Abstract
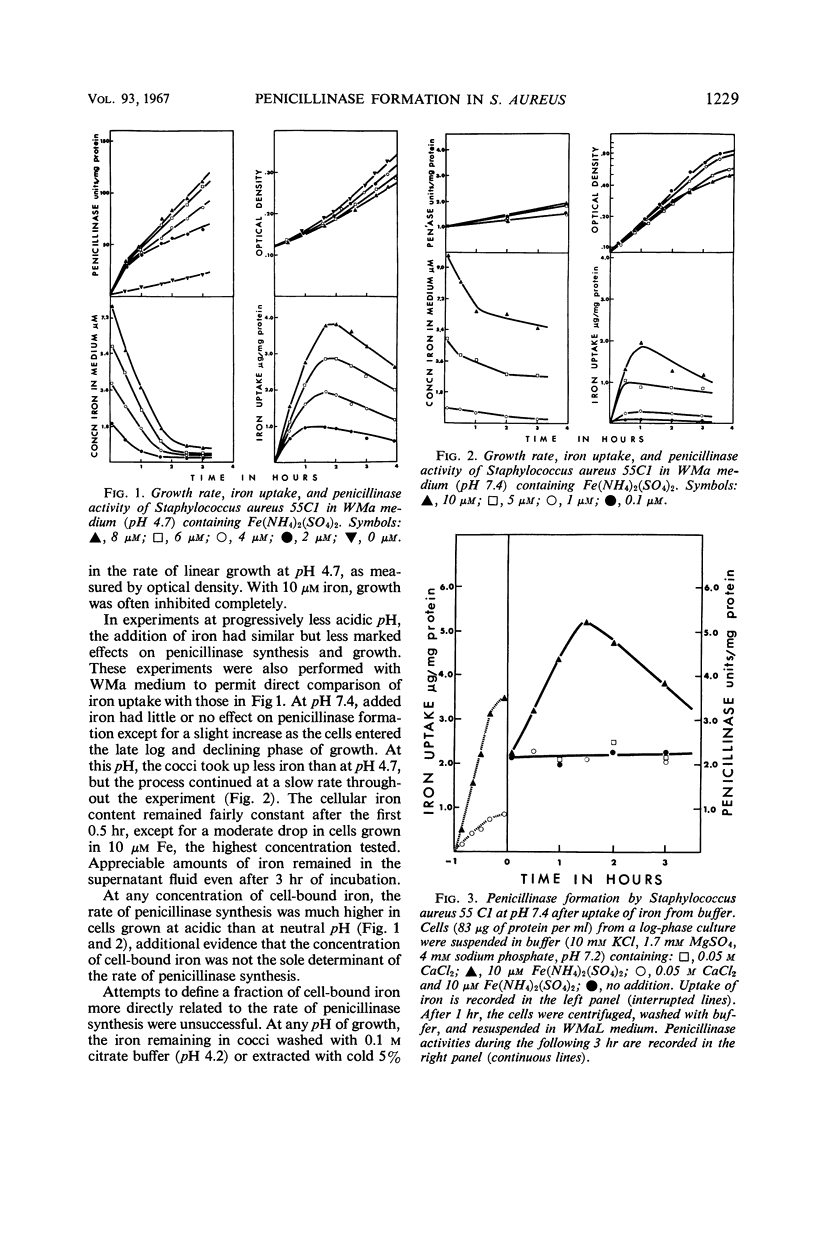
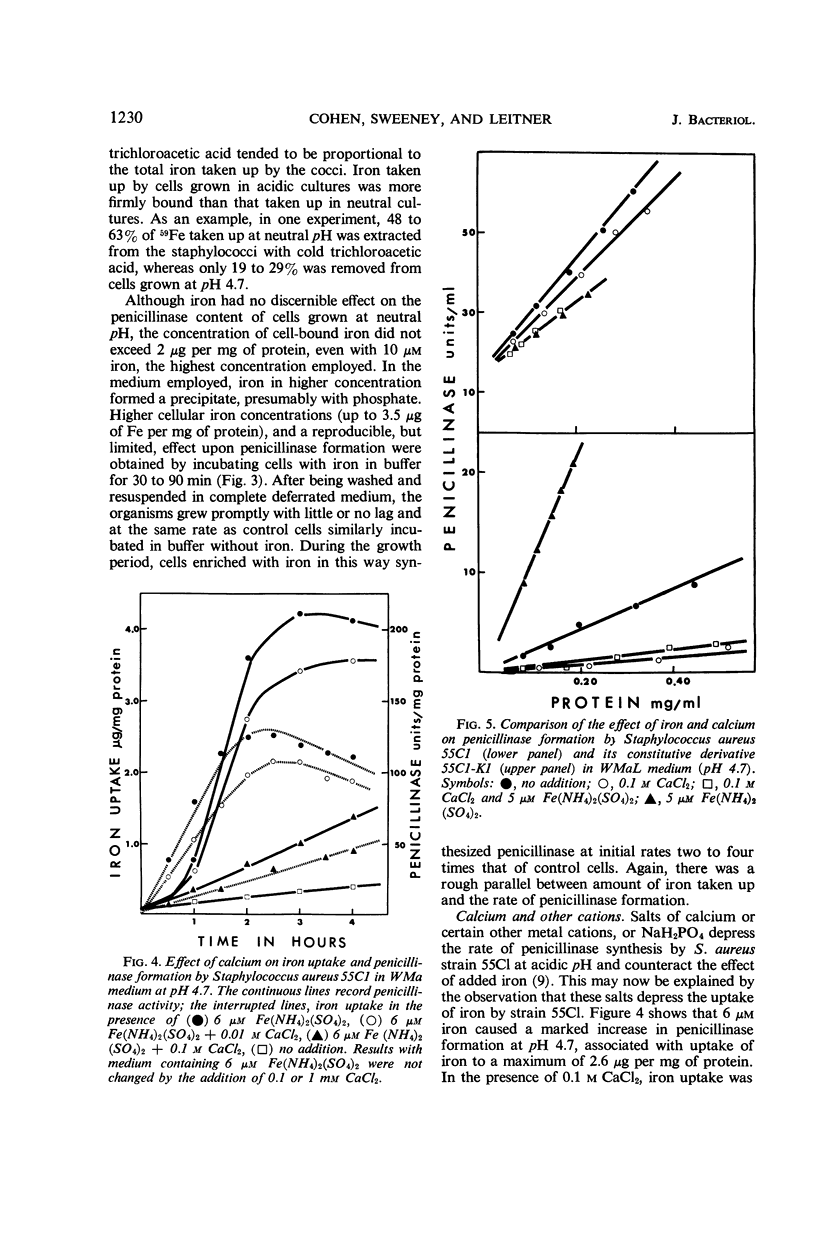
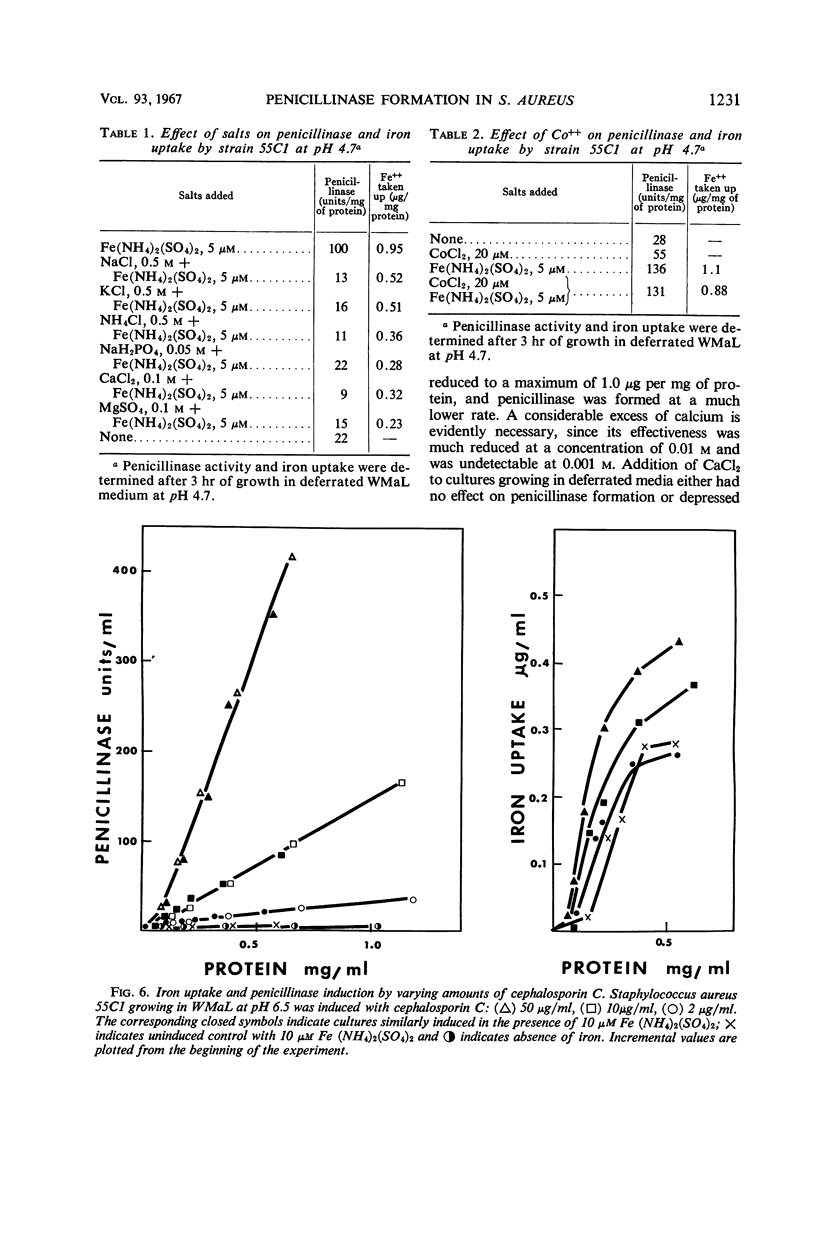
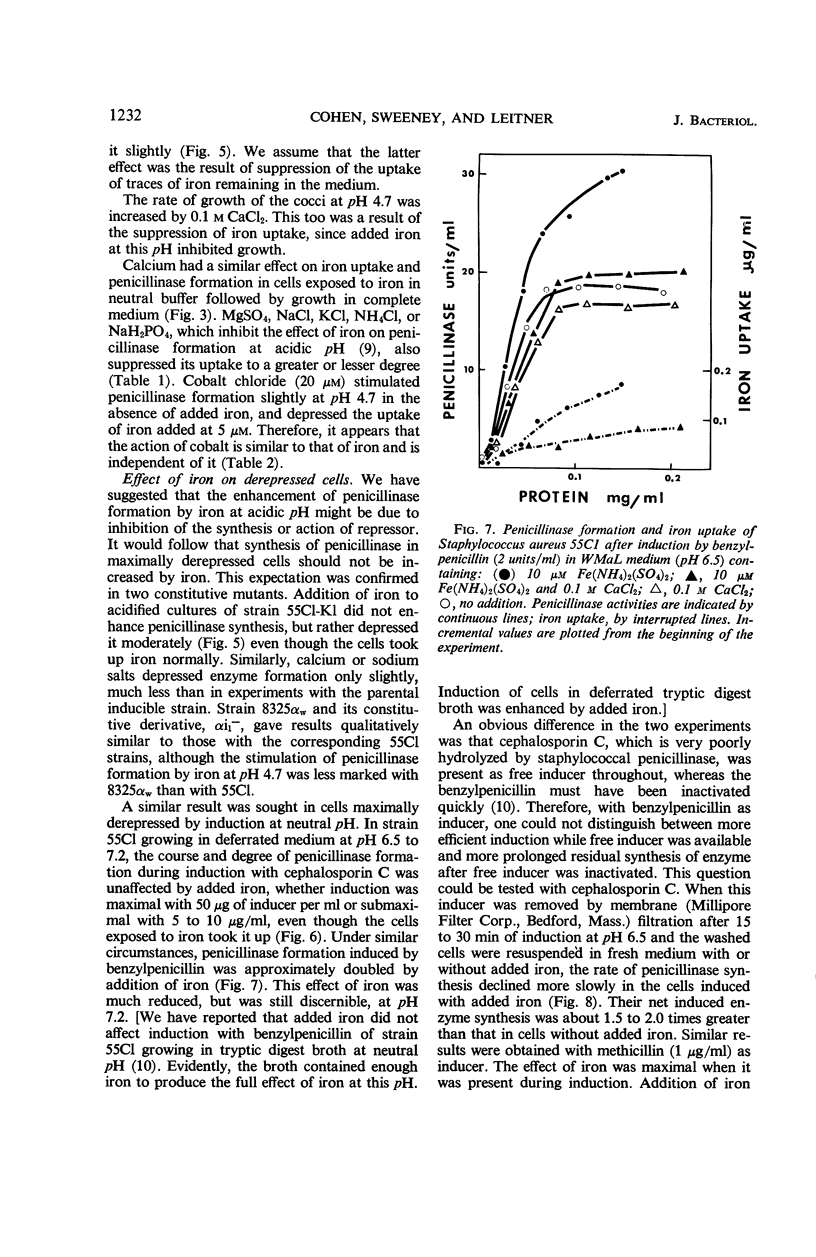
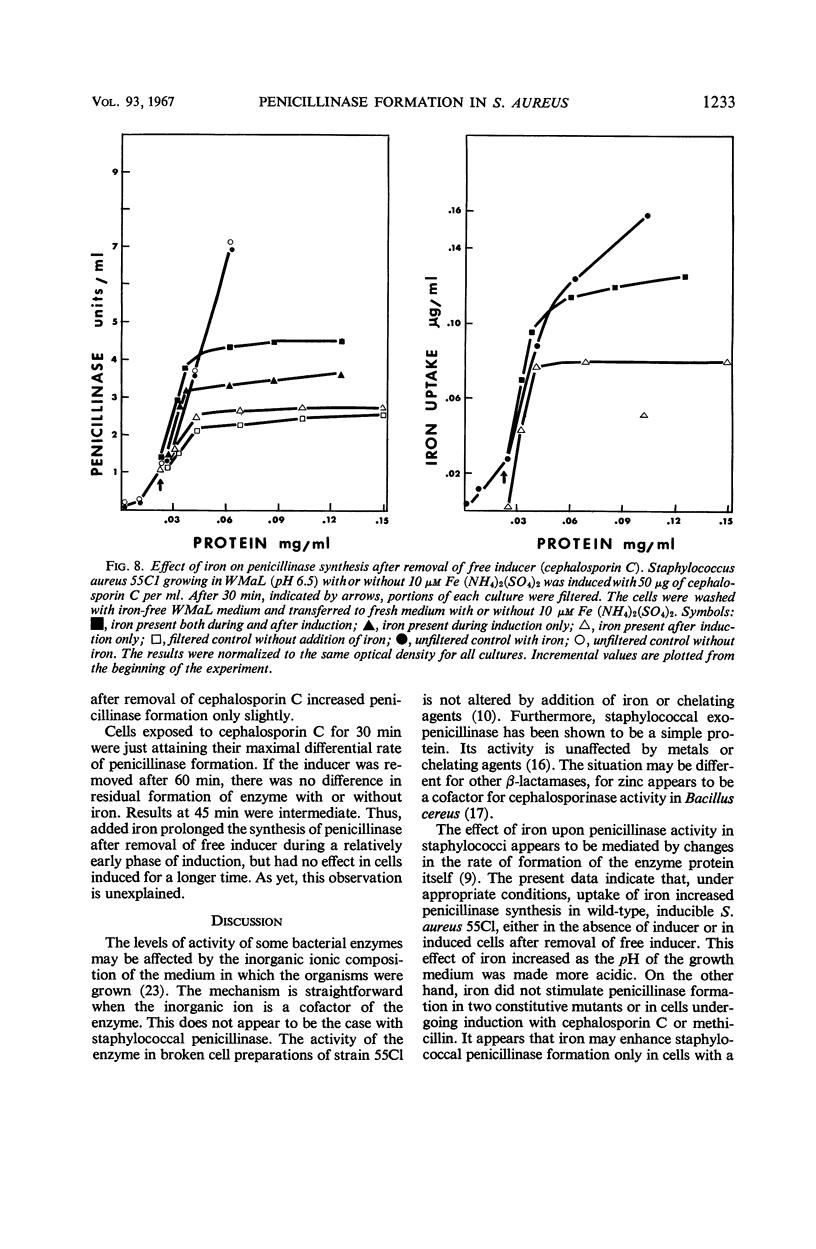
The uptake of iron and the formation of penicillinase was examined in cultures of wild-type Staphylococcus aureus. Uptake of iron was about twice as great at pH 4.7 as at pH 7.4 At pH 4.7, increase in iron uptake in the range of 1.0 to 4.0 μg per mg of bacterial protein was associated with a progressive increase in the rate of penicillinase formation, but a direct correlation between cellular iron content and rate of enzyme formation was not demonstrated. Addition of iron to deferrated medium enhanced penicillinase formation at pH 6.5 to 7.4 two- to fourfold in cultures induced with benzylpenicillin and in uninduced cultures. To demonstrate an effect on the uninduced cells, it was necessary to increase iron uptake by preliminary incubation of cells with iron in buffer. Calcium and certain other ions depressed