Abstract
Problem
Toll-like receptors (TLRs) recognize conserved sequences on the surface of pathogens and trigger effector cell functions. Previously, we described the expression of TLR3 by human trophoblast and their ability to respond to (Poly[I:C]). Here we evaluate the effect of Poly[I:C] on mouse pregnancy and characterize the local and systemic response.
Method of study
C57B/6 wild type (wt) and TLR3 knockout (TLR3KO) mice were treated with Poly[I:C] at 16.5 dpc and pregnancy outcome recorded. Morphologic changes, cytokines and chemokines levels in blood and utero-placental tissue were determined. NF-κB pathway was evaluated in vivo and in vitro.
Results
Poly[I:C] in C57B/6 wt mice caused preterm delivery within 24 hr (4.5 mg/kg). No effect was observed in TLR3KO mice. In addition, we observed local (placenta) and systemic (serum) response characterized by increased production of proinflammatory cytokines and chemokines. The NF-κB pathway was activated by Poly[I:C] in human and mice trophoblast cells.
Conclusion
We report that Poly[I:C] induces preterm delivery via TLR3-dependent manner. Furthermore, we demonstrate that the trophoblast is able to recognize Poly[I:C] through TLR3 and respond to viral infection, modulating the immune system at the feto-maternal interface.
Keywords: Infection, placenta, preterm delivery, TLR3, toll-like receptors, trophoblast
Introduction
Preterm birth accounts for 75% of perinatal mortality and more than half the long-term morbidity.1 In the USA, the preterm delivery rate is 12–13%, and the rate has risen over the last decade.2 Although most preterm babies survive, they are at increased risk of neuro-developmental impairments and respiratory and gastrointestinal complications.2 In addition, follow-up to middle age and beyond is warranted to identify the risks, especially for cardiovascular and metabolic disorders.3,4
Numerous studies have shown that infection is causally linked to preterm birth.5 Among them, bacterial infections in the genital tract and non-genital tract are well described in association with preterm birth. However, there is sparse evidence about viral infections, whether they are associated with or pre-dispose to preterm birth.2
Toll-like receptors (TLRs) are a family of innate immune receptors that have an essential role in the recognition of pathogen-associated molecular patterns (PAMP). To date, 10 functional human TLRs have been characterized and each TLR responds to a particular ligand.6 For example, TLR4 mediates responses towards Gram-negative bacterial lipopolysaccharide (LPS),7 whereas TLR3 mediates immune responses towards viral dsRNA.8 TLR signals must strike a delicate balance between clearing infection and causing pathologic effects on the host.
A number of animal studies have described an in vivo role of TLR4 for controlling bacterial infection and its impact on pregnancy outcome. For instance, Escherichia coli, lipopolysaccharide, and Fusobacterium nucleatum-induced preterm birth in mice is demonstrated to be dependent on TLR4 by using TLR4 mutant mice.9–11 However, little is known about the role of TLR3 in controlling viral infection during pregnancy.
During pregnancy, the placenta, contrary to previous dogma, functions not only as a simple mechanical barrier but also as an active functional barrier capable of recognizing self from non-self, and coordinates the maternal immune response to dangerous signals.12,13 Indeed, we have previously shown that the trophoblast expresses TLRs, and through TLRs recognizes pathogens and responds to them.14–17 Particularly, we demonstrated that trophoblasts are able to produce cytokines/chemokines and antiviral factors following TLR3 ligation in vitro, suggesting a potential active role of the trophoblasts in controlling viral infections.14,16
The objective of this study was to evaluate the potential effect of viral infections during pregnancy and characterize the response of the trophoblast to viral infection. Therefore, we established a mouse model consisting of pregnant mice receiving intraperitoneal injection of Poly[I:C], a synthetic analog of viral dsRNA that has been used extensively to mimic viral infection. We report that Poly[I:C] induces preterm delivery and its effect is TLR3-dependent. In addition, we demonstrate that the placenta, or the trophoblast, has a capacity to recognize Poly[I:C] through TLR3, and respond to viral infection by modulating the immune system at the feto-maternal interface.
Materials and methods
Reagents
MEM with d-valine was purchased from Caisson laboratories (North Logan, UT, USA). Type IV collagen was obtained from Becton Dickinson (Franklin Lakes, NJ, USA). Lymphocyte separation medium was purchased from MP Biomedicals (Solon, OH, USA). Poly[I:C], the synthetic analog of viral dsRNA was purchased from InvivoGen (San Diego, CA, USA). Biotinylated-Lectin DBA was obtained from Sigma-Aldrich (St. Louis, MO, USA). The rat anti-mouse F4/80 antibody was obtained from eBioscience (San Diego, CA, USA), and the mouse anti-NF-κB p65 antibody was purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, CA, USA). Alexa Fluor 546 anti rat/mouse IgG was purchased from Invitrogen (Carlsbad, CA, USA). Lysis buffer and assay buffer for Bio-Plex 100 IS system were obtained from Bio-Rad (Hercules, CA, USA).
Animals
C57BL/6 mice were obtained from The Jackson laboratory (Bar Harbor, ME, USA). TLR3 knock out mice were kindly provided by Dr. Richard A. Flavell, Yale University. Adult mice between 8 and 12 weeks of age were used for experiments. Timing of pregnancy in this study was determined by visual inspection of vaginal plug, which is defined as 0.5 dpc. All animals were maintained in the Yale University School of Medicine Animal Facility under specific pathogen-free conditions. All experiments were approved by the Yale Animal Resource Committee.
Poly[I:C] Injection and Tissue Collection
On 16.5 dpc, mice received intraperitoneal (i.p.) injections of 100 μL of either PBS or Poly [I:C] at 2.25–18 mg/kg. For evaluating systemic change and pregnancy outcome, mice were kept in individual cages and were observed closely for any sign of morbidity (piloerection, decreased movements, tachypnea). Preterm delivery was defined by delivery of at least one pup within the 24 hr after injection.
For immunohistochemical analysis and cytokine study, animals were killed 2, 4, or 48 hr after injection and tissue samples were collected. Blood was drawn via cardiac puncture. For morphologic and immunohistochemical analysis, intact mouse utero-placental units were isolated and were fixed with 4% paraformaldehyde, processed in paraffin blocks with an orientation that would provide cross sections. For cytokine study, murine placental tissues were carefully detached from decidua. The cord and the membrane were completely removed from the placenta. Tissues were snap frozen and kept in –80°C until further analysis.
Trophoblast Cells Isolation and Culture
Murine placental trophoblast cells were isolated and cultured as previously described.18 Mice were killed on 11.5–13.5 dpc. Placental tissues were minced and incubated with PBS containing 0.2 mg/mL of collagenase, 10 U/mL DNAse I for 1 hr at 37°C with mild agitation. The suspension was filtered through a 100-μm nylon cell strainer, and the cells were centrifuged to obtain a cell pellet, which was resuspended in MEM with d-valine with 10% FBS. The suspension was layered onto lymphocyte separation medium and centrifuged at 150 × g for 15 min. Trophoblast cells recovered from the interface were washed and resuspended in MEM with d-valine with 10% FBS and placed in a type IV collagen-coated plate and kept at 37°C/5% CO2. To evaluate the effect of Poly[I:C] on trophoblast cells, the cells were subsequently incubated with Poly[I:C] at an indicated concentration and supernatants were collected after an indicated period of time.
Human Trophoblast Cell Culture
The SV40-transformed HTR8 cells were a gift from Dr. C. Graham (Queens University, Kingston, Canada).19 HTR8 cells were maintained at 37°C/5% CO2 in RPMI 1640 supplemented with 10% FBS, 10 mm Hepes, 0.1 mm MEM non-essential amino acids, 1 mm sodium pyruvate, 100 nm penicillin/streptomycin. First trimester trophoblast cells were isolated and cultured as described.20,21 Briefly, after a brief wash, tissues were minced and incubated in PBS containing 0.125% trypsin, and 30 U/mL DNAse I for 1 hr at 37°C with mild agitation. The suspension was filtered and red blood cells were removed using lymphocyte separation medium as described above. Trophoblast cells were cultured in MEM with d-valine with 10% human serum and placed in a type IV collagen-coated plate and kept at 37°C/5% CO2. To evaluate the effect of Poly[I:C] on trophoblast cells, either HTR8 cells or primary trophoblast cells were subsequently incubated with Poly[I:C] at an indicated concentration and supernatants were collected following a culture of hours indicated. The supernatants were centrifuged to remove cell debris and stored at –80°C until analysis was performed.
