Abstract
Recruitment of receptor proteins to lipid rafts has been proposed as an important mechanism to regulate their cellular function. In particular, rafts have been implicated in regulation of integrin-mediated cell adhesion, although the underlying mechanism remains elusive. We used single-molecule near-field optical microscopy (NSOM) with localization accuracy of approximately 3 nm, to capture the spatio-functional relationship between the integrin LFA-1 and raft components (GPI-APs) on immune cells. Dual color nanoscale imaging revealed the existence of a nanodomain GPI-AP subpopulation that further concentrated in regions smaller than 250 nm, suggesting a hierarchical prearrangement of GPI-APs on resting monocytes. We previously demonstrated that in quiescent monocytes, LFA-1 preorganizes in nanoclusters. We now show that integrin nanoclusters are spatially different but reside proximal to GPI-AP nanodomains, forming hotspots on the cell surface. Ligand-mediated integrin activation resulted in an interconversion from monomers to nanodomains of GPI-APs and the generation of nascent adhesion sites where integrin and GPI-APs colocalized at the nanoscale. Cholesterol depletion significantly affected the reciprocal distribution pattern of LFA-1 and GPI-APs in the resting state, and LFA-1 adhesion to its ligand. As such, our data demonstrate the existence of nanoplatforms as essential intermediates in nascent cell adhesion. Since raft association with a variety of membrane proteins other than LFA-1 has been documented, we propose that hotspots regions enriched with raft components and functional receptors may constitute a prototype of nanoscale inter-receptor assembly and correspond to a generic mechanism to offer cells with privileged areas for rapid cellular function and responses to the outside world.
Keywords: integrin LFA-1, membrane nanocompartments, near-field scanning optical microscopy (NSOM), single molecule detection
During the past decade it is becoming clear that cell membranes are compartmentalized in domains that are diverse in size and composition (1). However, the mechanisms orchestrating this degree of organization remain enigmatic. Several models have been put forward to explain, at least partially, the variety in the clustering exhibited by most proteins and lipids in biological membranes (1–4). One of these proposed mechanisms concerns the presence of cholesterol-dependent lipid assemblies on the membrane, so-called lipid rafts, containing a variety of proteins including glycosylphosphatidylinositol anchored proteins (GPI-APs) (2, 5, 6). Yet, the existence of lipid rafts, let alone their contribution in a multitude of cellular functions, continues under scrutiny (7). This is largely due to their nanometer-scale dimensions making them not accessible to conventional fluorescence microscopy and to their highly dynamic character (5). In recent years, novel microscopy techniques provided more persuasive data favoring the existence of nanoscale rafts in resting and activated cells (8–12). Also, ligand-induced clustering of small rafts components was sufficient to induce signaling (13). Albeit rafts are supposedly involved in a diversity of cellular functions, including cell adhesion (14–18), the spatial-functional relationship at the relevant nanoscale between rafts, and specialized membrane receptors has not yet been explored.
Within the context of raft regulation in integrin-mediated adhesion, one of the most studied receptors is lymphocyte function-associated antigen-1 (LFA-1; CD11a/CD18) and its ligand ICAM-1. LFA-1 is a leukocyte-specific integrin that mediates migration of cells of the immune system and formation of the immunological synapse (19). The importance of rafts on LFA-1 activation on thymocytes was initially proposed after observing that cross-linking of GPI-APs rapidly induced LFA-1 mediated-binding (15). On T cells, LFA-1 mediated adhesion depended upon intact lipid rafts (16) and dynamic partitioning of LFA-1 in rafts controlled its endo-exocytic cycle on chemotactic neutrophils (17). Recently, we showed that on monocytes, LFA-1 preorganizes in ligand-independent nanoclusters (18). This well-defined organization correlated with integrin activity and its recruitment in rafts indicating a direct relationship between membrane organization and LFA-1 adhesive properties (18). However, technical challenges have prevented so far a direct visualization of these interactions at the relevant spatial scales.
Near-field scanning optical microscopy (NSOM) belongs to the family of superresolution optical techniques that provides simultaneous optical and topographic lateral resolution at the nanoscale (20, 21). The technique is based on scanning a subwavelength light source a few nanometers above the specimen. Due to the evanescent character of the excitation field, the technique is particularly suitable for investigating the distribution of cell surface proteins with nm precision at the single molecule level (20, 21). Recently, the potential of NSOM has been demonstrated by imaging nanodomains of different types of receptors on cardiac myocytes and cells of the immune system (22–24). In contrast to other recently developed super-resolution optical methodologies (25), NSOM guarantees simultaneous dual-color detection with equal spatial resolution, sensitivity and position accuracy for both colors. Here we used combined confocal/NSOM microscopy in aqueous conditions to simultaneously visualize the nanoscale organization of raft components and LFA-1 at the plasma membrane of monocytes before and after LFA-1 ligand binding.
Results and Discussion
LFA-1 Forms Nanoclusters on Monocytes Before Ligand Binding.
