Abstract
Psychopathy is a complex personality disorder that includes interpersonal and affective traits such as glibness, lack of empathy, guilt or remorse, shallow affect, and irresponsibility, and behavioral characteristics such as impulsivity, poor behavioral control, and promiscuity. Much is known about the assessment of psychopathy; however, relatively little is understood about the relevant brain disturbances. The present review integrates data from studies of behavioral and cognitive changes associated with focal brain lesions or insults and results from psychophysiology, cognitive psychology and cognitive and affective neuroscience in health and psychopathy. The review illustrates that the brain regions implicated in psychopathy include the orbital frontal cortex, insula, anterior and posterior cingulate, amygdala, parahippocampal gyrus, and anterior superior temporal gyrus. The relevant functional neuroanatomy of psychopathy thus includes limbic and paralimbic structures that may be collectively termed ‘the paralimbic system’. The paralimbic system dysfunction model of psychopathy is discussed as it relates to the extant literature on psychopathy.
Keywords: Psychopathy, Cognitive neuroscience, Affective neuroscience, Review, fMRI, ERP
1. Introduction
Psychopathy is a serious mental health disorder. Psychopathy is believed to affect approximately 1% of the general population, 15–25% of the male and female prison population (Hare, 1991, 2003), and 10–15% of substance abuse populations (Alterman and Cacciola, 1991; Alterman et al., 1993, 1998). Over the last 20 years, much has been learned about the assessment and characterization of the forensic and legal aspects of psychopathy. However, compared with other psychiatric disorders of similar prevalence, relatively little is known about the neural systems implicated in psychopathy. This review will draw on information from multiple disciplines, including neurology, psychiatry, psychology, cognitive neuroscience, psychophysiology, and epileptology. The literatures will be integrated and a new model of the functional neuroanatomy underlying psychopathy will be presented. The review is offered in several parts. First, the assessment and classification of psychopathy is reviewed. The second part of the review will draw upon indirect evidence from studies of how insults or damage to regions of the brain may lead to symptoms and cognitive abnormalities consistent with those observed in psychopathy suggesting that these latter circuits may be implicated in the disorder. The third part of the review focuses on the cognitive and affective neuroscience studies of psychopathy. Finally, a new model of the functional neuroanatomy underlying psychopathy will be presented.
2. The construct and assessment of psychopathy
The modern concept of psychopathy can be traced back to the psychiatrist Pinel (1792 as cited in Cleckley, 1941), who labeled the condition ‘madness without delirium’. This term was used to denote the lack of morality and behavioral control in these individuals that occurred despite the absence of any psychotic symptoms or defects in intellectual function. In the 200 years that followed, the condition has been through an evolution in terminology, but many of the defining characteristics have remained unchanged. These characteristics were most clearly delineated, and the current diagnostic criteria established, by the writings of the psychiatrist Hervey Cleckley (1941). In his 40 years of clinical work, Cleckley came to narrow the syndrome he called psychopathy to 16 characteristics. Affectively, psychopaths are callous, shallow, and superficial, and they lack insight and empathy for the effect their poor behavior has on others; behaviorally, psychopaths are impulsive, nomadic, and have weak behavioral control.
In subsequent years, Hare and colleagues operationalized and transformed Cleckley's characteristics into items on the Hare Psychopathy Checklist (Hare, 1980) and its successor, the Hare Psychopathy Checklist-Revised (PCL-R; Hare, 1991, 2003). The PCL-R is now the most widely accepted diagnostic instrument for psychopathy. There is a substantial literature attesting to the reliability and validity of the PCL-R as a measure of psychopathy in incarcerated offenders, forensic patients, psychiatric patients, and substance abuse patients (see Hare, 2003, for a review). The items on the PCL-R are listed in Table 1.
Table 1.
The 20 items listed on the Psychopathy Checklist-Revised (Hare, 1991, 2003)
| Item | Two-factor model | Four-factor model | |
|---|---|---|---|
| 1 | Glibness/superficial charm | 1 | 1 |
| 2 | Grandiose sense of self-worth | 1 | 1 |
| 3 | Need for stimulation | 2 | 3 |
| 4 | Pathological lying | 1 | 1 |
| 5 | Conning/manipulative | 1 | 1 |
| 6 | Lack of remorse or guilt | 1 | 2 |
| 7 | Shallow affect | 1 | 2 |
| 8 | Callous/lack of empathy | 1 | 2 |
| 9 | Parasitic lifestyle | 2 | 3 |
| 10 | Poor behavioral controls | 2 | 4 |
| 11 | Promiscuous sexual behavior | – | – |
| 12 | Early behavioral problems | 2 | 4 |
| 13 | Lack of realistic, long-term goals | 2 | 3 |
| 14 | Impulsivity | 2 | 3 |
| 15 | Irresponsibility | 2 | 3 |
| 16 | Failure to accept responsibility | 1 | 2 |
| 17 | Many marital relationships | – | – |
| 18 | Juvenile delinquency | 2 | 4 |
| 19 | Revocation of conditional release | 2 | 4 |
| 20 | Criminal versatility | – | 4 |
The items corresponding to the early two-factor conceptualization of psychopathy (Harpur et al., 1988, 1989) and current four-factor model are listed (Hare, 2003). The two-factor model labels are Interpersonal/Affective (Factor 1) and Social Deviance (Factor 2) and the four-factor model labels are Interpersonal (Factor 1), Affective (Factor 2), Lifestyle (Factor 3), and Antisocial (Factor 4). Items followed by a dash did not load on any factor.
The PCL-R assessment is performed by reviewing the participant's institutional records, including intake assessments, social worker assessments, and reports of institutional adjustment and transgressions. A semi-structured interview covering school adjustment, employment, intimate relationships, family, friends, and criminal activity is conducted. Explicit criteria detailing each item are reviewed from the PCL-R manual, and each item is scored on a three point scale: 0—does not apply, 1—applies somewhat, and 2—definitely applies, to the individual. The resulting scores range from 0 to 40, and the recommended diagnostic cutoff for psychopathy is 30 (Hare, 1991, 2003). Interviews are typically video taped so that an independent rating can be obtained. The total assessment time typically ranges from 2 to 5 h, it is rigorous, and training is required.
Early factor analyses of the PCL-R items revealed two correlated factors (see Table 1; Harpur et al., 1988, 1989). Factor 1 included items related to emotional and interpersonal relationships. Factor 2 items reflected impulsive and antisocial behaviors. This latter factor is most closely related to the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) classification of Antisocial Personality Disorder (ASPD; American Psychiatric Association, 1994). It is important to note that ASPD has been criticized for overly relying on antisocial behaviors, while excluding many of the affective and interpersonal characteristics considered to be central to the construct of psychopathy (Alterman et al., 1998; Hare, 1996; Hare et al., 1991; Hart and Hare, 1996; Widiger et al., 1996). ASPD also has been questioned on grounds of specificity in forensic populations (Hart and Hare, 1996). Nearly 80–90% of inmates in a maximum security prison fulfill the criteria for ASPD, while only 15–25% score above the diagnostic criteria for psychopathy. In addition, the diagnostic confusion of psychopathy as putatively measured by ASPD and the complex relationships of these constructs with substance disorders have led to considerable confusion and hampered research efforts. It is important to stress that proper psychometric assessment of a condition is crucial, particularly when trying to relate it to psychophysiological or cognitive neuroscience data. The review below will largely be constrained to studies that employed psychometric measures consistent with the classic conceptualization of psychopathy. That is, the preferred conceptualization of psychopathy is a disorder that includes both interpersonal and affective characteristics and deviant behavior.
Recent psychometric analyses, using Item Response Theory (IRT), of PCL-R scores from large diverse multicultural samples have revealed a two-factor four-facet model (see Table 1; Hare, 2003). This model includes facets labeled, Interpersonal (Factor 1), Affective (Factor 2), Lifestyle (Factor 3), and Antisocial (Factor 4) (but see also Cooke and Michie, 1997, for a three-factor model). There have been few studies that have examined the neurocognitive correlates of the factor models of psychopathy (Patrick et al., 1993, 1994; Raine et al., 2004; Soderstrom et al., 2002; Sutton et al., 2002). However, as in complex disorders such as schizophrenia, relating specific symptom clusters to patterns of cerebral activity may clarify the relevant brain disturbances and help reconcile discrepant findings (Liddle, 2001). It may be fruitful therefore to relate psychophysiological and cognitive neuroscience measures to the factors of psychopathy. A potential problem with this method, however, is that the factors of psychopathy are often strongly correlated, making it difficult to relate neural correlates to the symptom profiles.
In summary, research over the last 20 years has made great strides in characterizing the psychometric assessment and classification of psychopathy. The classic conceptualization of psychopathy is a disorder that includes both interpersonal and affective characteristics and behavioral traits.
3. Neurology and psychopathy
One way to examine the possible neural regions implicated in psychopathic behavior is to draw from studies of behavioral changes and cognitive impairments associated with damage to specific brain circuits. It is important to recognize that this method provides only indirect evidence of the possible regions implicated in psychopathic symptomatology; nevertheless, some interesting and compelling data have accumulated. The most notable neurological case study is that of the railroad worker Phineas Gage (Harlow, 1848). Gage suffered penetrating trauma to the prefrontal cortex (Damasio et al., 1994). He was transformed by this accident from a responsible railroad manager to an impulsive, irresponsible, sexually promiscuous, verbally abusive individual (Harlow, 1848). Many of Gage's symptoms are consistent with those classically associated with psychopathy.
Subsequent studies of patients with prefrontal lobe damage suggest that the orbital frontal cortex plays a role in mediating some behaviors related to psychopathy (Blumer and Benson, 1975; Damasio, 1994). Damage to the orbital frontal cortex leads to a condition termed ‘pseudopsychopathy’ (Blumer and Benson, 1975) or ‘acquired sociopathic personality’ (Damasio, 1994) characterized by problems with reactive aggression, motivation, empathy, planning and organization, impulsivity, irresponsibility, insight, and behavioral inhibition (Malloy et al., 1993; Stuss et al., 1983). In some cases, patients may become prone to grandiosity (Blumer and Benson, 1975) and confabulation (Malloy et al., 1993; Schnider, 2001). Recent studies suggest that bilateral damage to orbital frontal cortex is necessary to elicit changes in social behavior (Hornak et al., 2003). Moreover, patients with gross, extensive damage due to stroke or closed head injury are more likely to exhibit ‘acquired sociopathic’ symptomology than are patients with focal, circumscribed surgical lesions to orbital frontal cortex (Hornak et al., 2003). These data suggest that some aspects of psychopathic symptomatology may map onto the (dys)function of orbital frontal cortex and adjacent regions. However, the ‘pseudopsychopathy’ or ‘acquired sociopathy’ model does not appear to fully account for the constellation of symptoms observed in psychopathy. For example, patients with orbital frontal damage rarely show instrumental or goal-directed aggression — a cardinal feature of psychopathy (Blair, 2001; Hare, 1993). Orbital frontal patients also do not typically exhibit the callousness commonly observed in psychopathic individuals. Similarly, patients with ‘acquired sociopathy’, unlike psychopathic individuals, are characterized by lack of motivation, hoarding behavior, mood disturbances, incontinence, and failure or inability to make long-term plans (Blumer and Benson, 1975). Psychopathic individuals, on the other hand, often enjoy making grandiose life plans — they just fail to follow through with them.
Patients with orbital frontal lesions show impairment on affective voice and face expression identification tasks (Hornak et al., 1996, 2003), response reversal or extinction (Blair and Cipolotti, 2000; Rolls et al., 1994), and decision-making (Bechara et al., 2000). The animal literature shows that lesions to orbital frontal cortex impair inhibitory performance on No Go trials of Go/No Go paradigms (Iversen and Mishkin, 1970). Psychopaths have problems with processing certain aspects of affective speech and face stimuli (Blair et al., 1997; Kosson et al., 2002; Louth et al., 1998). Psychopaths, under certain contextual demands, also show poor response inhibition (Kiehl, 2000; Lapierre et al., 1995), response modulation (Newman and Kosson, 1986; Newman et al., 1987, 1992; Newman and Schmitt, 1998) and more recently response reversal (Mitchell et al., 2002). Boys with psychopathic tendencies and adult psychopaths tend to show impairments on the Bechara gambling test of decision making (Blair, 2001; Mitchell et al., 2002), though not all studies have found this effect (Schmitt et al., 1999).
