Abstract
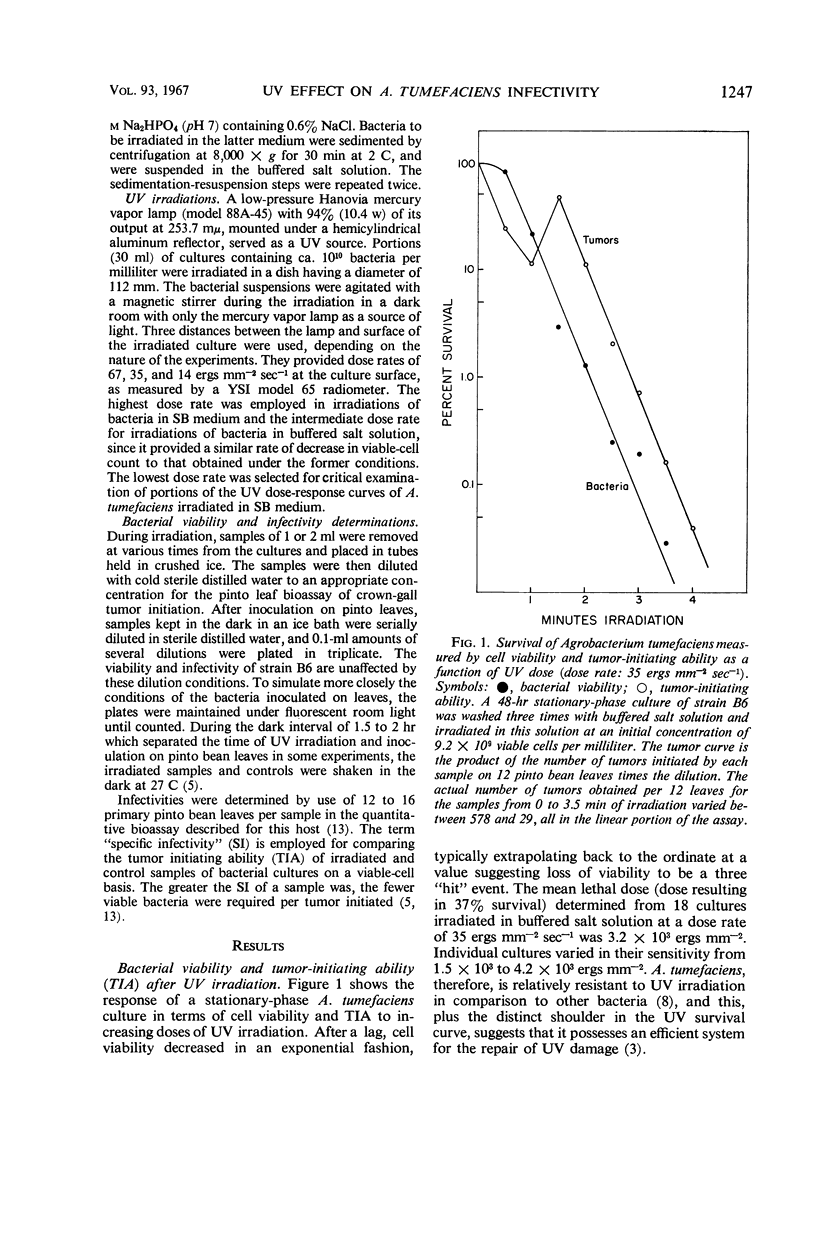
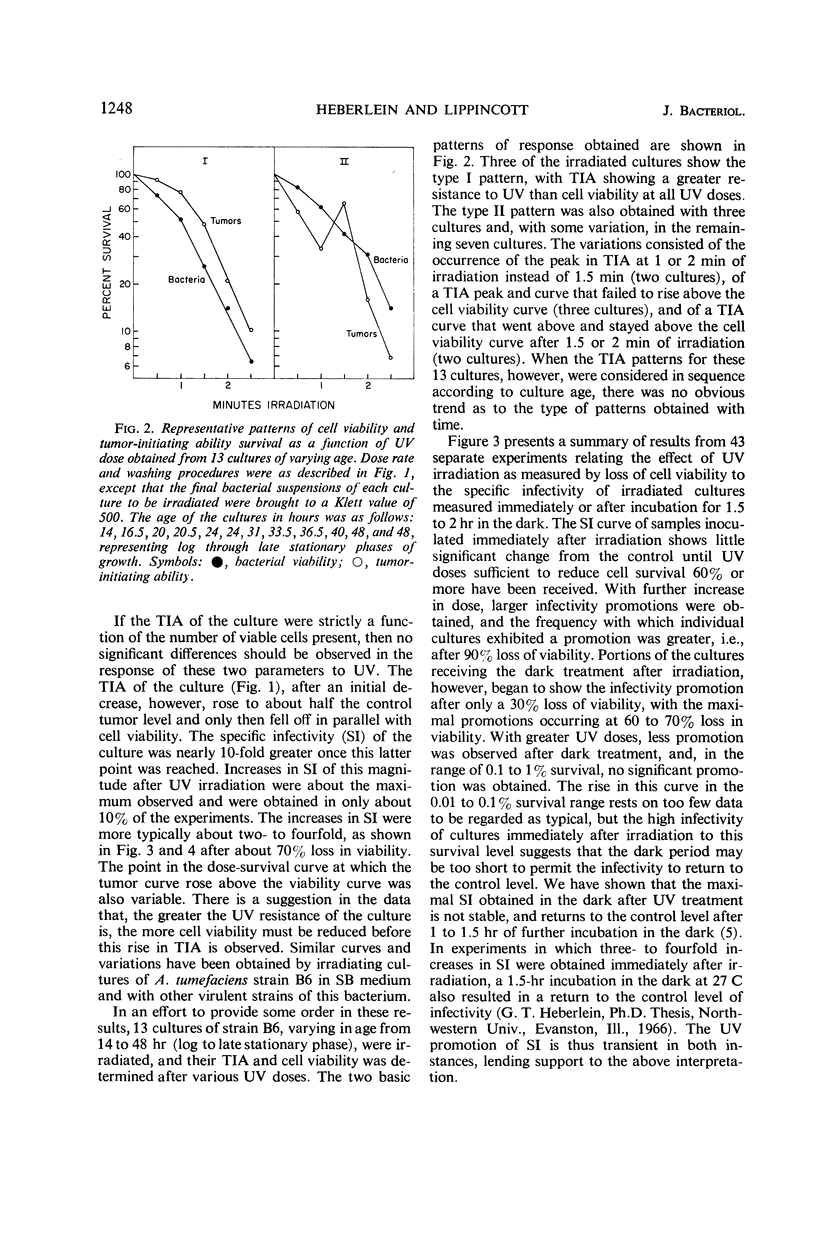
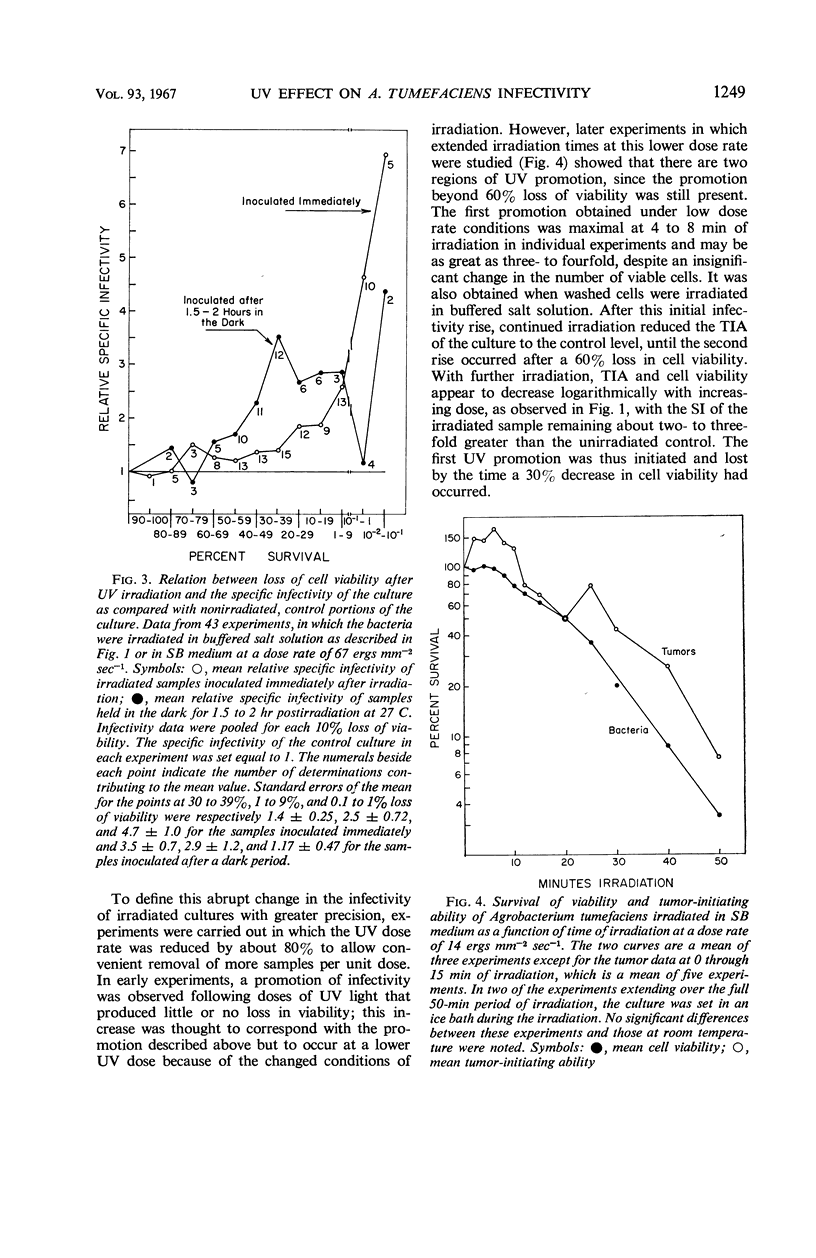
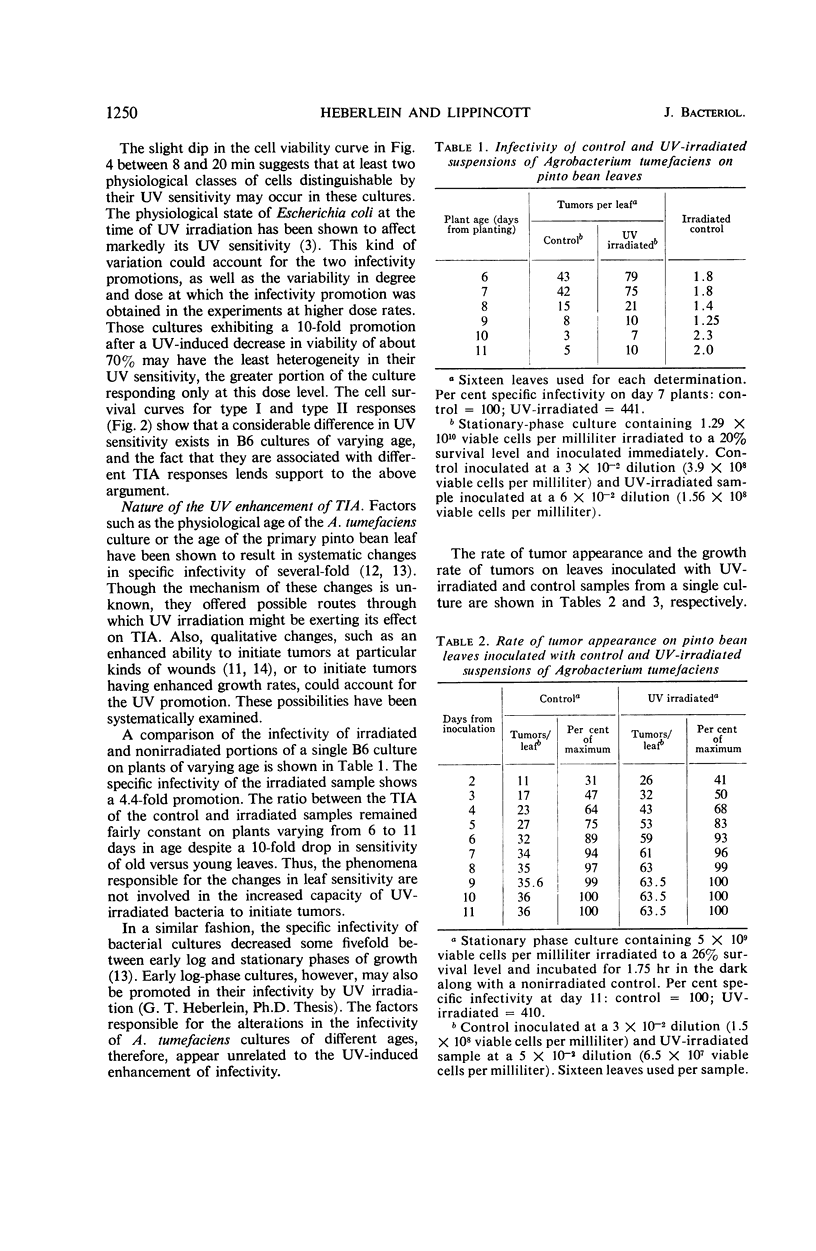
The infectivity of Agrobacterium tumefaciens strain B6 irradiated with short-wavelength ultraviolet light was followed as a function of dose. Previously reported enhancements of B6 infectivity by ultraviolet irradiation, in samples inoculated after 1.75 hr of dark incubation at 27 C, or immediately following irradiation, were found to occur most frequently after losses in cell viability of 60% and of 90% or more, respectively. Changes in colony-forming ability and tumor-initiating ability with increasing dose showed no obvious correlation until the maximal infectivity promotion of samples inoculated immediately after irradiation was reached. Thereafter, both bacterial responses typically decreased in parallel. With low dose rates, infectivity promotions were obtained with less than 10% loss in cell viability. Data for tumor appearance and tumor growth resulting from inoculations with irradiated cultures showed no significant differences