Abstract
The neuronal nicotinic receptor has gained considerable recognition as a target, not just for combating drug addiction but also for treating a number of illnesses ranging from neurodegenerative diseases to psychotic disorders like schizophrenia. This recognition has led to a burgeoning field examining the receptor at all levels. A class of nicotinic receptors that contains the α7 gene product, apparently as a homomer, illustrates this multidisciplinary approach. Here, we review recent progress in our understanding of this class of receptors based on data from molecular, structural, physiological and patho-physiological studies. These studies have set the stage for rational drug design to combat disorders of the central nervous system. The studies also exemplify the cautious approach needed in developing CNS therapies and the importance of physiology in tempering drug design.
INTRODUCTION
Nicotine is a drug of abuse that places an enormous burden on the healthcare system. On an average, smokers are twice as likely to die of a number of illnesses, ranging from coronary heart disease and stroke, to lung cancer, than their non-smoking counterparts [1]. At the same time, the drug has been shown to be neuroprotective for diseases like Alzheimer’s (AD) and Parkinson’s [2–5] suggesting a potential therapeutic application for the drug. While the strategy of developing drugs that preserve the protective effects of nicotine without the addictive ones seems obvious, the wide-spread action of nicotine in the brain should give us pause lest we are left with unanticipated and unintended consequences.
A rational approach to drug design that targets the nicotinic system, must involve understanding of the bioavailability of the drug, drug targets- the nicotinic acetylcholine receptors (nAChRs), ligand binding kinetics, drug – receptor interactions based on structural data and, perhaps most importantly, the pharmacological, physiological, and pathological signaling mechanisms utilized by nicotine.
Here, we review the actions of this drug from view points ranging from its chemistry to its role in central nervous system (CNS) pathology. The aim of this review is not to delve into extensive details (for which the reader is referred to a number of excellent reviews), but to highlight the plethora of issues that present themselves in the process of combating CNS disorders and in the development of effective therapeutic strategies for these illnesses. We focus on a nAChR sub-type containing the α7 subunit (α7-nAChR), a predominant subtype in the brain, as an example to illustrate these points.
1. CHEMISTRY AND BIO-AVAILABILITY OF NICOTINE
When a cigarette is smoked the peak arterial concentration of nicotine rises to about 500 nM [6]. However, the drug tends to accumulate in various compartments in the body. For example, the concentrations of nicotine in amniotic fluid, placenta and in breast milk [7–9], as well as the brain [10] reach significantly higher levels than in the serum. Therefore while evaluating nicotinic effects at compartments like synapses in the brain, the actual nicotine exposure of these structures to the drug is not easily determinable.
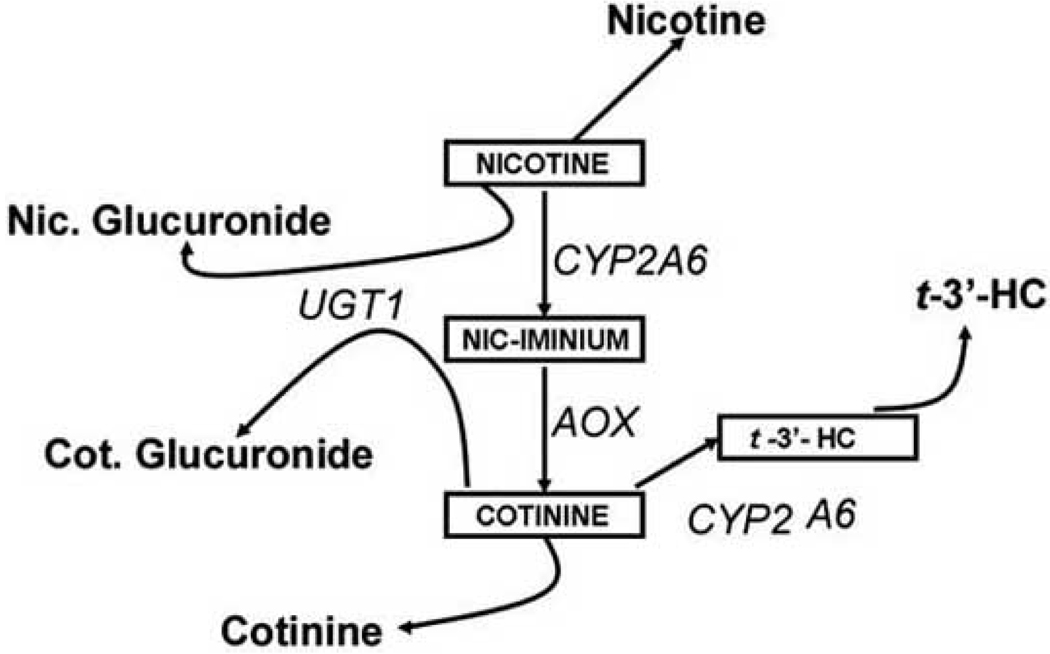
The clearance rate of nicotine from to the blood stream depends on its degradation, mainly by the liver. The individual variability in nicotine clearance would determine the plasma nicotine concentration and, by extrapolation, smoking behavior. The major pathways that account for most of nicotine metabolism is shown in Fig. (1), based on data from urinary excretion [11].
Fig. (1). Pathways for nicotine degradation.
Once absorbed in the bloodstream nicotine is degraded, in the liver, by a variety of enzymatic pathway to give rise to identifiable and measurable reaction products. Abbreviations: CYP2A6 - cytochrome P450 2A6; AOX – Aldehyde oxidase; UGT1 – UDP-glucuronosyltransferase; t’-3’-HC – 3’-hydroxy cotinine.
1.1. The Cytochrome P450 System
The main site of nicotine breakdown is the liver, where most of nicotine is broken down into cotinine. The first step in this reaction is the conversion of nicotine to Δ1’(5’) - iminium ion. This step is catalyzed by the Cytochrome P450 2A6 enzyme (CYP2A6). CYP2A6 is a ∼50KDa protein that was characterized as the P450 enzyme with the major coumarin 7-hydroxylase activity [12,13]. The enzyme has a major catalytic role in the conversion of nicotine to cotinine and cotinine to 3’-hydroxycotinine (3’-HC). The levels of these metabolites are highly correlated with coumarin 7-hydroxylase activity from human liver microsomes as well as the CYP2A6 protein levels [14,15] and coumarin acts as a competitive inhibitor for nicotine oxidation.
A second site for the action of CYP2A6 is the hydroxylation of cotinine to 3’-HC. Therefore the levels of cotinine and 3’-HC act as good indicators of nicotine clearance. Indeed the clearance of oral nicotine correlates well with the plasma 3’-HC/cotinine ratios [16] and can be taken as a good indicator of CYP activity as well as act as a predictor of cigarette consumption in humans [17]. 8-methoxypsoralen, an inhibitor of CYP2A6 has been shown to facilitate smoking cessation, suggesting another target for therapeutics aimed at quitting the habit [18,19]. The availability of crystal structure for CYP2A6 at < 2 angstrom resolution [20] as well as the determination of characteristics of effective CYP2A6 inhibitors [21] will assist in the rational design of smoking cessation aids using these enzymes as targets.
While it appears that CYP2A6 is the major enzyme responsible for the C-oxidation of nicotine, patients lacking the enzyme still metabolize nicotine suggesting that other cytochrome P450 enzymes might be able to compensate. CYP2B6 shows significant nicotine oxidation activity while other enzymes CYP2D6 and CYP2E1 appear to have lesser roles in nicotine metabolism [22–25]. In a situation, analogous to the alcohol dehydrogenase, a number of polymorphisms have been described for CYP2A6. More than 25 different alleles have been reported in the literature for this enzyme (for a compilation of these alleles see [11]). These range from whole gene deletions to gene duplications showing increased enzyme activity. The occurrence of these alleles, coupled with data on 8-methoxypsoralen (see above) also raises the possibility that genetic variants could be good predictors of an individual’s susceptibility to nicotine addiction.
1.2. Aldehyde Oxidase
In 1960 a second enzyme responsible for the conversion of nicotine to cotinine was characterized and termed to be an aldehyde oxidase [26]. It was later shown that this enzyme converts the Δ1’(5‘) iminium ion to cotinine suggesting that iminium oxidase might be a more appropriate name for this enzyme [27]. In the absence of this enzyme or in the presence of inhibitors, the iminium ion accumulates. Raloxifene, a selective estrogen receptor modulator that is used in the treatment of postmenopausal women in order to reduce risks of osteoporosis and breast cancer, is a potent inhibitor of the aldehyde oxidase [28]. The compound acts as a non-competitive inhibitor for the enzyme with a Ki of 1–2 nM. Results also suggest that this effect is very specific for aldehyde oxidase as the compound has very little effect on xanthine oxidase, a closely related enzyme [28]. Whether this step in nicotine metabolism could represent a target for candidate drugs, remains to be seen.