iron uptake at acidic and at neutral pH, and, presumably as a result of this action, depressed the formation of penicillinase. Iron did not enhance penicillinase formation at pH 4.7 by two penicillinase constitutive mutants nor by wild-type cells undergoing induction at pH 6.5 by cephalosporin C or methicillin. After removal of cephalosporin C or methicillin during an early phase of induction, residual synthesis of enzyme was increased by prior uptake of iron. The results are considered compatible with the concept that uptake of iron, especially at acidic pH, interferes with the formation or function of penicillinase repressor.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABELSON P. H., ALDOUS E. Ion antagonisms in microorganisms; interference of normal magnesium metabolism by nickel, cobalt, cadmium, zinc, and manganese. J Bacteriol. 1950 Oct;60(4):401–413. doi: 10.1128/jb.60.4.401-413.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellamy W. D., Klimek J. W. Some Properties of Penicillin-resistant Staphylococci. J Bacteriol. 1948 Feb;55(2):153–160. doi: 10.1128/jb.55.2.153-160.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN M., HORIBATA K. Analysis of the differentiation and of the heterogeneity within a population of Escherichia coli undergoing induced beta-galactosidase synthesis. J Bacteriol. 1959 Nov;78:613–623. doi: 10.1128/jb.78.5.613-623.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLLINS J. F. THE DISTRIBUTION AND FORMATION OF PENICILLINASE IN A BACTERIAL POPULATION OF BACILLUS LICHENIFORMIS. J Gen Microbiol. 1964 Mar;34:363–377. doi: 10.1099/00221287-34-3-363. [DOI] [PubMed] [Google Scholar]
- GERONIMUS L. H., COHEN S. Induction of staphylococcal penicillinase. J Bacteriol. 1957 Jan;73(1):28–34. doi: 10.1128/jb.73.1.28-34.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAMINSKI Z. C. Maximum synthesis of staphylocococcal penicillinase in the absence of penicillin. Arch Biochem Biophys. 1962 Jun;97:578–581. doi: 10.1016/0003-9861(62)90126-1. [DOI] [PubMed] [Google Scholar]
