/ehp.8690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu WA, Okino MS, Lipscomb JC, Evans MV. Issues in the pharmacokinetics of trichloroethylene and its metabolites. Environ Health Perspect. 2006b;114:1450–1456. doi: 10.1289/ehp.8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commandeur JN, Vermeulen NP. Identification of N-acetyl(2,2-dichlorovinyl)- and N-acetyl(1,2-dichlorovinyl)-L-cysteine as two regioisomeric mercapturic acids of trichloroethylene in the rat. Chem Res Toxicol. 1990;3:212–218. doi: 10.1021/tx00015a005. [DOI] [PubMed] [Google Scholar]
- Cummings BS, Lash LH. Metabolism and toxicity of trichloroethylene and S-(1,2-dichlorovinyl)-L-cysteine in freshly isolated human proximal tubular cells. Toxicol Sci. 2000;53:458–466. doi: 10.1093/toxsci/53.2.458. [DOI] [PubMed] [Google Scholar]
- Dekant W, Koob M, Henschler D. Metabolism of trichloroethene--in vivo and in vitro evidence for activation by glutathione conjugation. Chem Biol Interact. 1990;73:89–101. doi: 10.1016/0009-2797(90)90110-9. [DOI] [PubMed] [Google Scholar]
- Delinsky AD, Bruckner JV, Bartlett MG. A review of analytical methods for the determination of trichloroethylene and its major metabolites chloral hydrate, trichloroacetic acid and dichloroacetic acid. Biomed Chromatogr. 2005a;19:617–639. doi: 10.1002/bmc.488. [DOI] [PubMed] [Google Scholar]
- Delinsky AD, Delinsky DC, Muralidhara S, Fisher JW, Bruckner JV, Bartlett MG. Analysis of dichloroacetic acid in rat blood and tissues by hydrophilic interaction liquid chromatography with tandem mass spectrometry. Rapid Commun Mass Spectrom. 2005b;19:1075–1083. doi: 10.1002/rcm.1890. [DOI] [PubMed] [Google Scholar]
- Fariss MW, Reed DJ. High-performance liquid chromatography of thiols and disulfides: dinitrophenol derivatives. Methods Enzymol. 1987;143:101–109. doi: 10.1016/0076-6879(87)43018-8. [DOI] [PubMed] [Google Scholar]
- Finkelstein MB, Patel NJ, Anders MW. Metabolism of [14C]- and [35S]S-(1,2-dichlorovinyl)-L-cysteine in the male Fischer 344 rat. Drug Metab Dispos. 1995;23:124–128. [PubMed] [Google Scholar]
- International Agency for Research on Cancer. Dry cleaning, some chlorinated solvents and other industrial chemicals. World Health Organization; Lyon, France: 1995. [Google Scholar]
- Ketcha MM, Stevens DK, Warren DA, Bishop CT, Brashear WT. Conversion of trichloroacetic acid to dichloroacetic acid in biological samples. J Anal Toxicol. 1996;20:236–241. doi: 10.1093/jat/20.4.236. [DOI] [PubMed] [Google Scholar]
- Kim S, Vermeulen R, Waidyanatha S, Johnson BA, Lan Q, Rothman N, Smith MT, Zhang L, Li G, Shen M, Yin S, Rappaport SM. Using urinary biomarkers to elucidate dose-related patterns of human benzene metabolism. Carcinogenesis. 2006;27:772–781. doi: 10.1093/carcin/bgi297. [DOI] [PubMed] [Google Scholar]
- Kuklenyik Z, Ashley DL, Calafat AM. Quantitative detection of trichloroacetic acid in human urine using isotope dilution high-performance liquid chromatography-electrospray ionization tandem mass spectrometry. Anal Chem. 2002;74:2058–2063. doi: 10.1021/ac011250g. [DOI] [PubMed] [Google Scholar]
- Larson JL, Bull RJ. Species differences in the metabolism of trichloroethylene to the carcinogenic metabolites trichloroacetate and dichloroacetate. Toxicol Appl Pharmacol. 1992;115:278–285. doi: 10.1016/0041-008x(92)90333-n. [DOI] [PubMed] [Google Scholar]