We previously demonstrated by antibody (Ab) co-patching and confocal microscopy that on monocytes LFA-1 fully colocalized with the raft marker GM1 and that raft disruption affected LFA-1-binding to its ligand, ICAM-1 (18). To investigate whether the impaired binding of LFA-1 to ICAM-1 upon cholesterol extraction was due to LFA-1 nanocluster disruption, we analyzed the LFA-1 distribution upon treatment with methyl-β-cyclodextrin (MCD), a lipid raft-disrupting agent that extracts membrane cholesterol (see Fig. S1). Interestingly, this treatment had no effect on nanocluster integrity neither on the expression level and/or conformational state of LFA-1 (Fig. S1). These results suggest that intact rafts are required for LFA-1 adhesion to its ligand, but not for maintaining ligand-independent LFA-1 nanoclustering. Moreover rafts are not a prerequisite for the high affinity expression of the receptor (Fig. S1). From this we hypothesized that a different mechanism might regulate the distribution of LFA-1 and rafts in the absence and the presence of the ligand at a spatial scale beyond the resolution of standard optical microscopy.
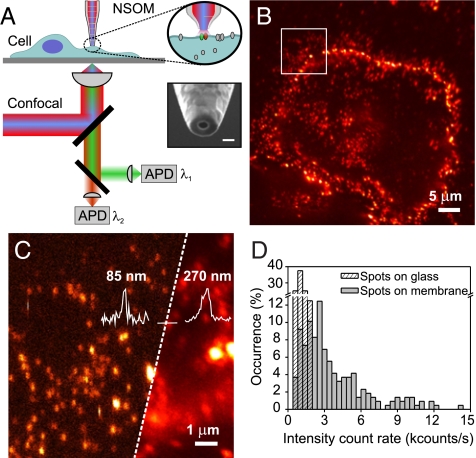
To examine the nanometer-scale organization of LFA-1 in relation to rafts on intact quiescent cells we performed extensive nanoscale imaging of LFA-1 using a combined single molecule confocal/NSOM set-up working in aqueous conditions. A scheme of the set-up is shown in Fig. 1A (see Materials and Methods and SI Text). Representative confocal and NSOM images of LFA-1 on the cell membrane of fixed monocytes are shown in Fig. 1 B and C, and Fig. S2. The resolving capability of NSOM reveals individual LFA-1 spots well-separated on the cell membrane (Fig. 1C, left). Multiple images were analyzed by measuring the brightness of individual LFA-1 spots on the cell membrane (Fig. 1D). For comparison, the brightness of individual fluorescent spots of non-specifically adsorbed anti-LFA-1 Abs scarcely found on the substrate next to the cell surface were also included in the same plot (Fig. 1D). The larger number of occurrences at high intensity values on the cell surface indicates that multiple LFA-1 molecules are present in each spot consistent with its organization in nanoclusters. To provide further evidence for nanoclustering of LFA-1 we compared the experimental data with simulations to estimate the random probability of “apparent” clustering taking into account the NSOM probe size and the experimental LFA-1 density (Fig. S3A and SI Text). At the given experimental conditions, the simulations result in negligible cluster probability confirming that the clusters observed are indeed real, in agreement with our previous electron microscopy studies (18). NSOM also showed an apparent cluster diameter of 85 nm (σ = 20) (Fig. S2). This value is close to the aperture probe size reflecting that the real nanocluster size may be somewhat smaller (SI Text).
Fig. 1.
LFA-1 forms nanoclusters on monocytes before ligand binding. (A) Principle of operation: the sample is imaged either by confocal or NSOM. In NSOM, the probe raster scans the sample laterally while locally exciting fluorophores exclusively on the cell membrane. The inset shows a representative NSOM probe as used in our experiments. (Scale bar, 100 nm.) (B) Confocal image of LFA-1 at the cell surface using mAb TS2/4-Alexa647. (C) Composed confocal (Right) and NSOM in aqueous conditions (Left) image of the region highlighted in B. The increased spatial resolution of NSOM (85 nm) compared to confocal (270 nm) is further emphasized by the line profiles over the same individual spot. (D) Intensity count rate distribution of LFA-1 nanoclusters (220 spots) (gray) over multiple cells and of individual Abs. non-specifically attached to the glass surface (dashed).
Hierarchical Nanoscale Organization of GPI-APs on Resting Monocytes.
