Abstract
Objective
Melanoma-associated antigen gene B2 (MAGE-B2) encodes an embryonic antigen normally silenced after birth except in testis and placenta. We identified the MAGE-B2 gene and autoantibodies in pediatric patients with systemic lupus erythematosus (SLE) glomerulonephritis. Our purpose herein was to determine the prevalence of MAGE-B2 autoantibodies in association with active SLE, as well as to infer a pathogenetic role of MAGE-B2 protein through its distribution in cells and tissues.
Methods
A cross-sectional study analyzed the frequency of MAGE-B2 autoantibodies in 40 pediatric SLE patients, 23 adult controls, and 16 pediatric juvenile rheumatoid arthritis (JRA) patients using Western blots containing recombinant MAGE-B2. SLE disease activity index 2000 (SLEDAI-2K) and British Isles Lupus Assessment Group (BILAG) index measured SLE disease activity. Tissue distribution of MAGE-B2 protein was also assessed by immunohistochemistry, immunofluorescence, and Western blots.
Results
Seventeen (43%) of 40 pediatric SLE patients had MAGE-B2 autoantibodies as compared to 0 of 16 JRA patients and 2 of 23 adult controls. SLE disease activity was significantly higher in MAGE-B2 autoantibody-positive vs. autoantibody-negative patients (SLEDAI-2K: mean 10.9 vs. 5.2, p=0.013; BILAG: mean 15.3 vs. 6.3, p=0.023). Active nephritis was more prevalent (60% vs. 24%) in MAGE-B2 autoantibody-positive SLE patients. MAGE-B2 protein was visualized in SLE kidney proximal convoluted tubules and in tumor epithelial cells, but not in lymphoblastoid cells.
Conclusion
MAGE-B2 autoantibody appears to be a clinically relevant biomarker for pediatric SLE disease activity and nephritis.
Key Index Terms: Systemic lupus erythematosus, MAGE-B2, autoantibody, disease biomarker, glomerulonephritis, pediatric
Introduction
Systemic lupus erythematosus (SLE) is a life-long autoimmune illness that can potentially affect every organ in the body. The disease course is one of intermittent remissions and exacerbations, with exacerbations often precipitated by ultraviolet radiation, infections, or drugs.1 Genetic and environmental components contribute to the SLE disease process, but its etiology remains elusive despite over 50 years of intensive research. Current pathophysiologic models suggest that cryptic antigen expression may be induced after an initial triggering event, causing a downstream cascade of antigen recognition, activation of the innate and adaptive immune systems, autoantibody production, chronic inflammation, and organ damage.2,3
Our group was the first to report melanoma-associated antigen gene B2 (MAGE-B2) autoantibodies in patients with SLE.4 In search of autoantigens that might provoke an autoimmune response, MAGE-B2 (National Center for Biotechnology Information accession number NM_002364) was cloned from a human epithelioma cell line (HEp-2) protein expression library, using uncharacterized serum autoantibodies from two pediatric patients with SLE.4,5 These patients had Class IV glomerulonephritis according to the World Health Organization (WHO) classification,6 high anti-nuclear antibody titers, and high double-stranded deoxyribonucleic acid (dsDNA) antibody titers. They did not have a prior diagnosis of malignancy and have remained cancer-free for the past 10 years.
The large MAGE gene family is categorized alphabetically (A through L), with the majority clustering on the X chromosome.7 MAGE-A1 was the first MAGE antigen described, notably for its ability to activate cytotoxic T lymphocytes in the context of major histocompatibility complex (MHC) presentation.8 Other MAGE relatives, including MAGE-B2, were later identified by their sequence homology and intronless open reading frame to the MAGE A genes.9–11 The MAGE A, B, and C families belong to a larger cancer-testis gene family that has characteristic expression in normal testis and in various cancers such as melanoma, non-small cell lung carcinoma, ductal breast carcinoma, and testicular carcinoma.11,12 MAGE antigens are expressed in developing fetal ovaries and normal placenta, and may have important roles in embryogenesis and gametogenesis.11–16 The MAGE-B2 gene, located on the short arm of the X-chromosome, has 4 exons with the single open reading frame in exon 4.4,7,11 The MAGE-B2 protein has 319 amino acids and a molecular weight of 35 kDa.4 The biologic function of MAGE-B2 is still unknown.15
The discovery of MAGE-B2 autoantibodies in pediatric SLE patients prompted us to perform a cross-sectional study to ascertain the prevalence and clinical relevance of this autoantibody in a pediatric SLE cohort. We determined for the first time that an association exists between the presence of MAGE-B2 autoantibodies and SLE disease activity and nephritis.
PATIENTS AND METHODS
Patients
Forty pediatric patients with SLE were enrolled into the study from the outpatient clinics and inpatient wards of the Children’s Hospital of Orange County (CHOC) and the Mattel Children’s Hospital at the University of California, Los Angeles (UCLA) between January 2002 and February 2007. Inclusion criterion included the diagnosis of SLE by the presence of 4 out of 11 clinical and laboratory criteria as defined in 1997 by the American College of Rheumatology (ACR).17 Patients with SLE who had undergone renal transplantation were excluded from the study as their renal function and urine studies more likely reflected transplant-related changes rather than SLE-induced changes and their immunosuppressive regimens differed from those of SLE patients without transplant. Clinical data (age, gender, ethnicity, date of diagnosis, history, physical examination, medications, laboratory results, and radiographic reports) were collected prospectively at UCLA and retrospectively at CHOC.