- Lash LH, Fisher JW, Lipscomb JC, Parker JC. Metabolism of trichloroethylene. Environ Health Perspect. 2000a;108 2:177–200. doi: 10.1289/ehp.00108s2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lash LH, Parker JC, Scott CS. Modes of action of trichloroethylene for kidney tumorigenesis. Environ Health Perspect. 2000b;108 2:225–240. doi: 10.1289/ehp.00108s2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lash LH, Putt DA, Parker JC. Metabolism and tissue distribution of orally administered trichloroethylene in male and female rats: identification of glutathione- and cytochrome P-450-derived metabolites in liver, kidney, blood, and urine. J Toxicol Environ Health A. 2006;69:1285–1309. doi: 10.1080/15287390500360133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lash LH, Xu Y, Elfarra AA, Duescher RJ, Parker JC. Glutathione-dependent metabolism of trichloroethylene in isolated liver and kidney cells of rats and its role in mitochondrial and cellular toxicity. Drug Metab Dispos. 1995;23:846–853. [PubMed] [Google Scholar]
- Lin YS, Smith TJ, Kelsey KT, Wypij D. Human physiologic factors in respiratory uptake of 1,3-butadiene. Environ Health Perspect. 2001;109:921–926. doi: 10.1289/ehp.01109921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney LL, Picken JC, Weakley FB, Eldridge AC, Campbell RE, Cowan JC, Biester HE. Possible toxic factor of trichloroethylene-extracted soybean oil mea. J Am Chem Soc. 1959;81:909–915. [Google Scholar]
- Merdink JL, Gonzalez-Leon A, Bull RJ, Schultz IR. The extent of dichloroacetate formation from trichloroethylene, chloral hydrate, trichloroacetate, and trichloroethanol in B6C3F1 mice. Toxicol Sci. 1998;45:33–41. doi: 10.1006/toxs.1998.2500. [DOI] [PubMed] [Google Scholar]
- National Academy of Science. Assessing the human health risks of trichloroethylene: Key scientific issues. The National Academies Press; Washington, D.C.: 2006. [Google Scholar]
- National Toxicology Program. 11th report on carcinogens. 2004 [PubMed] [Google Scholar]
- Olsen BA. Hydrophilic interaction chromatography using amino and silica columns for the determination of polar pharmaceuticals and impurities. J Chromatogr A. 2001;913:113–122. doi: 10.1016/s0021-9673(00)01063-3. [DOI] [PubMed] [Google Scholar]
- Pastino GM, Yap WY, Carroquino M. Human variability and susceptibility to trichloroethylene. Environ Health Perspect. 2000;108 2:201–214. doi: 10.1289/ehp.00108s2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaport SM, Kupper LL. Quantitative exposure assessment. Rappaport, SM; El Cerrito, CA, U.S.A.: 2008. [Google Scholar]
- Shou WZ, Naidong W. Simple means to alleviate sensitivity loss by trifluoroacetic acid (TFA) mobile phases in the hydrophilic interaction chromatography-electrospray tandem mass spectrometric (HILIC-ESI/MS/MS) bioanalysis of basic compounds. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;825:186–192. doi: 10.1016/j.jchromb.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Withey JR, Collins BT, Collins PG. Effect of vehicle on the pharmacokinetics and uptake of four halogenated hydrocarbons from the gastrointestinal tract of the rat. J Appl Toxicol. 1983;3:249–253. doi: 10.1002/jat.2550030506. [DOI] [PubMed] [Google Scholar]
- Wu C, Schaum J. Exposure assessment of trichloroethylene. Environ Health Perspect. 2000;108 2:359–363. doi: 10.1289/ehp.00108s2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi J, Ohmichi M, Hasegawa M, Yoshida H, Ogawa N, Higuchi S. Identification of rat urinary and biliary metabolites of esonarimod, a novel antirheumatic drug, using liquid chromatography/electrospray ionization tandem mass spectrometry with postcolumn addition of 2-(2-methoxyethoxy)ethanol, a signal-enhancing modifier. Drug Metab Dispos. 2001;29:806–812. [PubMed] [Google Scholar]
- Yamaguchi J, Ohmichi M, Jingu S, Ogawa N, Higuchi S. Utility of postcolumn addition of 2-(2-methoxyethoxy)ethanol, a signal-enhancing modifier, for metabolite screening with liquid chromatography and negative ion electrospray ionization mass spectrometry. Anal Chem. 1999;71:5386–5390. doi: 10.1021/ac990664v. [DOI] [PubMed] [Google Scholar]