from controls, nor did the age of the bacterial culture or age of the host plant influence the response. The infectivity promotion appears to result from an increase in the proportion of viable cells that will subsequently initiate tumors. The characteristics of this ultraviolet infectivity promotion are shown to be most similar to those found in prophage and bacteriocin induction.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALPER T., GILLIES N. E. The relationship between growth and survival after irradiation of Escherichia coli strain B and two resistant mutants. J Gen Microbiol. 1960 Feb;22:113–128. doi: 10.1099/00221287-22-1-113. [DOI] [PubMed] [Google Scholar]
- HEBERLEIN G. T., LIPPINCOTT J. A. PHOTOREVERSIBLE ULTRAVIOLET ENHANCEMENT OF INFECTIVITY IN AGROBACTERIUM TUMEFACIENS. J Bacteriol. 1965 Jun;89:1511–1514. doi: 10.1128/jb.89.6.1511-1514.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL R. F. Dose-mutation relationships in ultraviolet-induced reversion from auxotrophy in Escherichia coli. J Gen Microbiol. 1963 Feb;30:281–287. doi: 10.1099/00221287-30-2-281. [DOI] [PubMed] [Google Scholar]
- Hanawalt P. C. The U.V. sensitivity of bacteria: its relation to the DNA replication cycle. Photochem Photobiol. 1966 Jan;5(1):1–12. [PubMed] [Google Scholar]
- Harm W. Host-cell reactivation of non-lethal ultraviolet-effects. Photochem Photobiol. 1965 Jun;4(3):575–585. doi: 10.1111/j.1751-1097.1965.tb09776.x. [DOI] [PubMed] [Google Scholar]
- Hill R. F. Ultraviolet-induced lethality and reversion to prototrophy in Escherichia coli strains with normal and reduced dark repair ability. Photochem Photobiol. 1965 Jun;4(3):563–568. doi: 10.1111/j.1751-1097.1965.tb09774.x. [DOI] [PubMed] [Google Scholar]
- JAGGER J. Photoreactivation. Bacteriol Rev. 1958 Jun;22(2):99–142. doi: 10.1128/br.22.2.99-142.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIEB M. DARK REPAIR OF UV INDUCTION IN K12 (LAMBDA). Virology. 1964 Jul;23:381–388. doi: 10.1016/0042-6822(64)90260-0. [DOI] [PubMed] [Google Scholar]
- LIPPINCOTT J. A., HEBERLEIN G. T. THE INDUCTION OF LEAF TUMORS BY AGROBACTERIUM TUMEFACIENS. Am J Bot. 1965 Apr;52:396–403. [PubMed] [Google Scholar]