In summary, damage to the orbital frontal cortex appears to be associated with some symptoms and cognitive impairments that may also be found in psychopaths. However, despite these apparent similarities, no studies have actually explicitly investigated how orbital frontal patients score on psychopathy measures. Similarly, many of the studies of cognitive function in psychopathy and orbital frontal patients have used different cognitive tests, making inferences between studies problematic. It is only recently that identical tasks (i.e., response reversal) have been examined in both orbital frontal patients and in samples of psychopathic individuals (Mitchell et al., 2002). Nevertheless, it appears that lesions to orbital frontal cortex appear to elicit behaviors that most consistently map onto the Affective (Factor 2) and Lifestyle (Factor 3) factors of the four-factor psychopathy model. These factors of psychopathy include symptoms of impulsivity, irresponsibility, and stimulation seeking as well as a general lack of empathy. In some cases, orbital frontal patients’ symptomatology may be similar to the Interpersonal items (Factor 1), which include superficial charm, grandiose sense of self-worth, and pathological lying. Thus, while the ‘pseudopsychopathy’ or ‘acquired sociopathy’ model appears to mimic some features of psychopathy, the two disorders differ in many respects. This raises the possibility that disturbances in brain regions other than orbital frontal cortex may contribute to psychopathy.
Other brain regions that may be implicated in psychopathy include the anterior cingulate. The anterior cingulate is a multifaceted complex structure that is commonly divided into at least two distinct functional regions (Devinsky et al., 1995). The rostral aspect, often termed the ‘affective’ division, is known to be involved in pain perception and affect regulation (Bush et al., 2000). The caudal region, termed the ‘cognitive’ division, is known to be involved with response conflict, error monitoring, and task switching, among other processes (Kiehl et al., 2000a). Selective lesions to the anterior cingulate are rare, but when they do occur, they tend to be related to emotional unconcern (Mesulam, 2000), hostility, irresponsibility, and disagreeableness (Swick, 2003). Recently, Hornak and colleagues have shown that selective lesions to bilateral anterior cingulate cortex produce disturbances in personality functioning similar to those observed in patients with orbital frontal lesions (Hornak et al., 2003). In humans, anterior cingulate lesions lead to perseveration (Mesulam, 2000), difficulties in affective face and voice identification (Hornak et al., 2003), error monitoring (Swick and Jovanovic, 2002; Swick and Turken, 2002; Turken and Swick, 1999) and response inhibition abnormalities (Degos et al., 1993; Tekin and Cummings, 2002). Psychopathy has long been associated with perseveration (see review by Newman, 1998), apathy (Cleckley, 1941; McCord and McCord, 1964), difficulties in identifying some affective face stimuli (Blair et al., 1997; Kosson et al., 2002), and more recently, error monitoring (Bates et al., submitted for publication) and response inhibition abnormalities (Kiehl et al., 2000b; Lapierre et al., 1995). Studies also have shown that the volume of the right anterior cingulate is positively correlated with harm avoidance (Pujol et al., 2002). Psychopaths are known to score low on harm-avoidance measures (Hare, 1991). Thus, it would appear that bilateral damage to the anterior cingulate and/or orbital frontal cortex may lead to symptoms and cognitive impairments similar to those observed in psychopathy. The facets of psychopathy that appear to map onto anterior cingulate dysfunction include the Affective (Factor 2) and Lifestyle (Factor 3) factors of the four-factor psychopathy model (Hare, 2003). These latter facets include symptoms of lack of empathy, shallow affect, impulsivity, and irresponsibility. These symptoms appear to be prevalent in patients with anterior cingulate damage (Mesulam, 2000; Swick, 2003).
In addition to regions of the frontal cortex, regions in the temporal lobe may be linked to some symptoms of psychopathy. Damage to the medial temporal lobe in general (Kluever and Bucy, 1938, 1939), and neural tracts that pass through the amygdala in particular (Aggleton, 1992), have long been associated with emotional and behavioral changes in monkeys. These changes include an unnatural propensity for approach behavior or fearlessness and unusual tameness. Medial temporal lobe lesioned monkeys also show excessive fascination with objects, often placing them in the mouth, and general hyperactivity and hypersexual activity. This collection of behaviors has been termed the Kluever–Bucy syndrome. Only a small minority of humans with damage to bilateral amygdala exhibit the full manifestation of the Kluever–Bucy syndrome, while the majority developed less severe emotional changes (Adolphs and Tranel, 2000). One case study reported that bilateral amygdala damage due to calcification associated with Urbach–Wiethe disease may be related to mild antisocial behavior, including rebelliousness, disregard for social convention, and lack of respect for authorities (Adolphs and Tranel, 2000).
Detailed psychological and personality assessments of patients with temporal lobe epilepsy suggests a high incidence of psychopathic-like behavior. Indeed, some studies have reported that pre-operatively the prevalence of psychopathic-like behaviors is as high as 70% of patients with anterior temporal lobe epilepsy (Blumer, 1975; Hill et al., 1957). Structures that are commonly implicated in temporal lobe epilepsy include the amygdala, hippocampus, parahippocampal gyrus, and anterior superior temporal gyrus (temporal pole). Interestingly, removal of the anterior temporal lobe appears to alleviate these behavioral problems in the majority of cases. Hill and colleagues (1957) reported that improvements in personality functioning following temporal lobectomy include reduced hostility and violence, more appropriate sexual behavior (e.g., reduced use of prostitutes and sexual fetishes), increased warmth in social relationships, and increased empathy (see also Hood et al., 1983). Note that removal of the anterior temporal lobe reduced the psychopathic symptoms, suggesting that these behaviors might reflect pathological activity or disruption in the circuits that involve the anterior temporal lobes.
In a similar vein, elective amygdalotomies have been performed on patients with severe aggressive disorders. In general, studies suggest that bilateral amygdalotomy reduces the severity and frequency of aggressive behavior and helps to restore emotional control (Bagshaw et al., 1972; Lee et al., 1988, 1998). Amygdalectomized monkeys fail to show the heart rate and respiratory components of the orienting response, and their skin conductance responses are markedly depressed (Bagshaw et al., 1972).
Cognitive studies of patients with amygdala damage have revealed that these patients have difficulty in processing certain classes of affective stimuli. The right amygdala and adjacent medial temporal lobe regions appear to be involved with face-processing difficulties, particularly those associated with affective states of withdrawal-avoidance (Anderson et al., 2000). Patients with bilateral amygdala damage judged unfamiliar individuals to be more approachable and trustworthy than control subjects — especially for faces rated as unapproachable and untrustworthy by control subjects (Adolphs et al., 1998). Similarly, bilateral damage to the amygdala is associated with impairments in recognizing angry and fearful faces and affective intonations (Scott et al., 1997).
The amygdala appears to be involved in extracting emotional salience from linguistic stimuli (Anderson and Phelps, 2001; Funayama et al., 2001; Isenberg et al., 1999; Strange et al., 2000). Similarly, studies suggest that the fear-potentiated startle during emotional picture processing is dependent upon the medial temporal lobe (Angrilli et al., 1996) as is fear-potentiated startle during fear conditioning with verbal stimuli (Funayama et al., 2001). The amygdala, in particular on the left, appears to be implicated in aversive conditioning (Bechara et al., 1995; Funayama et al., 2001; LaBar et al., 1995). Imaging studies have also linked the orbital frontal cortex to aversive conditioning (Morris et al., 1998). In addition, as with patients with orbital frontal damage, patients with amygdala damage are also impaired on the Bechara gambling test of decision making (Bechara et al., 1999).
Psychopathy is associated with difficulties in processing some face stimuli, particularly distress (Blair et al., 1997) and disgust cues (at least when using their left hands; Kosson et al., 2002). Distress cues are believed to be involved in aversive conditioning and are thought to rely upon the amygdala (Blair, 1995). Processing of disgust cues is not believed to rely upon amygdala; rather the relevant circuitry is thought to be the anterior insular cortex (Phillips et al., 1997, 1998). Adult psychopaths (Williamson et al., 1991) and children with psychopathic tendencies (Loney et al., 2003) show reduced facilitation for processing emotional words during affective lexical decision tasks. These latter results suggest amygdala involvement in psychopathy. Psychopaths also do not show the same pattern of fear-potentiated startle during emotional picture processing, supporting the notion of amygdala (and orbital frontal) abnormality (Levenston et al., 2000; Patrick et al., 1993; Sutton et al., 2002). This latter effect appears to be particularly related to the interpersonal and affective components of psychopathy (Patrick et al., 1993; Sutton et al., 2002).
It appears that damage to the amygdala and regions of the antero-lateral temporal lobe are involved in certain symptoms of psychopathy, including aggression, impulsivity and poor behavioral control, and empathy and emotional unconcern. The psychopathy factor scores that appear to map onto these regions include Affective (Factor 2), Lifestyle (Factor 3) and Antisocial (Factor 4). These factors include items that reflect empathy, shallow affect, poor decision making, impulsivity, and irresponsibility. It does not appear that medial temporal lobe insults result in ‘pseudopsychopathic’ symptomatology similar to that observed in patients with anterior cingulate and orbital frontal lesions.
It is noteworthy to mention that damage to other regions of the frontal cortex (i.e., superior frontal or dorsolateral prefrontal), parietal cortex, or occipital cortex, are not known to lead to behavioral symptoms or cognitive abnormalities consistent with psychopathy. Of note, damage to dorsolateral frontal cortex classically leads to problems in the control, regulation, and integration of cognitive functions. Patients with dorsolateral prefrontal damage are often passive, and they display poor attention and working memory function. These functions do not appear to be disrupted in psychopathy. Furthermore, patients with dorsolateral prefrontal damage are not impaired on affective face or voice identification tasks as are psychopaths (Hornak et al., 2003).
In summary, studies of behavioral changes and cognitive impairments associated with focal brain damage suggest that the orbital frontal cortex, anterior insula, and anterior cingulate of the frontal lobe and the amygdala and adjacent regions of the anterior temporal lobe are implicated in psychopathic symptomatology.
4. Neuropsychological test findings in psychopathy
Reviews of neuropsychological function in psychopathy show no consistent relationship of standard measures of IQ, visuospatial ability, memory, selective attention, or simple motor control to psychopathy ratings on the PCL-R (Hare, 1984, 2003). Thus, it can be argued that traditional neuropsychological assessment is not particularly sensitive to those cognitive abnormalities that have been measured in psychontal), parietal cortex, or occipital cortex, are not known to lead to behavioral symptoms or cognitive abnormalities consistent with psychopathy. It may be possible, however, that neuropsychological information such as IQ or other cognitive domains may provide insight when examined in the context of psychopathy factor scores instead of the total PCL-R score. For example, Christian et al. (1997) found a correlation of IQ to the impulsive/antisocial characteristics of children with psychopathic tendencies, but not to the affective and interpersonal characteristics. A recent meta-analysis suggests that deficits in executive functions can be detected in groups defined by high levels of antisocial behavior (Morgan and Lilienfeld, 2000). This review found that performance on the Porteus Maze test, a test of the integration of cognitive and motor control, was particularly related to antisocial behavior. However, this latter study was unable to differentiate specific associations of neuropsychological impairment to childhood conduct disorder or adult psychopathy.
5. Abnormalities in neurocognitive function in psychopathy
Using the PCL-R (or its predecessors) for assessment, researchers have found that psychopathy is associated with performance abnormalities in several affective and cognitive domains. The extant psychophysiological and cognitive neuroscience literature on psychopathy can be broadly classified into three general areas: 1) language, 2) attention and orienting processes, and 3) affect and emotion. Each of these areas will be reviewed with particular attention to studies that have used electrophysiology and brain-imaging techniques. The findings from these areas will be synthesized and the common threads in each of the different cognitive domains will be linked to the same functional neural architecture.
6. Language processes and psychopathy
Early research sought to identify impairments in cognitive functioning by examining the relationship between psychopathy and hemispheric lateralization for language function (Day and Wong, 1996; Hare, 1979; Hare and Jutai, 1988; Hare and McPherson, 1984; Jutai et al., 1987; Raine et al., 1990). These studies generally found that psychopathy appeared to be related to abnormalities in the cerebral lateralization for some language stimuli, particularly for language functions of the left hemisphere.
In one of the first event-related potential (ERP) language studies in psychopathy, Jutai et al. (1987) observed a peculiar late positive wave occurring at 600 ms post-stimulus over left temporal lobe sites in psychopaths’ waveforms for target stimuli (phonemes) during a dual task procedure. It was suggested that the result may indicate that psychopaths have unusual lateralization of the cerebral hemispheres for language function. Anomalous perceptual asymmetries in language and attention continue to be an area of research in psychopathy, although it is not yet clear how these abnormalities might lead to psychopathic behavior.