- LEITNER F., COHEN S. Effect of pH and inorganic salts on penicillinase formation in Staphylococcus aureus. J Bacteriol. 1962 Feb;83:314–323. doi: 10.1002/path.1700830139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEITNER F., SWEENEY H. M., MARTIN T. F., COHEN S. INDUCTION OF STAPHYLOCOCCAL PENICILLINASE BY BENZYLPENICILLIN: EFFECT OF PH, CONCENTRATION OF FERROUS ION AND INDUCER, AND DURATION OF EXPOSURE OF CELLS TO INDUCER. J Bacteriol. 1963 Oct;86:717–727. doi: 10.1128/jb.86.4.717-727.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVICK R. P. ANALYSIS BY TRANSDUCTION OF MUTATIONS AFFECTING PENICILLINASE FORMATION IN STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1963 Oct;33:121–136. doi: 10.1099/00221287-33-1-121. [DOI] [PubMed] [Google Scholar]
- NOVICK R. P., RICHMOND M. H. NATURE AND INTERACTIONS OF THE GENETIC ELEMENTS GOVERNING PENICILLINASE SYNTHESIS IN STAPHYLOCOCCUS AUREUS. J Bacteriol. 1965 Aug;90:467–480. doi: 10.1128/jb.90.2.467-480.