1.3. UDP-Glucuronosyltransferase (UGT)
N-Glucuronidation of both nicotine and cotinine occur and have been shown to account for 3–4% and 9–17% of the metabolites excreted in the urine, respectively [29]. At the same time 3’-HC undergoes O-glucuronidation. Available evidence suggests that different UGTs are involved in the formation of N- and O-glucuronides [30,31]. While the existence of glucuronidation is unequivocal from a number of studies, the exact enzymes involved are less well characterized. Based on the inhibition profile using imipramine (which is UGT1A4 specific), propofol (specific for UGT1A9), and bilirubin (a substrate for UGT1A1), it was determined that UGT 1A4 might be the primary enzyme involved in the N-glucuronidation reactions with the other two playing a minor role [32,33]. On the other hand the existence of a specific enzyme for 3’-HC glucuronidation is yet to be determined. While polymorphisms of UGTs are known, their effects on nicotine and cotinine clearance are not clear.
1.4. Other Pathways for Nicotine Metabolism
Incoming nicotine gets converted to a number of other minor metabolites in the liver all of which, taken together, form about 15–20% of the metabolites found in the urine. Flavin containing monooxygenase 3 (FMO3) in liver microsomes converts nicotine to nicotine N oxide (NNO). Blocking FMO activity with methimazole, a selective inhibitor, prevented NNO formation from nicotine [34]. CYP2A6 in addition to its main role in the formation of cotinine and 3’-HC, also catalyzes the conversion of nicotine to nornicotine and cotinine to norcotinine, relatively minor metabolites in nicotine degradation. Lastly, a species specific N-methyltransferase activity resulting in formation of the nicotine isomethonium ion, has been reported from guinea pig and human liver cytosol but not from rat liver cytosol [35–37].
Alterations in the half life of nicotine using inhibitors of enzymes in the pathway forms one avenue in therapeutic strategies to combat nicotine dependence.
2. NICOTINIC RECEPTORS- TARGETS OF NICOTINE ACTION
Biological actions of nicotine are mediated by its ability to bind and open nicotinic nAChRs. These receptors are widely distributed in the central and peripheral nervous system and consist of multiple subtypes with differing pharmacologies. A total of 12 genes have been cloned and shown to code for the different nAChR subunits. These are classified into α subunits and β subunits, the α subunit being the ligand binding subunit. There are 9 α(α2–α10) and 3 β(β2–β4) subunits that comprise the nAChRs in the nervous system. The nAChR is a pentamer consisting of either five alpha subunits (homomeric) or combinations of an alpha and a beta subunit (heteromeric). The major homomeric receptor in the brain is made up of 5 α7 subunits (α7-nAChRs) while the major heteromeric receptor consists of the α4 and β2 combination (α4β2-nAChRs). Nomenclature of nAChRs is based on the prototypic receptor at the neuromuscular junction where the ligand binding subunit is the α1 subunit with one of the structural subunits that form the pentamer being the β1 subunit. The pentameric receptor, viewed from the top, is arranged in a doughnut like shape with the subunits surrounding a central pore which is the ion channel through which receptor signals are transmitted. Binding of the agonist, nicotine or acetylcholine, the endogenous transmitter, results in a conformation change leading to the opening of the ion channel. The receptor pore forms a cationic channel that allows the influx of sodium ions and, to a lesser extent, calcium ions. The current due to calcium influx through the pore forms about 1–5% of the total ion flux but is extremely important in transducing receptor signals. This is especially true for the α7-nAChRs which have a high calcium permeability (see below).
2.1. Ligand Binding Properties of nAChRs
nAChRs can be considered as prototypic allosteric proteins, with the receptor existing in several interconvertible conformational states [38], varying in their ligand affinities and their open/closed state. The ligand binding site and the channel domain are separate, thus leading to the possibility that the two can be independently modified. Ligand (in this case nicotine or acetylcholine, the endogenous ligand) binding stabilizes one or more receptor states resulting in altering the net behavior of the nAChRs. Using an adaptation of the Monod Wyman Changeux (MWC) model for allosteric interactions [39] given by the equation below, one could arrive at a simplistic model for receptor kinetics transitioning between states A (open) and state B (closed). In the equation, A, is the fraction of receptors in the open state, L is the isomerization constant between states, Ka and Kb are the respective dissociation constants for nicotine for the two states, n is the number of ligands bound.
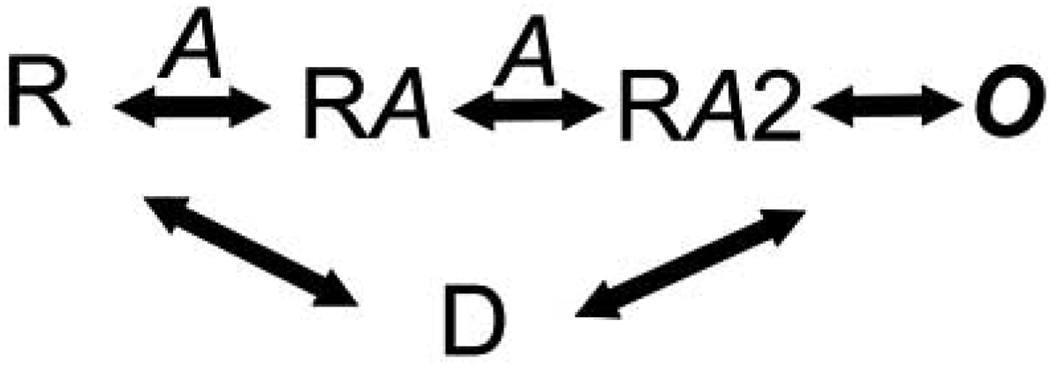
What is also known about most ligand-gated ion channels is that longer exposure to agonists can drive the receptors to a third state, the desensitized state, which represents a closed conformation but with a high affinity for the agonist Fig. (2). The receptor in the closed state (R) sequentially binds two nicotine molecules leading to the opening of the ion channel (O). The agonist bound state now is driven to D, the desensitized form. From D the receptor returns to the unliganded form or possibly, re-traverses the open state. While this model provides a brief description of ligand binding and channel opening, in reality, the process is likely to be much more complex.
Fig. (2). A simple kinetic model for receptor activation.
R- unbound state of the receptor; A – agonist; O- open state; D – desensitized state.
Kinetic models from point mutations followed by single-channel analyses suggest the existence of multiple sub-states e.g. see [40]. Indeed cryo-EM studies on the ligand bound conformation of the Torpedo nAChRs suggests the possible existence of at least 10 ligand bound states [41]. This indicates that these states might exist as a continuum of conformations rather than discrete states as depicted below. However, for the understanding of the receptor in terms relevant to physiological and pathological signaling mechanisms, the 3 kinetic states described here might suffice, though the contention is open to debate.
From a therapeutic view, the idea of stabilizing the channel in the open, closed, or desensitized state, in order to control synaptic signaling is an attractive one and one that has considerable promise in the treatment of illnesses involving α7-nAChRs. This possibility is currently being exploited (see below).
2.2. Information from Site-Directed Mutagenesis
nAChRs belong to the family of Cys-loop proteins that have a signature sequence of 13 amino acids flanked by cysteines in the N-terminal region. The cysteines at the two ends are linked covalently to each other forming a closed loop that couples the ligand binding domain from the channel domain.