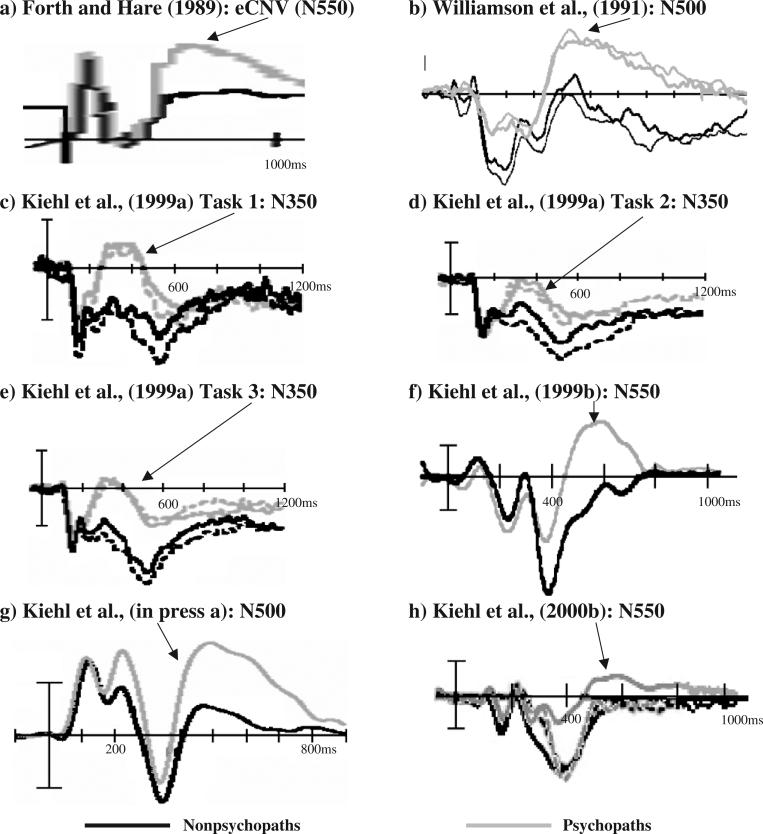
Subsequent research found that abnormalities in language processes are most prevalent when psychopathic individuals are required to perform tasks involving semantic processing (Gillstrom and Hare, 1988; Hare and Jutai, 1988; Hare and McPherson, 1984). For example, Hare and Jutai (1988) observed that psychopathic individuals made more errors than did nonpsychopathic control participants in an abstract semantic categorization task. More recently, Kiehl et al. (1999a) examined psychopathic persons’ ability to process abstract (e.g., justice) and concrete (e.g., table) words during a lexical decision task and during an abstract or concrete discrimination task. Consistent with the hypothesis that psychopathic persons have difficulty in processing abstract information, Kiehl et al. (1999a) found that criminal psychopaths made more errors than did criminal nonpsychopaths when they had to classify word stimuli as abstract. Psychopaths also failed to show the normal ERP differentiation between concrete and abstract words. Kiehl et al. (1999a) also found that psychopaths’ ERP waveforms to all linguistic stimuli (all words and non-words) were associated with a prominent late negativity maximal over frontal midline sites (see Fig. 1).
Fig. 1.
Illustration of event-related potential (ERP) data from studies in criminal psychopaths depicting the late ERP negativities observed during a motor preparation task (Forth and Hare, 1989), an emotional lexical decision task (Williamson et al., 1991), a concrete/abstract lexical decision task (Kiehl et al., 1999a), a concrete/abstract discrimination task (Kiehl et al., 1999a), an emotional polarity discrimination task (Kiehl et al., 1999a), a visual oddball task (Kiehl et al., 1999b), an auditory oddball task (Kiehl et al., in press-a), and a Go/No Go response inhibition task (Kiehl et al., 2000b). All figures are adapted to approximately the same time scale and amplitude (negative plotted up), and are from frontal or central electrode sites. eCNV=early contingent negative variation.
Neuroimaging studies found that processing of abstract words was associated with greater activity in the right anterior superior temporal gyrus and surrounding cortex than was processing of concrete words (Kiehl et al., 1999c, 2004). These data suggest that the behavioral and ERP abnormalities observed in psychopaths for processing of abstract words during the context of lexical decision tasks may be related to the function of the right anterior temporal lobe. To directly examine this hypothesis, Kiehl et al. (2004) used fMRI to elucidate the abnormal functional neuroanatomy in a group of criminal psychopaths during performance of a concrete–abstract lexical decision task. Consistent with hypotheses, psychopaths failed to show the normal pattern of hemodynamic differentiation between abstract and concrete stimuli in the right anterior temporal lobe. Indeed, the right anterior superior temporal gyrus failed to activate during processing of abstract words relative to baseline, suggesting that this brain region is functionally impaired in psychopaths — at least during performance of lexical decision tasks (Kiehl et al., 2004).
Abnormalities in language processing to emotional stimuli have also been observed in psychopathic individuals. Day and Wong (1996) found that psychopathic individuals did not show the same hemispheric laterality in behavioral performance as did control participants for processing negatively valenced word stimuli. Psychopaths have difficulty attributing guilt to the characters in a story passage (Blair et al., 1995). Similarly, when asked to identify the emotion of the speaker based on prosody, psychopaths appear to be impaired in the recognition of fearful vocal affect (Blair et al., 2002). Psychopaths also have difficulty categorizing emotional metaphors (Herve et al., 2003). Evidence for abnormalities in language processing also comes from analyses of the speech of psychopathic individuals. Psychopathic individuals do not express differentially, in voice analyses, affective and neutral words (Louth et al., 1998).
Williamson et al. (1991) studied psychopaths during performance of an emotional lexical decision task. Studies have shown that healthy control participants respond faster and more accurately to letter strings that form emotional words (positive and negative in affect) than letter strings that form neutral words (Graves et al., 1981). Williamson et al. found that psychopaths failed to show any difference in reaction time between emotional and neutral words. Psychopaths failed to show the normal ERP differentiation between emotional and neutral words, while criminal nonpsychopaths showed the expected ERP differentiation. Williamson et al. concluded that these data support the hypothesis that psychopaths have difficulty processing affective stimuli in the linguistic domain. Studies of patients with brain damage and functional brain-imaging studies have linked the amygdala and the anterior and posterior cingulate with affective word processing (Anderson and Phelps, 2001; Isenberg et al., 1999; Maddock and Buonocore, 1997).
Using single photon emission computed tomography (SPECT), Intrator et al. (1997) found that psychopathic individuals showed greater activation for affective than for neutral stimuli bilaterally in temporo-frontal cortex. This latter effect was not predicted and was interpreted post hoc as supporting the notion that psychopaths may require more cognitive resources to process and evaluate affective stimuli than do comparison subjects (Intrator et al., 1997). An alternative hypothesis, albeit speculative, is that psychopaths tend to recruit alternative brain regions to process affective material than do nonpsychopathic control participants, presumably because the brain regions normally engaged in processing emotional stimuli are dysfunctional in psychopaths. It is important to note that Intrator et al. only reported data from a single axial slice of cortex, raising the possibility that other brain regions may be implicated in the disorder.
Another interesting finding from the ERP data of Williamson et al. (1991) was that psychopaths’ ERPs to all linguistic stimuli (positive, negative, neutral words and pseudowords) were characterized by a prominent late negativity at 500 ms (N500) post-stimulus at frontal and central electrode sites (see Fig. 1). Williamson et al. offered two possible explanations for the psychopaths’ N500 finding. First it was suggested that because the task employed required a Go/No Go decision, it was possible that the prominent N500 of the psychopaths was related to poor response inhibition. However, Kiehl et al. (1999a) reported that psychopaths exhibited a large centro-frontal negative wave with latency about 350 ms during three different language tasks, all of which employed a Go/Go design (see Fig. 1). Moreover, other studies have observed fronto-central ERP negativities in psychopaths to Go trials but not during No Go trials during a response inhibition study (Kiehl et al., 2000b). It is unlikely therefore that the abnormal late fronto-central negative waves exhibited by psychopaths can be attributed entirely to difficulties in response inhibition. While both Williamson et al. (1991) and Kiehl et al. (1999a) used tasks that demanded linguistic processing, the late negative wave in both studies was elicited for all word types [i.e., positive, negative, and neutral words in Williamson et al. (1991); concrete and abstract words (Tasks 1 and 2) and positive and negative words (Task 3) in Kiehl et al. (1999a)], raising the possibility that it is independent of stimulus content.
Based on similarities in the morphology and topography of the psychopathic individuals’ N350 (Kiehl et al., 1999a) and N500 components (Williamson et al., 1991), Kiehl et al. (1999a) suggested a possible explanation for the functional significance of these components. The tasks employed by Williamson et al. (1991) and Kiehl et al. (1999a) both involved lexico-semantic processing and required a concurrent behavioral response. Thus, in the 300–600 ms after a word stimulus is presented, both semantic and decision-making processes are engaged and will elicit overlapping ERP components of opposite polarity. In general, presentation of a word stimulus in the absence of any online task demands will elicit a large ERP negativity in the 300- to 500-ms time window (N4 or N400) rather than an ERP positivity. Thus, one interpretation of the psychopaths’ fronto-central ERP negativities offered by Kiehl et al. (1999a) and Williamson et al. (1991) was that they may be related to an abnormally large N400. To the extent that the amplitude of the N400 reflects cognitive operations involved in processing the semantic meanings of words, these data are consistent with the hypothesis that psychopathy is associated with abnormal semantic processing. That interpretation is strengthened by the fact that abnormally large N400s have been reported in other psychopathological conditions with conceptual and empirical links to psychopathy (Niznikiewicz et al., 1997). Studies from intracranial recordings and from event-related fMRI have shown that the putative generators underlying the N400 include both infero-medial and lateral temporal lobe structures (Kiehl et al., 2002; McCarthy et al., 1995). Functional abnormalities have been observed in these structures in psychopaths during language and cognitive tasks (Kiehl et al., 2004; Intrator et al., 1997). Thus, at first glance, there is considerable evidence that the late fronto-central ERP negativities observed in psychopaths during linguistic tasks may be related to abnormally large N400s. However, one study examining the N400 component in psychopaths during performance of a classic sentence-processing task (Kutas and Hillyard, 1980) failed to find any evidence for abnormalities (Kiehl et al., in press-b). Thus, the N500 and N350 abnormalities observed in psychopaths do not appear to be related to the processes associated with the generation of the N400 component. This conclusion is tentative however, pending further research.
In summary, studies of language processes suggest that psychopathy is associated with abnormalities in processing semantic and affective material. These abnormalities appear to be greatest when psychopaths are processing abstract stimuli and emotional stimuli. Processing of abstract word stimuli during lexical decisions tasks is believed to rely upon the right anterior superior temporal gyrus. Processing of emotional word stimuli appears to rely upon the anterior and posterior cingulate and the amygdala. Thus, the extant language literature suggests that reduced activity is observed in psychopaths during language processing in the right anterior superior temporal gyrus, amygdala, and anterior and posterior cingulate.
7. Attention and orienting processes in psychopaths
Studies of attention and orienting can be traced back to the earliest days of psychophysiological research in psychopathy. In a classic study, Lykken (1957) found that psychopathic individuals [defined by ratings on Cleckley (1941) criteria] failed to show conditioned autonomic increases in skin conductance in an aversive conditioning paradigm using electric shock (Lykken, 1957). Dozens of studies examining the relationship between psychopathy and peripheral measures of autonomic nervous system functioning followed this study. These studies included examination of resting levels of skin conductance and heart rate as well as psychophysiological changes associated with punishment, classical conditioning, and processing of noxious and appetitive stimuli. However, these early studies failed to produce reliable results and were widely criticized on procedural and methodological grounds. The most common criticisms levied against this literature were that a wide variety of procedures were used to assess psychopathy, many with dubious validity, and no consensus was reached on how to best measure or quantify peripheral measures of autonomic function (Hare, 1978). Fortunately, some general conclusions can be drawn from the body of data that addressed the methodological issues. Studies largely found that psychopathic individuals tended to show lower basal levels of skin conductance and to exhibit smaller changes in skin conductance under a wide variety of experimental manipulations compared with nonpsychopathic inmates. In particular, psychopaths tended to show relatively small skin conductance changes to otherwise noxious stimuli, such as loud tones (Hare et al., 1978), insertion of a hypodermic needle (Hare, 1972), and slides of mutilated faces (Mathis, 1970). Psychopaths also failed to show normal skin conductance increases in anticipation of receiving painful stimuli (Hare, 1965; Hare and Quinn, 1971). Veit et al. (2002) showed that psychopaths, relative to controls, had reduced hemodynamic activity in orbital frontal cortex, anterior cingulate, and amygdala during an aversive conditioning study (Veit et al., 2002). Psychopaths did show transient hemodynamic activity in the amygdala, but this effect was small compared with that observed in healthy controls. It should be noted, however, that only four psychopathic individuals participated and apparently only one or two met the criteria for psychopathy (i.e., PCL-R score greater than 30). Also, it appears that this latter study used within-subject or fixed effects statistical analyses that limit the inferences that can be drawn to that of a case study.