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVICK R. P. Staphylococcal penicillinase and the new penicillins. Biochem J. 1962 May;83:229–235. doi: 10.1042/bj0830229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick A., Weiner M. ENZYME INDUCTION AS AN ALL-OR-NONE PHENOMENON. Proc Natl Acad Sci U S A. 1957 Jul 15;43(7):553–566. doi: 10.1073/pnas.43.7.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRET C. J. Iodometric assay of penicillinase. Nature. 1954 Nov 27;174(4439):1012–1013. doi: 10.1038/1741012a0. [DOI] [PubMed] [Google Scholar]
- RICHMOND M. H. PURIFICATION AND PROPERTIES OF THE EXOPENICILLINASE FROM STAPHYLOCOCCUS AUREUS. Biochem J. 1963 Sep;88:452–459. doi: 10.1042/bj0880452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWALLOW D. L., SNEATH P. H. Studies on staphylococcal penicillinase. J Gen Microbiol. 1962 Jul;28:461–469. doi: 10.1099/00221287-28-3-461. [DOI] [PubMed] [Google Scholar]
- Sabath L. D., Abraham E. P. Zinc as a cofactor for cephalosporinase from Bacillus cereus 569. Biochem J. 1966 Jan;98(1):11C–13C. doi: 10.1042/bj0980011c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H., Kato