Sixteen pediatric patients meeting the 1986 ACR diagnostic criteria for juvenile rheumatoid arthritis (JRA)18 were enrolled into the autoimmune disease control group from CHOC between April 2003 and February 2005. Due to difficulties in the recruitment of age-matched healthy pediatric controls, 23 adult volunteer controls were enrolled from both institutions between June 2004 and December 2006. These volunteers self-reported normal health without autoimmune disease or malignancy; their medical records were not obtained. All subjects and/or their respective parents or guardians gave written informed consent and/or assent (for children between the ages of 7 to 13 years of age) approved by the UCLA Institutional Review Board and the CHOC Institutional Review Board. This study was performed in accordance with the Declaration of Helsinki guidelines.19
Disease activity measurement
Disease activity was measured by the SLE Disease Activity Index 2000 (SLEDAI-2K)and the British Isles Lupus Assessment Group (BILAG) index (version 3).20,21 BILAG index “A” received 9 points, “B” received 4 points, “C” received 1 point, and “D” and “E” received 0 points. Disease activity measurements were performed by one investigator (ADCH) and confirmed by a second blinded investigator (DKM). Renal SLEDAI scores encompassed the 4 renal categories in the SLEDAI-2K: hematuria (>5 red blood cells/high power field), pyuria (>5 white blood cells/high power field), urinary casts (heme-granular or red blood cell casts), and proteinuria (24-hour urine protein >0.5 grams). “Active nephritis” was described in patients with proteinuria (as defined by the Renal SLEDAI criteria or by the random urine protein to creatinine ratio of >0.5), and/or any of the other Renal SLEDAI categories.22 The “all nephritis” category included all patients with past or current SLE glomerulonephritis, diagnosed either clinically or by renal biopsy. The total number of patients whose data were available for disease activity analysis was 32 (n=15 in the MAGE-B2 autoantibody-positive group, n=17 in the MAGE-B2 autoantibody-negative group).
Since complement levels and anti-dsDNA antibody titers are often used clinically to monitor SLE disease activity, MAGE-B2 autoantibody status was compared to anti-dsDNA antibody titers and the presence of low complement levels. Patients were stratified into high-titer or low-titer dsDNA antibody groups. High-titer anti-dsDNA antibody was defined as an indirect immunofluorescence titer of ≥ 1:320 on Crithidia luciliae substrate or an enzyme immunoassay value 3-fold greater than the established laboratory negative cut-off value. Patients with decreased complement values in C3, C4, or both, were placed in the “low complement” group. Possible correlations of MAGE-B2 autoantibodies with other SLE-associated autoantibodies (i.e. Smith, ribonucleoprotein, Ro, La, cardiolipin, scleroderma-70, ribosomal P, histone, and anti-neutrophil cytoplasmic antibodies) were also analyzed.
Recombinant MAGE-B2 protein synthesis
PBluescript plasmids containing MAGE-B2 complementary DNA (cDNA) were extracted from a HEp-2 cDNA expression library using the ZAP-II lambda phage system, per the manufacturer’s protocol (Stratagene, La Jolla, CA).4 MAGE-B2 cDNA was excised from the PBluescript plasmid with Xho I and BAM HI restriction enzymes (New England Biolabs, Ipswich, MA) and ligated into a glutathione S-transferase (GST)-containing vector, PGEX 6p-1 (Sigma-Aldrich, St. Louis, MO). The PGEX-MAGE-B2 vector was transformed into BL21-DE3 Escherichia Coli (Invitrogen, Carlsbad, CA) and the MAGE-B2-GST fusion protein was obtained after isopropyl-beta-D-thiogalactopyranoside induction, sonication, and GST column extraction (General Electric Healthcare, Piscataway, NJ). GST tags were excised from MAGE-B2 proteins with thrombin (General Electric Healthcare).
MAGE-B2 autoantibody detection via Western Blot
Four milliliters of blood were collected from each patient, and plasma was separated and frozen (−80°C) in aliquots. Prior to use on immunoblots, patient plasma was pre-absorbed with E. coli lysate (Stratagene) to minimize non-specific binding to recombinant MAGE-B2 proteins synthesized in an E. coli system. We followed an immunoblot protocol previously established in our lab using 7.5% polyacrylamide gel electrophoresis and 0.4 micrograms (μg) of recombinant MAGE-B2 protein per lane.23 Each polyvinylidene difluoride (PVDF, Bio-Rad, Hercules, CA) membrane strip containing a single lane of recombinant MAGE-B2 was incubated with a pre-absorbed plasma sample (1:250 dilution),24 followed by incubation with horseradish peroxidase-conjugated anti-human IgG secondary antibody (1:100,000 dilution, Sigma-Aldrich). “Positivity” was defined by visual inspection for a single band at approximately 36 kDa. PVDF membranes were re-used for screening after efficient stripping (30 minutes at 65°C in 20% sodium dodecyl sulfate/7.8%β-mercaptoethanol) was confirmed by a 1–2 hour film exposure following secondary antibody incubation and enhanced chemiluminescence (General Electric). PVDF membranes were probed with commercial MAGE-B2 antibody after the 5th and 11th strippings and demonstrated MAGE-B2 protein immunoreactivity. PVDF membranes were not used after the 11th stripping.
Specificity of commercial MAGE-B2 antibody and MAGE-B2 autoantibodies
Commercial goat polyclonal anti-MAGE-B2 antibody (1:500 dilution, Santa Cruz Biotechnology, Santa Cruz, CA) served as a positive control on immunoblots loaded with recombinant MAGE-B2 proteins (0.4μg). To establish binding specificity, the commercial MAGE-B2 antibody was absorbed at 4°C with blocking peptide (Santa Cruz) at 50 times the molar concentration of the antibody. The commercial antibody was also absorbed with our recombinant MAGE-B2 protein. In parallel experiments, recombinant MAGE-B2 protein was used to absorb patient autoantibodies. All absorbed antibodies were then tested on immunoblots.
Subcellular MAGE-B2 expression
Using the NE-PER kit (Pierce, Rockford, IL) for subcellular fractionation, nuclear and cytosolic lysates (25μg each) from HEp-2, a SLE lymphoblastoid cell line (LCL), and a normal LCL, along with recombinant MAGE-B2 protein (0.9μg), were analyzed by Western blot using commercial MAGE-B2 antibody (1:500, Santa Cruz). Subcellular fractionation controls included Lamin A&C antibodies (1:500, catalog number ab58529, Abcam, Cambridge, MA) as a nuclear marker, and heat shock protein-90 (HSP-90) antibody (1:30,000, Novus Biologicals, Littleton, CO) as a cytosolic marker.