The hippocampus is also believed to be involved in aversive conditioning. One study has reported a negative correlation between psychopathy scores and dorsal hippocampal volumes in violent offenders co-morbid with alcoholism (Laakso et al., 2001). Laakso et al. (2001) studied 18 violent male offenders with a history of alcoholism and found that there was a strong negative correlation between dorsal hippocampal volume and PCL-R scores. This latter effect appeared to be stronger for Factor 1 scores (interpersonal/affective) than for Factor 2 scores (impulsive/behavioral). The data were interpreted as support for the model that hippocampal dysfunction may be implicated in the poor aversive learning and acquisition of conditioned fear that characterizes psychopathy. Recently, Raine et al. (2004) found that unsuccessful psychopaths (individuals with a history of incarceration) compared with ‘successful’ psychopaths (individuals without a history of incarceration) and healthy controls had abnormal asymmetry in the anterior hippocampus. These data were interpreted as support for a neurodevelopmental perspective on psychopathy. This study was the first brain-imaging work to compare ‘unsuccessful’ to ‘successful’ psychopaths. However, it appears that there were few individuals with PCL-R scores above the diagnostic cutoff (30 on the PCL-R) for psychopathy (Raine et al., 2004). Thus, it remains to be determined if the results would generalize to individuals (successful or unsuccessful) with high scores on the PCL.
This collection of abnormalities suggests that psychopaths have difficulty in fear conditioning, are relatively lacking in fear, and do not respond well in classical conditioning paradigms with punishment contingencies. An alternative interpretation, first proposed by Hare (1968), is that these results suggest abnormalities in orienting processes. That is, in all the psychophysiological studies listed above, an orienting response would be elicited by the salient cues or stimuli. The canonical orienting response is associated with concert of peripheral and neuronal changes, including skin-conductance increases, heart-rate decreases, and blood-pressure modulation. Sokolov (1963) is largely credited with describing the myriad of experimental conditions under which orienting processes are engaged. In general, unpredictable, novel, or task-relevant salient stimuli, broadly defined, will lead to an orienting response (Sokolov, 1963). Indeed, Hare has suggested that deficient or abnormalities in orienting processes might be a fundamental aspect of psychopathy (Hare, 1978).
ERP studies have been used to study aspects of the orienting response for many years in health and psychopathology. Processing of novel and salient (target) stimuli known to elicit an orienting response has often been studied in the context of ‘oddball’ paradigms. In a typical oddball paradigm, low-probability, task-irrelevant novel and low-probability, task-relevant target stimuli are presented against a background of frequent or standard stimuli. Both novel and target stimuli are associated with a sequence of electrical components, the most prominent of which is a large broadly distributed positive wave termed the P3 or P300. Novel stimuli elicit a P3 with a fronto-central maximum that has an earlier latency than the parietally distributed P3 elicited by salient or target stimuli. The P3 elicited by novel and target stimuli are believed to be related to processes involving attentional capture, allocation of cognitive resources, and contextual updating — all components linked to ‘orienting processes’.
There have been 10 ERP studies on psychopathy defined according to PCL or PCL-R scores (Flor et al., 2002; Forth and Hare, 1989; Jutai and Hare, 1983; Jutai et al., 1987; Kiehl, 2000; Kiehl et al., 1999a,b, 2000; Raine and Venables, 1988; Williamson et al., 1991). Nine studies have reported information about P3s in psychopathy, although only five studies used paradigms in which the salience of stimuli was manipulated in a manner expected to elicit a canonical P3 response (Jutai et al., 1987; Kiehl, 2000; Kiehl et al., 1999a, 2000b; Raine and Venables, 1988).
Jutai et al. (1987) found no significant difference between psychopaths and nonpsychopaths in the amplitude or latency of the P3. In contrast, Raine and Venables (1988) reported that the amplitude of parietal P3 to visual target stimuli elicited during a continuous performance task was greater in psychopaths than in nonpsychopaths. More recently, studies have shown that the P3 elicited during visual and auditory oddball tasks is slightly smaller over frontal, central and parietal sites in psychopaths than in nonpsychopaths (Kiehl, 2000; Kiehl et al., 1999a). Similar effects were observed in psychopaths during performance of a response-inhibition task (Kiehl et al., 2000b). In the remaining studies that reported information about P3, there was little evidence indicating that P3 amplitude was abnormal in psychopaths. However, these latter studies did not use paradigms that manipulated the salience of the stimuli.
Thus, of the studies that have manipulated the salience of the stimuli in a manner expected to elicit a P3 response, one reported a null finding (Jutai et al., 1987), one reported enlarged P3 responses in psychopaths (Raine and Venables, 1988), and three studies, from independent samples, reported reduced P3 responses in psychopaths (Kiehl, 2000; Kiehl et al., 1999a, 2000b). Thus, it is not clear whether the P3 is abnormal in psychopaths. However, perhaps more interesting and illuminating than the P3 in psychopathy is that there were a number of other ERP abnormalities observed in psychopaths during processing of salient task-relevant target stimuli.
Recall that psychopaths’ ERPs elicited by linguistic stimuli were characterized by an abnormal late negativity that was maximal over frontal and central sites (Kiehl et al., 1999a; Williamson et al., 1991). In the studies that have required online decision-making but made no explicit demands on linguistic processing (and have recorded from frontal electrode sites), late ERP negativities have also been observed in psychopaths (Kiehl, 2000; Kiehl et al., 1999a, 2000b). In two visual (nonlinguistic) target-detection tasks, psychopaths’ waveforms were characterized by a late ERP negativity in the 300- to 500-ms time window (Kiehl et al., 1999a, 2000b). This ERP negativity was similar in morphology and topography to those observed in previous language tasks in psychopaths (see Fig. 1). In the auditory modality, Kiehl et al. (in press-a) also found that psychopaths had a larger N2b and smaller P3 components than did nonpsychopaths. Similarly, Forth and Hare (1989) reported that the early contingent negative variation (CNV), which occurs at 300–800 ms post-stimulus, was abnormally large in psychopaths relative to nonpsychopaths at frontal sites (see Fig. 1). In the task used by Forth and Hare, the stimulus that elicited the early CNV was highly salient as participants had to prepare to make a fast finger movement following the stimulus. It is therefore plausible that an alternative explanation for the presence of the large early CNV is that this waveform is similar to that found in oddball and language tasks. This interpretation is strengthened by the fact that the early CNV is similar to the late ERP negativities in psychopaths in both the temporal envelope and scalp topography.
In summary, studies have shown that psychopathy is associated with late fronto-central ERP negativities for a variety of stimuli. These abnormal ERP waveforms have been elicited by word stimuli (Kiehl et al., 1999a; Williamson et al., 1991), salient cues (Forth and Hare, 1989), simple visual stimuli (Kiehl et al., 1999b, 2000b), and task-relevant auditory stimuli (Kiehl et al., in press-a). Indeed, every study that has reported ERPs from frontal electrode sites to salient task-relevant stimuli in a well-defined group of psychopaths has observed a late ERP negativity that was larger in psychopaths than in nonpsychopaths. The consistency of these results is illustrated in Fig. 1. At least one common thread in these paradigms is that each is associated with orienting processes. Thus, a plausible interpretation for the functional significance of the fronto-central ERP negativities in psychopaths is that they are related to abnormal orienting processes. However, the neural systems implicated in these processes in psychopathy are still unclear. The ERP data, from the oddball task in particular, have yielded precise information about the temporal stages of information processing in psychopaths. Studies have shown that ERP differences between psychopaths and nonpsychopaths emerge as early as 200 ms post-stimulus with the psychopaths’ enlarged N2b at frontal sites. The psychopaths’ enlarged N2b is followed by a slightly smaller, broadly distributed P3 component and punctuated by the appearance of a late fronto-central negativity that is several times larger than that observed in nonpsychopaths. It is temping to infer from the ERP data that the neural generators associated with these abnormal components are located in frontal brain regions — since the topography of the scalp-recorded ERP are maximal over frontal electrode sites. However, localizing ERP generators, particularly those occurring in later time windows, is problematic. Determining the neural generators underlying scalp-recorded ERPs is referred to as the ‘inverse problem’ and there are an infinite number of combinations of electrical sources that could lead to the recordings observed at the scalp. This is particularly true for localizing late components of the oddball task (Halgren and Marinkovic, 1996; Halgren et al., 1998). However, there are several additional avenues available to explore the neural systems underlying processing of salient stimuli. A better understanding of which systems are engaged in processing oddball stimuli might lead to a better understanding of the neural circuits that are abnormal in psychopathy.
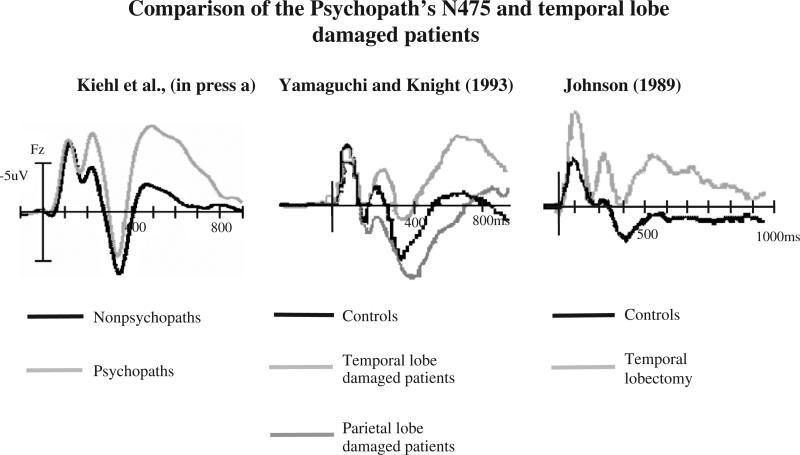
One of the first methods used to investigate the potential generators underlying the processing of salient or oddball stimuli was to record ERPs in patient populations with localized brain insults. The main tenet here is that if the circuits involved in salient stimulus processing are damaged, it should lead to observable abnormalities in the scalp-recorded ERPs. These studies found that frontal, temporal, parietal, and limbic structures are engaged during processing of oddball stimuli (see review by Soltani and Knight, 2000). Interestingly, in patients with temporal lobe damage, several studies found clear evidence for fronto-central ERP negativities during processing of salient target stimuli in oddball paradigms (Johnson, 1993; Yamaguchi and Knight, 1993). Yamaguchi and Knight (1993) found that patients with temporal lobe damage, relative to controls or patients with parietal lobe damage, had enlarged N2b components, smaller P3 components, and late fronto-central negativities (see Fig. 2). Similar effects were observed in epilepsy patients following resection of the anterior medial temporal lobe (amygdala and anterior superior temporal gyrus) for the treatment of intractable epilepsy (Johnson, 1993). Paller and colleagues (1988) have also shown that fronto-central ERP negativities are elicited by auditory oddball stimuli in monkeys following temporal lobe lesions (Paller et al., 1988, 1992). These data suggest that enlarged N2b, reduced P3, and late negativity at frontal sites elicited during processing of oddball stimuli appear to be characteristic of patients with temporal–limbic damage and criminal psychopaths (see Fig. 2 for comparison). Thus, it would appear that the most parsimonious interpretation for the late ERP negativities observed in psychopaths during processing of task-relevant salient stimuli is that they appear to be an electrophysiological signature of temporal–limbic brain abnormalities. It is temping to conclude that these ERP components are linked to problems with orienting processes. Studies of patients with medial temporal lobe damage have shown that ERP abnormalities and autonomic responding elicited by orienting stimuli are impaired — despite the fact that these latter patients can reliably discriminate target and novel stimuli (Knight, 1996). These data suggest that the autonomic and neural components of the orienting response are impaired in patients with medial temporal lobe damage and in psychopathic individuals. It is also clear that diminished orienting processes (i.e., heart rate modulations, skin conductance changes, electrocortical responses) may not lead to impairment in task performance. That is, patients with medial temporal lobe damage and criminal psychopaths are not impaired in performing oddball tasks. Indeed, some data suggest that a lack of automatic orienting to salient stimuli may lead psychopaths to perform better than nonpsychopaths (Newman et al., 1997). More studies are needed to examine the functional (and behavioral) significance of these orienting processes in psychopaths.
Fig. 2.
Comparison of the late ERP negativities elicited by auditory oddball stimuli in criminal psychopaths (Kiehl et al., in press-a), patients with temporal lobe damage (Yamaguchi and Knight, 1993) and patients who had undergone temporal lobectomy for the treatment of intractable epilepsy (Johnson, 1989). All three groups are typified by an enhanced N2b, diminished frontal P3, and enlarged late negativity (N500), relative to control participants.