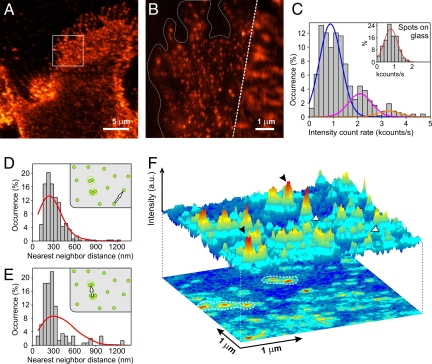
To map the organization of raft components on resting monocytes we focused on GPI-APs collectively labeled with monovalent bacterial toxin pro-aerolysin (FLAER) at saturating labeling conditions (SI Text and Fig. S4). Representative confocal and NSOM images are shown in Fig. 2 A and B. The intensity distribution of individual GPI-AP spots on the cell surface is shown in Fig. 2C together with Gaussian fittings. Since the first part of the distribution centered at 873 counts/s (σ = 450) overlaps well with that of individual FLAER on the glass [that is, 775 counts/s (σ = 370) (see inset)], we assign this distribution to that of monomers. From Gaussian fittings of the main distribution it follows that approximately 70% of the GPI-APs organized as monomers on the membrane, with the residual 30% forming nanodomains containing 2–4 molecules (Fig. 2C). Furthermore, simulations of random organization at the experimental packing densities and NSOM resolution confirm that this percentage of nanodomains is real (Fig. S3B and SI Text). These data support earlier findings of similar monomer and nanodomain ratios of GPI-APs in quiescent cells (8). Interestingly, the non-raft associated transferrin receptor (CD71) follows a comparable organization, with approximately 88% of CD71 being monomeric and the remaining 12% forming small oligomers on the cell surface (see Fig. S5 A–C).
Fig. 2.
GPI-AP nanodomains form hotspots on the cell membrane. (A) Confocal image of GPI-APs at the cell surface of a fixed monocyte. (B) Composed confocal (Right) and NSOM (Left) image of the region highlighted in A. Cell borders are highlighted to easier visual inspection. (C) Intensity distribution of GPI-AP fluorescent spots (473 spots) over multiple cells. The percentage of monomers, dimers and tetramers is derived from Gaussian fittings to the GPI-AP distribution: monomers (71%, blue with 873 counts/s, σ = 450), dimers (magenta, 19%, with 2,100 counts/s, σ = 475), trimers (orange, 5%, 3,300 counts/s, σ = 450), and the remaining 5% forming higher order GPI-AP oligomers. The inset shows the intensity distribution of individual fluorescent spots found on the glass surface, as fitted by a single Gaussian 775 counts/s, σ = 371; and thus corresponding to single FLAER. (D) Nearest neighbor distance distribution of monomeric GPI-APs (330 spots) (bars) and simulations of random monomer organization (red). Experimental and simulation distributions are significantly similar: P = 0.88. (E) Similar distributions as D, compiling only distances between GPI-AP nanodomains (150 spots). Experimental and simulation distributions in (E) deviate significantly, P < 0.005. (F) 3D projection of an NSOM image with nanodomains (black arrows) and monomers (white arrows) of GPI-APs. Contour dashed lines on the 2-D image illustrate the preference of nanodomains to concentrate on specific sites as hotspots.
We next examined the overall distribution of GPI-AP monomers and nanodomains on the resting cell surface by nearest-neighbor distribution analysis and compared the experimental data with simulations of random organization (see SI Text). The monomeric and nanodomain subpopulations were separated using a threshold (1.5 kcounts/s) as determined from Fig. 2C (see SI Text). While GPI-AP monomers are randomly scattered at the cell surface (Fig. 2D), approximately 47% of GPI-AP nanodomains preferentially reside in regions less than or equal to 250 nm from each other in a pronounced nonrandom fashion (Fig. 2 E and F). These results directly show the occurrence of a GPI-AP hierarchical organization on the cell surface. Similar type of GPI-AP distribution on resting CHO cells has been also recently inferred by fluorescence anisotropy, albeit at lower spatial resolution (<450 nm), and linked to cortical actin activity (26). Importantly, our data directly demonstrate and quantify the existence of GPI-AP “hotspots” on the resting cell membrane, resulting from both a preexisting nanodomain organization and an increase in their local surface density. As such, our results together with those of Mayor's group (26) suggest a generic mechanism of hierarchical GPI-AP organization on the cell membrane, regardless of the cell type investigated. Interestingly, a heterogeneous distribution of GPI-APs on different cell types has been also observed by electron microscopy (27).
In Resting Monocytes, LFA-1 Nanoclusters Are Spatially Segregated but Proximal to GPI-APs.