MAGE-B2 indirect immunofluorescence
HEp-2 cells and ductal epithelial breast cancer cells (MDA-231, gift from John Colicelli, Ph.D.) were sequentially incubated with primary and secondary antibodies at 37°C prior to fixation, based on a cell surface indirect immunofluorescence (IF) protocol by Fujimoto.25 Primary antibodies included polyclonal goat IgG anti-MAGE-B2 (4μg/mL, Santa Cruz) and monoclonal mouse IgG2a anti-HLA ABC antibodies (10μg/mL, Abcam). Secondary antibodies included fluorescein isothiocyanate (FITC) conjugated anti-goat IgG (1:125, Sigma-Aldrich) and tetramethyl rhodamine isothiocyanate (TRITC) conjugated anti-mouse IgG (1:100, Sigma-Aldrich). Normal goat and mouse IgG controls (goat IgG, 4μg/mL, and mouse IgG, 10μg/mL, Santa Cruz) were sequentially incubated at identical conditions as above, followed by respective secondary antibodies. Nuclei were stained blue with 4′6-diamidino 2-phenylindole (DAPI) after fixation with 4% paraformaldehyde. For intracellular indirect IF, fixed HEp-2 cells were permeabilized with 0.5% Triton X-100 before proceeding with the above protocol.
MAGE-B2 Immunohistochemistry
Samples of core renal biopsies obtained from SLE patients with WHO Class IV or Class V glomerulonephritis6 (6 each) and a histologically normal region of resected kidney from a patient with renal cell carcinoma were obtained anonymously from the UCLA Tissue Procurement Core Laboratory. Immunohistochemistry was performed after incubating slides at 95°C in 10 mM sodium citrate (pH 6.0) for 25 minutes, using commercial MAGE-B2 antibodies (1μg/mL, Santa Cruz) or goat IgG control (1μg/mL, Invitrogen), Vectastain ABC Kit (Vector Laboratories, Burlingame, CA), and diaminobenzidine (Vector Laboratories) according to the manufacture’s protocol. Digital photographs of immunohistochemistry and immunofluorescence slides were taken with an Axiocam camera on an Axioskop 2 microscope, using AxioVision software (Carl Zeiss Microimaging, Inc., Thornwood, NY).
Statistical Analysis
The p values for comparing population proportions were computed with the Fisher’s exact test. The p values for comparing continuous data with a normal distribution were calculated using the Student’s t test; the p values for comparing continuous data with a skewed distribution were calculated using the Wilcoxon/Kruskal Wallis Rank Sum test. A Spearman rank correlation was computed for assessing the strength of the possibly non-linear association between disease duration and activity. P values <0.05 were considered statistically significant. Computations were made with JMP version 6 (SAS Institute Inc., Cary, NC).
RESULTS
SLE Patients
We tested pre-absorbed plasmas from 40 pediatric patients with SLE enrolled in the cross-sectional study. Using Western blot strips loaded with 0.4μg of recombinant MAGE-B2 protein, we observed single bands at approximately 36 kDa in 17 (43%) samples. We interpreted these bands as MAGE-B2 autoantibodies. Age, gender, and ethnicity were not significantly different between the two groups (Table 1). Female to male ratio was 5.7 to 1 (34 females, 6 males). The ethnic distribution of the cohort was 51% Hispanic, 29% Asian, 12.5% African American, and 7.5% Caucasian. None of the SLE patients had a current or past diagnosis of cancer.
Table 1.
SLE Patient demographics
| MAGE-B2 Ab positive | MAGE-B2 Ab negative | P value* | |
|---|---|---|---|
| (n=17) | (n=23) | ||
| AGE (years) | |||
| Mean ± SD | 14.1 ± 4.2 | 15.7 ± 3.6 | 0.19** |
| Range | 3 – 19 | 9 – 23 | |
| Median | 15 | 15 | |
| Gender (%) | |||
| Male | 17.6 | 4.3 | 0.29 |
| Female | 82.4 | 95.7 | |
| Ethnicity (%) | |||
| Asian | 32.3 | 26.1 | 0.97 |
| African American | 11.8 | 13 | |
| Hispanic | 50 | 52.2 | |
| Caucasian | 5.9 | 8.7 | |
| Disease duration (months) | |||
| Mean ± SD | 31.5 ± 42.3 | 56.7 ± 35.6 | 0.007† |
| Median | 23 | 51 | |
| Biopsy proven nephritis (%) | |||
| Total | 52.9 | 56.5 | 0.57 |
| WHO Class II | 5.9 | 0 | |
| WHO Class III | 5.9 | 8.7 | |
| WHO Class IV | 17.6 | 26.1 | |
| WHO Class V‡ | 23.5 | 21.7 | |
| No nephritis | 35.3 | 39.1 | |
| Medication*** | |||
| Prednisone (mean ± SD mg) | 23.4 ± 15 | 21.8 ± 17.2 | 0.79** |
| Cyclophosphamide (%) | 40 | 35.7 | 1 |
| Mycophenolate Mofetil(%) | 33.3 | 71.4 | 0.07 |
| Azathioprine (%) | 6.7 | 7.1 | 1 |
| Methotrexate (%) | 0 | 14.3 | 0.22 |
| Hydroxychloroquine (%) | 53.3 | 92.9 | 0.04 |
| ACE inhibitor (%) | 46.7 | 57.1 | 0.72 |
| Aspirin (%) | 40 | 28.6 | 0.70 |
| NSAID (%) | 13.3 | 21.4 | 0.65 |
P values calculated using Fisher’s exact test except where noted.
Student’s t-test.
Medication analysis included 29 patients whose medication records were available.
Wilcoxon/Kruskal Wallis Rank sum test.
Includes mixed proliferative and membranous glomerulonephritis
Abbreviations: n= number; SD= standard deviation; Ab= autoantibody, ACE= angiotensin-converting enzyme; NSAID= non-steroidal anti-inflammatory drugs; WHO= World Health Organization.
Disease duration from the date of diagnosis was shorter in the MAGE-B2 antibody-positive SLE patient group as compared with the antibody-negative SLE patient group (Table 1). The mean disease duration was 31.5 months (range 0–171) in the antibody-positive group and 56.7 months (range 2–153) in the antibody-negative group (p=0.007). Seven patients (41%) in the former group had disease duration of less than six months, as compared to one patient (4%) in the latter group (p= 0.048). MAGE-B2 autoantibody was present in 7 of 8 (88%) patients whose SLE disease duration was less than six months, as compared to 10 of 32 (31%) patients whose SLE disease duration was greater than six months (p=0.006).