Immunohistochemistry
Paraffin embedded tissues were sectioned (5 μm), adhered to glass slides and deparaffinized. The hydrated sections were stained with hematoxylin and eosin by standard procedures for morphologic study. Localization of uterine NK cells, macrophages and NF-κB p65 expression in mouse utero-placental units was carried out as follows.
Uterine NK cells detection was performed as previously reported.22,23 Endogenous peroxides were blocked by immersing slides in 0.1% H2O2 for 30 min. Slides were subsequently incubated with 3% BSA in 0.1 m PB 0.01% Triton X-100 for 30 min at room temperature. Following two washes with 0.1 m PB 0.01% Triton X, slides were incubated overnight at 4°C with either biotinylated-lectin DBA diluted at 1:200 in 1% BSA 0.1 m PB or diluent alone for negative control. After two washes in 0.1 m Tris, slides were developed with streptavidin-HRP followed by DAB substrate and counterstained with hematoxylin (Sigma-Aldrich) before dehydration. Slides were subsequently mounted and visualized by light microscopy.
For macrophage detection, antigen retrieval was performed with Retrievagen A (pH 6.0) (BD Biosciences, San Diego, CA, USA) for 30 min in boiling water in a steamer. Slides were blocked with BSA as described above. After washing, slides were incubated overnight at 4°C with either the rat anti mouse F4/80 antibody at 1:20 in 1% BSA 0.1 m PB or diluent alone for negative control. After washing, specific staining was detected by incubating with Alexa Fluor 546 anti rat IgG at a 1:200 dilution for 30 min at room temperature. After the washing step, slides were counterstained with Hoechst 33342 (Invitrogen) in 1:5000 and mounted with Aquamount (Lerner Laboratories, Pittsburgh, PA, USA) and visualized by fluorescent microscopy.
For NF-κB p65 detection, antigen retrieve was performed as described above. Slides were blocked with mouse-on-mouse (MOM) blocking reagent (Vector Laboratories, Burlingame, CA, USA) for 1 hr at room temperature. Following washes, slides were incubated in MOM diluent for 5 min and subsequently incubated overnight at 4°C with either the mouse anti- NF-κB p65 antibody at a 1:100 dilution in MOM diluent or diluent alone for negative control. After washes, specific staining was detected as described above with secondary antibody (Alexa Fluor 546 anti-mouse IgG). Slides were counter-stained and visualized by fluorescent microscopy.
Cytokine Studies
The amount of cytokine/chemokine content in murine placental tissue and serum, and supernatant from either mouse or human trophoblasts culture were determined by Multiplex. Placental tissues were processed as follows. Tissues were homogenized by homogenizer in 1 mL lysis buffer (Beadlyte)/1 g weight total protein containing 0.2 μg/mL of PMSF and a protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN, USA). Samples were centrifuged for 30 min at 20,000 × g/4°C and supernatants were collected. Protein concentrations were determined using BCA assay (Pierce, Rockford, IL, USA) and 100 μg of total protein was diluted in assay diluent. Serum samples were prepared by centrifugation of coagulated blood for 15 min at 500 × g/room temperature and diluted at 1:5 in assay diluent. The Bio-Plex Mouse Cytokine 23-Plex Panel (Cat no. 171-F11241) was used for the murine tissue. Human samples were measured using the Bio-Plex custom Human Cytokine 17-plex (no. X500FHB86U) also from Bio-Rad Laboratories and read on the Bio-Rad 100 IS system as recently described.24
NF-κB Activity Assay
The effect of Poly[I:C] on trophoblast cell NF-κB activity was determined using a luciferase reporter construct pBII-LUC containing two NF-κB sites before a Fos essential promoter (a kind gift from Dr. S. Ghosh, Yale University, New Haven, CT, USA).25 Trophoblast cells were transiently transfected with 2 μg pBII-LUC using Fugene 6 (Roche Diagnostics, Nutley, NJ, USA) as previously described.26 Following transfection, cells were allowed to recover in growth media for 24 hr. The cells were then treated with or without 25 μg/mL Poly[I:C], lysed for protein and luciferase activity was measured using the Luciferase Assay System (Promega, Madison, WI, USA) according to the manufacturer's protocol. Briefly, 10 g of protein in a total volume of 100 μL was mixed with 20 μL of the Luciferase Assay Reagent and luminescence was measured using TD 20/20 Luminometer (Turner Designs, Sunnyvale, CA, USA). Relative activity was calculated based on readings measured from untreated cells after subtracting blank values. Each sample was assayed in triplicate.
Statistical Analysis
Data are expressed as mean ± standard error for in vitro study and median ± first or third quartiles for in vivo study. Statistical significance (P < 0.05) was determined using either t-tests or Mann–Whitney U-test. Unless stated otherwise, all experiments were performed in triplicate and a representative of at least three independent experiments is shown.
Results
Poly[I:C] Administration Induced Preterm Delivery in Wild Type Mice, but not in TLR3 knock Out Mice
Our first objective was to characterize the effect of Poly[I:C] administration on pregnancy outcome. Thus, we evaluated the result of increasing concentrations of Poly[I:C] (2.25–18 mg/kg) administered i.p. to pregnant wild type C57 BL/6 (wt) mice on 16.5 dpc. As shown in Table Ia, pregnant mice treated with either the control PBS or the low dose of Poly[I:C] (2.25 mg/kg) had normal and complete pregnancies; however, 80% of pregnant mice receiving 4.5 mg/kg Poly[I:C] and 100% of pregnant mice receiving 18 mg/kg Poly[I:C] underwent preterm delivery. The number of dead pups/dam was significantly higher in the group of mice treated with either 4.5 or 18 mg/kg Poly[I:C] compared with either PBS or 2.25 mg/kg Poly[I:C] group. There was no difference in the number of resorptions among groups. Of note, Poly[I:C] at a dose of 4.5 mg/kg did not induce any apparent systemic adverse effects in the dam, except inducing labor, whereas Poly[I:C] at a dose of 18 mg/kg affected the well being of the dam by inducing piloerection, tachypnea and decreased movement.
Table I.
(a) Pregnancy Outcome in wt Mice Treated with Poly[I:C]. (b) Pregnancy Outcome in TLR-3KO Mice Treated with Poly[I:C]
| Treatment | Dose (mg/kg) | Preterm delivery within 24 hr (%) | Number of implantation site/dam (mean) | Number of resorption/dam (mean) | Number of dead pups/dam‡ (mean) |
|---|---|---|---|---|---|
| (a) Wildtype* | |||||
| PBS | na | 0% (0/3) | 6.67 | 1.00 | 0.00a |
| Poly[I:C] | 2.25 | 0% (0/4) | 8.25 | 1.60 | 0.00a |
| Poly[I:C] | 4.5 | 80% (4/5) | 8.33 | 0.00 | 6.00b |
| Poly[I:C] | 18 | 100% (3/3) | 8.00 | 0.30 | 7.00b |
| (b) TLR3 knockout† | |||||
| Poly[I:C] | 4.5 | 0% (0/3) | 9.33 | 0.00 | 0.00 |
| Poly[I:C] | 18 | 0% (0/5) | 7.20 | 0.40 | 0.83 |
Pregnant wild type C57 BL/6 (wt) mice were treated with either the control PBS or increasing concentrations of Poly[I:C] (2.25–18 mg/kg) administered i.p. on 16.5 dpc. Mice were killed 24 hr after the injection. The percentage of preterm delivery was recorded if occurred during the following 24 hr after the injection. The mean of implantation sites/dam, mean of dead pups/dam and the mean of resorptions among groups were recorded.
Pregnant TLR3 Knockout TLR3(KO) mice were treated with Poly[I:C] (4.5–18 mg/kg) administered i.p. on 16.5 dpc. Mice were killed 24 hr after the injection. The percentage of preterm delivery was recorded if occuring during the following 24 hr after the injection. The mean of implantation sites/dam, mean of dead pups/dam and the mean of resorptions among groups were recorded.
Values with different superscript letters are significantly different (P < 0.05).
To determine whether Poly[I:C] induced preterm labor is TLR3 dependent, we treated pregnant TLR3 knockout mice (TLR3KO) with the same protocol as described for the wild type (wt) pregnant mice. Neither the 4.5 nor 18 mg/kg dose of Poly[I:C] was able to affect pregnancy or induce preterm delivery (Table Ib). These results indicate that the effects of Poly[I:C] on inducing preterm delivery is TLR3-dependent.
Poly[I:C] at a dose of 4.5 mg/kg induces preterm delivery in the wt mice; however, it did not appear harmful to the dam and the mice did not show any ill-effects. Therefore, we used the dose of 4.5 mg/kg for further characterization, unless stated otherwise.