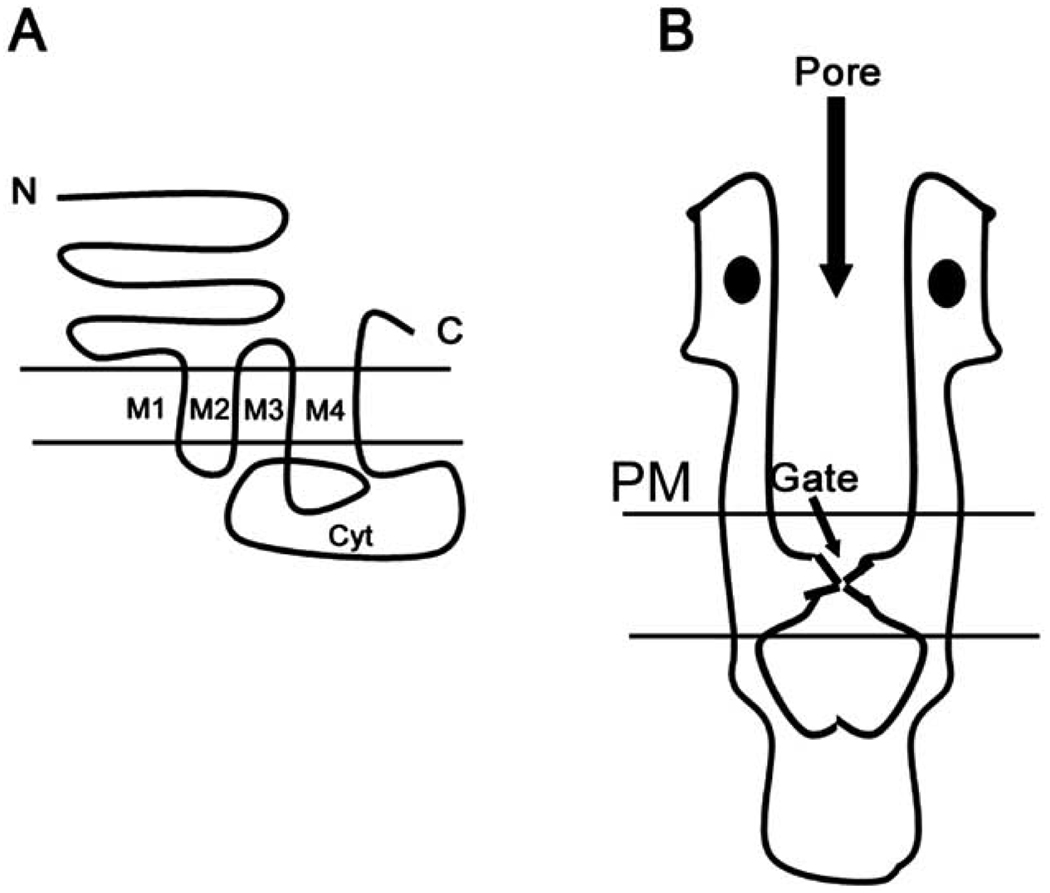
Hydropathy plots indicate four transmembrane domains for each subunit, given by M1–M4 in Fig. (3). The M2 loops (one from each subunit) form the channel domain. The next transmembrane segment (M3) is separated from the last transmembrane domain by a large cytoplasmic loop. This loop is the target of regulation by intracellular second messengers. Regulation of receptor function by the phosphorylation of serine/threonine, as well as tyrosine, residues occurs at this loop [42].
Fig. (3). Basic Structure of nAChRs.
A. The figure shows a typical 4 transmembrane topology of nAChRs from the N- to C- termini (N & C). Transmembrane domains are denoted (M1–M4). There is a large cytoplasmic loop separating M4 from M3 (cyt).
B. A low resolution model of nAChRs based on cryo-EM and X-ray crystallographic studies. There is a large N-terminal portion of the protein that forms the extracellular portion of the receptor. The distance between residues lining the ion channel pore opening narrows as the protein spans the plasma membrane. The narrowest portion of the pore lining region, lined by a ring of leucine residues, forms the channel gate. This is followed by the large cytoplasmic region. The two ligand-binding domains are depicted as filled ovals in the figure.
Considerable insight into the kinetics and allosteric mechanisms of nAChRs has been gleaned by studies using site-directed mutagenesis. Distinct modular organization of the receptor was shown using a chimeric protein which contained the ligand binding domain of α7-nAChR and the channel domain of the serotonergic 5HT3 receptor. The chimeric receptor channel now was activated with nAChR agonists and blocked by competitive antagonist for the receptors [43]. Substitution of a single amino acid in the M2 region of α7-nAChRs (L247T) altered the desensitization kinetics of the receptors [44,45]. The mutation was shown to decrease the rate of desensitization of the receptor. In addition, the channel exhibited an additional high affinity conduction state at low agonist concentrations suggesting, as depicted in the state diagram above, the possibility that the receptor could traverse directly from a desensitized to an open state, circumventing the closed, unbound conformation. A third characteristic of the mutant, consistent with the allosteric nature of the transitions is that they exhibit a significant number of spontaneous openings even in the absence of the agonist [46], not predicted by the Koshland induced-fit hypothesis [47]. Paradoxically, the L247T mutant now exhibited an altered pharmacology as well, considering that the point mutation was located in the M2 channel domain [46]. The nAChR competitive antagonist dihydro-β-erythroidine (DHβE) now functioned as a full agonist while another competitive antagonist of α7-nAChRs, methyllycaconitine (MLA) did not. Once again this is consistent with the state diagram above by postulating that DHβE inhibits the receptor activity by locking the receptors in the desensitized state while MLA’s antagonism comes from stabilizing the closed state conformation. The gating of nAChRs, i.e. the agonist-mediated transition to the open state occurs in microsecond time scales demonstrating a very efficient coupling between the two domains [48]. This was demonstrated to require specific residues in the cysteine loop [49] by using chimeras between two fast gating channels the α7-nAChR and the anionic glycine receptor (GlyR). Inefficient coupling between the two resulted in slow activation rate which was overcome by reinstating two specific cys-loop amino acids that belonged to the receptor contributing the channel domain [49].
A key feature of the α7-nAChR channel is its high calcium permeability [50,51]. In addition the receptor uses multiple calcium amplification pathways to very effectively raise intracellular calcium levels [52–54]. Indeed much of the physiological effects of the receptor are due to its ability to mediate calcium-dependent signal transduction in neurons. Another key feature of the receptor consistent with the allosteric model is its modulation by extracellular calcium concentrations. The receptor currents are potentiated by increasing external calcium concentrations [55]. Mutations in the canonical EF-hand domain located at the N-terminal portion of the receptor alter this calcium-mediated potentiation [56]. nAChRs are also regulated by covalent modification of amino acids in the cytoplasmic loop; for example see [57]. While not examined in detail there is evidence for changes in α7-nAChR function by phosphorylation. Neuregulins, a family of neuronal proteins that regulate tyrosine kinase activity, cause rapid internalization of α7-nAChRs via tyrosine phosphorylation by an EGF receptor related kinase [58]. Interestingly, a naturally occurring mutation in the human α7-nAChR (G423S) caused rapid desensitization of receptor currents [59]. Other calcium-dependent serine/threonine kinases also have been shown to affect α7-nAChR desensitization [60]. These studies show yet another process that can be therapeutically exploited.
2.3. Insights into the Structure of α7-nAChRs
Much of what we know about the structure of nAChRs comes from the study of the receptor at the neuromuscular junction and its counterparts in electric organs of fish. Inherent problems in crystallizing membrane proteins has necessitated a more circuitous and tedious process trying to determine the structure of these receptors [61–64].
Cryo-EM examination of receptor arrays that are present in electric organs of fish like the electric eel and the Torpedo marmorata, revealed some of the finer structure of the receptor up to a resolution of about 4 Angstroms [62–65]. A serendipitous finding brought our understanding of the ligand binding domain structure of the receptor closer to or atomic resolution. It was discovered that the sea mollusk Lymnaea stagnalis has glial cells that release and acetylcholine binding protein (AChBP) that consists of the nAChR ligand binding domain in a soluble form and hence amenable to crystallization [66,67]. Combining these results with those elucidated by Cryo-EM a clearer picture of the nAChR structure has emerged. Considering that the nAChRs on neurons are likely to be similar, much of the structural information obtained from these studies is likely to be true for the receptors on neurons as well. Indeed the AChBP has much greater homology to the α7-nAChR, a major nAChR in the brain [66,68].
Following is a brief summary of what is known about nAChR structure from pioneering studies on the muscle type receptor and extrapolation to what the structure of α7-nAChRs might be from the fact that much of the receptor properties are conserved across subtypes. The nAChR channel structure deduced from the Torpedo receptors shows a large channel spanning a length of about 160 angstroms normal to the membrane plane. A 20 angstrom ligand-binding domain forms part of a central vestibule and contains two agonist binding sites [65]. Each subunit in this domain is organized around two sets of β-sheets joined via a disulfide bridge that forms a part of the cys-loop [68].
Loops M1–M4, as shown in Fig. (3) are organized as α-helices in such a fashion that the M2 helix from all five subunits form a ring lining a narrow water filled pore of the ion channel. Deep within the ion pore is a ring of leucine residues that form a hydrophobic constriction in the pore which form the ‘gate’ of the channel. In the absence of the agonist this ‘gate’ is closed and prevents ion flux through the channel. When agonist binds to the channel, as evidenced from elegant studies performed by trapping the receptor in an agonist bound state [41], the α-subunits M2 helices change their configuration widening the pore and thus opening the ‘gate’. Cations now permeate the channel resulting in membrane depolarization.