Finally, a recent SPECT study in violent offenders institutionalized in a psychiatric facility found that the interpersonal factor, but not the social deviance factor, of psychopathy was negatively correlated with perfusion in the temporal lobe and the orbital frontal cortex (Soderstrom et al., 2002). This was the first study to examine the factors of psychopathy and measures of hemodynamic activity. Unfortunately, the range of psychopathy scores was from 2 to 31, suggesting that few offenders were actually above the diagnostic cutoff of 30 for psychopathy (Hare, 1991, 2003).
To recap, studies of language, attention, and orienting processes suggest that the neural circuitry involved in psychopathy includes the amygdala, anterior superior temporal gyrus, orbital frontal cortex, and anterior cingulate gyrus.
8. Affective processes in psychopathy
A variety of different methods have been used to assess affective processes in psychopaths. Previously the studies examining affective processes in psychopathy in the context of linguistic tasks were reviewed. These studies largely found that psychopaths do not differentiate the subtleties between affective and neutral stimuli in the same way that criminal nonpsychopaths and healthy individuals do. Studies using the startle–reflex methodology have shown that psychopaths do not show the normal pattern of blink modulation when processing negatively valenced stimuli as do nonpsychopaths and healthy individuals (Patrick et al., 1993). In healthy controls and in nonpsychopathic prisoners, startle probes presented during viewing of negative picture stimuli (e.g., mutilated faces) elicit a larger blink reflex response than that elicited during viewing of neutral or positive stimuli. However, this pattern of responding was not observed in psychopaths with high scores on the interpersonal and affective features of psychopathy. That is, psychopaths’ blink-reflex magnitude was similar for processing of positive and negative stimuli, suggesting that negative stimuli did not elicit the same defensive response in psychopaths as they do in nonpsychopaths (see also Levenston et al., 2000; Sutton et al., 2002). Indeed, one interpretation of the abnormal startle response in psychopaths is that they had an appetitive response to the negative stimuli. The neural circuitry of the startle response includes structures in the medial and lateral temporal lobe, in particular, the right amygdala.
Few other studies have directly examined the neural correlates of emotional processing in psychopaths. One study used fMRI to examine the neural systems associated with emotional processing in psychopaths within the context of an affective memory task (Kiehl et al., 2001). Consistent with previous research in healthy controls, criminal nonpsychopaths and noncriminal controls had better memory for affective stimuli than for neutral stimuli. Psychopaths showed a trend in this direction. Importantly, there were no group differences in overall performance, suggesting that psychopaths were just as engaged in performance of the task as were the criminal nonpsychopaths and noncriminal controls. The results of the imaging data indicated that psychopaths failed to show the normal pattern of differentiation between affective and neutral stimuli in amygdala, ventral striatum, rostral and caudal anterior cingulate, and posterior cingulate. Recall, however, that psychopaths did show a trend for better memory recall for negative than for neutral words. Thus, there should be some brain regions in which psychopaths show greater activity for processing negative than neutral stimuli than do controls. Psychopaths, but not controls, showed differences in brain activation for affective relative to neutral stimuli in right and left lateral frontal cortex. These areas are normally associated with semantic processing. Thus, it appears that psychopathy is associated with hypofunctioning of temporo-limbic circuits that are normally associated with affective processing and that lateral frontal brain regions may be engaged in some compensatory manner. These data also provide support for the hypothesis that the excessive affect-related activity observed in psychopaths in lateral fronto–temporal cortex (Intrator et al., 1997) may be compensating for reduced activity in limbic regions. However, this interpretation is still tentative pending further replication.
All of the ERP and brain-imaging studies summarized above examined affective processes in psychopaths in the context of a task that involved language stimuli (Intrator et al., 1997; Kiehl et al., 1999a, 2001; Williamson et al., 1991). This raises the possibility that some of the observed differences may be related to linguistic processes per se. However, Muller and colleagues (2003) reported that psychopaths had disordered activity during a non-linguistic task that involved emotional slide processing in orbital frontal regions, medial temporal (amygdala, parahippocampal gyrus) and rostral and caudal anterior cingulate (Muller et al., 2003). In particular, the authors reported that criminal psychopaths, relative to noncriminal controls, showed reduced activation in subgenual and dorsal cingulate and temporal gyrus for passive viewing of negative slides, and excessive activity was observed in right prefrontal regions and amygdala. The reductions in anterior and posterior cingulate for processing of negative slides support the notion that these systems are abnormal in psychopathy. However, the excessive activity in the amygdala appears to contradict the notion that this structure is hypofunctioning in psychopathy. This discrepancy raises the issue that differences in methodology between studies may have influenced the results. With respect to tasks, most of the studies that either directly or indirectly implicated amygdala hypofunctioning in psychopathy employed procedures that ensured all participants were actively engaged in the task and processing all stimuli (Flor et al., 2002; Kiehl et al., 2001, 2003; Levenston et al., 2000; Patrick, 1994; Patrick et al., 1993; Sutton et al., 2002). The Muller et al. study employed a passive viewing paradigm in which participants were required to ‘feel the emotions the pictures suggest’ (p. 154). Thus, it is not possible to determine from this paradigm if the controls and psychopaths were equally participating in the task. Moreover, research has shown that drawing attention towards processing affect in the emotional stimuli leads to reduced amygdala activity, at least in healthy controls, compared with tasks that require processing of the emotional stimuli but do not require evaluating the affect in the stimulus (Hariri et al., 2003). With respect to participants, Muller et al. acknowledged the small sample size of six psychopaths and six noncriminal controls. It is also noteworthy that the psychopaths from Muller et al. were patients at a maximum security psychiatric facility who were found ‘not guilty by reason of mental defect’ (p. 154) for their criminal activity. However, no information was provided on whether these patients had co-morbid Axis I psychopathology, such as schizophrenia or other psychotic disorders. In addition, no information was reported on whether the control group differed from the psychopathic group on IQ, education, or visual acuity. With respect to image analysis, Muller et al. employed a fixed effects model in which variance between groups was ignored. Fixed effects analyses in functional imaging are known to lead to very high rates of false positives since a large response from a single participant can result in a significant finding. Thus, replication is needed to confirm these latter results.
In summary, cognitive neuroscience studies of affective processing have found that the neural circuits embracing the temporo-limbic system are either dysfunctional or hypofunctioning in psychopathy. In particular, sites implicated include the amygdala, parahippocampal regions (Laakso et al., 2001; Raine et al., 2004), anterior superior temporal gyrus, rostral and caudal anterior cingulate, and posterior cingulate. Similar abnormalities are observed in these neural circuits in psychopaths during performance of language, attention, and orienting tasks.
9. Psychopathy as a disorder of the paralimbic system
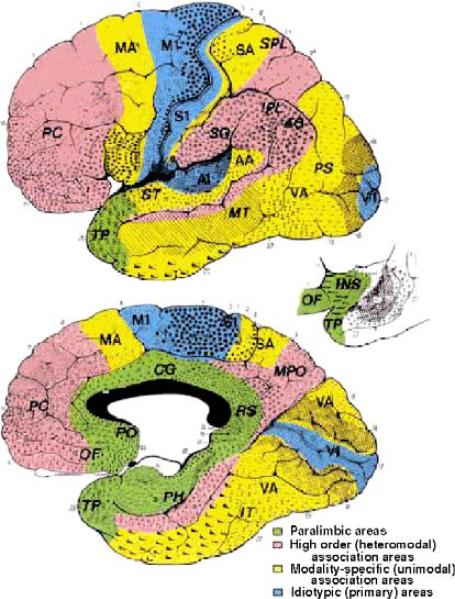
Studies of patients with brain damage suggest that regions of the frontal lobe, including the orbital frontal cortex and anterior cingulate, and regions of the temporal lobe, including the amygdala, parahippocampal gyrus, and anterior superior temporal gyrus, are implicated in psychopathic symptomatology. Psychophysiological studies employing ERPs have repeatedly shown that psychopaths’ brain potentials elicited by salient stimuli are associated with aberrant late negativities (see Fig. 1). These aberrant brain potentials are also observed in patients with temporal lobe damage in general, and with the amygdala and anterior superior temporal gyrus in particular (see Fig. 2). Cognitive neuroscience studies of psychopathy suggest that the anterior and posterior cingulate, insula, orbital frontal cortex, amygdala, and anterior superior temporal gyrus are dysfunctional or hypofunctioning in psychopathy during language, attention and orienting, and affective processing tasks. At first glance, it might appear that these neural circuits implicated in psychopathy come from a heterogeneous and spatially remote collection of brain regions. However, neuroanatomists and cytoarchitectologists have grouped the anterior superior temporal gyrus (temporal pole), rostral and caudal anterior cingulate, posterior cingulate, orbital frontal cortex, insula, and parahippocampal regions into the paralimbic cortex (Brodmann, 1909; 1994; Mesulam, 2000). The paralimbic cortex, also referred to as the mesocortex, is interposed between the neocortex and the allocortex (Fig. 3). The paralimbic cortex provides a gradual transition from primary limbic regions, including the septal region, substantia innominata, and the amygdaloid complex, to higher neocortical regions. It is important to note that there are dense connections between the paralimbic cortex and core limbic structures, in particular the amygdala. The amygdaloid complex comprises both nuclear and cortical layers. These cortical features of the amygdala often extend into the paralimbic areas, blurring the boundaries between limbic and paralimbic regions (Mesulam, 2000). Thus, these regions may collectively be termed the ‘paralimbic system’.
Fig. 3.
Illustration of the cytoarchitectural maps of Brodmann (1909, adapted from Mesulam, 2000). The components of the paralimbic system include the orbital frontal cortex, medial (amygdala and parahippocampal gyrus) and lateral (anterior superior temporal gyrus) temporal lobe, and rostral, caudal and posterior cingulate gyrus.
These data suggest that the relevant functional neuroanatomy implicated in psychopathy is the paralimbic system. Now it is not known how or when in development these abnormalities in psychopathy arise. Clinical (Cleckley, 1941; Hare, 1993) and recent research data suggest that psychopathic symptoms are present at a very early age (Frick, 1995, 1998; Frick et al., 2000). Given that the brain structures implicated in psychopathy are linked based on cytoarchitectural similarities, it is tempting to argue that psychopathy may be neurodevelopmental in nature. However, at present, there are little data speaking to structural brain alternations in psychopathy. Two structural MRI studies have shown that psychopathy is associated with hippocampal abnormalities (Laakso et al., 2001; Raine et al., 2004). Clearly, more research on functional and structural brain changes in psychopathy is needed.
It is important to recognize that the hypothesis that limbic and paralimbic structures are implicated in psychopathy is not new. Indeed, clinicians have speculated since the time of Phineas Gage (1800s) that frontal brain regions are implicated in the disorder (Harlow, 1848). Similarly, Fowles (1980) postulated that the basis of psychopathy was a deficiency in the behavioral inhibition system — a system that is believed to rely upon septo-hippocampal brain regions (see also Gorenstein and Newman, 1980). Raine (1993) has emphasized the role of orbital frontal cortex in antisociality and psychopathy. Other researchers have argued that amygdala dysfunction is central to models of psychopathy (Blair, 2001, 2002, 2003). The argument that the paralimbic system is abnormal in psychopathy is consistent with these perspectives. However, the review offered above from the neurological literature suggests that selective lesions to the septal–hippocampal system, orbital frontal cortex, or amygdala do not lead to the complete manifestation of psychopathy. Moreover, the functional neuroimaging data suggest that in addition to these latter structures the anterior and posterior cingulate are implicated in psychopathy. Thus, the argument presented here is that a broader view, encompassing all of the paralimbic system, is warranted.
It is also relevant to recognize that a potential limitation of this model is that most of the supporting evidence comes from studies examining brain function or from indirect evidence of behavioral changes in patients following brain damage or lesions. The paralimbic system is defined based on similarities in the cytoarchitecture or brain structure; however, there is a relative paucity of studies that have examined gray matter abnormalities in psychopathy. The few structural brain-imaging studies in psychopathy suggest that hippocampal regions (i.e., paralimbic) are implicated in the disorder (Laakso et al., 2001; Raine et al., 2004). Future structural imaging studies may lead to a better understating of whether the observed abnormalities in brain function in psychopaths map onto structural brain pathology. Studies of brain metabolism (i.e., positron emission tomography) may also provide insights into the relevant neuroanatomy implicated in psychopathy.
