Abstract
Olfactory cues play decisive roles in the lives of most insect species, providing information about biologically relevant resources, such as food, mates, and oviposition sites. The nocturnal moth Manduca sexta feeds on floral nectar from a variety of plants (and thus serves as a pollinator), but females oviposit almost exclusively on solanaceous plants, which they recognize on the basis of olfactory cues. Plants, however, respond to herbivory by releasing blends of volatiles that attract natural enemies of herbivores. Thus, oviposition behavior probably results from the sensory evaluation not only of attractive host plant volatiles but also of repellent volatiles that indicate the acceptability or inappropriateness, respectively, of host plants for the females’ offspring. Here we describe results from chemical-ecological, neurophysiological, and behavioral experiments aimed at understanding the neural mechanisms that control oviposition behavior in M. sexta.
Keywords: olfaction, insect, herbivory, moth, Manduca sexta, oviposition, insect
Olfactory cues play decisive roles in the lives of most insect species, providing information about biologically relevant resources, such as food, mates, and oviposition sites.1, 2 Neurobiological experimentation in a naturalistic context (e.g., using behaviorally relevant stimuli) is essential for discovering how neural circuits produce behavior. Here we discuss results from ongoing experiments aimed at discovering the neural representations of olfactory stimuli that elicit oviposition behavior in the sphinx moth Manduca sexta.
M. sexta is a nocturnally active insect that uses olfactory cues to find mates, flowers on which to feed, and appropriate host plants on which to lay eggs. Although male and female adults of this moth feed on nectar from a variety of plants, females oviposit almost exclusively on solanaceous plants,3-6 a behavior mediated primarily by olfactory cues.7-9 In the south-western United States, Datura wrightii plants attract M. sexta as pollinators, and adults have a strong innate feeding preference for its flowers.10 D. wrightii also is a host plant for M. sexta larvae.6
In another Datura species, the annual D. stramonium, artificially increasing the nectar levels in flowers increases oviposition rates by M. sexta and increases fruit and seed set.11 Although larvae consume and develop on these plants and have physiological adaptations to cope with the toxic alkaloids that they contain,12-14 the plants benefit from being cross-pollinated by the adults11 and can tolerate high levels of defoliation and quickly regrow after herbivory. Thus, the Datura-Manduca relationship is a mutualistic system in which plant-herbivore and plant-pollinator interactions are linked11 but still are far from being completely understood. We have asked which sensory stimuli control oviposition behavior in the specialist herbivorous insect M. sexta and how they are represented in the female moth’s brain.
Neural Basis of Oviposition Behavior and the Importance of [+]Linalool
Two large female-specific glomeruli (LFGs) are prominent in the antennal lobes of adult female M. sexta.15 Because they are present only in females, the LFGs are expected to subserve female-specific behaviors, such as the location and/or selection of appropriate host plants for oviposition. Glomerular output (projection) neurons (PNs) in the two LFGs (Fig. 1A, B) respond to volatile organic chemicals (VOCs) from plants.16 In particular, PNs in the lateral LFG (latLFG) respond preferentially to antennal stimulation with [±]linalool,16 and particularly, to the [+] enantiomer of this odor compound17 (Fig.1C). In males, both the two main glomeruli of the male-specific macroglomerular complex are sites of primary processing of olfactory information about one of the two main components of the conspecific females’ sex pheromone. Those glomeruli interact through synaptic connections among local interneurons and PNs that have arborizations in the glomeruli18, 19 and apparently “bind” features of those individual pheromone components for further processing in higher-order brain centers downstream from the antennal lobes.18 In females, we found a similar relationship between the LFGs, as medial LFG (medLFG) PNs are hyperpolarized by stimulation with [±]linalool (Fig. 1D, middle panel), presumably via lateral synaptic input from the adjacent latLFG. We have not yet found an odor substance that excites the medLFG PNs at physiological concentrations, however, and therefore have not been able to test for reciprocal interactions between these identified glomeruli. Nevertheless, our findings suggest that interactions between the two LFGs may parallel those between the two main macroglomerular complex glomeruli and that the LFGs play significant roles in the processing of specific host plant volatiles possibly involved in oviposition behavior.