- LWOFF A. Lysogeny. Bacteriol Rev. 1953 Dec;17(4):269–337. doi: 10.1128/br.17.4.269-337.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott B. B., Lippincott J. A. Characteristics of Agrobacterium tumefaciens auxotrophic mutant infectivity. J Bacteriol. 1966 Oct;92(4):937–945. doi: 10.1128/jb.92.4.937-945.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott J. A., Heberlein G. T. The quantitative determination of the infectivity of Agrobacterium tumefaciens. Am J Bot. 1965 Sep;52(8):856–863. [PubMed] [Google Scholar]
- Lippincott J. A., Lippincott B. B. Timing of events in crown-gall tumor development on Pinto bean leaves. Dev Biol. 1965 Oct;12(2):309–327. doi: 10.1016/0012-1606(65)90033-3. [DOI] [PubMed] [Google Scholar]
- MELECHEN N. E., SKAAR P. D. The provocation of an early step of induction by thymine deprivation. Virology. 1962 Jan;16:21–29. doi: 10.1016/0042-6822(62)90198-8. [DOI] [PubMed] [Google Scholar]
- Mennigmann H. D. Induction in Escherichia coli 15 of the colicinogenic factor by thymine-less death. Biochem Biophys Res Commun. 1964 Jul 1;16(4):373–378. doi: 10.1016/0006-291x(64)90043-9. [DOI] [PubMed] [Google Scholar]