Effect of Poly[I:C] on Placental Morphology
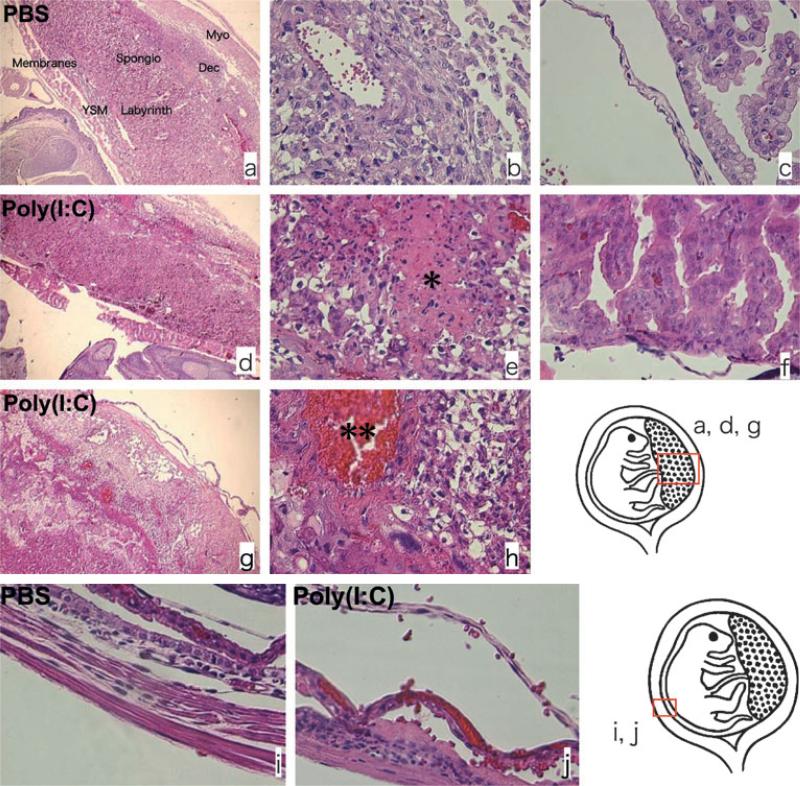
Our next objective was to characterize the morphologic changes associated with Poly[I:C] induced pre-term delivery. First, we performed histopathologic analysis to determine the effect of Poly[I:C] injection on morphologic integrity at the feto-maternal interface. Utero-placental units of wt animals at 4 hr post injection of 4.5 mg/kg of Poly[I:C] were collected and hematoxylin and eosin staining was performed. The feto-maternal interface from PBS-treated animals kept its morphologic integrity characterized by well-defined layers at the maternal and the fetal side (Fig. 1a–c and i). On the contrary, Poly[I:C] treated mice showed significant changes on the integrity of the feto-maternal interface (Fig. 1d–h and j) with a loss of the characteristic layers. Furthermore, necrosis (Fig. 1e), inflammation (Fig. 1e–g), hemorrhage (Fig. 1h) and edema (Fig. 1f,j) were apparent in the decidua, the yolk sac membrane, chorion and the amniotic membrane of Poly[I:C] treated animals. Inflammation was accompanied by polymorphonu-clear leukocyte infiltration (Fig. 1e–g).
Fig. 1.
Poly[I:C] injection induced morphologic change at feto-maternal interface; 4.5 mg/kg Poly[I:C] or PBS was injected i.p. to wild type mice on 16.5 dpc and killed after 4 hr. Hematoxylin and eosin staining was performed for histopathologic analysis. The feto-maternal interface from PBS-treated animals kept its morphologic integrity (a–c and i). On the contrary, the feto-maternal interface from Poly[I:C] treated mice lost the integrity (d–h and j). Necrosis (e*), inflammation (e–g), hemorrhage (h**) and edema (f and j) were apparent in the decidua, the yolk sac membrane, chorion and amniotic membrane of poly(I:C) treated animals. The inflammation is accompanied by polymorphonuclear leukocytes infiltration (e–g). V: blood vessel, am: amniotic membrane, ysm; yolk sac membrane, ch: chorion, dec: decidua, myo: myometrium. Original magnification; a, d, g: 40×, b, c, e, f, h, i, j: 400×. Data are representative of at least three mice from same treatments.
None of these changes was evident in the TLR3KO mice treated with same concentration of Poly[I:C] (data not shown).
Effect of Poly[I:C] on Immune Cells Distribution at the Feto-maternal Interface
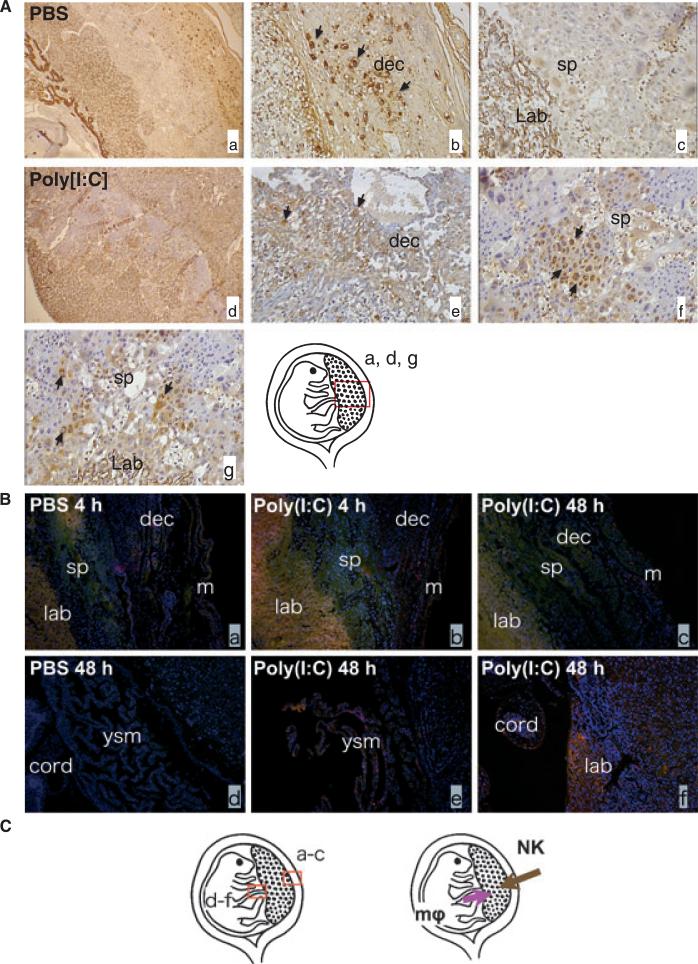
As we observed a robust inflammatory response in pregnancies treated with Poly[I:C], our next objective was to characterize changes on the phenotype and distribution of immune cells at the maternal/fetal interface. In normal murine pregnancies, uterine NK cells are localized only in the decidua and normally absent in the placenta.24 That was indeed the case in PBS-treated mice, where NK cells were restricted to the decidua (Fig. 2Ab) and no NK cells were seen in spongiotrophoblast and labyrinth, consistent with normal distribution of NK cells. In contrast, in Poly[I:C] treated mice few NK cells were found in the decidua (Fig. 2Ae), but a high number of NK cells were observed in spongiotrophoblast (Fig. 2Af) and labyrinth zone (Fig. 2Ag), which indicates that Poly[I:C] treatment triggered NK cells infiltration from the decidua into the placental zone.
Fig. 2.
Poly[I:C] injection induced NK cells and macrophages infiltration into the placenta; 4.5 mg/kg Poly[I:C] or PBS was injected i.p. to wild type mice on 16.5 dpc. Mice were killed after either 4 or 48 hr. Utero-placental units were collected. Immunohistochemical analysis of the feto-maternal interface using lectin (for uNK cells; brown) or anti f4/80 antibody (for Macrophages; pink) were performed. (A) In PBS-treated mice, uterine NK cells were restricted to the decidua (Ab) and no NK cells were seen in spongiotrophoblast and labyrinth, (a–c). The distribution of NK cells changed in Poly[I:C] treated mice (d). Fewer NK cells were found in decidua (e) and more cells were found in spongiotrophoblast (f) and labyrinth zone (g). Slides were counterstained with hematoxylin (purple). (B) Macrophages were identified mainly in the outer myometrium and rarely in the decidua and were never observed in spongiotrophoblast and labyrinth in PBS-treated control animals (a). Macrophage infiltration from the maternal side toward the placental zone was not observed up to 48 hr after Poly[I:C] injection (b and c). Instead, when we observed the fetal side of the placenta at 48 hr post injection, we found macrophages infiltrated from the fetal side (umbilical cord and yolk sac membrane) toward the placenta zone (e and f). No such infiltration was found in PBS-treated animals (d). Slides were counterstained with Hoechst (blue nuclei). ysm, yolk sac membrane; m, myometrium; dec, decidua; sp, spongio; trophoblast, lab, labyrinth; cord, umbilical cord. (C) Schematic localization for uterine NK cells and macrophages and the direction of their infiltrations toward placental zone. Original magnification; 2A a and d: 40X, 2Ab, c, e, f and g: 2B a–f; 400X. Data are representative of at least three mice from same treatments.