Some evidence for the structure of α7-nAChRs comes from studies comparing simulated receptor conformation based on data from the receptor in unliganded state and in the presence of multiple bound ligands [69–71]. Key findings from these studies are as follows. The apoprotein and the receptor in the presence of an antagonist (d-tubocurarine; dTC) show a considerable amount of asymmetry presumably resulting in a narrow pore. However, the asymmetry in the two cases arises from different motions of the ligand-binding domain. The apoprotein shows an assymmetric but tightly packed subunits whereas in the dTC bound asymmetry, the packing is much looser. In either case one would postulate that the resultant narrow pore would be impermeable to ion flow across the membrane. The binding of ACh results in a loosely packed but symmetric arrangement and a resultant larger pore. Binding of calcium, the positive allosteric modulator, results in a looser conformation. Once again these structures presumably lead to a wider, permeant pore. However, in spite of the fact that the receptor is a homomer containing five ligand binding subunits, the Hill coefficient (nH) was shown to be closer to 2; e.g see [72] suggesting a stochastic binding of two agonist molecules was sufficient to open the channels. Though there is no convincing evidence for functional non-equivalence of the binding sites, it remains a possibility that differential binding of agonists to two of the five sites might confer differential properties or that the channel opens with partial occupancy. There is also a possibility that receptor desensitization is proportional to occupancy and that low receptor occupancy by the ligand might result in a more tonic, slowly desensitizing current [73,74]. Such an idea would be consistent with a very slow rising calcium signal upon receptor activation, not completely explainable by downstream amplification [75].
3. SIGNALING BY α7-nAChRs
As discussed above, a key feature of the α7-nAChRs is their high permeability to calcium [50,76] comparable to that of the NMD A class of glutamate receptors, the gold standard for high calcium permeable ligand-gated ion channels. Perhaps, more importantly, the receptor is very efficient at raising intracellular free calcium levels ([Ca]i) [53,54,75]. This efficiency arises from the fact that calcium signals through the receptors are amplified by subsequent activation of voltage-gated calcium channels as well as calcium release from the endoplasmic reticulum (ER). The latter means of amplification happens via a process called calcium-induced calcium release (CICR), where flux through the receptor opens ryanodine receptors (RyRs) on the ER. RyRs are ligand-gated ion channels that use calcium as the ligand. As these receptors have a relatively low affinity for calcium (∼ 10–100 µM), it implies that the α7-nAChRs and RyRs must be in close proximity to each other. This is because most cells have a very high calcium buffering capacity (∼ equivalent of 2 mM BAPTA [77]) which rapidly attenuates calcium concentrations such that [Ca]i drops to 1µM or less about 250 nm from the influx site. In addition, the calcium signaling can be further amplified by release through inositol trisphosphate receptor (IP3Rs) on the ER, thus resulting in a calcium cascade that propagates along the entire cell [53].
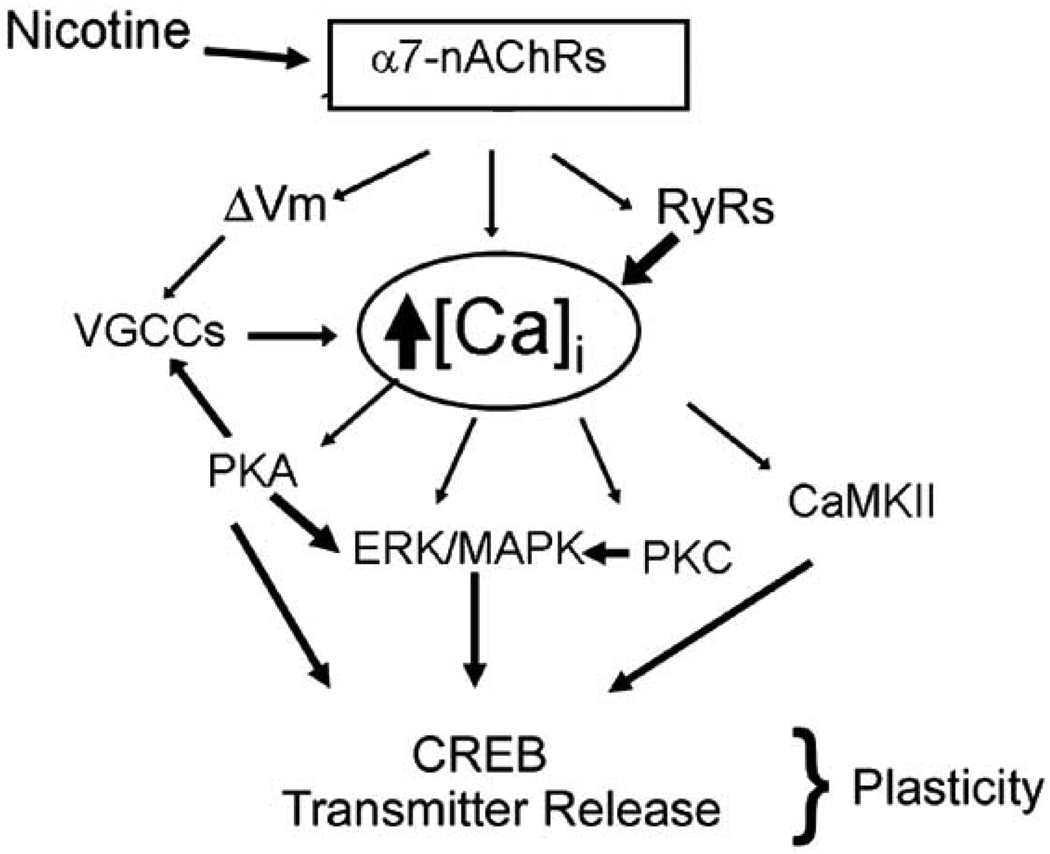
Efficient modulation of [Ca]i results in the activation of a host of calcium-dependent signaling cascades that mediate short- and long-term effects of α7-nAChRs. Fig. (4) shows a simplified diagram illustrating major pathways mediating signal transduction upon α7-nAChR activation. There is evidence for the activation of almost all calcium-mediated second messengers. This complex cascade of downstream enzymes mediate changes in synaptic strength and plasticity affecting a number of effects ranging from addiction to attention, A major endpoint of this signaling that results in a long-term change in gene expression and synaptic strength is the activation by phosphorylation of cyclic AMP response element binding protein (CREB). A number of calcium-activated enzymes lead to CREB activation. The activation of VGCCs by α7-nAChRs leads to production of cAMP [78]. This second messenger can activate protein kinase A leading to CREB phosphorylation, activation of the mitogen-activated protein kinase (MAPK) and a feedback regulation of VGCCs and potentially the α7-nAChRs [57]. MAPK further amplifies the activation of CREB; Fig. (4).
Fig. (4). α7-nAChRs and calcium-dependent signal transduction.
Activation of α7-nAChRs by nicotine, triggers an efficient calcium-dependent signal transduction cascade. Calcium flux through upon activation of α7-nAChRs by nicotine is amplified downstream by membrane depolarization (ΔVm) and activation of VGCCs as well as by CICR from the endoplasmic reticulum via RyRs. The rise in intracellular free calcium levels results in activation of multiple calcium-dependent kinases resulting in short- and long-term changes in synaptic plasticity.
A second important cascade starts with the activation of calcium/calmodulin-dependent protein kinase II (CaMKII) which converges on CREB activation as well as phosphorylates a number of substrates. In the postsynaptic neuron, CaMKII regulates glutamate receptor on the postsynaptic neuron leading to the facilitation of long-term potentiation (LTP), thought to be the cellular correlate of learning and memory [79–82]. Similarly the role of protein kinase C (PKC) has been documented as well [83–87].
What these data tell us is that α7-nAChRs are indeed positioned and capable of effectively altering neuronal functions and synaptic strength in the CNS.
One long-term consequence of α7-nAChR-mediated calcium changes is the regulation of neuronal survival in the CNS. Nicotine, acting via this receptor, can be cytotoxic to developing neurons and adult progenitor cell [88–90] while being neuroprotective for differentiated neurons [91,92]. Both these effects of the receptor activation are mediated via changes in [Ca]i. This apparent paradox can be explained by the finding that calcium signaling is coded by narrow spatial and temporal patterns. Immature and undifferentiated neurons have poor calcium buffering capabilities thus causing large and long-lived changes in [Ca]i resulting in apoptotic implications for the role of this receptor in neurodegenerative diseases like Alzheimer’s disease (AD) and Parkinson’s (PD). This is discussed below.
Another major function of α7-nAChRs is their modulation of transmitter release. These receptors, also present on terminals of heterologous transmitter systems potentiate the release of glutamate [52,75,93], and GABA [94–97] in the brain, in addition to other transmitters. In a hippocampal synapse this presynaptic release mediated by α7-nAChRs can mediate synaptic transmission in the absence of presynaptic action potentials (AP) suggesting that activation of these receptors can actually usurp normal signaling pathways in the brain [52,75]. Another important way in which α7-nAChRs modulate synaptic function is by the activation of GABAergic interneurons [94,98,99].