Because the presence of autoantibodies could be influenced by medications, we compared the therapy received by patients in the cohort. The mean daily dosage of prednisone was similar between groups: 23.4 mg vs. 21.8 mg (Table 1). Angiotensin converting enzyme inhibitor and cyclophosphamide exposure were also similar. There was a trend towards increased mycophenolate mofetil (MMF) treatment in the MAGE-B2 autoantibody-negative subset (p=0.07). Hydroxychloroquine (HCQ) treatment was more frequent in the same subset (p=0.04). The types of nephritis and prevalence of biopsy-proven nephritis were comparable in both SLE patient groups (Table 1).
SLE disease activity and nephritis
SLE disease activity was significantly higher in patients with MAGE-B2 autoantibodies (Figure 1A). The mean SLEDAI-2K score was 10.9 in the antibody-positive group as compared to 5.2 in the antibody-negative group (p=0.013); the mean BILAG score was 15.3 in the antibody-positive group as compared to 6.3 in the antibody-negative group (p=0.023). Renal SLEDAI scores were not significantly different between the patient groups (p=0.21) (Table 2). When the SLEDAI-2K, BILAG, and renal SLEDAI scores were adjusted using urine protein to creatinine ratio >0.5 as a surrogate for a 24-hour urine protein excretion > 0.5 grams, the significance of the p values remained relatively unchanged (p=0.027, p=0.013, and p=0.25, respectively).
Figure 1.
A. Scatter plot showing disease activity distribution of SLE patients with and without the MAGE-B2 autoantibody. The horizontal line in each column depicts the mean value. P values were obtained using Wilcoxon/Kruskal Wallis Rank Sum Test. B. Comparison of active nephritis and all nephritis in patients with and without MAGE-B2 autoantibody. The “active nephritis” category was defined as any positive Renal SLEDAI and/or a urine protein to creatinine ratio > 0.5 or proteinuria >0.5 grams per 24 hours. The “all nephritis” category included patients with past or current lupus nephritis, diagnosed clinically or by renal biopsy. P values were obtained via Fisher’s exact test.
Table 2.
Comparison of SLE disease activity
| MAGE-B2 Ab Positive | MAGE-B2 Ab Negative | P Value* | |
|---|---|---|---|
| (n=15) | (n=17) | ||
| SLEDAI-2K | |||
| Mean ± SD | 10.9 ± 7.5 | 5.2 ± 5.7 | 0.013 |
| Median | 8 | 2 | |
| BILAG | |||
| Mean ± SD | 15.3 ± 13.2 | 6.3 ± 5.5 | 0.023 |
| Median | 13 | 5 | |
| Renal SLEDAI | |||
| Mean ± SD | 2.7 ± 4.5 | 1.2 ± 2.7 | 0.21 |
| Median | 0 | 0 | |
P values were obtained with Wilcoxon/Kruskal Wallis Rank Sum test.
Abbreviations: n= number; SLEDAI-2K= Systemic Lupus Erythematosus Disease Activity Index 2000; BILAG= British Isles Lupus Assessment Group Index; SD= standard deviation; Ab= autoantibody.
Since SLE disease activity is generally higher in newly diagnosed patients, we repeated the analysis above after excluding all patients with disease duration of less than six months. Seven patients from the antibody-positive group and one patient from the antibody-negative group were excluded. The resulting data showed a mean SLEDAI-2K score ± standard deviation of 10.1 ± 8.4 in the antibody-positive group and 5.4 ± 5.9 in the antibody-negative group (p=0.08); mean BILAG score ± standard deviation was 10.9 ± 11.6 in the antibody-positive group and 6.5 ± 5.6 in the antibody-negative group (p=0.42). Spearman’s correlation showed an inverse relationship between SLE disease activity and disease duration, but it did not reach statistical significance: r= −0.24 (p=0.18) and r= − 0.33 (p=0.07) comparing SLE disease duration to SLEDAI-2K and BILAG indices, respectively.
The cohort of pediatric patients with MAGE-B2 autoantibody had a higher prevalence of active lupus nephritis (60%) as compared to those without the antibody (24%) (p= 0.07) (Table 3, Figure 1B). Both patient groups had a high prevalence of glomerulonephritis (71% and 65%, p=1.0), approaching the 75% reported in previous series.1 The proportion of patients with positive anti-dsDNA antibody titers was similar between the two groups: 67% vs. 65% (p=1.0). Sub-analysis showed that 60% of MAGE-B2 antibody-positive patients had high-titer dsDNA antibodies, as compared to 35% of the MAGE-B2 antibody-negative patients (p=0.29). Low complement levels were more prevalent in the MAGE-B2 autoantibody-positive group (60% vs. 29%) (p=0.15). Low complement levels were found in approximately 80% of the patients with high-titer anti-dsDNA antibodies. There was also no serological correlation between MAGE-B2 autoantibodies and other SLE-associated autoantibodies: Smith, ribonucleoprotein, Ro, La, cardiolipin, scleroderma-70, ribosomal P, histone, and anti-neutrophil cytoplasmic antibodies (data not shown).
Table 3.
Comparison of dsDNA Ab titers, hypocomplementemia, and nephritis
| MAGE-B2 Ab Positive | MAGE-B2 Ab Negative | P Value* | |
|---|---|---|---|
| (n=15) | (n=17) | ||
| Active nephritis %(n) | 60 (9) | 24 (4) | 0.07 |
| All nephritis** %(n) | 71 (12) | 65 (15) | 1.0 |
| Anti-dsDNA Ab positive %(n) | 67 (10) | 65 (11) | 1.0 |
| High-titer dsDNA Ab %(n) | 60 (9) | 35 (6) | 0.29 |
| Low Complement % (n) | 60 (9) | 29 (5) | 0.15 |
P values were computed with Fisher’s Exact test.
All nephritis category: n=17 in the MAGE-B2 Ab positive group and n=23 in the MAGE-B2 Ab negative group.
Abbreviations: n= number, dsDNA= double-stranded deoxynucleic acid; Ab=autoantibody.