Figure 1.
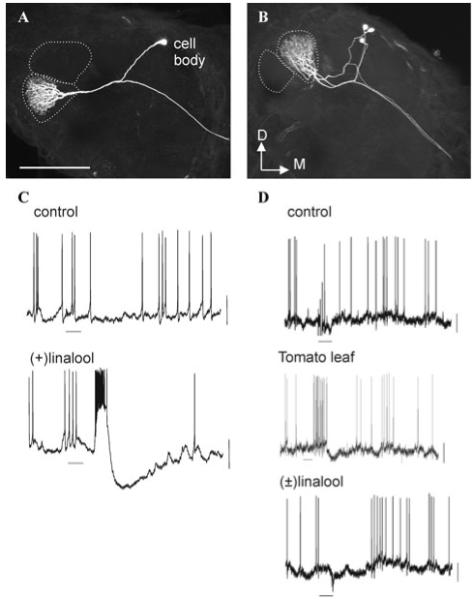
Laser-scanning confocal microscopic images of female-specific output neurons with arborizations confined to the lateral large female glomerulus (latLFG, A) and the medial large female glomerulus (medLFG, B). Dotted lines indicate the outline of the female-specific glomeruli. Scale bar, 200 μm. D, dorsal; M, medial. C, D: Electrophysiological recordings obtained from neurons in a latLFG (C) and a medLFG (D) in response to the odors indicated at a concentration of 10-3 vol/vol. Neurons were stimulated as described previously.17
What is the behavioral significance of linalool for M. sexta moths? Linalool is found among the many VOCs emitted by plants, including host plants of M. sexta.20, 21 Although the LFGs probably participate in the sensory assessment of potential oviposition sites, these glomeruli might also process information about components of a putative pheromone produced by a metathoracic androconial organ in the male moth.22 [+]Linalool is a component of a male pheromone in another moth species,23 but our chemical analysis of the VOCs produced by the male scent organ in M. sexta has found para-and meta-cresol (among other components) but not linalool (unpublished findings).
Oviposition Deterrents Emitted by Plants: A Contrast to [+]Linalool
VOCs released by host plants change in response to environmental factors, such as herbivory and time of day. For instance, plant species may respond to herbivory by producing defensive proteins that slow larval growth24 and by releasing blends of VOCs that provide host location cues for insects that are natural enemies of the herbivores.25-31 Thus, females avoid ovipositing on such “induced” plants because they are likely to host herbivores that would compete with their own offspring and to have attracted parasitoids that attack eggs and larvae.32 Evidence from several systems,33-35 including the Manduca quinquemaculata-Nicotiana attenuata (wild tobacco) relationship,32 has shown that females avoid ovipositing on plants damaged by larval feeding and that olfactory cues mediate this avoidance (but see Ref. 36). Thus, oviposition behavior probably results from the sensory evaluation not only of attractive host plant VOCs but also of repellent VOCs that indicate the acceptability or inappropriateness, respectively, of host plants for the females’ offspring.