Macrophages were identified mainly in the outer myometrium, rarely in the decidua and were never observed in spongiotrophoblast and labyrinth in PBS-treated control animals (Fig. 2Ba). In contrast to uterine NK cells, we did not observe macrophage infiltration from the maternal side toward the placental zone up to 48 hr after Poly[I:C] injection (Fig. 2Bb and c). However, when we evaluated the fetal side of the placenta at 48 hr post injection, we found macrophages that infiltrated from the fetal side (umbilical cord and yolk sac membrane) toward the placental zone (Fig. 2Be and f). Fig. 2C indicates the difference between uterine NK cells and macrophages in the direction of their infiltrations toward the placental zone.
Cytokine/Chemokine Response to Poly[I:C] in vivo
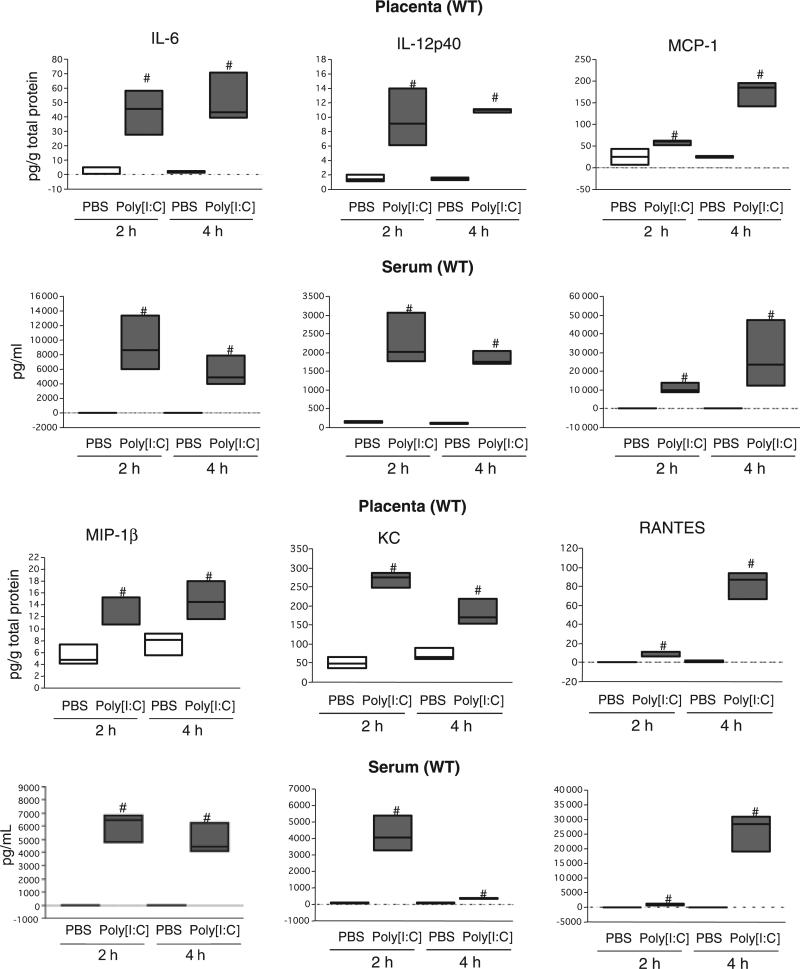
Previously we have shown that the trophoblast have the capacity to recognize pathogen-associated molecular patterns such as Poly[I:C] and LPS through TLRs and respond to them by producing cytokines/chemokines.14–16 To determine whether this is the case in vivo, we evaluated the cytokine/chemokine response by the placenta to Poly[I:C] treatment. In addition, we compared the local response (placenta) versus the systemic response (serum). Placenta and serum samples were collected either 2 or 4 hr after Poly[I:C] administration and cytokine/chemokine content were analyzed by Multiplex. From 37 analyzed cytokines, we observed in wt animals, a significant increase in the levels of IL-6, IL-12p40 and the chemokines MCP-1, MIP-1β, KC and RANTES, (Fig. 3a–f and Figure S1). A second wave of cytokines was observed at 4 hr and characterized by high levels of IL-1β, TNF-α and IFN-γ (Figure S1a–c). All of these cytokines and chemokines were also elevated in the serum of animals treated with Poly[I:C]. In addition, levels of IL-12p70 and IL-10 were increased in the serum, but not in the placenta (Figure S1d and e).
Fig. 3.
Poly[I:C] injection induced robust local and systemic inflammatory response in wild type pregnant mice; 4.5 mg/kg Poly[I:C] or PBS was injected i.p. to wild type mice on 16.5 dpc. Mice were killed after 2 and 4 hr and placenta and sera were collected. Cytokine/chemokine concentrations in placental lysate (Placenta) and sera (Serum) were measured. Boxes represent the distance between the first (25%) and third (75%) quartiles and horizontal lines in the boxes represent medians. #P > 0.05. Data are representative of at least six mice per group.
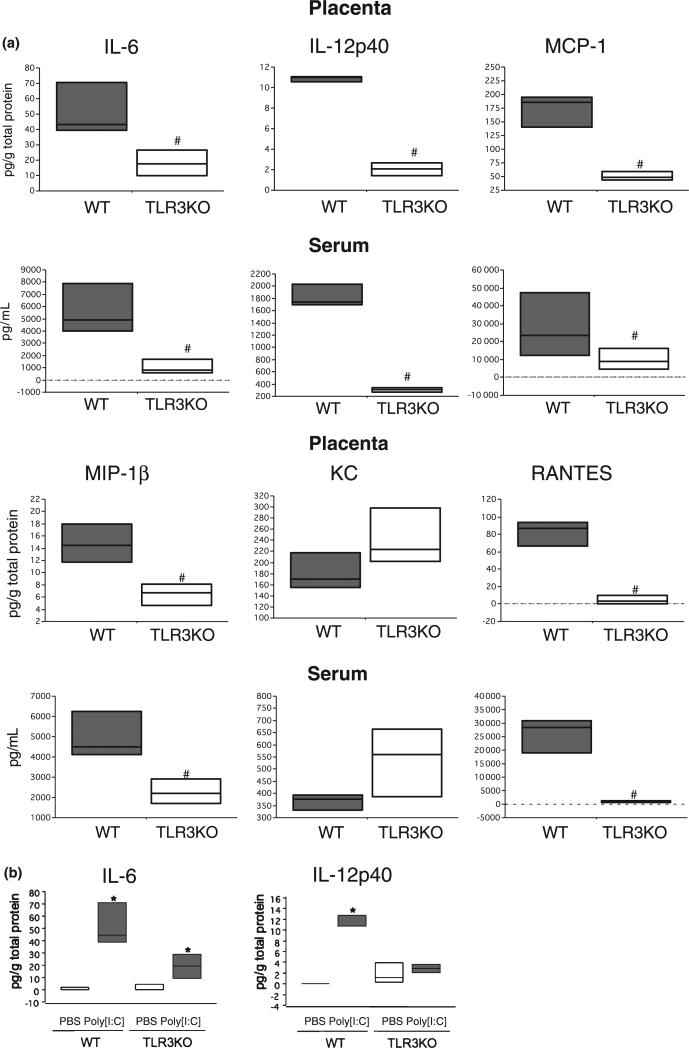
We then evaluated whether the cytokine/chemokine response is TLR3-dependent by collecting placenta and serum samples from TLR3KO mice following the same treatment and time points as described for wt mice. Following treatment with Poly[I:C] (4.5 mg/kg), all the cytokine/chemokine levels, with the exception of KC, were significantly lower in TLR3KO mice compared with the wt, which indicates the effect of Poly[I:C] on cytokine/chemokine production is TLR3-dependent (Fig. 4a, Figure S2). Interestingly, in the TLR3 KO mice, when compared to the total concentration levels of IL-6 between the control PBS and treated Poly[I:C], there were significant differences; although the levels were still much lower than those observed in the wt mice. These results suggest the presence of a TLR3-independent pathways which enables Poly[I:C]-induced IL-6 production (Fig. 4b).
Fig. 4.