Plasma samples from JRA patients were included to determine whether MAGE-B2 autoantibodies occur in other autoimmune diseases. Using immunoblot strips containing our recombinant MAGE-B2 proteins, none of the 16 patients with JRA had detectable autoantibody to MAGE-B2, as compared to 43% of SLE patients (p=0.001). MAGE-B2 autoantibodies were present in 2 of the 23 adult controls (p=0.005 vs. SLE patients).
Specificity of commercial MAGE-B2 antibodies and autoantibodies
Using PVDF membrane strips loaded with our recombinant MAGE-B2 protein (0.4μg), the commercial antibody signal was abrogated by absorption with commercial MAGE-B2 blocking peptide (Figure 2A,2B) and with recombinant MAGE-B2 protein (Figure 2C) demonstrating antigen-binding specificity of the commercial anti-MAGE-B2 antibody. Plasma MAGE-B2 autoantibody signal was also blocked by recombinant MAGE-B2 protein (Figure 2D).
Figure 2.
Specificity of MAGE-B2 antibodies using PVDF strips loaded with 0.4μg of recombinant MAGE-B2 protein. A. The commercial MAGE-B2 antibody signal was no longer observed after absorption with blocking peptide (+BP). B. The same PVDF membranes as in A were stripped and re-probed with MAGE-B2 antibody at the same dilution. The weaker signal in − BP membrane in section A most likely reflects the fact that the centrifugation step was omitted in section B immunoblotting. C. The commercial MAGE-B2 signal was blocked by full-length recombinant MAGE-B2 protein (+rMB2). D. A SLE patient’s MAGE-B2 autoantibody signal was blocked after absorption with recombinant MAGE-B2 protein (+rMB2).
MAGE-B2 expression
MAGE-B2 was seen in immunoblots of cytosolic and nuclear HEp-2 lysates (Figure 3). It was appreciated predominantly in the cytosolic compartment, but given the comparative lower beta-actin control expression in the nuclear lysate sample, it is possible that the HEp-2 nuclear MAGE-B2 expression may be similar to that seen in the cytosol. No MAGE-B2 expression was seen in either the SLE or normal LCLs. Nuclear marker lamin A&C demonstrated no nuclear contamination of the cytoplasmic fractions. HSP-90, mainly a cytosolic protein, demonstrated some cytoplasmic contamination of the HEp-2 nuclear preparation, but not of the LCLs.
Figure 3.
Immunoblot loaded with cytosolic (C) and nuclear (N) lysates of HEp-2 cells, SLE lymphoblastoid cell line (LCL), and normal LCL. Recombinant MAGE-B2 (rMAGE-B2) is in the last lane. Note MAGE-B2 expression in both subcellular compartments of HEp-2 cells but not in LCLs. Second panel shows beta-actin antibody loading control. Third panel shows the degree of cross contamination of cytosolic fractions with nuclear protein marker, Lamin A&C. Fourth panel shows the same blot for nuclear fraction contamination, using a cytosolic protein marker, HSP-90.
Surface indirect immunofluorescence of HEp-2 cells showed distinct surface MAGE-B2 expression, which colocalized with class I MHC (Figure 4). MDA231 ductal epithelial cell IF showed similar surface MAGE-B2 expression and colocalization with class I MHC (data not shown). MAGE-B2 was mainly distributed in the HEp-2 cytoplasm on intracellular IF.
Figure 4.
Indirect surface immunofluorescence (IF) of HEp-2 cells shows A. MAGE-B2 stained green with FITC, B. HLA-ABC stained red with TRITC, and C. colocalization of MAGE-B2 and HLA-ABC (yellow) on the cell membrane. D. Goat serum IgG (control for MAGE-B2 antibodies) and E. mouse serum IgG (control for HLA-ABC antibodies) are stained with FITC and TRITC, respectively. F. Overlay of D and E shows no specific staining or colocalization. G. Within HEp-2 cells, MAGE-B2, stained green with FITC, is distributed mainly in the cytoplasm. H. Intracellular IF with goat serum IgG, labeled with FITC, shows minimal staining. Nuclei are stained blue with 4′,6-diamidino-2-phenylindole.
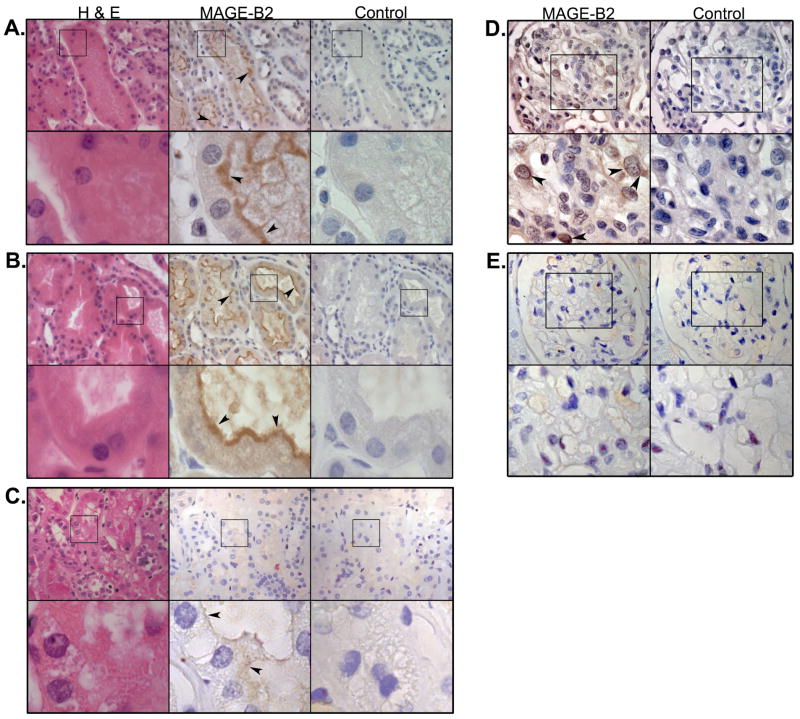
In the kidney, all 6 biopsy samples of Class IV glomerulonephritis and 5 of 6 biopsy samples of Class V glomerulonephritis showed strong MAGE-B2 expression in the brush borders of the proximal convoluted tubules (See Figure 5A, 5B as an example). In contrast, proximal convoluted tubules of normal kidney sections of a renal carcinoma patient showed minimal staining (Figure 5C). There was mild MAGE-B2 staining in the SLE glomerular epithelial (podocyte) cells, with predominant staining of the cytoplasm and nuclear membrane (Figure 5D). Normal glomeruli did not stain for MAGE-B2 (Figure 5E). This provides evidence for MAGE-B2 expression in the kidney tissues and is compatible with the observations in Figure 4 that the protein is strongly expressed on cell surfaces.