In summary, converging evidence suggests that psychopathy is associated with paralimbic system dysfunction. This hypothesis is supported by indirect evidence from studies of behavioral changes following lesions or damage to the paralimbic system, bolstered by findings from analog studies, and further supported by the extant cognitive neuroscience literature in psychopathy. The particular neural regions implicated include the orbital frontal cortex, insula, amygdala, parahippocampal regions, anterior superior temporal gyrus, and rostral, caudal and posterior cingulate. It is important to note that the studies reviewed employed the PCL-R or its derivatives to assess psychopathy (Hare, 2003). The PCL-R is widely considered to be the best metric for assessing psychopathy in forensic and clinical contexts. The replicability and consistency of the neurobiological findings are a further testament to the psychometric robustness of the PCL-R and the construct of psychopathy in general. To conclude, this review has examined data from psychological, electrophysiological, and brain-imaging studies in psychopathy during language, attention and orienting, and affective tasks. In addition, this review has considered indirect evidence from behavioral and cognitive changes associated with brain damage or insults. These converging results suggest that the relevant functional neural architecture implicated in psychopathy is the paralimbic system.
Acknowledgements
The author thanks his mentors, Drs. Michael R. Levenson, George R. Mangun, Robert D. Hare, and Peter F. Liddle for their tutelage. This work was supported by NIMH grants 1 R01 MH0705539-01 (PI: Kiehl) and 1 R01 MH072681-01 (PI: Kiehl).
References
- Adolphs R, Tranel D. Emotion, recognition, and the human amygdala. In: Aggleton JP, editor. The Amygdala: A Functional Analysis. Vol. 2. Oxford University Press; New York: 2000. pp. 587–630. [Google Scholar]
- Adolphs R, Tranel D, Damasio AR. The human amygdala in social judgment. Nature. 1998;393(6684):470–474. doi: 10.1038/30982. [DOI] [PubMed] [Google Scholar]
- Aggleton JP. The functional effects of amygdala lesions in humans: a comparison with findings in monkeys. In: Aggleton JP, editor. The Amygdala: Neurobiological Aspects of Emotion, Memory, and Dysfunction. Wiley-Liss; New York: 1992. pp. 485–503. [Google Scholar]
- Alterman AI, Cacciola JS. The antisocial personality disorder diagnosis in substance abusers: problems and issues. Journal of Nervous and Mental Disease. 1991;1797:401–409. doi: 10.1097/00005053-199107000-00003. [DOI] [PubMed] [Google Scholar]
- Alterman AI, Cacciola JS, Rutherford MJ. Reliability of the Revised Psychopathy Checklist in substance abuse patients. Psychological Assessment. 1993;54:442–448. [Google Scholar]
- Alterman AI, McDermott PA, Cacciola JS, Rutherford MJ, Boardman CR, McKay JR, Cook TG. A typology of antisociality in methadone patients. Journal of Abnormal Psychology. 1998;1073:412–422. doi: 10.1037//0021-843x.107.3.412. [DOI] [PubMed] [Google Scholar]
- American Psychological Association . Diagnostic and Statistical Manual for Mental Disorders. 4th ed. APA Press; New York: 1994. [Google Scholar]
- Anderson AK, Phelps EA. Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature. 2001;4116835:305–309. doi: 10.1038/35077083. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Spencer DD, Fulbright RK, Phelps EA. Contribution of the anteromedial temporal lobes to the evaluation of facial emotion. Neuropsychology. 2000;144:526–536. doi: 10.1037//0894-4105.14.4.526. [DOI] [PubMed] [Google Scholar]
- Angrilli A, Mauri A, Palomba D, Flor H, Birbaumer N, Sartori G, di Paola F. Startle reflex and emotion modulation impairment after a right amygdala lesion. Brain. 1996;119:1991–2000. doi: 10.1093/brain/119.6.1991. [DOI] [PubMed] [Google Scholar]
- Bagshaw MH, Mackworth NH, Pribram KH. The effect of resections of the inferotemporal cortex or the amygdala on visual orienting and habituation. Neuropsychologia. 1972;102:153–162. doi: 10.1016/0028-3932(72)90054-1. [DOI] [PubMed] [Google Scholar]
- Bates AT, Liddle PF, Kiehl KA. Error monitoring abnormalities in criminal psychopaths. submitted for publication.
- Bechara A, Tranel D, Damasio H, Adolphs R, Rockland C, Damasio AR. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science. 1995;269(5227):1115–1118. doi: 10.1126/science.7652558. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. Journal of Neuroscience. 1999;1913:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain. 2000;12311:2189–2202. doi: 10.1093/brain/123.11.2189. [DOI] [PubMed] [Google Scholar]
- Blair RJR. A cognitive developmental approach to mortality: investigating the psychopath. Cognition. 1995;571:1–29. doi: 10.1016/0010-0277(95)00676-p. [DOI] [PubMed] [Google Scholar]
- Blair RJ. Neurocognitive models of aggression, the antisocial personality disorders, and psychopathy. Journal of Neurology, Neurosurgery and Psychiatry. 2001;716:727–731. doi: 10.1136/jnnp.71.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJR. Neuro-cognitive models of acquired sociopathy and developmental psychopathy. In: Glicksohn J, editor. The Neurobiology of Criminal Behavior Neurobiological Foundation of Aberrant Behaviors. Kluwer Academic Publishers; Dordrecht, The Netherlands: 2002. pp. 157–186. [Google Scholar]
- Blair RJ. Neurobiological basis of psychopathy. British Journal of Psychiatry. 2003;182:5–7. doi: 10.1192/bjp.182.1.5. [DOI] [PubMed] [Google Scholar]
- Blair RJR, Cipolotti L. Impaired social response reversal. A case of ‘acquired sociopathy’. Brain. 2000;123(Pt 6):1122–1141. doi: 10.1093/brain/123.6.1122. [DOI] [PubMed] [Google Scholar]
- Blair RJR, Sellars C, Strickland I, Clark F. Emotion attributions in the psychopath. Personality and Individual Differences. 1995;194:431–437. [Google Scholar]
- Blair BJR, Jones L, Clark F, Smith M. The psychopathic individual: a lack of responsiveness to distress cues? Psychophysiology. 1997;342:192–198. doi: 10.1111/j.1469-8986.1997.tb02131.x. [DOI] [PubMed] [Google Scholar]
- Blair RJR, Mitchell DG, Richell RA, Kelly S, Leonard A, Newman C, Scott SK. Turning a deaf ear to fear: impaired recognition of vocal affect in psychopathic individuals. Journal of Abnormal Psychology. 2002;1114:682–686. doi: 10.1037//0021-843x.111.4.682. [DOI] [PubMed] [Google Scholar]
- Blumer D. Temporal lobe epilepsy and its psychiatric significance. In: Benson DF, Blumer D, editors. Psychiatric Aspects of Neurological Disease. Grune and Stratton; New York: 1975. pp. 171–198. [Google Scholar]
- Blumer D, Benson DF. Personality changes with frontal lobe lesions. In: Benson DF, Blumer D, editors. Psychiatric Aspects of Neurological Disease. Grune and Stratton; New York: 1975. pp. 151–170. [Google Scholar]
- Brodmann K. Vergleichende Lokalisationlehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. J.A., Barth; Leipzig: 1909. [Google Scholar]
- Brodmann K. Localisation in the Cerebral Cortex. Smith-Gordon; London: 1994. [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Science. 2000;46:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Christian RE, Frick PJ, Hill NL, Tyler L. Psychopathy and conduct problems in children: II. Implications for subtyping children with conduct problems. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;362:233–241. doi: 10.1097/00004583-199702000-00014. [DOI] [PubMed] [Google Scholar]
- Cleckley H. The Mask of Sanity. 1st ed. Mosby; St. Louis, MO: 1941. [Google Scholar]
- Cooke D, Michie C. An item response theory: analyses of the Hare Psychopathy Checklist-Revised. Psychological Assessment. 1997;9:3–14. [Google Scholar]
- Damasio AR. Decartes’ Error: Error, Reason, and the Human Brain. Grosset/Putnam; New York: 1994. [Google Scholar]
- Damasio H, Grabowski T, Frank R, Galaburda AM, Damasio AR. The return of Phineas Gage: clues about the brain from the skull of a famous patient. Science. 1994;264(5162):1102–1105. doi: 10.1126/science.8178168. [DOI] [PubMed] [Google Scholar]
- Day R, Wong S. Anomalous perceptual asymmetries for negative emotional stimuli in the psychopath. Journal of Abnormal Psychology. 1996;1054:648–652. doi: 10.1037//0021-843x.105.4.648. [DOI] [PubMed] [Google Scholar]
- Degos JD, da Fonseca N, Gray F, Cesaro P. Severe frontal syndrome associated with infarcts of the left anterior cingulate gyrus and the head of the right caudate nucleus. A clinico-pathological case. Brain. 1993;116(Pt 6):1541–1548. doi: 10.1093/brain/116.6.1541. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate to behaviour. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Flor H, Birbaumer N, Hermann C, Ziegler S, Patrick CJ. Aversive Pavlovian conditioning in psychopaths: peripheral and central correlates. Psychophysiology. 2002;394:505–518. doi: 10.1017.S0048577202394046. [DOI] [PubMed] [Google Scholar]
- Forth AE, Hare RD. The contingent negative variation in psychopaths. Psychophysiology. 1989;266:676–682. doi: 10.1111/j.1469-8986.1989.tb03171.x. [DOI] [PubMed] [Google Scholar]
- Fowles DC. The three arousal model: implications of Gray's two-factor learning theory for heart rate, electrodermal activity, and psychopathy. Psychophysiology. 1980;172:87–104. doi: 10.1111/j.1469-8986.1980.tb00117.x. [DOI] [PubMed] [Google Scholar]
- Frick PJ. Callous-unemotional traits and conduct problems: a two-factor model of psychopathy in children. Issues in Criminological and Legal Psychology. 1995;24:47–51. [Google Scholar]
- Frick PJ. Callous-unemotional traits and conduct problems: applying the two-factor model of psychopathy to children. In: Cooke DJ, Forth AE, Hare RD, editors. Psychopathy: Theory, Research, and Implications for Society. Kluwer Academic Publishers; Dordrecht, The Netherlands: 1998. pp. 161–188. [Google Scholar]
- Frick PJ, Barry CT, Bodin SD. Applying the concept of psychopathy to children: implications for the assessment of antisocial youth. In: Gacono CB, editor. The Clinical and Forensic Assessment of Psychopathy: A Practitioner's Guide. Lawrence Erlbaum Associates, Inc., Publishers; Mahwah, NJ: 2000. pp. 3–24. [Google Scholar]
- Funayama ES, Grillon C, Davis M, Phelps EA. A double dissociation in the affective modulation of startle in humans: effects of unilateral temporal lobectomy. Journal of Cognitive Neuroscience. 2001;136:721–729. doi: 10.1162/08989290152541395. [DOI] [PubMed] [Google Scholar]
- Gillstrom BJ, Hare RD. Language-related hand gestures in psychopaths. Journal of Personality Disorders. 1988;21:21–27. [Google Scholar]
- Gorenstein EE, Newman JP. Disinhibitory psychopathology: a new perspective and a model for research. Psychological Reviews. 1980;873:301–315. [PubMed] [Google Scholar]
- Graves R, Landis T, Goodglass H. Laterality and sex differences for visual recognition of emotional and non-emotional words. Neuropsychologia. 1981;19:95–102. doi: 10.1016/0028-3932(81)90049-x. [DOI] [PubMed] [Google Scholar]
- Halgren E, Marinkovic K. General principles for the physiology of cognition as suggested by intracranial. In: Ogura C, Koga Y, Shimokochi M, editors. Recent Advances in Event-Related Brain Potential Research. Elsevier; New York: 1996. pp. 1072–1084. [Google Scholar]
- Halgren E, Marinkovic K, Chauvel P. Generators of the late cognitive potentials in auditory and visual oddball tasks. Electroencephalography and Clinical Neurophysiology. 1998;1062:156–164. doi: 10.1016/s0013-4694(97)00119-3. [DOI] [PubMed] [Google Scholar]
- Hare RD. Psychopathy, fear arousal and anticipated pain. Psychological Reports. 1965;162:499–502. doi: 10.2466/pr0.1965.16.2.499. [DOI] [PubMed] [Google Scholar]