We next focused on the simultaneous nanoscale imaging of LFA-1 and GPI-APs using dual color excitation/detection NSOM with identical optical resolution for both colors (Fig. 3). Unexpectedly, upon high-resolution optical inspection, LFA-1 nanoclusters do not significantly colocalize with GPI-APs as evidenced by the low occurrence of yellow spots on the images (Fig. 3A). Indeed, Pearsons's correlation coefficient C over multiple NSOM images rendered values of 0.13 ± 0.08, where C = 1 corresponds to full colocalization and C = 0 to no colocalization. Nevertheless, the absence of spatial overlap does not exclude the possibility of a more complex underlying organization at this spatial scale. To test this hypothesis, we directly measured the nearest inter-domain distances between LFA-1 nanoclusters and GPI-APs and contrasted the data with simulations assuming random organization of LFA-1 nanoclusters with respect to GPI-APs, at the experimental surface densities (see Materials and Methods and Fig. S6). For the analysis, we rigorously quantify spots (on the green and red channels) that can be clearly separated as individual entities, and disregard the minor fraction of spots that are too close to each other to avoid biasing on the analysis. Interestingly, the distribution of inter-domain distances between LFA-1 nanoclusters and GPI-APs showed a clear deviation toward shorter values when compared to simulations (Fig. 3B). Indeed, there is a significant number of occurrences at distances <150 nm with a subpopulation of approximately 25% of LFA-1 nanoclusters being in <50–100 nm neighborhood of GPI-APs. As control, we investigated the nanoscale organization of CD71 with respect to GPI-APs (Fig. S5 D and E). As expected, the experimental distribution overlapped well with that of random organization of the receptor with respect to GPI-APs, entirely consistent with the classification of CD71 as non-raft marker.
Fig. 3.
LFA-1 nanoclusters are spatially segregated but proximal to GPI-AP hotspots. (A) Representative dual color excitation/detection NSOM image of LFA-1 nanoclusters (red) and GPI-APs (green) in steady state. (B) Nearest inter-domain distance distributions of LFA-1 nanoclusters to its closest GPI-AP (bars) together with simulations of random spatial distribution of LFA-1 nanoclusters and GPI-APs (red). The inset corresponds to the difference (Δi in %) between experimental data and simulations. At shorter distances (< cross-over point in Δi) both distributions are significantly different with P < 0.01. Nearest inter-domain distance distributions of: (C) GPI-AP monomers to its closest LFA-1 nanocluster (bars); and (D) GPI-AP nanodomains to its closest LFA-1 nanocluster (bars), together with the corresponding simulations (red). Experimental and simulation distributions in (C) are significantly similar with P = 0.22 while in (D) are significantly different, P < 0.008. Cartoons and arrows direction next to the distributions indicate how the interdomain distances were measured.
LFA-1 exists in different conformational states that reflect its degree of binding affinity to the ligand (28). Earlier reports related the state of LFA-1 activation to raft association as the extended, high affinity form of the integrin was seen to preferentially colocalize with rafts (16). Furthermore, we previously reported that on quiescent monocytes, 25–30% of the LFA-1 nanoclusters contained primed LFA-1 molecules expressing the specific activation-dependent L16 epitope (18). Thus, we hypothesized that the spatial proximity between LFA-1 and GPI-APs could be related to this fraction of ligand-independent primed nanoclusters and expected a massive increase in the occurrence of proximal events when simultaneously imaging L16+-LFA-1 and GPI-APs. In contrast to our expectations, no significant proximity between L16+-LFA-1 and GPI-APs was observed (see Fig. S7 A and B), indicating that the active fraction of LFA-1 bears no specific relation with rafts. As additional control, we visualized the total population of LFA-1 in relation to GPI-APs using the alternative NKI-15 Ab against LFA-1. Similar proximity values as to the ones using TS2/4 were obtained (Fig. S7 C and D). Altogether, these results imply that LFA-1 is spatially segregated but proximal to GPI-APs irrespective of its conformational state.
LFA-1 Nanoclusters Reside Proximal to GPI-AP Hotspots.
Considering that monomers and nanodomains of GPI-APs distribute differently on the cell membrane, we enquired on the correlation between the distinct GPI-AP organization patterns and LFA-1 nanoclusters. In here, nearest inter-domain distance analysis was determined from monomeric (nanodomains of) GPI-APs to their closest LFA-1 nanocluster, and the data contrasted with simulations according to a Poisson process. We found that the overall organization of monomeric GPI-APs is largely uncorrelated with respect to that of LFA-1 nanoclusters (Fig. 3C). In strong contrast, a large pool of GPI-AP nanodomains (≈50%) resides in close proximity; that is, <200 nm, to LFA-1 nanoclusters (Fig. 3D). These striking results show that a significant population of LFA-1 nanoclusters, irrespective of their conformational state, resides close to GPI-AP hotspots. Together, these data demonstrate the existence of well-defined ligand-independent supramolecular platforms composed by distinct entities on the plasma membrane.
LFA-1 and GPI-AP Reorganization upon Ligand Binding Activation.
To investigate the behavior of these preassemblies upon integrin-ligation, we allowed LFA-1 on monocytes to interact with ICAM-1 nanoaggregates and subsequently imaged ICAM-1 and GPI-APs simultaneously. Importantly, the use of ICAM-1 nanoaggregates allowed us to follow the very initial stages of integrin-mediated adhesion (see Fig. S8C). Colocalization between ICAM-1 and GPI-APs is clearly apparent on the confocal image (Fig. 4A). This association is specifically mediated by LFA-1 since binding of ICAM-1 in the presence of the LFA-1 blocking Ab L15 is completely abolished (Fig. S8 A and B). NSOM imaging confirmed the increased colocalization between ICAM-1 and GPI-APs (Fig. 4B), with C = 0.32 ± 0.14. Strikingly, a pronounced switch in the organization of GPI-APs was found upon LFA-1 binding to ICAM-1 nanoaggregates. Indeed, the percentage of GPI-AP nanodomains increased from approximately 30% in the unbound state to approximately 80% in the ligand-bound state of LFA-1 (Fig. 4C and Fig. S8D). These results demonstrate that ligand-binding of LFA-1 induces nanoscale changes of GPI-APs on the cell membrane.