The presence of α7-nAChRs in non-neuronal cells has remained a puzzle over the years. The receptor is expressed in a wide range of cell types throughout the body [100] where they might serve different functions, like modulation of cell death, migration and signaling, mainly by their ability to modulate calcium levels. For example in T-lymphocytes, nAChR-mediated calcium change is correlated with T-cell anergy [101]. Nicotine exposure increases the proliferation of vascular endothelial cells [102], stimulates the expression of nitric oxide synthase [103] and has a net positive effect on angiogenesis [104]. Increased vascularization by nAChR-activation has implicated a role for the drug in tumor promotion.
An unusual finding is that non-neuronal cells in the brain, especially astrocytes, appear to possess α7-nAChRs [53,100,105,106] suggesting that the effects of the receptor might be much more widespread than discussed above. Taken together, the wide distribution of α7-nAChRs, and the efficiency with which they modulate transmitter release and calcium signaling, makes them likely candidates to mediate the effects of nicotine in addiction and neurodegenerative diseases. At the same time, their localization throughout the body makes it unlikely that one could develop strategies to specifically target these receptors in a particular area.
4. NICOTINE ADDICTION
The fact that nicotine is addictive is not in question, at least among neurobiologists. The mechanism of addiction, however, remains an open question. More than five decades ago James Olds discovered that electrical stimulation of certain regions of the brain resulted in positive reinforcing effects, and that rats could be trained to self-stimulate as long as electrodes were placed in these regions e.g. see [107,108]. This ‘pleasure drive’ was strong enough to override the effects of hunger as well as sexual stimulation [107]. The idea that these areas, traced to the mesolimbic dopaminergic pathway, could be involved in the positive reinforcement of drug addiction, came later with studies demonstrating that animals could be trained to self administer drugs of abuse when delivered to the same mesolimbic system [109]. Since then much of addiction research has been focused on the modulation of dopamine release [110–112]. α7-nAChRs have been shown to be located on glutamatergic terminals of the ventral tegmental area (VTA), the starting point of the mesolimbic dopaminergic system [113]. There is some evidence that the α7-nAChRs enhance glutamate release and suppress GABA release on to dopaminergic neurons [114,115]. This would argue for the direct action of α7-nAChRs on the mesolimbic reward system. At the same time nicotine also modulates dopamine release [116–118]. Taken together, these results suggest a direct action of nAChRs in general, and α7-nAChRs in particular, on the brain reward system thus providing a link between the drug and addiction.
It must be noted that the mesolimbic dopaminergic pathway is not the only one likely to mediate addictive effects. It is being recognized that addiction is a non-linear process, involving homeostatic dysregulation rather than stimulation or inhibition of specific pathways [119,120]. Thus α7-nAChRs in many other brain areas could influence the addictive nature of nicotine. This viewpoint makes sense as the number of possible drugs that are addictive and the few instances of cross-addiction make it unlikely that there would be a single reward pathway. However, it is likely that addiction does involve the mesolimbic dopaminergic pathway, though its alteration might arise by inputs from a number of brain areas.
Studies with the nAChR gene knockouts (KOs) suggest differential roles for different receptor subtypes. While β2-KOs result in the loss of nicotine self administration [121], α7-gene KOs might show a decrement in nicotine withdrawal symptoms [122,123]. These results further emphasize the notion that nicotine addiction involves a complex interplay of various nAChR subunits.
5. α7-NICOTINIC RECEPTORS IN NEURODEGERATIVE DISEASES
Nicotine has been shown to positively modulate a number of brain functions linked to cognition. It improves attention, arousal, learning, learnt discrimination, and memory functions [124–127]. Many of these effects of the drug are mediated by α7-nAChRs activated by the drug. These effects have been linked to learning as well as in neurodegenerative diseases. A key finding over the years has been the role of α7-nAChRs in neuronal survival [88–92] increasing the plausibility of a role for these receptors in neurodegenerative diseases.
5.1. Nicotine and Alzheimer’s Disease
Nicotine has been shown to increase memory and attention in normal humans [128]. In AD the drug has been shown to improve memory deficits. This idea is supported by epidemiological data suggesting that incidence of AD among smokers is significantly less than in non-smokers [129]. The cholinergic hypothesis for AD has been prevalent for a long time based on the finding that loss of basal forebrain cholinergic neurons is one of the early symptoms of AD. This led to the use of acetylcholine esterase (AChE) inhibitors for treatment of the disease [130]. The results from this line of therapy have been disappointing, thus undermining the idea as a whole. Upon reflection, however, these findings are not contradictory to the cholinergic hypothesis. The efficacy of the AChE inhibitors, whose function is to increase the lifetime of the transmitter in the extracellular space, depends on the presence of cholinergic projections. If these are the earliest neurons to die, as suggested, the loss of projections to would render inhibition of AChE ineffective. More recent data suggests that nAChR agonists and antagonists might be a better, more effective, therapeutic approach to the disease. Some attempts at drug development based on this idea have been made. This is summarized in the next section.
A feature of α7-nAChRs is their modulation by the beta amyloid 1–42 peptide (Aβ), the key component of plaques found in the brain of AD patients. Some evidence exists showing that Aβ by activating α7-nAChRs, can modulate the MAPK pathway and CREB activation [131]. At the same time the receptor is downregulated in AD brains [132]. Further, in transgenic mouse models of AD, nicotine via the MAPK pathway also increases the hyperphosphorylation of the tau protein, the cause of neurofibrillary tangles, which is a part of the AD pathology as well [132,133]. Thus the exact mechanisms which mediate the enhanced cognitive functions seen upon nicotine treatment remains unclear.
5.2. Parkinson’s Disease
Nicotine has been shown to be protective against PD as well. In PD, there is a specific loss of the dopaminergic neurons of substantia nigra, which provide inhibitory control to the neurons of the striatum. Once again the role of nAChRs is likely to be complex, involving differential modulation of a number of pathways. In mouse models of PD, where selective lesions of dopaminergic neurons were made by injection of 6-hydroxy dopamine (6-OHDA) selectively into the striatum or the substantia nigra, the levels of a number of nAChRs showed dramatic decline though the α7-nAChR levels remained unchanged [134]. In patients with PD there is again a selective increase in α7-nAChRs while levels of heteromeric nAChRs decline [135,136]. α7-nAChRs have been shown to trigger an anti-inflammatory pathway in brain microglia [137]. The activation of the receptor can suppress the inhibition of pro-inflammatory transcription factors NFkappaB and c-myc [138].
In neurons, dopamine is oxidized by monoamine oxidases (MAOs). One class of MAO; MAO-B, oxidizes dopamine and various primary and tertiary amines to their corresponding aldehyde and free amines, resulting in the release of hydrogen peroxide a source of free radicals. The oxidation of dopamine to dihydroxy phenyl acetic acid, via a series of reactions, generates a number of reactive oxygen species (ROS). As the brain has a more limited capacity to clear ROS than other tissues, these species can trigger a cytotoxic cycle, wherein in the presence of solvated Fe(II) and H2O2, the toxic 6-OHDA is formed (Fenton Reaction). The 6-OHDA, in turn, is able to mobilize more Fe(II) from stored forms of iron in proteins, thus propagating neurotoxicity and neuronal death. Nicotine has been shown to be neuroprotective by blocking MAO activity, thus acting as a protective antioxidant [139].
Thus there are both epidemiological, as well as potential mechanistic bases, for the protective role of nAChRs in neurodegenerative diseases and it is likely that α7-nAChRs play an important role.
6. α7-nAChRs IN SCHIZOPHRENIA
The involvement of α7-nAChRs in schizophrenia is backed up by a copious amount of literature. The schizophrenic population shows a much greater incidence of smoking than the general population e.g. see [140]. In addition, patients show a comparatively poor level of auditory sensory gating and smoking helps compensate for this deficit. These findings led to the examination of nAChRs in schizophrenia. [141]. Auditory gating is measured as changes in a specific peak in EEG recordings. This response, known as the P50 auditory evoked response, is seen about 40–80 ms after the presentation of the auditory stimulus. In normal population presentation of two stimuli closely spaced in time (∼500 ms) results in the attenuation of the P50 response to the second stimulus. This relative suppression of the P50 response is an indicator of sensory gating. There is much less suppression of the P50 evoked response in schizophrenics [142], leading to the idea that defects in this process contribute to schizophrenic symptoms. Consistent with epidemiological data, smoking restores, to a large extent, the P50 ratios in schizophrenics [143] implying a role for nAChRs in this process. A number of studies indicate that P50 deficits show significant correlation with the level of α7-nAChRs in the brain [144]. Further, infusion of α7-nAChR antagonists decrease P50 ratios (i.e. response to the second tone not suppressed) while agonists increase them [145]. This effect is mimicked by the atypical antipsychotic, clozapine, in a manner consistent with its effects being via α7-nAChRs [145]. Linkage analyses showed that the P50 changes were mapped to the chromosomal locus 15q13-q14 [146]. The α7 gene lies within this locus thus providing good correlation between α7-nAChRs, P50 deficits and schizophrenia [147]. These results also suggest that therapeutic interventions based on modulating α7-nAChR function might be useful in the treatment of certain schizophrenic symptoms as well.