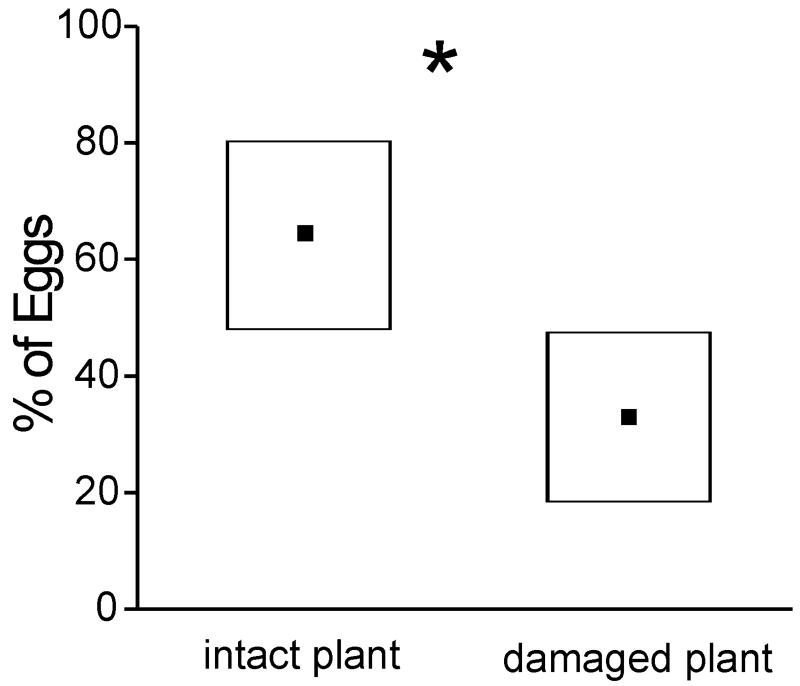
Host-derived olfactory cues mediate oviposition behavior in M. sexta.4, 7, 9, 21 Do M. sexta females also avoid ovipositing on plants damaged by larval feeding, and if so, what olfactory cues mediate this behavior? To investigate this possibility, we mated M. sexta adult females 1.5 days posteclosion and tested them individually during the first 2-2.5 h of the scotophase of the following night in 2 × 2 × 2 m flight cages. In each trial, two Lycopersicon esculentum (tomato) plants were positioned in the arena 1.5 m apart. Each female was allowed to lay eggs during 10 min after takeoff, and we collected and counted the eggs on each plant. Each female was used only once, and we discarded those that did not oviposit. Each moth was presented with an intact tomato plant and one that had been damaged by larval feeding for 4 days by 5-10 first-to-fourth-instar M. sexta larvae. We used the tomato plant because it is a preferred host plant for M. sexta but does not offer nectar—that is, adult moths use it only for oviposition and not for feeding. We found that 70% of females laid eggs first on the undamaged plant and that overall they laid statistically significant more eggs on the undamaged plant than on the plant damaged by larval herbivory (Fig. 2).
Figure 2.
Proportion of eggs (black symbols, median; boxes, 25% percentiles; n = 18) laid by individual mated females presented with an intact tomato plant and a larva-damaged tomato plant. *, statistically significant differences (P < 0.001, Wilcoxon matched pairs test).
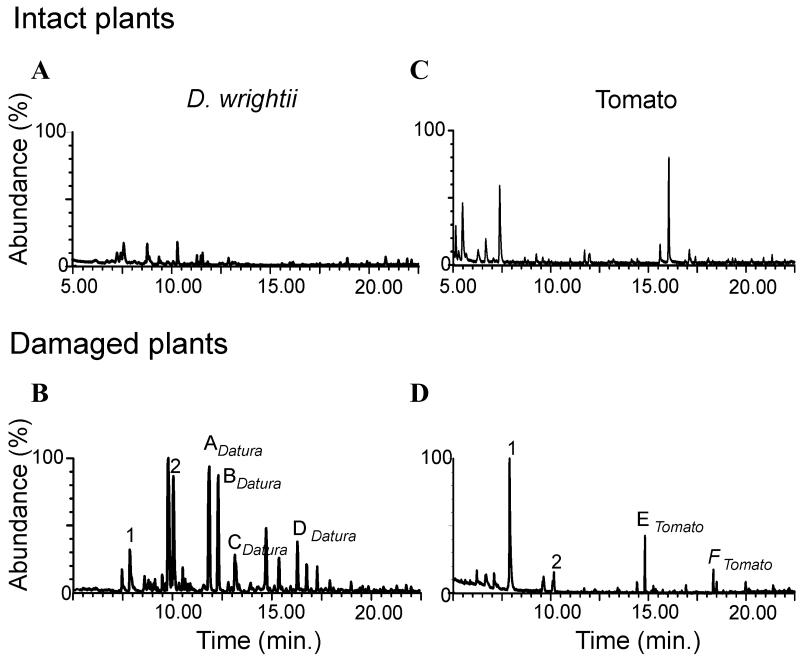
We investigated the VOCs released by undamaged D. wrightii and L. esculentum plants and larva-damaged plants on which M. sexta larvae had been allowed to feed for 72 h. Potted, undamaged or larva-damaged plants were placed in the glass chamber of a volatile collection system (Analytical Research Systems, Gainesville, FL), andclean filteredair was forced through an adsorbent-cartridge trap as described previously.21 We trapped VOCs overnight, eluted them with n-hexane, and analyzed them by gas chromatography with mass spectrometry. We verified the identities of compounds by matching retention times with purified synthetic standards and by