Poly[I:C] injection failed to induce local and systemic inflammatory response in TLR3 knockout pregnant mice; 4.5 mg/kg Poly[I:C] or PBS was injected i.p. to either wild type (wt) or TLR3 knockout (TLR3KO) mice on 16.5 dpc. Mice were killed after 2 and 4 hr and placenta and sera were collected. Cytokine/chemokine concentrations in placental lysate (Placenta) and sera (Serum) were measured. Boxes represent the distance between the first (25%) and third (75%) quartiles and horizontal lines in the boxes represent medians. #Significantly lower (P > 0.05) than wild type treated mouse, *Significantly higher (P > 0.05) than PBS-treated mouse.
Poly[I:C] Induced Murine Trophoblast Cytokine/Chemokine Secretion in vitro
As we observed a robust cytokine/chemokine response in the placenta, our next objective was to determine whether the trophoblast has an active contribution on this response. For this, we isolated trophoblast cells from mouse placenta (wt and TLR3KO mice) and evaluated in vitro its response to Poly[I:C] treatment. Thus, cells were treated either with vehicle or with 25 μg/mL Poly[I:C] for 48 hr and cytokine/chemokine concentrations were determined in the supernatants using Multiplex. Consistent with the in vivo findings, trophoblast isolated from wt animals responded to Poly[I:C] and secreted higher levels of cytokines/chemokines compared with vehicle-treated cells. This response was absent in trophoblast cells isolated from TLR3KO mice (Table II). These findings further support the premise that trophoblast cells respond to Poly[I:C] and the effect of Poly[I:C] on cytokine/chemokine production by trophoblasts is TLR3-dependent.
Table II.
Poly[I:C] induced murine trophoblast cytokine/chemokine secretion in vitro
| Fold increases |
||
|---|---|---|
| Chemokine/cytokine | WT | KO |
| IL-1β | 4.76 ± 2.0* | ND |
| IL-6 | 5.72 ± 2.5* | 1.19 ± 0.11 |
| IL-12p40 | 3.99 ± 2.5* | ND |
| IL-12p70 | 2.68 ± 1.6* | ND |
| TNF-α | 0.94 ± 0.24 | 1.03 ± 0.14 |
| G-CSF | 1.55 ± 0.18 | 0.96 ± 0.19 |
| MCP-1 | 2.49 ± 0.75* | 1.15 ± 0.045 |
| MIP-1α | 3.79 ± 1.0* | 0.72 ± 0.27 |
| MIP-1β | 3.62 ± 0.9* | 1.45 ± 0.23 |
| KC | 2.42 ± 0.79* | 1.11 ± 0.069 |
| RANTES | 52.3 ± 46* | 1.32 ± 0.20 |
Isolated trophoblast cells from mouse placenta (wt and TLR3KO mice) were cultured and treated either with vehicle or 25 μg/mL Poly[I:C] for 48 hr and cytokine/chemokine concentrations were determined in the supernatants using Multiplex. Fold increases of (mean ± S.E.M.) cytokine/chemokine secretion (with Poly[I:C] treatment over non treatment) either wild type (WT) or TLR3 knockout (TLR3KO) mice sample are shown.
Mean ± S.E.M.
P < 0.05.
Poly[I:C] Injection Promoted NF-κB Activation in Murine Placenta in vivo
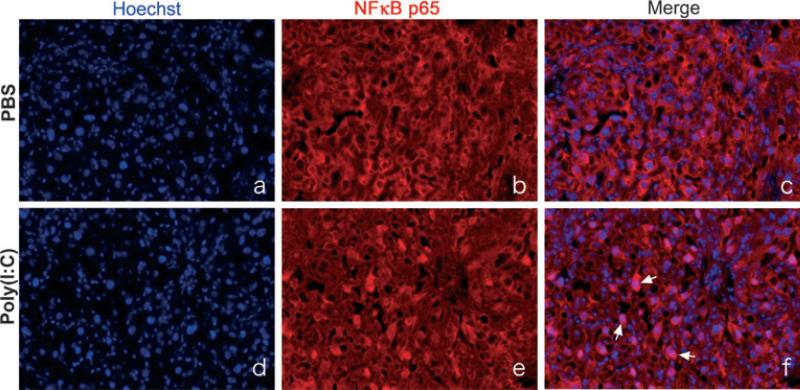
As TLR-induced cytokine/chemokine production is mediated through NF-κB activation,27 we then determined NF-κB activity following Poly[I:C] injection in mouse placental tissue by determining nuclear translocation of NF-κB p50-p65 dimer unit using immunohistochemistry with specific antibody for NF-κB p65. The placenta of wild type animals at 2 hr post injection was collected and immunohistochemical study carried out. p65 was rarely found within the nucleus of cells in tissue from PBS-treated animals (Fig. 5c). On the contrary, markedly higher positive reactivity for p65 was observed in nuclei of placenta tissue obtained from Poly[I:C] treated animals (Fig. 5f), indicative of NF-κB activation in the placental trophoblast following Poly[I:C] treatment.
Fig. 5.
Poly[I:C] injection promoted NF-κB activation in murine placenta in vivo; 4.5 mg/kg Poly[I:C] or PBS was injected i.p. to wild type mice on 16.5 dpc. Mice were killed after 2 hr and placenta was collected. Immunohistochemistry with anti NF-κB p65 antibody was performed. Slides were counterstained with Hoechst (nuclear staining). In tissues from PBS-injected animals (c), NF-κB p65 was mainly detected in the cytoplasm of cells. On the contrary, a markedly higher number of stained nuclei was observed in tissue from Poly[I:C] treated animal (f). Arrows are indicating nuclear localization which has double color. Original magnification was 200×. Data are representative of at least three mice from same treatments.
Poly[I:C] Induced Human Trophoblast Cytokine/Chemokine Secretion in vitro
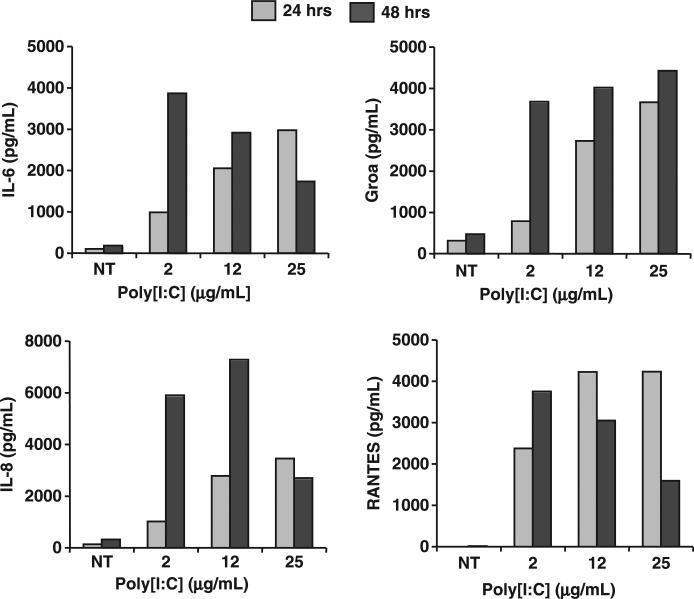
Having confirmed the capacity of murine placenta and trophoblast to respond to Poly[I:C] treatment by producing cytokines and chemokines, we then evaluated whether similar response could be observed in human trophoblast cells. Either human primary trophoblast cell cultures or the first trimester trophoblast cell line HTR8 was cultured in the presence or absence of Poly[I:C] (25 μg/mL) and their cytokine/chemokine secretion was determined by Multiplex. Similar to findings observed with the murine trophoblast cultures, a significant increase in cytokines and chemokines was observed in human trophoblast cells following Poly[I:C] treatment (Fig. 6). Table III summarizes all the cytokines/chemokines significantly up-regulated in Poly[I:C] treated trophoblasts. The response was specific as demonstrated by a time- and dose-dependent increase in cytokine/chemokine secretion by human trophoblast following Poly[I:C] treatment (Fig. 6).
Fig. 6.
Poly[I:C] treatment induced cytokine/chemokine secretion from first trimester human trophoblast cells in a dose- and a time-dependent manner. First trimester trophoblast cell line HTR8 were treated with Poly[I:C] at the doses indicated. Supernatant were collected after either 24 or 48 hr. Cytokine/chemokine concentrations in supernatant were measured. Data are representative of 13 cytokine/chemokine data.
Table III.