Fig. 4.
Activation of LFA-1 by ICAM-1 nanoaggregates drives the conversion of GPI-APs from monomers to nanodomains, and formation of nascent adhesion sites. (A) Confocal image shows GPI-APs (green) and ICAM-1 nanoaggregates (red) binding to the cell membrane. (B) Dual color NSOM image on a selected region of the membrane. (C) Monomer and nanodomain percentages of GPI-APs in resting cells and upon LFA-1 activation (intensity distributions in Fig. S8D). (D) Surface correlation plot between the number of GPI-APs per fluorescent spot (x-axis) and its proximal ICAM-1 nanoaggregate (y-axis). Color code reflects the number of occurrences: blue (low) to red (high) over 34 occurrences (see SI Text).
To further relate this change of GPI-AP architecture to LFA-1 ligand-activation, we separated the GPI-AP fluorescent spots in two different subpopulations according to their spatial proximity to ICAM-1 binding sites. We then specifically focused on the GPI-AP subpopulation proximal (<200 nm) to ICAM-1 nanoaggregates and determined the number of GPI-AP molecules in each of those selected spots as well as the number of ICAM-1 nanoggregates per spot. Fig. 4D shows the correlation between the number of GPI-APs per spot (x axis) and its associated proximal ICAM-1 spot (y axis). The values at both axes correspond to the number of molecules per spot, that is, number of GPI-APs as x axis and number of ICAM-1 nanoaggregates as y axis, as extracted from the intensity distributions of GPI-APs and ICAM-1 nanoaggregates respectively (Fig. S8 C and D and SI Text). Dimers to tetramers of GPI-APs showed the highest correlation to ICAM-1 nanoaggregates, as evidenced by the large number of correlated events in the yellow-red-color coded region of Fig. 4D, while monomeric GPI-APs did not show significant correlation to ICAM-1 (blue-color coded region in Fig. 4D). These results indicate that LFA-1 activation by its ligand drives the interconversion of GPI-APs from monomers to nanodomains leading to the formation of localized adhesion platforms at the cell surface. Consistent with this, the subpopulation of GPI-AP spots unrelated to ICAM-1 (that is, further away >500 nm) exhibited an intensity distribution that effectively corresponded to that of monomers (Fig. S8E).
ICAM-1 Micrometer-Sized Patterns Promote the Formation of LFA-1 Microclusters and Co-Recruitment of GPI-APs.
We finally wished to follow the formation of stable adhesion platforms upon LFA-1 binding to ICAM-1. For this, dense ICAM-1 pattern surfaces (5-μm squares) surrounded by BSA regions were prepared using microcontact printing [see SI Text and (29)]. L16+-LFA-1 labeled monocytes were allowed to adhere to the patterns and after fixation GPI-APs were fluorescently labeled with FLAER. Clear preferential accumulation of both LFA-1 and GPI-APs occurred on the ICAM-1-rich areas (square patterns) as compared to the BSA regions (Fig. 5 A and B) consistent with the formation of stable and large mixed microclusters composed by LFA-1 and GPI-APs.
Fig. 5.
Functional cell adhesion and LFA-1:GPI-APs nanoassembly are affected by cholesterol depletion. (A) Representative confocal image of an individual cell seeded on ICAM-1/BSA patterns (LFA-1:red, GPI-AP:green). ICAM-1 regions are enriched by LFA-1 microclusters and GPI-APs (yellow patches). (B) Fluorescence accumulation index (ratio between the average intensity per pixel on the ICAM-1 areas and that of the ligand-free areas) for six different cells. For both LFA-1 and GPI-APs, the ratio is >1 indicating accumulation on ICAM-1 areas. (C) ICAM-1 bead binding essay for MCD-treated cells. (D and E) Representative dual-color NSOM images of MCD treated cells in the resting sate. (Scale bar, 1 μm.) (F) Nearest inter-domain distance distributions of LFA-1 to its closest GPI-AP for 220 LFA-1 spots (bars) together with simulations of random spatial distribution of the two proteins (red). Experimental and simulation distributions are significantly similar with P = 0.19.
To assess the functional significance of our findings we treated the cells with the raft-disturbing MCD agent (SI Text). Cholesterol extraction by MCD significantly affected the binding of these cells to fluorescent ICAM-1 beads, indicating that raft disruption perturbs LFA-1 mediated adhesion (Fig. 5C). Furthermore, we treated the cells at the steady state with MCD and analyzed by NSOM the reciprocal distribution of LFA-1 and GPI-APs (Fig. 5 D–F). As expected, the interdomain distances between LFA-1 and GPI-APs follow now a random distribution, in strong contrast to the proximity pattern observed on non-treated cells (Fig. 3B). Together, these results indicate that preassembled hotspots of LFA-1 and GPI-APs in the resting state are a prerequisite for LFA-1 mediated cell adhesion.