mass-spectrometric comparisons using the National Institute of Standards and Technology database. Herbivory-induced changes in the VOC profile revealed similarities between plant species (peaks 1-2, Fig. 3). Moreover, we observed differences in the profiles of induced VOCs between the two solanaceous species (Fig. 3), with jimsonweed plants producing aromatics (A, B, and CDatura, respectively) and a sesquiterpene (DDatura) and tomato plants producing sesquiterpenes (Etomato and Ftomato). We also collected and analyzed VOCs from damaged plants 1 week after larvae had been removed and found a VOC profile similar to that from undamaged plants (data not shown). Moreover, the VOCs released by larva-damaged plants were different from those released from mechanically damaged plants.
Figure 3.
Plants respond to herbivory by releasing characteristic VOCs. Representative ion chromatograms of the headspace from intact D. wrightii vegetation (A), larva-damaged D. wrightii vegetation (B), intact tomato (L. esculentum) (C), and larva-damaged L. esculentum (D). In C and D the same numbers indicate common compounds; letters indicate different compounds released by D. wrightii and L. esculentum.
The results presented here indicate that the VOCs produced by larva-damaged host plants are different from the VOCs emitted by intact plants and are species specific (Fig. 3). Also, female moths avoid oviposition on such “herbivory induced” plants (Fig. 2). These chemical-ecological and behavioral findings provide a biological basis for studies of the neural mechanisms that allow a specialist insect, such as M. sexta, to evaluate host-derived VOCs. The physiological and odor response characteristics of LFG PNs (Fig. 1) suggest that these neurons play substantial roles in the processing of host-derived VOCs that mediate attraction for oviposition or possibly repellency, deterrence, or unattractiveness for a moth seeking an oviposition site. We expect that our ongoing studies of these insect-plant systems will enable us to uncover the olfactory neural mechanisms underlying oviposition behavior in M. sexta, on the basis of the prediction that olfactory function of the moth’s brain is adapted to naturally occurring, behaviorally relevant, volatile stimuli.