A list of cytokine/chemokine secretion by human trophoblast upregulated by Poly[I:C]
| Cytokine/chemokine |
|---|
| Cytokines |
| IL-1β |
| IL-6 |
| TNF-α |
| IFN-γ |
| IL-2 |
| Chemokines |
| IL-8 |
| G-CSF |
| GM-CSF |
| MCP-1 |
| MIP-1α |
| MIP-1β |
| GROα |
| RANTES |
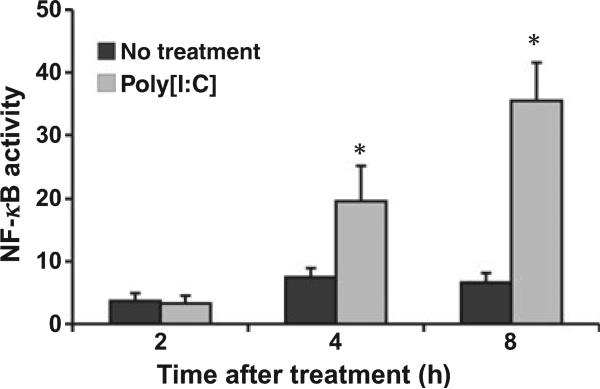
Poly[I:C] Promoted NF-κB Activation in Human Trophoblast in vitro
NF-κB activation upon Poly[I:C] treatment was also validated in human trophoblast cells. Activation of NF-κB in HTR8 cells was monitored using a lucifer-ase reporting system containing two NF-κB binding sites. As shown in Fig. 7, when trophoblast cells were treated with 25 μg/mL Poly[I:C], NF-κB activity was increased at 4 hr post-treatment.
Fig. 7.
Poly[I:C] promoted NF-κB activation in human trophoblast in vitro. HTR8 cells transfected with a NF-κB reporter construct were treated with Poly[I:C] (25 g/mL). NF-κB activity was increased at 4 hr post-treatment. *P < 0.05.
Discussion
Epidemiologic studies have reported an association between viral infection either in the genital tract or in the non-genital tract, and preterm labor.28–32 However, the potential mechanism by which viral infection might induce preterm labor has not been elucidated. In the present study, we demonstrated that intraperitoneal injection of Poly[I:C], a synthetic viral dsRNA, induced preterm birth in mice. Furthermore, we demonstrated that the placenta, and more specifically the trophoblast, plays an active role in the response to Poly[I:C] through the production of cytokines and chemokines, and this response is mediated through TLR3 expression and function.
Bacterial and viral infections pose a significant threat to pregnancy and well-being of the fetus by gaining access to the placenta through one of three major routes: by way of the maternal circulation; by ascending into the uterus from the lower reproductive tract or by descending into the uterus from the peritoneal cavity.33 Clinical studies have established a strong association between pregnancy complications and intrauterine infections.33–35 Indeed, infections have been reported as responsible for up to 40% of preterm labor cases. Furthermore, 80% of preterm deliveries occurring at less than 30 weeks of gestation have evidence of infection.34–36 In addition, other pregnancy complications, such as pre-eclampsia, may have an underlying infectious trigger.37–39 One of the main questions of this study was how a microorganism, in this case a virus, might initiate a response that would induce preterm labor or abortion, or even pre-eclampsia.
dsRNA is a universal viral PAMP produced by most viruses at some point during their replication40 and it is recognized by intracellular TLR3 expression. The response following binding of TLR3 is characterized by cytokines and chemokines that will mount active immune responses. We found that Poly[I:C] has a striking effect on the survival of pregnancy by inducing preterm labor in less than 24 hr following treatment. These results confirmed previous studies, which demonstrated that intrauterine injection of Poly[I:C] induced preterm birth.41 However, the doses of Poly[I:C] used in those studies were very high which could affect the well-being of the mother and therefore affect the fetus. In this study, we used a dose that had no effect on the maternal well-being; therefore, the outcome of the pregnancy would be the result of changes at the maternal-fetal interface.
Interestingly, the dose used in this study for Poly[I:C], which was able to induce preterm birth without inducing any maternal systemic adverse, is exceptionally lower (4.5 mg/kg) compared with 30 mg/kg previously reported for dsRNA induced disease in mice.42
To characterize the mechanism by which Poly[I:C] induced preterm delivery, we evaluated histologic changes at the feto-maternal interface in our mouse model. We observed a massive inflammatory process characterized by infiltration of immune cells (polymorphonuclear cells and NK cells), necrosis, hemorrhage and edema, mainly localized in the placenta, the amniotic membrane, and the umbilical cord. Some of these morphologic characteristics are observed in cases of human chorioamnionitis and funisitis.
At the normal murine feto-maternal interface, immune cells such as neutrophils, macrophages and NK cells are assumed to be excluded from the placenta and localized only in the decidua.12 Treatment with Poly[I:C] disrupt this normal distribution and induce a massive migration of immune cells, primarily NK cells from the decidua towards the placenta, invading the spongiotrophoblast and then the labyrinth. This type of migration is consistent with an immune response, which would exert adverse effects on pregnancy such as fetal demise or abortion.43 Interestingly, macrophages followed a different migration pattern; we did not observe any change in macrophage distribution at the decidua-placenta interface; however, we found a significant number of macrophages at the fetal side of the placenta. These results suggest that the fetal side could actively contribute to mounting an immune response that could endanger the fetus. Indeed, a recent study in humans described that in cases of villitis of unknown etiology (VUE), placentas from male neonates showed CISH+ signals from Y chromosomes in a majority of macrophages, but not in lymphocytes, indicating that the macrophages were of fetal origin.44 Therefore, we could hypothesize that a viral infection of the placenta could be the trigger for the migration of fetal macrophages.
The pattern of cytokines and chemokines observed in the placental tissue following Poly[I:C] treatment suggests that the placenta plays an active role in the response to viral infection. Indeed, the pattern correlates with the histologic findings. Chemokines, such as KC and G-CSF, are known to attract neutrophils; IL-12p40 traffics macrophages, MCP-1, MIP-1α and MIP-1β attract macrophages and NK cells. All these cytokines were significantly increased in their expression levels within the placenta upon Poly[I:C] administration. These chemokines may be responsible for attracting immune cells from either the maternal side or the fetal side towards the placenta.
We also found a significant increase in the levels of pro-inflammatory cytokines in the placenta following Poly[I:C] treatment. Pro-inflammatory cytokines such as IL-6, TNF-α, IL-1β and IFN-γ are known to be elevated in amniotic fluid or amnion/choriodecidua membrane from women with preterm labor.45–48 Pro-inflammatory cytokines are believed to regulate intrauterine prostaglandin production, which is an important aspect of the onset of labor in terms of inducing uterine contraction and cervical ripening.49 They also increase the levels of matrix metalloproteinases (MMPs) expression and activity in the membranes50 and may induce apoptosis of trophoblast cells.51 Therefore, it is plausible to conclude that the necrotic areas and damage observed in the placenta and membranes of mice treated with Poly[I:C] may be the result of the high levels of these pro-inflammatory cytokines.
Our next question was whether the trophoblast could be the origin of the signals controlling the migration of these immune cells. Recently, we proposed that the trophoblast functions as an immune regulator and has the capacity to influence, based on the type of signals, the differentiation and migration of immune cells.13 Indeed, we have shown that trophoblast cells can induce the migration of macrophages and NK cells14 and modulate macrophage response to bacterial products such as LPS.24 Our in vitro study demonstrated that upon Poly[I:C] stimulation, either mouse primary trophoblast or human trophoblast showed cytokine/chemokine production profiles similar to in vivo results, suggesting that trophoblasts were primarily responsible for the response to this PAMP, although we cannot exclude the possibility that other cell types present in the placenta may also contribute to this response. This finding supports the notion that trophoblasts play a role in coordinating the maternal innate immune response to infection at the feto-maternal interface12,52 and, especially in this case, in response to viral infection.
In terms of the molecular mechanism of action, we have demonstrated a critical role for TLR3 on Poly[I:C] induced pre-term labor. In contrast to wt mice, TLR3KO mice did not undergo preterm delivery following Poly[I:C] administration, clearly suggesting that TLR3, and the concomitant cytokine response, is required for the in vivo response to Poly[I:C] and inducing preterm delivery. Cytokine/chemokine production upon Poly[I:C] injection was also impaired in TLR3KO mice, with some exceptions such as IL-6, which showed a slight increase by Poly[I:C] in TLR3KO mice. This is consistent with recent findings that showed the presence of a TLR3 independent, MDA5 dependent recognition of Poly[I:C] for regulating IL-6 production.53 We also confirmed that dsRNA ligated TLR3 and activated the NF-κB signaling pathway, inducing cytokine/chemokine production in vivo and in vitro using human trophoblast cells.