I. Regulatory mechanisms of the formation of diphtheria toxin. I. Isolation and property of an inhibitory factor. Jpn J Exp Med. 1965 Jun;35(3):145–158. [PubMed] [Google Scholar]
- THEODORE T. S., SCHADE A. L. GROWTH OF STAPHYLOCOCCUS AUREUS IN MEDIA OF RESTRICTED AND UNRESTRICTED INORGANIC IRON AVAILABILITY. J Gen Microbiol. 1965 Apr;39:75–83. doi: 10.1099/00221287-39-1-75. [DOI] [PubMed] [Google Scholar]
- WEINBERG E. D. Trace-metal control of specific biosynthetic processes. Perspect Biol Med. 1962;5:432–445. doi: 10.1353/pbm.1962.0017. [DOI] [PubMed] [Google Scholar]
- WRIGHT E. S., MUNDY R. A. Defined medium for phenol coefficient tests with Salmonella typhosa and Staphylococcus aureus. J Bacteriol. 1960 Aug;80:279–280. doi: 10.1128/jb.80.2.279-280.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WYATT H. V., REED G. W., SMITH A. H. Calcium requirement for growth of staphylococcus pyogenes. Nature. 1962 Jul 7;195:100–101. doi: 10.1038/195100a0. [DOI] [PubMed] [Google Scholar]