Acknowledgments
This work received financial support from NIH Grant R01-DC-02751 to J.G.H. and NSF-IOS 0822709 to C.E.R. and J.A.R. We thank Abreeza Zeeger for rearing and maintaining the plants, Suzanne Mackzum for rearing M. sexta, and members of the Hildebrand laboratory for helpful discussions and suggestions.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Hildebrand JG. Analysis of chemical signals by nervous systems. Proc. Natl. Acad. Sci. USA. 1995;92:67–74. doi: 10.1073/pnas.92.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dahanukar A, Hallem EA, Carlson JR. Insect chemoreception. Curr. Opin. Neurobiol. 2005;15:423–430. doi: 10.1016/j.conb.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Madden AH, Chamberlin FS. Biology of the tobacco hornworm in the southern cigar tobacco district. U.S. Dep. Agric. Tech. Bull. 896. 1945 [Google Scholar]
- 4.Yamamoto RT, Fraenkel GS. The specificity of the tobacco hornworm, Protoparce sexta, to solanaceous plants. Ann. Entomol. Soc. Am. 1960;53:503–507. [Google Scholar]
- 5.Tichenor LH, Seigler DS. Electroantennogram and oviposition responses of Manduca sexta to volatile components of tobacco and tomato. J. Insect Physiol. 1980;26:309–314. [Google Scholar]
- 6.Mechaber W, Hildebrand JG. Novel, nonsolanaceous host-plant record for Manduca sexta (Lepidoptera: Sphingidae) in the southwestern United States. Ann. Entomol. Soc. Am. 2000;93:447–451. [Google Scholar]
- 7.Yamamoto RT, Jenkins RY, McClusky RK. Factors determining the selection of plants for oviposition by the tobacco hornworm Manduca sexta. Entomol. Exp. Appl. 1969;12:504–508. [Google Scholar]
- 8.Sparks MR. A surrogate leaf for oviposition by the tobacco hornworm. J. Econ. Entomol. 1969;63:537–540. [Google Scholar]
- 9.Mechaber WL, Capaldo CT, Hildebrand JG. Behavioral responses of adult female tobacco hornworms, Manduca sexta, to hostplant volatiles change with age and mating status. J. Insect Sci. 2002;2:5. [PMC free article] [PubMed] [Google Scholar]
- 10.Riffell JA, Alarcón L, Abrell JL, et al. Behavioral consequences of innate preferences and olfactory learning in hawkmoth-flower interactions. Proc. Natl. Acad. Sci. USA. 2008;105:3404–3409. doi: 10.1073/pnas.0709811105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adler LS, Bronstein JL. Attracting antagonists: does floral nectar increase leaf herbivory? Ecology. 2004;85:1529–1526. [Google Scholar]
- 12.Wink M, Theile V. Alkaloid tolerance in Manduca sexta and phylogenetically related sphingids (Lepidoptera: Sphingidae) Chemoecology. 2006;12:29–46. [Google Scholar]
- 13.Kester KM, Peterson SC, Hanson F, et al. The roles of nicotine and natural enemies in determining larval feeding site distributions of Manduca sexta L. and Manduca quinquemaculata (Haworth) on tobacco. Chemoecology. 2002;12:1–10. [Google Scholar]
- 14.Glendinning JI. How do herbivorous insects cope with noxious secondary plant compounds in their diet? Entomol. Exp. Appl. 2002;104:15–25. [Google Scholar]
- 15.Rospars JP, Hildebrand JG. Sexually dimorphic and isomorphic glomeruli in the antennal lobes of the sphinx moth Manduca sexta. Chem. Senses. 2000;25:119–129. doi: 10.1093/chemse/25.2.119. [DOI] [PubMed] [Google Scholar]
- 16.Roche King J, Christensen TA, Hildebrand JG. Response characteristics of an identified, sexually dimorphic olfactory glomerulus. J. Neurosci. 2000;20:2391–2399. doi: 10.1523/JNEUROSCI.20-06-02391.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reisenman CE, Christensen TA, Francke W, Hildebrand JG. Enantioselectivity of projection neurons innervating identified olfactory glomeruli. J. Neurosci. 2004;24:2602–2611. doi: 10.1523/JNEUROSCI.5192-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lei H, Christensen TA, Hildebrand JG. Local inhibition modulates odor-evoked synchronization of glomerulus-specific output neurons. Nat. Neurosci. 2002;5:557–565. doi: 10.1038/nn0602-859. [DOI] [PubMed] [Google Scholar]