- Hare RD. Psychopathy, autonomic functioning, and the orienting response. Journal of Abnormal Psychology. 1968;733(Pt 2):1–24. doi: 10.1037/h0025873. [DOI] [PubMed] [Google Scholar]
- Hare RD. Psychopathy and physiological responses to adrenalin. Journal of Abnormal Psychology. 1972;792:138–147. doi: 10.1037/h0032725. [DOI] [PubMed] [Google Scholar]
- Hare RD. Electrodermal and cardiovascular correlates of psychopathy. In: Hare RD, Schalling D, editors. Psychopathic Behavior: Approaches to Research. Wiley; Chichester, UK: 1978. pp. 107–143. [Google Scholar]
- Hare RD. Psychopathy and laterality of cerebral function. Journal of Abnormal Psychology. 1979;886:605–610. doi: 10.1037//0021-843x.88.6.605. [DOI] [PubMed] [Google Scholar]
- Hare RD. A research scale for the assessment of psychopathy in criminal populations. Personality and Individual Differences. 1980;12:111–119. [Google Scholar]
- Hare RD. Performance of pychopaths on cognitive tasks related to frontal lobe function. Journal of Abnormal Psychology. 1984;93:133–140. doi: 10.1037//0021-843x.93.2.133. [DOI] [PubMed] [Google Scholar]
- Hare RD. Manual for the Hare Psychopathy Checklist-Revised. Multi-Health Systems; Toronto: 1991. [Google Scholar]
- Hare RD. Without Conscience: The Disturbing World of the Psychopaths Among Us. Pocket Books; New York: 1993. [Google Scholar]
- Hare RD. Psychopathy — a clinical construct whose time has come. Criminal Justice and Behavior. 1996;231:25–54. [Google Scholar]
- Hare RD. Manual for the Hare Psychopathy Checklist-Revised. 2nd ed Multi-Health Systems; Toronto: 2003. [Google Scholar]
- Hare RD, Jutai JW. Psychopathy and cerebral asymmetry in semantic processing. Personality and Individual Differences. 1988;92:329–337. [Google Scholar]
- Hare RD, McPherson LM. Psychopathy and perceptual asymmetry during verbal dichotic listening. Journal of Abnormal Psychology. 1984;932:141–149. doi: 10.1037//0021-843x.93.2.141. [DOI] [PubMed] [Google Scholar]
- Hare RD, Quinn MJ. Psychopathy and autonomic conditioning. Journal of Abnormal Psychology. 1971;773:223–235. doi: 10.1037/h0031012. [DOI] [PubMed] [Google Scholar]
- Hare RD, Frazelle J, Cox DN. Psychopathy and physiological responses to threat of an aversive stimulus. Psychophysiology. 1978;152:165–172. doi: 10.1111/j.1469-8986.1978.tb01356.x. [DOI] [PubMed] [Google Scholar]
- Hare RD, Hart SD, Harpur TJ. Psychopathy and the DSM-IV criteria for antisocial personality disorder. Journal of Abnormal Psychology. 1991;100:391–398. doi: 10.1037//0021-843x.100.3.391. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR. Neocortical modulation of the amygdala response to fearful stimuli. Biological Psychiatry. 2003;536:494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- Harlow J. Passage of an iron rod through the head. Boston Medical and Surgical Journal. 1848;34:389–393. [PMC free article] [PubMed] [Google Scholar]
- Harpur TJ, Hakstian AR, Hare RD. Factor structure of the Psychopathy Checklist. Journal of Consulting and Clinical Psychology. 1988;565:741–747. doi: 10.1037//0022-006x.56.5.741. [DOI] [PubMed] [Google Scholar]
- Harpur TJ, Hare RD, Hakstian AR. Two-factor conceptualization of psychopathy: construct validity and assessment implications. Psychological Assessment. 1989;11:6–17. [Google Scholar]
- Hart SD, Hare RD. Psychopathy and antisocial personality disorder. Current Opinion in Psychiatry. 1996;92:129–132. [Google Scholar]
- Herve HF, Hayes P, Hare RD. Psychopathy and sensitivity to the emotional polarity of metaphorical statements. Personality and Individual Differences. 2003;357:1497–1507. [Google Scholar]
- Hill D, Pond DA, Mitchell W, Falconer MA. Personality changes following temporal lobectomy for epilepsy. Journal of Mental Science. 1957;103:18–27. doi: 10.1192/bjp.103.430.18. [DOI] [PubMed] [Google Scholar]
- Hood T, Siegfried J, Wieser H. The role of stereotactic amygdalotomy in the treatment of temporal lobe epilepsy associated with behavioral disorders. Applied Neurophysiology. 1983;461-4:19–25. doi: 10.1159/000101236. [DOI] [PubMed] [Google Scholar]
- Hornak J, Rolls ET, Wade D. Face and voice expression identification in patients with emotional and behavioural changes following ventral frontal lobe damage. Neuropsychologia. 1996;344:247–261. doi: 10.1016/0028-3932(95)00106-9. [DOI] [PubMed] [Google Scholar]
- Hornak J, Bramham J, Rolls ET, Morris RG, O'Doherty J, Bullock PR, Polkey CE. Changes in emotion after circumscribed surgical lesions of the orbitofrontal and cingulate cortices. Brain. 2003;126:1691–1712. doi: 10.1093/brain/awg168. [DOI] [PubMed] [Google Scholar]
- Intrator J, Hare RD, Stritzke P, Brichtswein K, Dorfman D, Harpur T, Bernstein D, Handelsman L, Schaefer C, Keilp J, Rosen J, Machac J. A brain imaging single photon emission computerized tomography study of semantic and affective processing in psychopaths. Biological Psychiatry. 1997;422:96–103. doi: 10.1016/S0006-3223(96)00290-9. [DOI] [PubMed] [Google Scholar]
- Isenberg N, Silbersweig D, Engelien A, Emmerich S, Malavade K, Beattie B, Leon AC, Stern E. Linguistic threat activates the human amygdala. Proceedings of the National Academy of Sciences of the United States of America. 1999;9618:10456–10459. doi: 10.1073/pnas.96.18.10456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen SD, Mishkin M. Perseverative interference in monkey following selective lesions of the inferior prefrontal convexity. Experimental Brain Research. 1970;11:376–386. doi: 10.1007/BF00237911. [DOI] [PubMed] [Google Scholar]
- Johnson RJ. Auditory and visual P300s in temporal lobectomy patients: evidence for modality-dependent generators. Psychophysiology. 1989;266:633–650. doi: 10.1111/j.1469-8986.1989.tb03165.x. [DOI] [PubMed] [Google Scholar]
- Johnson RJ. On the neural generators of the P300 component of the event-related potential. Psychophysiology. 1993;301:90–97. doi: 10.1111/j.1469-8986.1993.tb03208.x. [DOI] [PubMed] [Google Scholar]
- Jutai JW, Hare RD. Psychopathy and selective attention during performance of a complex perceptual–motor task. Psychophysiology. 1983;202:146–151. doi: 10.1111/j.1469-8986.1983.tb03280.x. [DOI] [PubMed] [Google Scholar]
- Jutai JW, Hare RD, Connolly JF. Psychopathy and event-related brain potentials: ERPs associated with attention to speech stimuli. Personality and Individual Differences. 1987;82:175–184. [Google Scholar]
- Kiehl KA. Ph.D. Dissertation. University of British Columbia; Vancouver, B.C.: 2000. A neuroimaging investigation of affective, cognitive, and language functions in psychopathy. Unpublished. [Google Scholar]
- Kiehl KA, Hare RD, McDonald JJ, Brink J. Semantic and affective processing in psychopaths: an event-related potential study. Psychophysiology. 1999;36:765–774. [PubMed] [Google Scholar]
- Kiehl KA, Hare RD, McDonald JJ, Liddle PF. Reduced P3 responses in criminal psychopaths during a visual oddball task. Biological Psychiatry. 1999;4511:1498–1507. doi: 10.1016/s0006-3223(98)00193-0. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Liddle PF, Smith AS, Mendrek A, Forster BB, Hare RD. Neural pathways involved in the processing of concrete and abstract words. Human Brain Mapping. 1999;7:225–233. doi: 10.1002/(SICI)1097-0193(1999)7:4<225::AID-HBM1>3.0.CO;2-P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl KA, Liddle PF, Hopfinger JB. Error processing and the rostral anterior cingulate: an event-related fMRI study. Psychophysiology. 2000;37:216–223. [PubMed] [Google Scholar]
- Kiehl KA, Smith AM, Hare RD, Liddle PF. An event-related potential investigation of response inhibition in schizophrenia and psychopathy. Biological Psychiatry. 2000;483:210–221. doi: 10.1016/s0006-3223(00)00834-9. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Smith AM, Hare RD, Forster BB, Brink J, Liddle PF. Limbic abnormalities in affective processing by criminal psychopaths as revealed by functional magnetic resonance imaging. Biological Psychiatry. 2001;50:677–684. doi: 10.1016/s0006-3223(01)01222-7. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Laurens KR, Liddle PF. Reading anomalous sentences: an event-related fMRI study of semantic processing. Neuroimage. 2002;17:842–850. [PubMed] [Google Scholar]
- Kiehl KA, Laurens KR, Celone K, Pearlson GD, Liddle PF. Abnormal affective picture processing in criminal psychopaths: evidence supporting the paralimbic dysfunction hypothesis.. Presented at the Society for Psychophysiological Research; Chicago, IL. 2003. [Google Scholar]
- Kiehl KA, Smith AM, Mendrek A, Forster BB, Hare RD, Liddle PF. Temporal lobe abnormalities in semantic processing by criminal psychopaths as revealed by functional magnetic resonance imaging. Psychiatry Research: Neuroimaging. 2004;130:27–42. doi: 10.1016/S0925-4927(03)00106-9. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Bates AT, Laurens KR, Hare RD, Liddle PF. Brain potentials implicate temporal lobe abnormalities in criminal psychopaths. Journal of Abnormal Psychology. doi: 10.1037/0021-843X.115.3.443. in press-a. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Laurens KR, Bates AT, Liddle PF. Psychopathy and semantic processing: an examination of the N400. Personality and Individual Differences. in press-b. [Google Scholar]
- Kluever H, Bucy PC. An analysis of certain effects of bilateral temporal lobectomy in the rhesus monkey, with special reference to “psychic blindness.”. Journal of Psychology. 1938;5:33–54. [Google Scholar]
- Kluever H, Bucy PC. Preliminary analysis of functions of the temporal lobes in monkeys. Archives of Neurology and Psychiatry. 1939;42:979–1000. doi: 10.1176/jnp.9.4.606. [DOI] [PubMed] [Google Scholar]
- Knight RT. Contribution of human hippocampal region to novelty detection. Nature. 1996;3836597:256–259. doi: 10.1038/383256a0. [DOI] [PubMed] [Google Scholar]
- Kosson DS, Suchy Y, Mayer AR, Libby J. Facial affect recognition in criminal psychopaths. Emotion. 2002;24:398–411. doi: 10.1037/1528-3542.2.4.398. [DOI] [PubMed] [Google Scholar]
- Kutas M, Hillyard SA. Reading senseless sentences: brain potentials reflect semantic incongruity. Science. 1980;207(4427):203–205. doi: 10.1126/science.7350657. [DOI] [PubMed] [Google Scholar]
- Laakso MP, Vaurio O, Koivisto E, Savolainen L, Eronen M, Aronen HJ, Hakola P, Repo E, Soininen H, Tiihonen J. Psychopathy and the posterior hippocampus. Behavioural Brain Research. 2001;1182:187–193. doi: 10.1016/s0166-4328(00)00324-7. [DOI] [PubMed] [Google Scholar]
- LaBar KS, LeDoux JE, Spencer DD, Phelps EA. Impaired fear conditioning following unilateral temporal lobectomy in humans. Journal of Neuroscience. 1995;1510:6846–6855. doi: 10.1523/JNEUROSCI.15-10-06846.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapierre D, Braun CMJ, Hodgins S. Ventral frontal deficits in psychopathy: neuropsychological test findings. Neuropsychologia. 1995;332:139–151. doi: 10.1016/0028-3932(94)00110-b. [DOI] [PubMed] [Google Scholar]
- Lee GP, Arena JG, Meador KJ, Smith JR. Changes in autonomic responsiveness following bilateral amygdalotomy in humans. Neuropsychiatry, Neuropsychology, and Behavioral Neurology. 1988;12:119–129. [Google Scholar]
- Lee GP, Bechara A, Adolphs R, Arena J, Meador KJ, Loring DW, Smith JR. Clinical and physiological effects of stereotaxic bilateral amygdalotomy for intractable aggression. Journal of Neuropsychiatry and Clinical Neurosciences. 1998;104:413–420. doi: 10.1176/jnp.10.4.413. [DOI] [PubMed] [Google Scholar]
- Levenston GK, Patrick CJ, Bradley MM, Lang PJ. The psychopath as observer: emotion and attention in picture processing. Journal of Abnormal Psychology. 2000;1093:373–385. [PubMed] [Google Scholar]
- Liddle PF. Disordered Mind and Brain: The Neural Basis of Mental Symptoms. Gaskell; London: 2001. [Google Scholar]