7. THERAPEUTIC STRATEGIES BASED ON nAChRs
The multifarious role of nAChRs in various physiological and pathological processes and their subtype specific actions make them ideal targets for rational drug design. At the same time, the ubiquitous distribution of nAChRs warrants a more cautious approach to therapeutics based on these receptors for fear of potential side effects. The potential importance of α7-nAChRs in neuro-degeneration has led to the search of specific drugs targeting this receptor subtype. One approach that has significant potential is based on rational design comes from modeling data based on nAChR structures taking advantage of different local binding properties of different subtypes of the receptors [148].
A second approach, based on chemical modification of known ligands, has yielded some success as well in the search for potential subtype specific drugs. The marine worm toxin anabasine has been shown to act as a specific ligand for nAChRs. The benzylidine derivatives of this toxin have been shown to have a high degree of selectivity for α7-nAChRs. Two of these derivatives, (3-(2,4-dimethoxybenzylidine) anabasine (DMXBA; GTS-21) and 4OH GTS-21 have specific agonistic properties for α7-nAChRs [149–151]. This compound is now at Phase II trials for the treatment of schizophrenia [152]. Tropisetron, an anti-emetic, which is an agonist for serotonin receptor subclass (5-HT3), is also a partial α7-nAChR agonist. This drug attenuates P50 gating deficits [153]. (R)-(-)-5'Phenylspiro[1-azabicyclo[2.2.2] octane-3,2'-(3'H)furo[2,3-b]pyridine (PSAB-OFP) is another agonist shown to be selective for α7-nAChRs [154,155]. Marrero et al. [156] showed that 2-(3-pyridyl)-1-azabicyclo[3.2.2]nonane (TC-1698) has significant neuro-protective action via its ability to act as an α7-nAChR agonist. Varenicline (6,7,8,9-Tetrahydro-6,10 methano-6Hpyrazino [2,3-h] [3] benzazepine]), a compound based on the structure of cytisine, an α4β2 agonist is a complete agonist at α7-nAChRs and is a drug now approved for smoking cessation treatment [157].
Allosteric modulators of α7-nAChRs provide yet another target for rational drug design. A novel positive allosteric modulator of α7-nAChRs, 1-(5-chloro-2,4-dimethoxyphenyl)-3-(5-methyl-isoxazol-3-yl)-urea (PNU-120596) [158], has been shown to increase the mean open time of the receptor channels an also increases the efficiency of α7-nAChR-mediated changes in calcium levels [75,158].
The above examples of specific targeting of compounds to the α7-nAChRs serve to illustrate the current progress in drug design based on nAChR subtype specificity. In addition to the α7-nAChRs, other subtype specific agents have shown significant promise for the treatment of a number of potential pathological conditions (for review see [159]). This dramatic increase in interest in nAChR-based drugs further serves to illustrate the importance of these receptors in the modulation of various physiological and pathological conditions in human CNS.
CONCLUSION
It is a truism, but one often underappreciated, that a close interaction between physiology, chemistry, and pharmacology is necessary for successful therapeutic strategies. What is not clearly resolvable is the relative emphasis that needs to be placed on the extent of our knowledge in each of these disciplines. On the one hand, it is impractical and highly imprudent to wait until we know all there is to know (if that is definable) about a disease before developing drugs to combat it. On the other hand, one has to deal with the possibilities of unintended consequences that always accompany drugs based on incomplete information, warranting a more cautious approach. Nowhere is this conundrum clearer than in the case of nAChRs, a class of neurotransmitter receptors ubiquitous in their distribution in the brain and elsewhere, and one that modulates a huge range of physiological functions. Research on a major subtype of this receptor, the α7-nAChRs, illustrates an approach by combining information from all these disciplines to arrive at strategies for rational drug design. The important roles played by this receptor in addiction and neurodegenerative diseases illustrates the promise of these drugs as a new set of weapons in our arsenal for the treatment of these devastating disorders.
Data from both metabolism of nicotine and structure, function, and signaling by nAChRs, suggest a number of possible sites of interest for rational drug design. Selective alterations in nicotine levels, as well as changing receptor signaling by either altering kinetics or signaling efficacy could result in drugs specific for one or the other consequences of nAChR activation. Though yet to be realized, these approaches have the realistic promise of effective therapies targeted at what are, perhaps, the most intractable of CNS disorders.
ACKNOWLEDGEMENT
Funding for this work was provided by grants from the National Institute on Drug Abuse (RO1 DA 10266 and a CEBRA Grant 5 R21 DA019453) and the National Institute for Deafness and Communication Disorders (RO1DC 008855) to S.V. and the American Heart Association Scientist Development grant to G.S.
REFERENCES
- 1.Woloshin S, Schwartz LM, Welch HG. The Risk of Death by Age, Sex, and Smoking Status in the United States: Putting Health Risks in Context. J. Natl. Cancer Inst. 2008 doi: 10.1093/jnci/djn124. (Epub a head of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bourin M, Ripoll N, Dailly E. Nicotinic receptors and Alzheimer's disease. Curr. Med. Res. Opin. 2003;19:169–177. doi: 10.1185/030079903125001631. [DOI] [PubMed] [Google Scholar]
- 3.Picciotto MR, Zoli M. Neuroprotection via nAChRs: the role of nAChRs in neurodegenerative disorders such as Alzheimer's and Parkinson's disease. Front. Biosci. 2008;13:492–504. doi: 10.2741/2695. [DOI] [PubMed] [Google Scholar]
- 4.Buccafusco JJ, Terry AV., Jr The potential role of cotinine in the cognitive and neuroprotective actions of nicotine. Life Sci. 2003;72:2931–2942. doi: 10.1016/s0024-3205(03)00226-1. [DOI] [PubMed] [Google Scholar]
- 5.O'Neill MJ, Murray TK, Lakics V, Visanji NP, Duty S. The role of neuronal nicotinic acetylcholine receptors in acute and chronic neurodegeneration. Curr. Drug Targets CNS. Neurol. Disord. 2002;1:399–411. doi: 10.2174/1568007023339166. [DOI] [PubMed] [Google Scholar]
- 6.Henningfield JE, Stapleton JM, Benowitz NL, Grayson RF, London ED. Higher levels of nicotine in arterial than in venous blood after cigarette smoking. Drug Alcohol Depend. 1993;33:23–29. doi: 10.1016/0376-8716(93)90030-t. [DOI] [PubMed] [Google Scholar]
- 7.Dobek D, Karmowski A, Sobiech KA, Terpilowski L, Mis-Michalek M. Average quantitative concentration of cotinine within the system pregnant woman-baby. Arch. Immunol. Ther. Exp. (Warsz.) 1998;46:59–61. [PubMed] [Google Scholar]
- 8.Lambers DS, Clark KE. The maternal and fetal physiologic effects of nicotine. Semin. Perinatol. 1996;20:115–126. doi: 10.1016/s0146-0005(96)80079-6. [DOI] [PubMed] [Google Scholar]
- 9.Luck W, Nau H, Hansen R, Steldinger R. Extent of nicotine and cotinine transfer to the human fetus, placenta and amniotic fluid of smoking mothers. Dev. Pharmacol. Ther. 1985;8:384–395. doi: 10.1159/000457063. [DOI] [PubMed] [Google Scholar]
- 10.Ghosheh OA, Dwoskin LP, Miller DK, Crooks PA. Accumulation of nicotine and its metabolites in rat brain after intermittent or continuous peripheral administration of [2'-(14)C]nicotine. Drug Metab. Dispos. 2001;29:645–651. [PubMed] [Google Scholar]