General Model to Account for the Spatio-Functional Relationship Between LFA-1 and GPI-APs.
Our results reveal distinct but proximal compartmentalization of functionally different protein receptors at the nanometer scale. In absence of ligand, a significant fraction of LFA-1 and GPI-APs reside in separate distinct but physically proximal nanodomains (hotspots) that upon LFA-1 ligand binding interlock forming larger functional supramolecular platforms facilitating cell adhesion. This well-defined architecture in distinct but proximal nanodomains may constitute a generic mechanism exploited by the cell to rapidly and efficiently aggregate and/or segregate distinct nanodomains into larger functional cell surface assemblies when required.
The hotspots regions, enriched by GPI-AP nanodomains and LFA-1 nanoclusters as observed on the cell membrane in the absence of ligand, are likely the result of interactions with the cytoskeleton via association of cortical actin and GPI-APs (6, 26). An activated rich cytoskeleton mesh brought about by affinity regulated outside-in signaling of LFA-1 upon ligand-induced activation (28, 30) will then assist on the interlocking of integrins and GPI-APs resulting in the formation of functional adhesion platforms. Once this nascent adhesion is generated, pools of recruited high-mobility integrins will further facilitate the formation of micrometer-sized clusters of LFA-1, leading to stable cell adhesion (Fig. 6). Indeed, when cells are seeded on ICAM-1 patterned surfaces (5 μm in size) microclustering of LFA-1 is observed together with a clear co-recruitment of GPI-APs (Fig. 5 A and B). Consistent with this model, we recently demonstrated that a large population of LFA-1 nanoclusters diffuses freely on the cell membrane in the absence of the ligand, without interaction with the cytoskeleton (29), neither association with talin (18). Moreover, using single molecule tracking and directional motion analysis, we observed that recruitment of the mobile LFA-1 nanoclusters to the enriched ligand regions proceeds by random diffusion (29). Once engaged to its ligand, complete immobilization of LFA-1 occurs (31) and colocalization with talin is then observed (18) consistent with a great degree of cytoskeletal association (31, 32).
Fig. 6.
Different stages leading to efficient integrin-mediated cell adhesion. (Left) In quiescent cells, LFA-1 and GPI-APs preorganize in separate by proximal nanocompartments forming hotspots sites on the cell surface. (Center) Subsequent activation of LFA-1 by ligand binding drives the formation of larger supramolecular platforms that serve as nucleation sites for nascent cell adhesion. (Right) LFA-1 and GPI-AP microclustering brought about by recruitment of pools of mobile LFA-1 to the nucleation sites result in firm cell adhesion.
The application of NSOM as shown in here underscores its distinctive potential for the study of cellular membrane processes demanding quantitative nanoscale imaging and simultaneous multicolor detection with equal spatial resolution and position accuracy for all colors. We directly visualize the existence of nanoscale hotspot regions of the cell membrane that function as nucleation sites for cell adhesion. As such, our data uncover a mechanism that involves crucial intermediates in the process leading to effective cell adhesion. The formation of these specific sites enriched with different receptors represents a unique prototype for inter-receptor assembly at the nanoscale. This far-from-arbitrary spatial orchestration likely corresponds to a general mechanism to offer cells with privileged areas for instantaneous cell binding and rapid responses to the outside world. This particular arrangement might also regulate cell adhesion mediated by other integrin receptors whose interaction with rafts has been documented.
Materials and Methods
Cell Culture.
The monocytic THP-1 cell line was cultured in RPMI 1640 Dutch modification medium supplemented with 10% fetal calf serum and antibiotic-antimycotic from Gibco.
Monoclonal Antibody Labeling.
LFA-1 was labeled with 10 μg/mL mAb TS2/4 (neutral monoclonal Ab), mAb NKI-L15 (blocking monoclonal Ab), or NKI-L16 [Ca2+-dependent epitope recognizing the extended form of LFA-1 (18)]. CD71 was labeled with the anti-CD71 Ab (10 μg/mL) and GPI-APs with 100 nM Alexa-488 conjugated to pro-aerolysin (FLAER). Details on cell labeling procedures are further described in SI Text and Figs. S4 and S9.
Activation of LFA-1 by ICAM-1 Nanoaggregates and Micrometer-Sized Patterns.
Nano-aggregates were prepared using ICAM-1-Fc chimeras crosslinked with polyclonal rabbit-anti-ICAM-1. Patterns were fabricated using microcontact printing (29). Further details are found in SI Text.
Near-Field Scanning Optical Microscopy.