- Loney BR, Frick PJ, Clements CB, Ellis ML, Kerlin K. Callous-unemotional traits, impulsivity, and emotional processing in adolescents with antisocial behavior problems. Journal of Clinical Child and Adolescent Psychology. 2003;321:66–80. doi: 10.1207/S15374424JCCP3201_07. [DOI] [PubMed] [Google Scholar]
- Louth SM, Williamson S, Alpert M, Pouget ER, Hare RD. Acoustic distinctions in the speech of male psychopaths. Journal of Psycholinguistic Research. 1998;273:375–384. doi: 10.1023/a:1023207821867. [DOI] [PubMed] [Google Scholar]
- Lykken DT. A study of anxiety in the sociopathic personality. Journal of Abnormal and Social Psychology. 1957;55:6–10. doi: 10.1037/h0047232. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Buonocore MH. Activation of left posterior cingulate gyrus by the auditory presentation of threat-related words: an fMRI study. Psychiatry Research: Neuroimaging. 1997;75:1–14. doi: 10.1016/s0925-4927(97)00018-8. [DOI] [PubMed] [Google Scholar]
- Malloy P, Bihrle A, Duffy J, Cimino C. The orbitomedial frontal syndrome. Archives of Clinical Neuropsychology. 1993;8:185–201. [PubMed] [Google Scholar]
- Mathis H. Emotional responsivity in the antisocial personality. George Washington University; Washington, DC: 1970. Unpublished Doctoral Dissertation. [Google Scholar]
- McCarthy G, Nobre AC, Bentin S, Spencer DD. Language-related field potentials in the anterior–medial temporal lobe: I. Intracranial distribution and neural generators. Journal of Neuroscience. 1995;152:1080–1089. doi: 10.1523/JNEUROSCI.15-02-01080.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord WM, McCord J. The Psychopath: An Essay on the Criminal Mind. Van Nostrand; Princeton, NJ: 1964. [Google Scholar]
- Mesulam MM, editor. Principles of Behavioral and Cognitive Neurology. 2nd ed. Oxford University Press; New York: [Google Scholar]
- Mitchell D, Colledge E, Leonard A, Blair RJR. Risky decisions and response reversal: is there evidence of orbitofrontal cortex dysfunction in psychopathic individuals? Neuropsychologia. 2002;4012:2013–2022. doi: 10.1016/s0028-3932(02)00056-8. [DOI] [PubMed] [Google Scholar]
- Morgan AB, Lilienfeld SO. A meta-analytic review of the relation between antisocial behavior and neuropsychological measures of executive function. Clinical Psychology Review. 2000;201:113–136. doi: 10.1016/s0272-7358(98)00096-8. [DOI] [PubMed] [Google Scholar]
- Morris JS, Friston KJ, Dolan RJ. Experience-dependent modulation of tonotopic neural responses in human auditory cortex. Proceedings of the Royal Society of London: Biological Sciences. 1998;265(1397):649–657. doi: 10.1098/rspb.1998.0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JL, Sommer M, Wagner V, Lange K, Taschler H, Roder CH, Schuierer G, Klein HE, Hajak G. Abnormalities in emotion processing within cortical and subcortical regions in criminal psychopaths: evidence from a functional magnetic resonance imaging study using pictures with emotional content. Biological Psychiatry. 2003;542:152–162. doi: 10.1016/s0006-3223(02)01749-3. [DOI] [PubMed] [Google Scholar]
- Newman JP. Psychopathic behavior: an information processing perspective. In: Cooke DJ, Forth AE, Hare RD, editors. Psychopathy: Theory, Research, and Implications for Society. Kluwer Academic Publishers; Dordrecht, The Netherlands: 1998. pp. 81–104. [Google Scholar]
- Newman JP, Kosson DS. Passive avoidance learning in psychopathic and nonpsychopathic offenders. Journal of Abnormal Psychology. 1986:953. [PubMed] [Google Scholar]
- Newman JP, Schmitt WA. Passive avoidance in psychopathic offenders: a replication and extension. Journal of Abnormal Psychology. 1998;1073:527–532. doi: 10.1037//0021-843x.107.3.527. [DOI] [PubMed] [Google Scholar]
- Newman JP, Patterson CM, Kosson DS. Response perseveration in psychopaths. Journal of Abnormal Psychology. 1987;962:145–148. doi: 10.1037//0021-843x.96.2.145. [DOI] [PubMed] [Google Scholar]
- Newman JP, Kosson DS, Patterson CM. Delay of gratification in psychopathic and nonpsychopathic offenders. Journal of Abnormal Psychology. 1992;1014:630–636. doi: 10.1037//0021-843x.101.4.630. [DOI] [PubMed] [Google Scholar]
- Newman JP, Schmitt WA, Voss WD. The impact of motivationally neutral cues on psychopathic individuals: assessing the generality of the response modulation hypothesis. Journal of Abnormal Psychology. 1997;1064:563–575. doi: 10.1037//0021-843x.106.4.563. [DOI] [PubMed] [Google Scholar]
- Niznikiewicz MA, O'Donnell BF, Nestor PG, Smith L, Law S, Karapelou M, Shenton ME, McCarley RW. ERP assessment of visual and auditory language processing in schizophrenia. Journal of Abnormal Psychology. 1997;1061:85–94. doi: 10.1037//0021-843x.106.1.85. [DOI] [PubMed] [Google Scholar]
- Paller KA, Zola-Morgan S, Squire LR, Hillyard SA. P3-like brain waves in normal monkeys and in monkeys with medial temporal lesions. Behavioral Neuroscience. 1988;1025:714–725. doi: 10.1037//0735-7044.102.5.714. [DOI] [PubMed] [Google Scholar]
- Paller KA, McCarthy G, Roessler E, Allison T. Potentials evoked in human and monkey medial temporal lobe during auditory and visual oddball paradigms. Electroencephalography and Clinical Neurophysiology. 1992;843:269–279. doi: 10.1016/0168-5597(92)90008-y. [DOI] [PubMed] [Google Scholar]
- Patrick CJ. Emotion and psychopathy: startling new insights. Psychophysiology. 1994;314:319–330. doi: 10.1111/j.1469-8986.1994.tb02440.x. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Bradley MM, Lang PJ. Emotion in the criminal psychopath: startle reflex modulation. Journal of Abnormal Psychology. 1993;1021:82–92. doi: 10.1037//0021-843x.102.1.82. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Cuthbert BN, Lang PJ. Emotion in the criminal psychopath: fear image processing. Journal of Abnormal Psychology. 1994;1033:523–534. doi: 10.1037//0021-843x.103.3.523. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Senior C, Brammer M, Andrew C, Calder AJ, Bullmore ET, Perrett DI, Rowland D, Williams SC, Gray JA, David AS. A specific neural substrate for perceiving facial expressions of disgust. Nature. 1997;389(6650):495–498. doi: 10.1038/39051. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Scott SK, Calder AJ, Andrew C, Giampietro V, Williams SC, Bullmore ET, Brammer M, Gray JA. Neural responses to facial and vocal expressions of fear and disgust. Proceedings of the Royal Society of London: Biological Sciences. 1998;265(1408):1809–1817. doi: 10.1098/rspb.1998.0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol J, Lopez A, Deus J, Cardoner N, Vallejo J, Capdevila A, Paus T. Anatomical variability of the anterior cingulate gyrus and basic dimensions of human personality. Neuroimage. 2002;154:847–855. doi: 10.1006/nimg.2001.1004. [DOI] [PubMed] [Google Scholar]
- Raine A. The Psychopathology of Crime. Academic Press; San Diego: 1993. [Google Scholar]
- Raine A, Venables PH. Enhanced P3 evoked potentials and longer P3 recovery times in psychopaths. Psychophysiology. 1988;25:30–38. doi: 10.1111/j.1469-8986.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- Raine A, O'Brien M, Smiley N, Scerbo A, Chan CJ. Reduced lateralization in verbal dichotic listening in adolescent psychopaths. Journal of Abnormal Psychology. 1990;993:272–277. doi: 10.1037//0021-843x.99.3.272. [DOI] [PubMed] [Google Scholar]
- Raine A, Ishikawa SS, Arce E, Lencz T, Knuth KH, Bihrle S, LaCasse L, Colletti P. Hippocampal structural asymmetry in unsuccessful psychopaths. Biological Psychiatry. 2004;552:185–191. doi: 10.1016/s0006-3223(03)00727-3. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Hornak J, Wade D, McGrath J. Emotion-related learning in patients with social and emotional changes associated with frontal lobe damage. Journal of Neurology, Neurosurgery and Psychiatry. 1994;57:1518–1524. doi: 10.1136/jnnp.57.12.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt WA, Brinkley CA, Newman JP. Testing Damasio's somatic marker hypothesis with psychopathic individuals: risk takers or risk averse? Journal of Abnormal Psychology. 1999;1083:538–543. doi: 10.1037//0021-843x.108.3.538. [DOI] [PubMed] [Google Scholar]
- Schnider A. Spontaneous confabulation, reality monitoring, and the limbic system — a review. Brain Research: Brain Research Reviews. 2001;362:150–160. doi: 10.1016/s0165-0173(01)00090-x. [DOI] [PubMed] [Google Scholar]
- Scott SK, Young AW, Calder AJ, Hellawell DJ, Aggleton JP, Johnson M. Impaired auditory recognition of fear and anger following bilateral amygdala lesions. Nature. 1997;3856613:254–257. doi: 10.1038/385254a0. [DOI] [PubMed] [Google Scholar]
- Soderstrom H, Hultin L, Tullberg M, Wikkelso C, Ekholm S, Forsman A. Reduced frontotemporal perfusion in psychopathic personality. Psychiatry Research: Neuroimaging. 2002;1142:81–94. doi: 10.1016/s0925-4927(02)00006-9. [DOI] [PubMed] [Google Scholar]
- Sokolov EN. Perception and the Conditioned Reflex. Macmillan; New York: 1963. [Google Scholar]
- Soltani M, Knight RT. Neural origins of the P300. Critical Reviews in Neurobiology. 2000;143:199–224. [PubMed] [Google Scholar]
- Strange BA, Henson RN, Friston KJ, Dolan RJ. Brain mechanisms for detecting perceptual, semantic, and emotional deviance. Neuroimage. 2000;124:425–433. doi: 10.1006/nimg.2000.0637. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Benson DF, Kaplan EF. The involvement of orbitofrontal cerebrum in cognitive tasks. Neuropsychologia. 1983;21:235–248. doi: 10.1016/0028-3932(83)90040-4. [DOI] [PubMed] [Google Scholar]
- Sutton SK, Vitale JE, Newman JP. Emotion among women with psychopathy during picture perception. Journal of Abnormal Psychology. 2002;1114:610–619. doi: 10.1037//0021-843x.111.4.610. [DOI] [PubMed] [Google Scholar]
- Swick D. Personal communication. 2003.
- Swick D, Jovanovic J. Anterior cingulate cortex and the Stroop task: neuropsychological evidence for topographic specificity. Neuropsychologia. 2002;408:1240–1253. doi: 10.1016/s0028-3932(01)00226-3. [DOI] [PubMed] [Google Scholar]
- Swick D, Turken AU. Dissociation between conflict detection and error monitoring in the human anterior cingulate cortex. Proceedings of the National Academy of Sciences of the Unites States of America. 2002;9925:16354–16359. doi: 10.1073/pnas.252521499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekin S, Cummings JL. Frontal–subcortical neuronal circuits and clinical neuropsychiatry: an update. Journal of Psychosomatic Research. 2002;532:647–654. doi: 10.1016/s0022-3999(02)00428-2. [DOI] [PubMed] [Google Scholar]
- Turken AU, Swick D. Response selection in the human anterior cingulate cortex. Nature Neuroscience. 1999;210:920–924. doi: 10.1038/13224. [DOI] [PubMed] [Google Scholar]
- Veit R, Flor H, Erb M, Hermann C, Lotze M, Grodd W, Birbaumer N. Brain circuits involved in emotional learning in antisocial behavior and social phobia in humans. Neuroscience Letters. 2002;3283:233–236. doi: 10.1016/s0304-3940(02)00519-0. [DOI] [PubMed] [Google Scholar]
- Widiger TA, Cadoret R, Hare RD, Robins L, Rutherford M, Zanarini M, Alterman A, Apple M, Corbitt E, Forth A, Hart SD, Kultermann J, Woody G, Frances A. DSM-IV antisocial personality disorder field trial. Journal of Abnormal Psychology. 1996;1051:3–16. doi: 10.1037//0021-843x.105.1.3. [DOI] [PubMed] [Google Scholar]
- Williamson S, Harpur TJ, Hare RD. Abnormal processing of affective words by psychopaths. Psychophysiology. 1991;283:260–273. doi: 10.1111/j.1469-8986.1991.tb02192.x. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Knight RT. Association cortex contributions to the human P3. In: Haschke W, Roitbak AI, Speckmann E-J, editors. Slow Potential Changes in the Brain. Birkhauser; Boston: 1993. pp. 71–84. [Google Scholar]