- 11.Hukkanen J, Jacob P, III, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol. Rev. 2005;57:79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- 12.Yamano S, Tatsuno J, Gonzalez FJ. The CYP2A3 gene product catalyzes coumarin 7-hydroxylation in human liver microsomes. Biochemistry. 1990;29:1322–1329. doi: 10.1021/bi00457a031. [DOI] [PubMed] [Google Scholar]
- 13.Yun CH, Shimada T, Guengerich FP. Purification and characterization of human liver microsomal cytochrome P-450 2A6. Mol. Pharmacol. 1991;40:679–685. [PubMed] [Google Scholar]
- 14.Nakajima M, Yamamoto T, Nunoya K, Yokoi T, Nagashima K, Inoue K, Funae Y, Shimada N, Kamataki T, Kuroiwa Y. Role of human cytochrome P4502A6 in C-oxidation of nicotine. Drug Metab. Dispos. 1996;24:1212–1217. [PubMed] [Google Scholar]
- 15.Nakajima M, Yamamoto T, Nunoya K, Yokoi T, Nagashima K, Inoue K, Funae Y, Shimada N, Kamataki T, Kuroiwa Y. Characterization of CYP2A6 involved in 3'-hydroxylation of cotinine in human liver microsomes. J. Pharmacol. Exp. Ther. 1996;277:1010–1015. [PubMed] [Google Scholar]
- 16.Dempsey D, Tutka P, Jacob P, III, Allen F, Schoedel K, Tyndale RF, Benowitz NL. Nicotine metabolite ratio as an index of cytochrome P450 2A6 metabolic activity. Clin. Pharmacol. Ther. 2004;76:64–72. doi: 10.1016/j.clpt.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 17.Benowitz NL, Peng M, Jacob P., III Effects of cigarette smoking and carbon monoxide on chlorzoxazone and caffeine metabolism. Clin. Pharmacol. Ther. 2003;74:468–474. doi: 10.1016/j.clpt.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Sellers EM, Ramamoorthy Y, Zeman MV, Djordjevic MV, Tyndale RF. The effect of methoxsalen on nicotine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) metabolism in vivo. Nicotine. Tob. Res. 2003;5:891–899. doi: 10.1080/14622200310001615231. [DOI] [PubMed] [Google Scholar]
- 19.Sellers EM, Tyndale RF, Fernandes LC. Decreasing smoking behaviour and risk through CYP2A6 inhibition. Drug Discov. Today. 2003;8:487–493. doi: 10.1016/s1359-6446(03)02704-1. [DOI] [PubMed] [Google Scholar]
- 20.Yano JK, Hsu MH, Griffin KJ, Stout CD, Johnson EF. Structures of human microsomal cytochrome P450 2A6 complexed with coumarin and methoxsalen. Nat. Struct. Mol. Biol. 2005;12:822–823. doi: 10.1038/nsmb971. [DOI] [PubMed] [Google Scholar]
- 21.Yano JK, Denton TT, Cerny MA, Zhang X, Johnson EF, Cashman JR. Synthetic inhibitors of cytochrome P-450 2A6: inhibitory activity, difference spectra, mechanism of inhibition, and protein cocrystallization. J. Med. Chem. 2006;49:6987–7001. doi: 10.1021/jm060519r. [DOI] [PubMed] [Google Scholar]
- 22.Fukami T, Katoh M, Yamazaki H, Yokoi T, Nakajima M. Human cytochrome P450 2A13 efficiently metabolizes chemicals in air pollutants: naphthalene, styrene, and toluene. Chem. Res. Toxicol. 2008;21:720–725. doi: 10.1021/tx700325f. [DOI] [PubMed] [Google Scholar]
- 23.Yamazaki H, Inoue K, Hashimoto M, Shimada T. Roles of CYP2A6 and CYP2B6 in nicotine C-oxidation by human liver microsomes. Arch. Toxicol. 1999;73:65–70. doi: 10.1007/s002040050588. [DOI] [PubMed] [Google Scholar]
- 24.Messina ES, Tyndale RF, Sellers EM. A major role for CYP2A6 in nicotine C-oxidation by human liver microsomes. J. Pharmacol. Exp. Ther. 1997;282:1608–1614. [PubMed] [Google Scholar]
- 25.Benowitz NL, Jacob P, III, Perez-Stable E. CYP2D6 phenotype and the metabolism of nicotine and cotinine. Pharmacogenetics. 1996;6:239–242. doi: 10.1097/00008571-199606000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Hucker HB, Gillette JR, Brodie BB. Enzymatic pathway for the formation of cotinine, a major metabolite of nicotine in rabbit liver. J. Pharmacol. Exp. Ther. 1960;129:94–100. [PubMed] [Google Scholar]
- 27.Brandange S, Lindblom L. The enzyme “aldehyde oxidase” is an iminium oxidase. Reaction with nicotine delta 1'(5') iminium ion. Biochem. Biophys. Res. Commun. 1979;91:991–996. doi: 10.1016/0006-291x(79)91977-6. [DOI] [PubMed] [Google Scholar]
- 28.Obach RS. Potent inhibition of human liver aldehyde oxidase by raloxifene. Drug Metab Dispos. 2004;32:89–97. doi: 10.1124/dmd.32.1.89. [DOI] [PubMed] [Google Scholar]
- 29.Nakajima M, Yokoi T. Interindividual variability in nicotine metabolism: C-oxidation and glucuronidation. Drug Metab. Pharmacokinet. 2005;20:227–235. doi: 10.2133/dmpk.20.227. [DOI] [PubMed] [Google Scholar]
- 30.Benowitz NL, Jacob P., III Effects of cigarette smoking and carbon monoxide on nicotine and cotinine metabolism. Clin. Pharmacol. Ther. 2000;67:653–659. doi: 10.1067/mcp.2000.107086. [DOI] [PubMed] [Google Scholar]
- 31.Benowitz NL, Jacob P, III, Fong I, Gupta S. Nicotine metabolic profile in man: comparison of cigarette smoking and transdermal nicotine. J. Pharmacol. Exp. Ther. 1994;268:296–303. [PubMed] [Google Scholar]
- 32.Nakajima M, Tanaka E, Kwon JT, Yokoi T. Characterization of nicotine and cotinine N-glucuronidations in human liver microsomes. Drug Metab. Dispos. 2002;30:1484–1490. doi: 10.1124/dmd.30.12.1484. [DOI] [PubMed] [Google Scholar]
- 33.Tricker AR. Nicotine metabolism, human drug metabolism polymorphisms, and smoking behaviour. Toxicology. 2003;183:151–173. doi: 10.1016/s0300-483x(02)00513-9. [DOI] [PubMed] [Google Scholar]
- 34.Nakayama H, Fujihara S, Nakashima T, Kurogochi Y. Formation of two major nicotine metabolites in livers of guinea pigs. Biochem. Pharmacol. 1987;36:4313–4317. doi: 10.1016/0006-2952(87)90677-0. [DOI] [PubMed] [Google Scholar]
- 35.Cundy KC, Godin CS, Crooks PA. Evidence of stereo-specificity in the in vivo methylation of [14C](+/−)-nicotine in the guinea pig. Drug Metab. Dispos. 1984;12:755–759. [PubMed] [Google Scholar]
- 36.Gairola C, Godin CS, Houdi AA, Crooks PA. Inhibition of histamine N-methyltransferase activity in guinea-pig pulmonary alveolar macrophages by nicotine. J. Pharm. Pharmacol. 1988;40:724–726. doi: 10.1111/j.2042-7158.1988.tb07004.x. [DOI] [PubMed] [Google Scholar]
- 37.Crooks PA, Godin CS. N-methylation of nicotine enantiomers by human liver cytosol. J. Pharm. Pharmacol. 1988;40:153–154. doi: 10.1111/j.2042-7158.1988.tb05207.x. [DOI] [PubMed] [Google Scholar]
- 38.Edelstein SJ, Schaad O, Henry E, Bertrand D, Changeux JP. A kinetic mechanism for nicotinic acetylcholine receptors based on multiple allosteric transitions. Biol. Cybern. 1996;75:361–379. doi: 10.1007/s004220050302. [DOI] [PubMed] [Google Scholar]
- 39.Monod J, Wyman J, Changeux JP. On the nature of allosteric transitions: a plausible model. J. Mol. Biol. 1965;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- 40.Salamone FN, Zhou M, Auerbach A. A re-examination of adult mouse nicotinic acetylcholine receptor channel activation kinetics. J. Physiol. 1999;516 (Pt 2):315–330. doi: 10.1111/j.1469-7793.1999.0315v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Unwin N. Acetylcholine receptor channel imaged in the open state. Nature. 1995;373:37–43. doi: 10.1038/373037a0. [DOI] [PubMed] [Google Scholar]
- 42.Swope SL, Moss SJ, Raymond LA, Huganir RL. Regulation of ligand-gated ion channels by protein phosphorylation. Adv. Second Messenger Phosphoprotein Res. 1999;33:49–78. doi: 10.1016/s1040-7952(99)80005-6. [DOI] [PubMed] [Google Scholar]
- 43.Eisele JL, Bertrand S, Galzi JL, Devillers-Thiery A, Changeux JP, Bertrand D. Chimaeric nicotinic-serotonergic receptor combines distinct ligand binding and channel specificities [see comments] Nature. 1993;366:479–483. doi: 10.1038/366479a0. [DOI] [PubMed] [Google Scholar]