- 19.Heinbockel T. Ph.D. thesis. University of Arizona; Tucson, AZ: 1997. Functional organization of male-specific olfactory glomeruli in the sphinx moth Manduca sexta. [Google Scholar]
- 20.Raguso RA, Levin RA, Foose SE, et al. Fragrance chemistry, nocturnal rhythms and pollination “syndromes” in Nicotiana. Phytochemistry. 2003;63:265–284. doi: 10.1016/s0031-9422(03)00113-4. [DOI] [PubMed] [Google Scholar]
- 21.Fraser AM, Mechaber W, Hildebrand JG. Electroantennographic and behavioral responses of the sphinx moth Manduca sexta to host plant headspace volatiles. J. Chem. Ecol. 2003;29:1813–1833. doi: 10.1023/a:1024898127549. [DOI] [PubMed] [Google Scholar]
- 22.Birch MC, Poppy GM, Baker TC. Scents and eversible scent structures of male moths. Annu. Rev. Entomol. 1990;35:25–58. [Google Scholar]
- 23.Landolt PJ, Heath RR. Sexual role reversal in mate-finding strategies of the cabbage looper moth. Science. 1990;249:1026–1028. doi: 10.1126/science.249.4972.1026. [DOI] [PubMed] [Google Scholar]
- 24.Hare J, Walling L. Constitutive and jasmonate-inducible traits of Datura wrightii. J. Chem. Ecol. 2006;32:29–47. doi: 10.1007/s10886-006-9349-8. [DOI] [PubMed] [Google Scholar]
- 25.Baldwin IT, Preston CA. The ecophysiological complexity of plant responses to insect herbivores. Planta. 1999;208:137–145. [Google Scholar]
- 26.Halitschke R, Kebler A, Kahl J, et al. Ecophysiological comparison of direct and indirect defenses in Nicotiana attenuata. Oecologia. 2000;124:408–417. doi: 10.1007/s004420000389. [DOI] [PubMed] [Google Scholar]
- 27.Dicke M, Van Loop JJA. Multitrophic effects of herbivore-induced plant volatiles in an evolutionary context. Ent. Exp. Appl. 2000;97:237–249. [Google Scholar]
- 28.Turlings TCJ, Bernasconi ML, Bertossa R, et al. The induction of volatile emissions in maize by three herbivore species with different feeding habits: possible consequences for their natural enemies. Biol. Control. 1998;11:122–129. [Google Scholar]
- 29.Paré PW, Tumlinson JH. Plant volatiles as a defense against insect herbivores. Plant Physiol. 1999;121:325–331. [PMC free article] [PubMed] [Google Scholar]
- 30.Schnee C, Kollner TG, Held M, et al. The products of a single maize sesquiterpene synthase form a volatile defense signal that attracts natural enemies of maize herbivores. Proc. Natl. Acad. Sci. USA. 2006;103:1129–1134. doi: 10.1073/pnas.0508027103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Moraes CM, Lewis WJ, Paré PW, et al. Herbivore-infested plants selectively attract parasitoids. Nature. 1998;393:570–573. [Google Scholar]
- 32.Kessler A, Baldwin IT. Defensive function of herbivore-induced plant volatile emissions in nature. Science. 2001;291:2141–2144. doi: 10.1126/science.291.5511.2141. [DOI] [PubMed] [Google Scholar]
- 33.Landolt PJ. Effects of host plant leaf damage on cabbage looper moth attraction and oviposition. Entomol. Exp. Appl. 1993;67:79–85. [Google Scholar]
- 34.De Moraes CM, Mescher MC, Tumlinson JH. Caterpillar-induced nocturnal plant volatiles repel conspecific females. Nature. 2001;410:577–580. doi: 10.1038/35069058. [DOI] [PubMed] [Google Scholar]
- 35.Anderson P, Alborn H. Effects on oviposition behaviour and larval development of Spodoptera littoralis by herbivore-induced changes in cotton plants. Entomol. Exp. Appl. 1999;92:45–51. [Google Scholar]
- 36.Adler LS, Wink MD, Lentz AJ. Leaf herbivory and nutrients increase nectar alkaloids. Ecol. Lett. 2006;9:960–967. doi: 10.1111/j.1461-0248.2006.00944.x. [DOI] [PubMed] [Google Scholar]