Experiments were performed on a home built confocal/NSOM microscope optimized for dual-color single molecule fluorescence excitation/detection. Home-made NSOM probes had apertures of 80–100 nm in diameter. Routine operation of NSOM in liquid was achieved using a “diving bell” concept (20). Cells were first imaged in confocal mode. A selected area of the cell surface was further inspected by confocal and then the excitation light was focused onto the back-end of the NSOM probe. Only well-stretched areas of the cell membrane were inspected by NSOM to exclude potential height artifacts on the fluorescence image (see SI Text). Typically, three to six images from different cells were taken at each experimental condition.
Image Analysis.
Unprocessed images were analyzed using custom-made software. Spot sizes were determined using the full-width-at-half-maximum (FWHM) of a 2-D Gaussian fit to the intensity profile. Brightness of each spot (kcounts/s) was defined as the background-corrected average over all pixels within the FWHM. The (x,y) coordinate of each individual spot was determined using the center of mass of the FWHM, with a localization accuracy of σ approximately ω/(S/B) where ω is the width of the Gaussian fit to the intensity profile and S/B is the signal-to-background of individual spots (33). In our case, the localization accuracy varied from approximately 3 nm for CD71, to approximately 4 nm for LFA-1 and approximately 7 nm for GPI-AP, depending on the S/B ratio of individual images.
Nearest Inter-Domain Distance Distribution and Simulations.
The nearest inter-domain distances were determined by measuring the shortest distance from the (x,y) coordinate of a protein to the (x,y) coordinate of the nearest GPI-AP. The statistical significance for the different distributions is given in SI Text. Simulations of random organization between different components were performed by generating images containing particles located at random positions, with all of the parameters adjusted identical to the experimental data (Fig. S6). Subsequently, each two sets of simulated images were overlaid and the inter-domain distances were measured as described above.
Supplementary Material
Acknowledgments.
We thank J. Korterik, C. Manzo, M.J. Lopez-Bosque, and R. Diez-Ahedo for technical assistance and O. Esteban and N.F. van Hulst for useful discussions. This work was supported by grants from the Stichting voor Fundamenteel Onderzoek der Materie (to M.K.), Netherlands Organization for Scientific Research Veni Grant 916.66.028, the Human Frontier Science Program (to A.C.), EC-RTN-IMMUNANOMAP and EC-NEST-BIO-LIGHT-TOUCH (to M.F.G.-P. and T.S.v.Z.), and Netherlands Organization for Scientific Research TOP Grant 9120.6030 (to C.G.F.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0905217106/DCSupplemental.
References
- 1.Jacobson K, Sheets ED, Simson R. Revisiting the fluid mosaic model of membranes. Science. 1995;268:1441–1442. doi: 10.1126/science.7770769. [DOI] [PubMed] [Google Scholar]
- 2.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 3.Kusumi A, et al. Paradigm shift of the plasma membrane concept from the two-dimensional comtinuum fluid to the partitioned fluid: High-speed single-molecule tracking of membrane molecules. Annu Rev Biophys Biomol Struct. 2005;34:351–378. doi: 10.1146/annurev.biophys.34.040204.144637. [DOI] [PubMed] [Google Scholar]
- 4.Douglass AD, Vale RD. Single-molecule microscopy reveals plasma membrane microdomains created by protein-protein networks that exclude or trap signaling molecules in T cells. Cell. 2005;121:937–950. doi: 10.1016/j.cell.2005.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobson K, Mouritsen OG, Anderson RGW. Lipid rafts: At a crossroad between cell biology and physics. Nat Cell Biol. 2007;9:7–14. doi: 10.1038/ncb0107-7. [DOI] [PubMed] [Google Scholar]
- 6.Viola A, Gupta N. Tether and trap: Regulation of membrane-raft dynamics by actin-binding proteins. Nat Rev Immunol. 2007;7:889–896. doi: 10.1038/nri2193. [DOI] [PubMed] [Google Scholar]
- 7.Shaw AS. Lipid rafts: Now you see them, now you don't. Nat Immunol. 2006;7:1139–1142. doi: 10.1038/ni1405. [DOI] [PubMed] [Google Scholar]
- 8.Sharma P, et al. Nanoscale organization of multiple GPI-anchored proteins in living cell membranes. Cell. 2004;116:577–589. doi: 10.1016/s0092-8674(04)00167-9. [DOI] [PubMed] [Google Scholar]
- 9.Marguet D, Lenne PF, Rigneault H, He HT. Dynamics in the plasma membrane: How to combine fluidity and order. EMBO J. 2006;15:3446–3457. doi: 10.1038/sj.emboj.7601204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caiolfa VR, et al. Monomer-dimer dynamics and distribution of GPI-anchored uPAR are determined by cell surface assemblies. J Cell Bio. 2008;179:1067–1082. doi: 10.1083/jcb.200702151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lasserre R, et al. Raft nanodomains contribute to Akt/PKB plasma membrane recruitment and activation. Nat Chem Bio. 2008;4:538–547. doi: 10.1038/nchembio.103. [DOI] [PubMed] [Google Scholar]
