Abstract
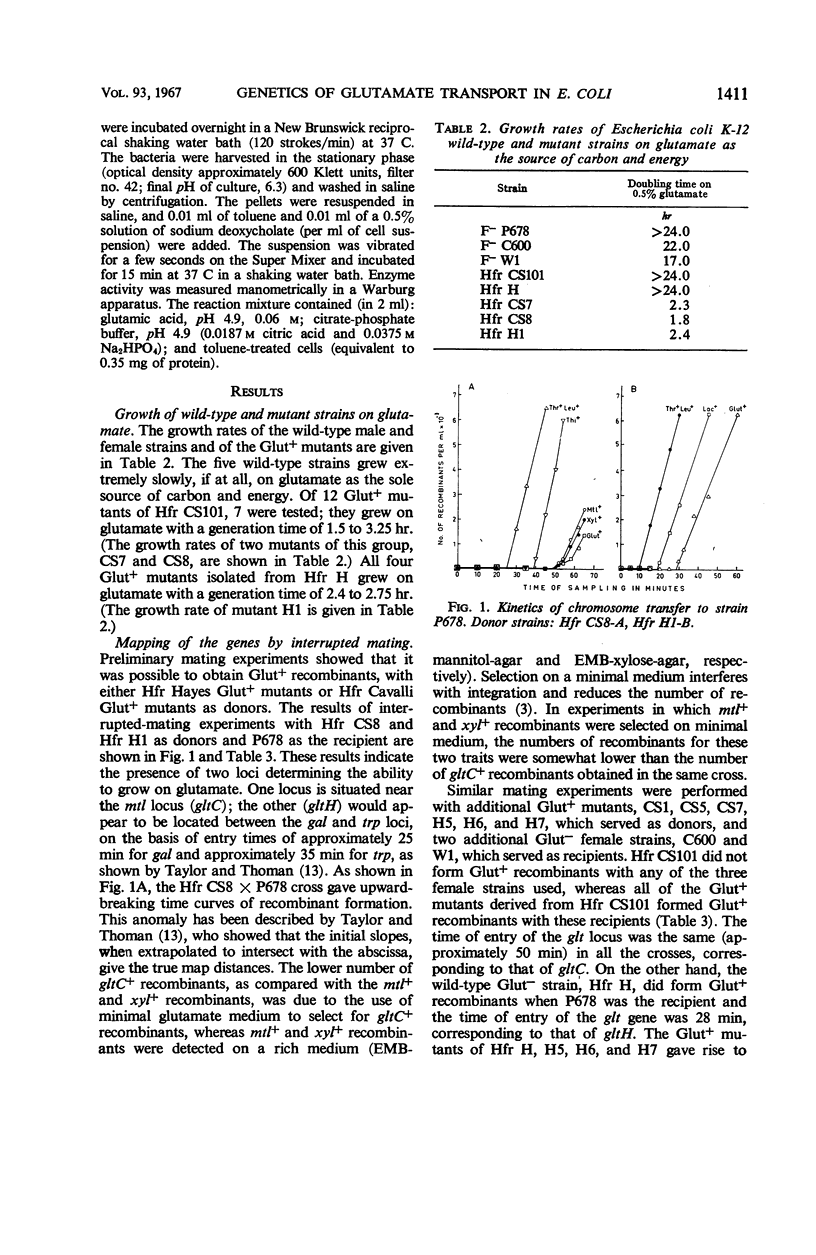
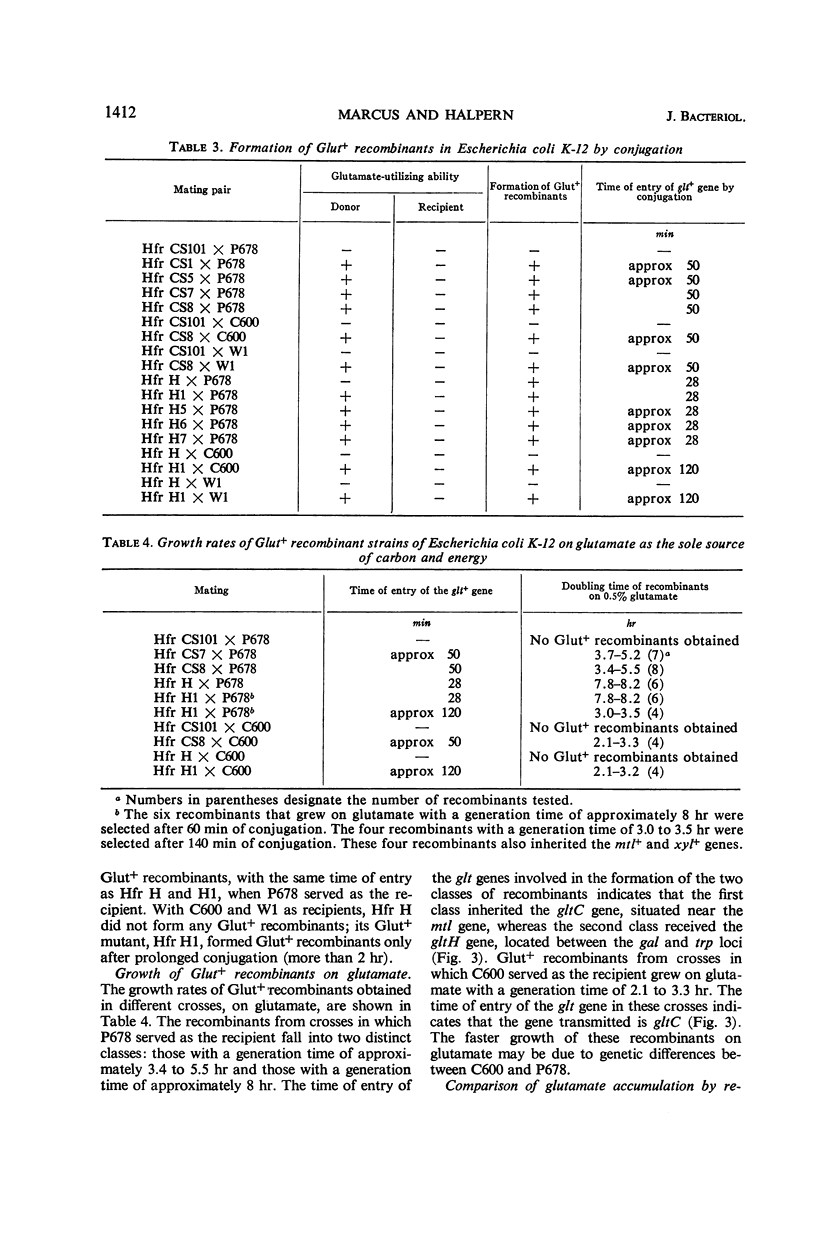
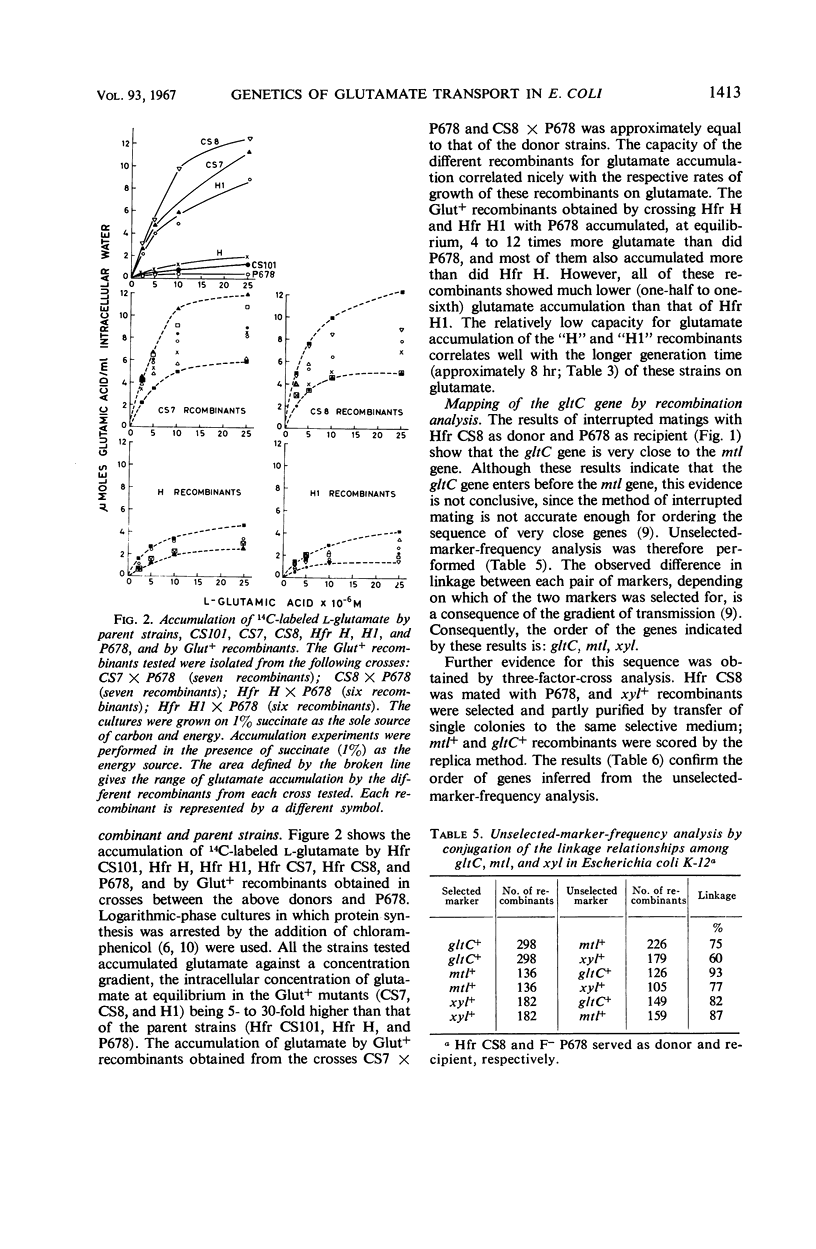
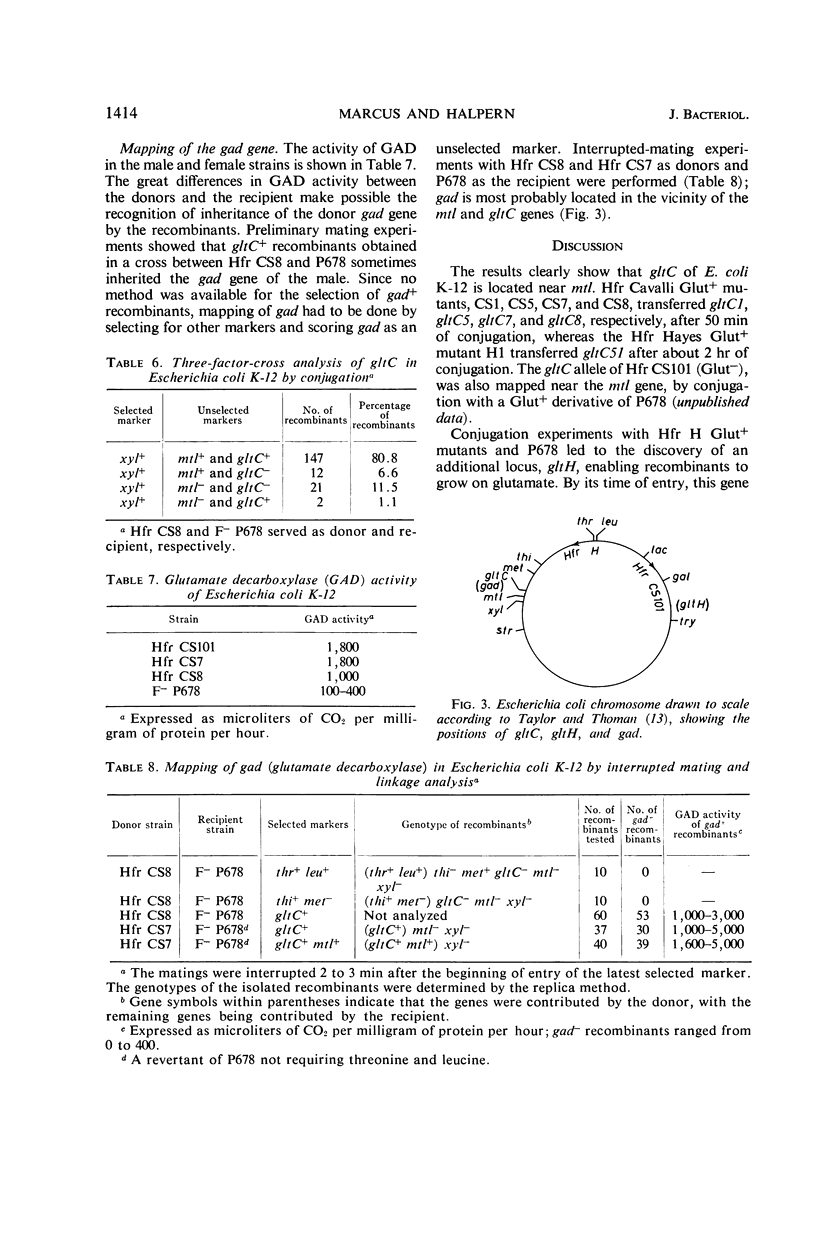
The location of the Escherichia coli K-12 genes determining or regulating glutamate transport, and the location of the gene determining glutamate decarboxylase synthesis, were established by conjugation. The ability to grow on glutamate as the sole source of carbon and energy was used to select for glutamate transport recombinants. Two genes determining the ability to grow on glutamate as the sole source of carbon and energy were mapped. One (gltC) is located near mtl (mannitol), and the other (gltH) appears to be located between the gal (galactose) and trp (tryptophan) loci. The