In summary, we have demonstrated that systemic administration of dsRNA induced preterm delivery through TLR3 activation in a mouse model. We show that in addition to the classical immune response mediated by the maternal immune system, the placenta, and more specifically the trophoblast, is able to recognize, through TLR3, the presence of dsRNA and mount a strong and specific immune response. Furthermore, the trophoblast may not only influence the maternal immune system, but may also induce the migration and differentiation of fetal immune cells. This contribution of the trophoblast in the maternal and fetal innate immune system supports the notion that the trophoblast acts as a component of the innate immune system. Our study raises the possibility of clinical significance of viral infections screening among pregnant or pre-pregnant women to identify a high-risk population for pre-term labor.
Supplementary Material
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Fig. S1. Poly[I:C] injection induced robust local and systemic inflammatory response in wild type pregnant mice 4.5 mg/kg Poly[I:C] or PBS was injected i.p. to either wild type mice on 16.5 dpc. Mice were killed after either 2 or 4 hr and placenta and sera were collected. Cytokine/chemokine concentrations in placental lysates (Placenta) and sera (Serum) were measured. Boxes represent the distance between the first (25%) and third (75%) quartiles and horizontal lines in the boxes represent medians. #Significantly higher than PBS-treated mouse.
Fig. S2. Poly[I:C] injection failed to induce local and systemic inflammatory response in TLR3 knockout pregnant mice. 4.5 mg/kg Poly[I:C] or PBS was injected i.p to either wild type (wt) or TLR3 knockout (TLR3KO) mice on 16.5 dpc. Mice were killed after either 2 or 4 hr and placenta and sera were collected. Cytokine/chemokine concentrations in placental lysates (Placenta) and sera (Serum) were measured. Boxes represent the distance between the first (25%) and third (75%) quartiles and horizontal lines in the boxes represent medians. #Significantly lower than wild type treated mouse, *Significantly higher than PBS-treated mouse.
Acknowledgment
This study was supported in part by the Perinatology Research Branch, Division of Intramural Research, NICHD, NIH, DHHS.
Footnotes
Citation
Koga K, Cardenas I, Aldo P, Abrahams VM, Peng B, Fill S, Romero R, Mor G. Activation of TLR3 in the trophoblast is associated with preterm delivery. Am J Reprod Immunol 2009; 61: 196–212
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.McCormick MC. The contribution of low birth weight to infant mortality and childhood morbidity. N Engl J Med. 1985;312:82–90. doi: 10.1056/NEJM198501103120204. [DOI] [PubMed] [Google Scholar]
- 2.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker D, Illsley R, Vagero D. Today or in the past? The origins of ischaemic heart disease. J Public Health Med. 1993;15:243–248. [PubMed] [Google Scholar]
- 4.Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371:261–269. doi: 10.1016/S0140-6736(08)60136-1. [DOI] [PubMed] [Google Scholar]
- 5.Romero R, Espinoza J, Chaiworapongsa T, Kalache K. Infection and prematurity and the role of preventive strategies. Semin Neonatol. 2002;7:259–274. doi: 10.1016/s1084-2756(02)90121-1. [DOI] [PubMed] [Google Scholar]
- 6.Kawai T, Akira S. Pathogen recognition with Toll-like receptors. Curr Opin Immunol. 2005;17:338–344. doi: 10.1016/j.coi.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 8.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 9.Wang H, Hirsch E. Bacterially-induced preterm labor and regulation of prostaglandin-metabolizing enzyme expression in mice: the role of toll-like receptor 4. Biol Reprod. 2003;69:1957–1963. doi: 10.1095/biolreprod.103.019620. [DOI] [PubMed] [Google Scholar]
- 10.Elovitz MA, Wang Z, Chien EK, Rychlik DF, Phillippe M. A new model for inflammation-induced preterm birth: the role of platelet-activating factor and Toll-like receptor-4. Am J Pathol. 2003;163:2103–2111. doi: 10.1016/S0002-9440(10)63567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu H, Redline RW, Han YW. Fusobacterium nucleatum induces fetal death in mice via stimulation of TLR4-mediated placental inflammatory response. J Immunol. 2007;179:2501–2508. doi: 10.4049/jimmunol.179.4.2501. [DOI] [PubMed] [Google Scholar]
- 12.Guleria I, Pollard JW. The trophoblast is a component of the innate immune system during pregnancy. Nat Med. 2000;6:589–593. doi: 10.1038/75074. [DOI] [PubMed] [Google Scholar]
- 13.Mor G. Inflammation and pregnancy: the role of toll-like receptors in trophoblast-immune interaction. Ann N Y Acad Sci. 2008;1127:121–128. doi: 10.1196/annals.1434.006. [DOI] [PubMed] [Google Scholar]
- 14.Abrahams VM, Visintin I, Aldo PB, Guller S, Romero R, Mor G. A role for TLRs in the regulation of immune cell migration by first trimester trophoblast cells. J Immunol. 2005;175:8096–8104. doi: 10.4049/jimmunol.175.12.8096. [DOI] [PubMed] [Google Scholar]
- 15.Abrahams VM, Bole-Aldo P, Kim YM, Straszewski-Chavez SL, Chaiworapongsa T, Romero R, Mor G. Divergent trophoblast responses to bacterial products mediated by TLRs. J Immunol. 2004;173:4286–4296. doi: 10.4049/jimmunol.173.7.4286. [DOI] [PubMed] [Google Scholar]
- 16.Abrahams VM, Schaefer TM, Fahey JV, Visintin I, Wright JA, Aldo PB, Romero R, Wira CR, Mor G. Expression and secretion of antiviral factors by trophoblast cells following stimulation by the TLR-3 agonist, Poly(I: C). Hum Reprod. 2006;21:2432–2439. doi: 10.1093/humrep/del178. [DOI] [PubMed] [Google Scholar]
- 17.Abrahams VM, Aldo PB, Murphy SP, Visintin I, Koga K, Wilson G, Romero R, Sharma S, Mor G. TLR6 modulates first trimester trophoblast responses to peptidoglycan. J Immunol. 2008;180:6035–6043. doi: 10.4049/jimmunol.180.9.6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schulz LC, Widmaier EP. The effect of leptin on mouse trophoblast cell invasion. Biol Reprod. 2004;71:1963–1967. doi: 10.1095/biolreprod.104.032722. [DOI] [PubMed] [Google Scholar]
- 19.Graham CH, Hawley TS, Hawley RG, MacDougall JR, Kerbel RS, Khoo N, Lala PK. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp Cell Res. 1993;206:204–211. doi: 10.1006/excr.1993.1139. [DOI] [PubMed] [Google Scholar]
- 20.Straszewski-Chavez SL, Abrahams VM, Mor G. The role of apoptosis in the regulation of trophoblast survival and differentiation during pregnancy. Endocr Rev. 2005;26:877–897. doi: 10.1210/er.2005-0003. [DOI] [PubMed] [Google Scholar]
- 21.Hirota Y, Osuga Y, Koga K, Yoshino O, Hirata T, Morimoto C, Harada M, Takemura Y, Nose E, Yano T, Tsutsumi O, Taketani Y. The expression and possible roles of chemokine CXCL11 and its receptor CXCR3 in the human endometrium. J Immunol. 2006;177:8813–8821. doi: 10.4049/jimmunol.177.12.8813. [DOI] [PubMed] [Google Scholar]
- 22.Kaitu'u-Lino TJ, Morison NB, Salamonsen LA. Neutrophil depletion retards endometrial repair in a mouse model. Cell Tissue Res. 2007;328:197–206. doi: 10.1007/s00441-006-0358-2. [DOI] [PubMed] [Google Scholar]
- 23.Nie G, Li Y, He H, Findlay JK, Salamonsen LA. HtrA3, a serine protease possessing an IGF-binding domain, is selectively expressed at the maternal-fetal interface during placentation in the mouse. Placenta. 2006;27:491–501. doi: 10.1016/j.placenta.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 24.Fest S, Aldo PB, Abrahams VM, Visintin I, Alvero A, Chen R, Chavez SL, Romero R, Mor G. Trophoblast-macrophage interactions: a regulatory network for the protection of pregnancy. Am J Reprod Immunol. 2007;57:55–66. doi: 10.1111/j.1600-0897.2006.00446.x. [DOI] [PubMed] [Google Scholar]