- 12.Eggeling C, et al. Direct observation of the nanoscale dynamics of membrane lipids in a living cell. Nature. 2009;457:1159–1163. doi: 10.1038/nature07596. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki KG, et al. GPI-anchored receptor clusters transiently recruit Lyn and G for temporary cluster immobilization and Lyn activation: Single-molecule tracking study 1. J Cell Biol. 2007;177:717–730. doi: 10.1083/jcb.200609174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.del Pozo MA, et al. Integrins regulate Rac targeting by internalization of membrane domains. Science. 2004;303:839–842. doi: 10.1126/science.1092571. [DOI] [PubMed] [Google Scholar]
- 15.Krauss K, Altevogt P. Integrin leukocyte function-associated antigen-1-mediated cell binding can be activated by clustering of membrane rafts. J Biol Chem. 1999;274:36921–36927. doi: 10.1074/jbc.274.52.36921. [DOI] [PubMed] [Google Scholar]
- 16.Leitinger B, Hogg N. The involvement of lipid rafts in the regulation of integrin function. J Cell Sci. 2002;115:963–972. doi: 10.1242/jcs.115.5.963. [DOI] [PubMed] [Google Scholar]
- 17.Fabbri M, et al. Dynamic partitioning into lipid rafts controls the endo-exocytic cycle of the alphaL/beta2 integrin, LFA-1, during leukocyte chemotaxis. Mol Biol Cell. 2005;16:5793–5803. doi: 10.1091/mbc.E05-05-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cambi A, et al. Organization of the integrin LFA-1 in nanoclusters regulates its activity. Mol Bio Cell. 2006;17:4270–4281. doi: 10.1091/mbc.E05-12-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dustin ML, Springer TA. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989;341:619–624. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- 20.Koopman M, et al. Near-field scanning optical microscopy in liquid for high resolution single molecule detection on dendritic cells. FEBS Lett. 2004;573:6–10. doi: 10.1016/j.febslet.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 21.de Lange F, et al. Cell biology beyond the diffraction limit: Near-field scanning optical microscopy. J Cell Sci. 2001;114:4153–4160. doi: 10.1242/jcs.114.23.4153. [DOI] [PubMed] [Google Scholar]
- 22.Ianoul A, et al. Imaging nanometer domains of β-adrenergic receptor complexes on the surface of cardiac myocytes. Nat Chem Biol. 2005;1:196–202. doi: 10.1038/nchembio726. [DOI] [PubMed] [Google Scholar]
- 23.de Bakker BI, et al. Nanoscale organization of the pathogen receptor DC-SIGN mapped by single-molecule high-resolution fluorescence microscopy. ChemPhys Chem. 2007;8:1473–1480. doi: 10.1002/cphc.200700169. [DOI] [PubMed] [Google Scholar]
- 24.de Bakker BI, et al. Nanometer scale organization of the alpha subunits of interleukin-2 and -15 receptors on Kit 225 FT7.10 human T lymphoma cells. J Cell Sci. 2008;121:627–633. doi: 10.1242/jcs.019513. [DOI] [PubMed] [Google Scholar]
- 25.Hell SW. Far-field optical nanoscopy. Science. 2007;316:1153–1158. doi: 10.1126/science.1137395. [DOI] [PubMed] [Google Scholar]
- 26.Goswami D, et al. Nanoclusters of GPI-anchored proteins are formed by cortical actin-driven activity. Cell. 2008;135:1085–1097. doi: 10.1016/j.cell.2008.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madore N, et al. Functionally different GPI proteins are organized in different domains on the neuronal surface. EMBO J. 1999;18:6917–6926. doi: 10.1093/emboj/18.24.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu Rev Immunol. 2007;25:619–647. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diez-Ahedo R, et al. Dynamic re-organization of individual adhesion nanoclusters in living cells by ligand patterned surfaces. Small. 2009;5:1258–1263. doi: 10.1002/smll.200801699. [DOI] [PubMed] [Google Scholar]
- 30.Alon R, Dustin ML. Force as a facilitator of integrin conformational changes during leukocyte arrest on blood vessels and antigen-presenting cells. Immunity. 2007;26:17–27. doi: 10.1016/j.immuni.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Cairo CW, Mirchev R, Golan DE. Cytoskeletal regulation couples LFA-1 conformational changes to receptor lateral mobility and clustering. Immunity. 2006;25:297–308. doi: 10.1016/j.immuni.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 32.Shamri R, et al. Lymphocyte arrest requires instantaneous induction of an extended LFA-1 conformation mediated by endothelium-bound chemokines. Nat Immunol. 2005;6:497–506. doi: 10.1038/ni1194. [DOI] [PubMed] [Google Scholar]
- 33.Thompson RE, et al. Precise nanometer localization analysis for individual fluorescence probes. Biophys J. 2002;82:2775–2783. doi: 10.1016/S0006-3495(02)75618-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.