glutamate decarboxylase gene (gad) is strongly linked to gltC. The gltC+ recombinants grow on glutamate much faster and accumulate this amino acid to a greater extent than do the gltH+ recombinants. The gltH+ gene functioned only in one female strain (P678), whereas the gltC gene functioned in all the female strains tested (P678, C600, W1).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALPERN Y. S. Induction and repression of glutamic acid decarboxylase in Escherichia coli. Biochim Biophys Acta. 1962 Dec 31;61:953–962. doi: 10.1016/0926-6550(62)90011-7. [DOI] [PubMed] [Google Scholar]
- HALPERN Y. S., UMBARGER H. E. Utilization of L-glutamic and 2-oxoglutaric acid as sole sources of carbon by Escherichia coli. J Gen Microbiol. 1961 Oct;26:175–183. doi: 10.1099/00221287-26-2-175. [DOI] [PubMed] [Google Scholar]
- HAYES W. The kinetics of the mating process in Escherichia coli. J Gen Microbiol. 1957 Feb;16(1):97–119. doi: 10.1099/00221287-16-1-97. [DOI] [PubMed] [Google Scholar]
- Halpern Y. S., Even-Shoshan A. Properties of the glutamate transport system in Escherichia coli. J Bacteriol. 1967 Mar;93(3):1009–1016. doi: 10.1128/jb.93.3.1009-1016.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern Y. S., Lupo M. Glutamate transport in wild-type and mutant strains of Escherichia coli. J Bacteriol. 1965 Nov;90(5):1288–1295. doi: 10.1128/jb.90.5.1288-1295.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KESSEL D., LUBIN M. STABILITY OF ALPHA-HYDROGEN OF AMINO ACIDS DURING ACTIVE TRANSPORT. Biochemistry. 1965 Mar;4:561–565. doi: 10.1021/bi00879a029. [DOI] [PubMed] [Google Scholar]
- LURIA S. E., ADAMS J. N., TING R. C. Transduction of lactose-utilizing ability among strains of E. coli and S. dysenteriae and the properties of the transducing phage particles. Virology. 1960 Nov;12:348–390. doi: 10.1016/0042-6822(60)90161-6. [DOI] [PubMed] [Google Scholar]
- TAYLOR A. L., THOMAN M. S. THE GENETIC MAP OF ESCHERICHIA COLI K-12. Genetics. 1964 Oct;50:659–677. doi: 10.1093/genetics/50.4.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatum E. L., Lederberg J. Gene Recombination in the Bacterium Escherichia coli. J Bacteriol. 1947 Jun;53(6):673–684. doi: 10.1128/jb.53.6.673-684.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]