Figure 5.
Immunohistochemistry of kidney biopsies from SLE patients with A. WHO Class IV and B. WHO Class V glomerulonephritis (GN), shows strong MAGE-B2 expression (brown staining) in the brush borders of proximal convoluted tubules (arrowheads). C. Biopsy from a normal kidney resection (from a patient with renal cancer) shows minimal staining of the proximal convoluted tubules (arrowheads). For sections A, B, and C: first column, hematoxylin and eosin (H&E) staining; second column, staining with anti-MAGE-B2 antibody (1μg/mL); third column, goat serum IgG control (1μg/mL). Top row: 400× magnification; bottom rows (insets): 1890× manignification. Immunohistochemistry of kidney biopsy from a SLE patient with WHO Class IV nephritis shows D. MAGE-B2 staining in glomerular epithelial nuclear membranes and cytoplasm (arrowheads). E. A normal glomerulus does not show any MAGE-B2 staining. For sections D and E: first column, anti-MAGE-B2 antibody (1μg/mL); second column, goat serum IgG control (1μg/mL). Top row: 630× magnification; bottom row (insets): 1260× magnification.
DISCUSSION
Currently, little is known about MAGE-B2 and its role in SLE. While patients with SLE are at increased risk of hematologic malignancies such as Non-Hodgkin’s lymphoma, melanoma is not a cancer that is typically associated with SLE.26 MAGE-B2, like many of the cancer/testis MAGE antigens, is transiently expressed during embryogenesis and is physiologically suppressed after birth, except in the testes and placenta.9,12,15,27 MAGE-B2 is fully capable of stimulating an immune response when it is presented to both T and B lymphocytes by the MHC.28–31 In non-SLE patients with non-small cell lung carcinoma, MAGE-B2 antibody titers are reported to reflect tumor burden, recurrence, and metastasis.31
Previous reports demonstrated MAGE-B2 expression at the transcriptional level in various cancers, but not protein expression.4,11 We show herein that the MAGE-B2 protein is expressed in four cell lineages: epithelial cancer cells (HEp-2), breast cancer ductal cells (MDA231), SLE kidney proximal convoluted tubules, and SLE kidney glomerular epithelial cells. HEp-2 cells expressed MAGE-B2 proteins mainly in the cytosolic fraction; on the cell surface, a weaker signal of MAGE-B2 protein co-localized with class I MHC molecules. Immunohistochemistry of WHO Class IV and Class V glomerulonephritis biopsies from SLE patients showed strong MAGE-B2 expression in the brush borders of proximal convoluted tubule cells and mild MAGE-B2 staining in glomerular epithelial cells, but not in normal tubules and glomeruli. The pathogenetic role of MAGE-B2 proteins and autoantibodies in the development of lupus nephritis is unknown; however, their presence in patients with lupus nephritis suggests a potential role in immune activation.
Genome-wide methylation abnormalities seen in SLE may explain why MAGE-B2 autoantibodies are found in our patient cohort.32,33 DNA methylation is a tightly regulated mechanism that controls gene transcription.32 Hypomethylation of DNA, invariably found in malignant cells, is thought to be a major contributor of carcinogenesis by activating silenced genes and proteins that disrupt normal cellular differentiation.34 MAGE-B2 mRNA transcription in non-MAGE-B2 expressing cell lines can be induced by chemical demethylation.11 Enhanced T lymphocyte autoreactivity with increased surface expression of B-lymphocyte co-stimulators have been attributed to aberrant methylation patterns in lupus.33,35 Perhaps the combination of dysregulated genomic methylation with presentation of usually hidden antigens activates autoreactive T lymphocytes, and sustains an autoimmune response, inflammation, and downstream pathology in SLE.
In this cross-sectional study, MAGE-B2 autoantibodies occurred in 43% of our pediatric SLE cohort. Both the SLEDAI-2K and BILAG scores were significantly higher in the MAGE-B2 autoantibody-positive patients. A large proportion of patients in this group (60%) also had active SLE nephritis. None of the 16 autoimmune control patients with JRA had MAGE-B2 autoantibodies. Two of 23 adult control patients were positive for this autoantibody. Since all adult control samples were collected from self-reported healthy volunteers without an option for obtaining follow-up medical information, we were unable to ascertain whether these individuals might have undiagnosed autoimmune disease or past/current malignancy.
The loss of statistical significance in the disease activity analysis after excluding patients with disease duration of less than 6 months suggested that the higher disease activity in MAGE-B2 autoantibody-positive patients may reflect the occurrence of the autoantibody early in the course of SLE disease or diagnosis, before adequate control of the disease was achieved with medical therapy. This is supported by the finding that the average SLE disease duration from diagnosis was shorter by 25 months in the antibody-positive group as compared to the antibody-negative group, and that 7 of 8 (88%) patients with disease duration <6 months had MAGE-B2 autoantibodies. MAGE-B2 autoantibody status may be influenced by disease progression and medications, such as HCQ (and possibly MMF). Correlation analysis also demonstrated an inverse relationship between disease activity and duration of illness, though it did not reach statistical significance.
The MAGE-B2 autoantibody-negative subset of patients was more likely to be treated with HCQ and had lower disease activity than the autoantibody-positive patients. While it is possible that HCQ alone may have suppressive effects on MAGE-B2 expression or autoantibody production, it is also possible that the suppression of this autoantibody reflects a positive treatment response or disease remission achieved with a combination of medications (including HCQ). Conversely, the presence of MAGE-B2 autoantibody may signify inadequate disease control or a higher risk of treatment resistance, with corresponding elevated SLE disease activity and active lupus nephritis.