- 25.Leung CH, Grill SP, Lam W, Gao W, Sun HD, Cheng YC. Eriocalyxin B inhibits nuclear factor-kappaB activation by interfering with the binding of both p65 and p50 to the response element in a noncompetitive manner. Mol Pharmacol. 2006;70:1946–1955. doi: 10.1124/mol.106.028480. [DOI] [PubMed] [Google Scholar]
- 26.Straszewski SL, Abrahams VM, Funai E, Mor G. XIAP confers human trophoblast cell resistance to Fas-mediated apoptosis. Mol Hum Reprod. 2004;10:33–41. doi: 10.1093/molehr/gah001. [DOI] [PubMed] [Google Scholar]
- 27.Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- 28.Hardy JM, Azarowicz EN, Mannini A, Medearis DN, Jr, Cooke RE. The effect of Asian influenza on the outcome of pregnancy, Baltimore, 1957-1958. Am J Public Health Nations Health. 1961;51:1182–1188. doi: 10.2105/ajph.51.8.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horn P. Poliomyelitis in pregnancy; a twenty-year report from Los Angeles County, California. Obstet Gynecol. 1955;6:121–137. [PubMed] [Google Scholar]
- 30.Gomez LM, Ma Y, Ho C, McGrath CM, Nelson DB, Parry S. Placental infection with human papillomavirus is associated with spontaneous preterm delivery. Hum Reprod. 2008;23:709–715. doi: 10.1093/humrep/dem404. [DOI] [PubMed] [Google Scholar]
- 31.Arechavaleta-Velasco F, Gomez L, Ma Y, Zhao J, McGrath CM, Sammel MD, Nelson DB, Parry S. Adverse reproductive outcomes in urban women with adeno-associated virus-2 infections in early pregnancy. Hum Reprod. 2008;23:29–36. doi: 10.1093/humrep/dem360. [DOI] [PubMed] [Google Scholar]
- 32.Eskild A, Bruu AL, Stray-Pedersen B, Jenum P. Epstein-Barr virus infection during pregnancy and the risk of adverse pregnancy outcome. BJOG. 2005;112:1620–1624. doi: 10.1111/j.1471-0528.2005.00764.x. [DOI] [PubMed] [Google Scholar]
- 33.Kim YM, Romero R, Chaiworapongsa T, Espinoza J, Mor G, Kim CJ. Dermatitis as a component of the fetal inflammatory response syndrome is associated with activation of Toll-like receptors in epidermal keratinocytes. Histopathology. 2006;49:506–514. doi: 10.1111/j.1365-2559.2006.02542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342:1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 35.Lamont RF. The role of infection in preterm labour and birth. Hosp Med. 2003;64:644–647. doi: 10.12968/hosp.2003.64.11.2343. [DOI] [PubMed] [Google Scholar]
- 36.Lamont RF. Infection in the prediction and antibiotics in the prevention of spontaneous preterm labour and preterm birth. BJOG. 2003;110(Suppl 20):71–75. doi: 10.1016/s1470-0328(03)00034-x. [DOI] [PubMed] [Google Scholar]
- 37.Hsu CD, Witter FR. Urogenital infection in preeclampsia. Int J Gynaecol Obstet. 1995;49:271–275. doi: 10.1016/0020-7292(95)02373-k. [DOI] [PubMed] [Google Scholar]
- 38.Raynor BD, Bonney EA, Jang KT, Coto W, Garcia MS. Preeclampsia and Chlamydia pneumoniae: is there a link? Hypertens Pregnancy. 2004;23:129–134. doi: 10.1081/PRG-120028284. [DOI] [PubMed] [Google Scholar]
- 39.von Dadelszen P, Magee LA. Could an infectious trigger explain the differential maternal response to the shared placental pathology of preeclampsia and normotensive intrauterine growth restriction? Acta Obstet Gynecol Scand. 2002;81:642–648. [PubMed] [Google Scholar]
- 40.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 41.Ilievski V, Lu SJ, Hirsch E. Activation of toll-like receptors 2 or 3 and preterm delivery in the mouse. Reprod Sci. 2007;14:315–320. doi: 10.1177/1933719107302959. [DOI] [PubMed] [Google Scholar]
- 42.Zhou R, Wei H, Sun R, Tian Z. Recognition of double-stranded RNA by TLR3 induces severe small intestinal injury in mice. J Immunol. 2007;178:4548–4556. doi: 10.4049/jimmunol.178.7.4548. [DOI] [PubMed] [Google Scholar]
- 43.Murphy SP, Fast LD, Hanna NN, Sharma S. Uterine NK cells mediate inflammation-induced fetal demise in IL-10-null mice. J Immunol. 2005;175:4084–4090. doi: 10.4049/jimmunol.175.6.4084. [DOI] [PubMed] [Google Scholar]
- 44.Kim JS, Romero R, Kim MR, Kim YM, Friel L, Espinoza J, Kim CJ. Involvement of Hofbauer cells and maternal T cells in villitis of unknown aetiology. Histopathology. 2008;52:457–464. doi: 10.1111/j.1365-2559.2008.02964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Romero R, Manogue KR, Mitchell MD, Wu YK, Oyarzun E, Hobbins JC, Cerami A. Infection and labor. IV. Cachectin-tumor necrosis factor in the amniotic fluid of women with intraamniotic infection and preterm labor. Am J Obstet Gynecol. 1989;161:336–341. doi: 10.1016/0002-9378(89)90515-2. [DOI] [PubMed] [Google Scholar]
- 46.El-Bastawissi AY, Williams MA, Riley DE, Hitti J, Krieger JN. Amniotic fluid interleukin-6 and preterm delivery: a review. Obstet Gynecol. 2000;95:1056–1064. [PubMed] [Google Scholar]
- 47.Romero R, Ceska M, Avila C, Mazor M, Behnke E, Lindley I. Neutrophil attractant/activating peptide-1/interleukin-8 in term and preterm parturition. Am J Obstet Gynecol. 1991;165:813–820. doi: 10.1016/0002-9378(91)90422-n. [DOI] [PubMed] [Google Scholar]
- 48.Huang SJ, Chen CP, Schatz F, Rahman M, Abrahams VM, Lockwood CJ. Pre-eclampsia is associated with dendritic cell recruitment into the uterine decidua. J Pathol. 2008;214:328–336. doi: 10.1002/path.2257. [DOI] [PubMed] [Google Scholar]
- 49.Hansen WR, Keelan JA, Skinner SJ, Mitchell MD. Key enzymes of prostaglandin biosynthesis and metabolism. Coordinate regulation of expression by cytokines in gestational tissues: a review. Prostaglandins Other Lipid Mediat. 1999;57:243–257. doi: 10.1016/s0090-6980(99)00008-8. [DOI] [PubMed] [Google Scholar]
- 50.Keelan JA, Blumenstein M, Helliwell RJ, Sato TA, Marvin KW, Mitchell MD. Cytokines, prostaglandins and parturition–a review. Placenta. 2003;24(Suppl A):S33–S46. doi: 10.1053/plac.2002.0948. [DOI] [PubMed] [Google Scholar]
- 51.Straszewski-Chavez SL, Visintin IP, Karassina N, Los G, Liston P, Halaban R, Fadiel A, Mor G. XAF1 mediates tumor necrosis factor-alpha-induced apoptosis and X-linked inhibitor of apoptosis cleavage by acting through the mitochondrial pathway. J Biol Chem. 2007;282:13059–13072. doi: 10.1074/jbc.M609038200. [DOI] [PubMed] [Google Scholar]
- 52.Mor G, Romero R, Aldo PB, Abrahams VM. Is the trophoblast an immune regulator?: the role of toll-like receptors during pregnancy. Crit Rev Immunol. 2005;25:375–388. doi: 10.1615/critrevimmunol.v25.i5.30. [DOI] [PubMed] [Google Scholar]
- 53.Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, Yamaguchi O, Otsu K, Tsujimura T, Koh CS, Reis CeSousa, Matsuura Y, Fujita T, Akira S. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Fig. S1. Poly[I:C] injection induced robust local and systemic inflammatory response in wild type pregnant mice 4.5 mg/kg Poly[I:C] or PBS was injected i.p. to either wild type mice on 16.5 dpc. Mice were killed after either 2 or 4 hr and placenta and sera were collected. Cytokine/chemokine concentrations in placental lysates (Placenta) and sera (Serum) were measured. Boxes represent the distance between the first (25%) and third (75%) quartiles and horizontal lines in the boxes represent medians. #Significantly higher than PBS-treated mouse.
Fig. S2. Poly[I:C] injection failed to induce local and systemic inflammatory response in TLR3 knockout pregnant mice. 4.5 mg/kg Poly[I:C] or PBS was injected i.p to either wild type (wt) or TLR3 knockout (TLR3KO) mice on 16.5 dpc. Mice were killed after either 2 or 4 hr and placenta and sera were collected. Cytokine/chemokine concentrations in placental lysates (Placenta) and sera (Serum) were measured. Boxes represent the distance between the first (25%) and third (75%) quartiles and horizontal lines in the boxes represent medians. #Significantly lower than wild type treated mouse, *Significantly higher than PBS-treated mouse.