- RUPERT C. S., GOODGAL S. H., HERRIOTT R. M. Photoreactivation in vitro of ultraviolet-inactivated Hemophilus influenzae transforming factor. J Gen Physiol. 1958 Jan 20;41(3):451–471. doi: 10.1085/jgp.41.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SETLOW J. K., DUGGAN D. E. THE RESISTANCE OF MICROCOCCUS RADIODURANS TO ULTRAVIOLET RADIATION. I. ULTRAVIOLET-INDUCED LESIONS IN THE CELL'S DNA. Biochim Biophys Acta. 1964 Aug 12;87:664–668. doi: 10.1016/0926-6550(64)90284-1. [DOI] [PubMed] [Google Scholar]
- SETLOW R. B., CARRIER W. L. THE DISAPPEARANCE OF THYMINE DIMERS FROM DNA: AN ERROR-CORRECTING MECHANISM. Proc Natl Acad Sci U S A. 1964 Feb;51:226–231. doi: 10.1073/pnas.51.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow R. B. Cyclobutane-type pyrimidine dimers in polynucleotides. Science. 1966 Jul 22;153(3734):379–386. doi: 10.1126/science.153.3734.379. [DOI] [PubMed] [Google Scholar]
- WITKIN E. M. Modification of mutagenesis initiated by ultraviolet light through postteatment of bacteria with basic dyes. J Cell Comp Physiol. 1961 Dec;58(3):135–144. doi: 10.1002/jcp.1030580413. [DOI] [PubMed] [Google Scholar]