The relatively high plasma dilution of 1:250 used in the Western blot screening was based on a protocol published by Lim et al.24 Since there were no prior data on MAGE-B2 autoantibody titers in SLE, we chose this dilution for our autoantibody screening to minimize the false positive rate (type I error), perhaps leading to a higher false negative rate (type II error). In addition, Western blot is more specific than sensitive, and may underestimate the prevalence of this autoantibody in our pediatric lupus cohort.
This exploratory investigation included a small cohort of pediatric SLE patients, autoimmune disease control patients (JRA), and normal controls. Despite finding statistically significant differences between the MAGE-B2 autoantibody-positive and autoantibody-negative SLE patients, this study did not possess sufficient power to detect small differences between groups. Because our pilot investigation was primarily focused on pediatric-onset SLE patients, we cannot generalize our results to the adult-onset SLE population.
In conclusion, MAGE-B2 autoantibody appears to be a potentially relevant serological biomarker for SLE disease activity in a pediatric cohort. This autoantibody is present relatively early in the SLE disease course and may fluctuate with disease activity. The presence of the MAGE-B2 autoantibody may be predictive of treatment response and may define a subset of SLE patients at higher risk for active lupus nephritis. Prospective controlled trials with periodic MAGE-B2 autoantibody titer monitoring will help establish its utility as a clinical disease biomarker. In addition, the physiological and pathogenetic roles of MAGE-B2 must be clarified. If the MAGE-B2 protein and its autoantibody are shown to be major participants in the pathogenesis of SLE, target-specific immunomodulatory therapies may then be envisioned and developed.
Acknowledgments
We thank Jeffrey Gornbein, Dr.P.H. for assisting with the data analyses and John Colicelli, Ph.D. for sharing the MDA231 cell line. We also thank Linda Baum, M.D., Ph.D., Charles Lassman, M.D., Ph.D., the members of Dr. Diane Nugent’s lab at CHOC, Dr. David Seligson’s lab, and Dr. Richard Gatti’s lab, and the members of the Pediatric Allergy, Immunology, and Rheumatology Division for all their advice and support on this project.
Funding: NIH/NICHD (T32 HD07512; PI: E. McCabe, M.D., Ph.D.) “Human and Molecular Development Training Grant”
NIH/NIAMS (1T32AR053463; PI: R. Singh, M.D.) “Academic Training in Rheumatology.”
Footnotes
Note: This is a pre-copy-editing, author-produced PDF of an article accepted for publication in The Journal of Rheumatology following peer review. The definitive publisher-authenticated version [Hoftman AD, Tai LQ, Tze S, Seligson D, Gatti RA, McCurdy DK. MAGE-B2 autoantibody: a new biomarker for pediatric systemic lupus erythematosus. J Rheumatol. 2008 Dec;35(12):2430-8.] is available online at: http://www.jrheum.org/content/35/12.
References
- 1.Petty RE, Laxer RM. Systemic Lupus Erythematosus. In: Cassidy J, Petty R, Laxer R, Lindsley C, editors. Textbook of Pediatric Rheumatology. 5. Philadelphia: Elselvier, Inc; 2005. pp. 342–383. [Google Scholar]
- 2.Quaratino S, Feldman M, Daylan CM, Acuto O, Londei M. Human self-reactive T cell clones expressing identical T cell receptor beta chains differ in their ability to recognize a cryptic self antigen. J Exp Med. 1996;183:349–58. doi: 10.1084/jem.183.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adorini L, Sinigaglia F. Pathogenesis and immunotherapy of autoimmune diseases. Trends Immunol. 1997;18:209–11. doi: 10.1016/s0167-5699(97)01031-1. [DOI] [PubMed] [Google Scholar]
- 4.McCurdy DK, Tai L-Q, Nguyen J, Wang Z, Yang H-M, Udar N, et al. MAGE Xp-2: A member of the MAGE gene family isolated from an expression library using systemic lupus erythematosus sera. Mol Genet Metabol. 1998;63:3–13. doi: 10.1006/mgme.1997.2639. [DOI] [PubMed] [Google Scholar]
- 5.Benson DA, Karsch-Mizrachi I, Lipman DJ, Ostell J, Wheeler DL. GenBank. Nucleic Acids Res. 2008;36:D25–30. doi: 10.1093/nar/gkm929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Churg J, Bernstein J, Glassock RJ, editors. Renal disease: classification and atlas of glomerular disease. 2. Tokyo: Igaku-Shoin; 1995. Lupus nephritis; pp. 151–5. [Google Scholar]
- 7.Chomez P, De Backer O, Bertrand M, De Plaen E, Boon T, Lucas S. An overview of the MAGE gene family with the identification of all human members of the family. Cancer Res. 2001;61:5544–51. [PubMed] [Google Scholar]
- 8.Van der Bruggen P, Traversari C, Chomez P, Lurquin C, DePlaen E, Van den Eynde B, et al. A gene encoding an antigen recognized by cytotoxic T lymphocytes on a human melanoma. Science. 1991;254:1643–7. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 9.Muscatelli F, Walker AP, DePlaen E, Stafford AN, Monaco AP. Isolation and characterization of a MAGE gene family in the Xp21.3 region. Proc Natl Acad Sci. 1995;92:4987–91. doi: 10.1073/pnas.92.11.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dabovic B, Zanaria E, Bardoni B, Lisa A, Bordignon C, Russo V, et al. A family of rapidly evolving genes from the sex reversal critical region in Xp21. Mammal Genome. 1995;6:571–580. doi: 10.1007/BF00352360. [DOI] [PubMed] [Google Scholar]
- 11.Lurquin C, De Smet C, Brasseur F, Muscatelli F, Martelange V, De Plaen E, et al. Two members of the human MAGEB gene family located in Xp21.3 are expressed in tumors of various histological origins. Genomics. 1997;46:397–408. doi: 10.1006/geno.1997.5052. [DOI] [PubMed] [Google Scholar]
- 12.Xiao J, Chen HS. Biological functions of melanoma-associated antigens. World J Gastroenterol. 2004;10:1849–53. doi: 10.3748/wjg.v10.i13.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gjerstorff MF, Kock K, Nielsen O, Ditzel HJ. MAGE-A1, GAGE and NY-ESO-1 cancer/testis antigen expression during human gonadal development. Human Reprod. 2007;4:953–60. doi: 10.1093/humrep/del494. [DOI] [PubMed] [Google Scholar]
- 14.Nelson PT, Zhang PJ, Spagnoli GC, Tomaszewski JE, Pasha TL, Frosina D, et al. Cancer testis (CT) antigens are expressed in fetal ovary. Cancer Immun. 2007;7:1–7. [PMC free article] [PubMed] [Google Scholar]
- 15.Simpson AJG, Caballero OL, Jungbluth A, Chen Y-T, Old LJ. Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer. 2005;5:615–25. doi: 10.1038/nrc1669. [DOI] [PubMed] [Google Scholar]
- 16.De Backer O, Verheyden AM, Martin B, Godelaine D, De Plaen E, Brasseur R, et al. Structure, chromosomal location, and expression pattern of three mouse genes homologous to the human MAGE genes. Genomics. 1995;28:74–83. doi: 10.1006/geno.1995.1108. [DOI] [PubMed] [Google Scholar]
- 17.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus [letter] Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 18.Cassidy JT, Levinson JE, Bass JC, Baum J, Brewer EJ, Jr, Fink CW, et al. A study of classification criteria for a diagnosis of juvenile rheumatoid arthritis. Arthritis Rheum. 1986;29:274–81. doi: 10.1002/art.1780290216. [DOI] [PubMed] [Google Scholar]
- 19.World Medical Organization. Declaration of Helsinki. BMJ. 1996;313:1448–9. [Google Scholar]
- 20.Gladman DD, Ibañez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol. 2002;29:288–91. [PubMed] [Google Scholar]
- 21.Hay EM, Bacon PA, Gordon C, Isenberg DA, Maddison P, Snaith ML, et al. The BILAG index: a reliable and valid instrument for measuring clinical disease activity in systemic lupus erythematosus. Q J Medicine. 1993;86:447–58. [PubMed] [Google Scholar]
- 22.Leung YY, Szeto CC, Tam LS, Lam CWK, Li EK, Wong KC, et al. Urine protein-to-creatinine ratio in an untimed urine collection is a reliable measure of proteinuria in lupus nephritis. Rheumatology. 2007;46:649–52. doi: 10.1093/rheumatology/kel360. [DOI] [PubMed] [Google Scholar]
- 23.Becker-Catania SG, Chen G, Hwang MJ, Wang Z, Sun X, Sanal O, et al. Ataxia-telangiectasia: phenotype/genotype studies of ATM protein expression, mutations, and radiosensitivity. Mol Genet Metab. 2000;70:122–33. doi: 10.1006/mgme.2000.2998. [DOI] [PubMed] [Google Scholar]
- 24.Lim Y, Lee DY, Lee S, Park SY, Kim J, Cho B, et al. Identification of autoantibodies associated with systemic lupus erythematosus. Biochem Biophys Res Commun. 2002;295:119–24. doi: 10.1016/s0006-291x(02)00637-x. [DOI] [PubMed] [Google Scholar]
- 25.Fujimoto T. GPI-anchored proteins, glycosphingolipids, and sphingomyelin are sequestered to caveolae only after crosslinking. J Histochemistry and Cytochemistry. 1996;44:929–41. 23. doi: 10.1177/44.8.8756764. [DOI] [PubMed] [Google Scholar]
- 26.Bernatsky S, Boivin JF, Joseph L, Rajan R, Zoma A, Manzi S, et al. An international cohort study of cancer in systemic lupus erythematosus. Arthritis Rheum. 2005;52:1481–90. doi: 10.1002/art.21029. [DOI] [PubMed] [Google Scholar]
- 27.Ohman Forslund K, Nordqvist K. The melanoma antigen genes--any clues to their functions in normal tissues? Exp Cell Res. 2001;265:185–94. doi: 10.1006/excr.2001.5173. [DOI] [PubMed] [Google Scholar]
- 28.Barnea E, Beer I, Patoka R, Ziv T, Kessler O, Tzehoval E, et al. Analysis of endogenous peptides bound by soluble MHC class I molecules: a novel approach for identifying tumor-specific antigens. Eur J Immunology. 2002;32:213–22. doi: 10.1002/1521-4141(200201)32:1<213::AID-IMMU213>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 29.Fleischhauer K, Gattinoni L, Dalerba P, Lauvau G, Zanaria L, Dabovic B, et al. The DAM family encodes a new group of tumor-specific antigens recognized by human leukocyte antigen A2-restricted cytotoxic T lymphocytes. Cancer Res. 1998;58:2969–82. [PubMed] [Google Scholar]
- 30.Novellino L, Castelli C, Parmiani G. A listing of human tumor antigens recognized by T cells: March 2004 update. Cancer Immunol Immunother. 2005;54:187–207. doi: 10.1007/s00262-004-0560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mizukami M, Hanagiri T, Baba T, Fukuyama T, Nagata Y, So T, et al. Identification of tumor associated antigens recognized by IgG from tumor-infiltrating B cells of lung cancer: correlation between ab titer of the patient’s sera and the clinical course. Cancer Sci. 2005;96:882–8. doi: 10.1111/j.1349-7006.2005.00119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ballestar E, Esteller M, Richardson BC. The epigenetic face of systemic lupus erythematosus. J Immunol. 2006;176:7143–7. doi: 10.4049/jimmunol.176.12.7143. [DOI] [PubMed] [Google Scholar]
- 33.Richardson B. DNA methylation and autoimmune disease. Clin Immunol. 2003;109:72–9. doi: 10.1016/s1521-6616(03)00206-7. [DOI] [PubMed] [Google Scholar]
- 34.Wilson AS, Power BE, Mollowy PL. DNA hypomethylation and human diseases. Biochim Biophys Acta. 2007;1775:138–62. doi: 10.1016/j.bbcan.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 35.Oelke K, Lu Q, Richardson D, Wu A, Deng C, Hanash S, et al. Overexpression of CD70 and overstimulation of IgG synthesis by lupus T cells and T cells treated with DNA methylation inhibitors. Arthritis Rheum. 2004;50:1850–60. doi: 10.1002/art.20255. [DOI] [PubMed] [Google Scholar]