- 44.Bertrand D, Galzi JL, Devillers-Thiery A, Bertrand S, Changeux JP. Mutations at two distinct sites within the channel domain M2 alter calcium permeability of neuronal alpha 7 nicotinic receptor. Proc. Natl. Acad. Sci. USA. 1993;90:6971–6975. doi: 10.1073/pnas.90.15.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Revah F, Bertrand D, Galzi JL, Devillers-Thiery A, Mulle C, Hussy N, Bertrand S, Ballivet M, Changeux JP. Mutations in the channel domain alter desensitization of a neuronal nicotinic receptor. Nature. 1991;353:846–849. doi: 10.1038/353846a0. [DOI] [PubMed] [Google Scholar]
- 46.Bertrand S, Devillers-Thiery A, Palma E, Buisson B, Edelstein SJ, Corringer PJ, Changeux JP, Bertrand D. Paradoxical allosteric effects of competitive inhibitors on neuronal alpha7 nicotinic receptor mutants. Neuroreport. 1997;8:3591–3596. doi: 10.1097/00001756-199711100-00034. [DOI] [PubMed] [Google Scholar]
- 47.Koshland DE, Jr, Nemethy G, Filmer D. Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry. 1966;5:365–385. doi: 10.1021/bi00865a047. [DOI] [PubMed] [Google Scholar]
- 48.Colquhoun D, Sakmann B. Fluctuations in the microsecond time range of the current through single acetylcholine receptor ion channels. Nature. 1981;294:464–466. doi: 10.1038/294464a0. [DOI] [PubMed] [Google Scholar]
- 49.Grutter T, de Carvalho LP, Dufresne V, Taly A, Edelstein SJ, Changeux JP. Molecular tuning of fast gating in pentameric ligand-gated ion channels. Proc. Natl. Acad. Sci. USA. 2005;102:18207–18212. doi: 10.1073/pnas.0509024102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seguela P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW. Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. J. Neurosci. 1993;13:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Castro NG, Albuquerque EX. alpha-Bungarotoxin-sensitive hippocampal nicotinic receptor channel has a high calcium permeability. Biophys. J. 1995;68:516–524. doi: 10.1016/S0006-3495(95)80213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sharma G, Vijayaraghavan S. Modulation of presynaptic store calcium induces release of glutamate and postsynaptic firing. Neuron. 2003;38:929–939. doi: 10.1016/s0896-6273(03)00322-2. [DOI] [PubMed] [Google Scholar]
- 53.Sharma G, Vijayaraghavan S. Nicotinic cholinergic signaling in hippocampal astrocytes involves calcium-induced calcium release from intracellular stores. Proc. Natl. Acad. Sci. USA. 2001;98:4148–4153. doi: 10.1073/pnas.071540198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vijayaraghavan S, Pugh PC, Zhang ZW, Rathouz MM, Berg DK. Nicotinic receptors that bind alpha-bungarotoxin on neurons raise intracellular free Ca2+ Neuron. 1992;8:353–362. doi: 10.1016/0896-6273(92)90301-s. [DOI] [PubMed] [Google Scholar]
- 55.Vernino S, Amador M, Luetje CW, Patrick J, Dani JA. Calcium modulation and high calcium permeability of neuronal nicotinic acetylcholine receptors. Neuron. 1992;8:127–134. doi: 10.1016/0896-6273(92)90114-s. [DOI] [PubMed] [Google Scholar]
- 56.Galzi JL, Bertrand S, Corringer PJ, Changeux JP, Bertrand D. Identification of calcium binding sites that regulate potentiation of a neuronal nicotinic acetylcholine receptor. EMBO J. 1996;15:5824–5832. [PMC free article] [PubMed] [Google Scholar]
- 57.Vijayaraghavan S, Schmid HA, Halvorsen SW, Berg DK. Cyclic AMP-dependent phosphorylation of a neuronal acetylcholine receptor alpha-type subunit. J. Neurosci. 1990;10:3255–3262. doi: 10.1523/JNEUROSCI.10-10-03255.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chang Q, Fischbach GD. An acute effect of neuregulin 1 beta to suppress alpha 7-containing nicotinic acetylcholine receptors in hippocampal interneurons. J. Neurosci. 2006;26:11295–11303. doi: 10.1523/JNEUROSCI.1794-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tsuneki H, Kobayashi S, Takagi K, Kagawa S, Tsunoda M, Murata M, Matsuoka T, Wada T, Kurachi M, Kimura I, Sasaoka T. Novel G423S mutation of human alpha7 nicotinic receptor promotes agonist-induced desensitization by a protein kinase C-dependent mechanism. Mol. Pharmacol. 2007;71:777–786. doi: 10.1124/mol.106.030866. [DOI] [PubMed] [Google Scholar]
- 60.Klein RC, Yakel JL. Paired-pulse potentiation of alpha7-containing nAChRs in rat hippocampal CA1 stratum radiatum interneurones. J. Physiol. 2005;568:881–889. doi: 10.1113/jphysiol.2005.096081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kersh GJ, Tomich JM, Montal M. The M2 delta transmembrane domain of the nicotinic cholinergic receptor forms ion channels in human erythrocyte membranes. Biochem. Biophys. Res. Commun. 1989;162:352–356. doi: 10.1016/0006-291x(89)92003-2. [DOI] [PubMed] [Google Scholar]
- 62.Miyazawa A, Fujiyoshi Y, Stowell M, Unwin N. Nicotinic acetylcholine receptor at 4.6 A resolution: transverse tunnels in the channel wall. J. Mol. Biol. 1999;288:765–786. doi: 10.1006/jmbi.1999.2721. [DOI] [PubMed] [Google Scholar]
- 63.Unwin N. Nicotinic acetylcholine receptor at 9 A resolution. J. Mol. Biol. 1993;229:1101–1124. doi: 10.1006/jmbi.1993.1107. [DOI] [PubMed] [Google Scholar]
- 64.Unwin N. Neurotransmitter action: opening of ligand-gated ion channels. Cell. 1993;72 Suppl:31–41. doi: 10.1016/s0092-8674(05)80026-1. [DOI] [PubMed] [Google Scholar]
- 65.Unwin N. Structure and action of the nicotinic acetylcholine receptor explored by electron microscopy. FEBS Lett. 2003;555:91–95. doi: 10.1016/s0014-5793(03)01084-6. [DOI] [PubMed] [Google Scholar]
- 66.Smit AB, Syed NI, Schaap D, van Minnen J, Klumperman J, Kits KS, Lodder H, van der Schors RC, van Elk R, Sorgedrager B, Brejc K, Sixma TK, Geraerts WP. A gliaderived acetylcholine-binding protein that modulates synaptic transmission. Nature. 2001;4 11:261–268. doi: 10.1038/35077000. [DOI] [PubMed] [Google Scholar]
- 67.Brejc K, van Dijk WJ, Smit AB, Sixma TK. The 2.7 A structure of AChBP, homologue of the ligand-binding domain of the nicotinic acetylcholine receptor. Novartis. Found. Symp. 2002;245:22–29. [PubMed] [Google Scholar]
- 68.Brejc K, van Dijk WJ, Klaassen RV, Schuurmans M, van Der Oost J, Smit AB, Sixma TK. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature. 2001;4 11:269–276. doi: 10.1038/35077011. [DOI] [PubMed] [Google Scholar]
- 69.Henchman RH, Wang HL, Sine SM, Taylor P, McCammon JA. Ligand-induced conformational change in the alpha7 nicotinic receptor ligand binding domain. Biophys. J. 2005;88:2564–2576. doi: 10.1529/biophysj.104.053934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gao F, Bren N, Burghardt TP, Hansen S, Henchman RH, Taylor P, McCammon JA, Sine SM. Agonist-mediated conformational changes in acetylcholine-binding protein revealed by simulation and intrinsic tryptophan fluorescence. J. Biol. Chem. 2005;280:8443–8451. doi: 10.1074/jbc.M412389200. [DOI] [PubMed] [Google Scholar]
- 71.Henchman RH, Wang HL, Sine SM, Taylor P, McCammon JA. Asymmetric structural motions of the homomeric alpha7 nicotinic receptor ligand binding domain revealed by molecular dynamics simulation. Biophys. J. 2003;85:3007–3018. doi: 10.1016/S0006-3495(03)74720-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Uteshev VV, Stevens DR, Haas HL. Alpha-bungarotoxin-sensitive nicotinic responses in rat tuberomammillary neurons. Pflugers Arch. 1996;432:607–613. doi: 10.1007/s004240050176. [DOI] [PubMed] [Google Scholar]
- 73.Uteshev VV, Meyer EM, Papke RL. Activation and inhibition of native neuronal alpha-bungarotoxin-sensitive nicotinic ACh receptors. Brain Res. 2002;948:33–46. doi: 10.1016/s0006-8993(02)02946-3. [DOI] [PubMed] [Google Scholar]
- 74.Papke RL, Meyer E, Nutter T, Uteshev VV. alpha7 receptor-selective agonists and modes of alpha7 receptor activation. Eur. J. Pharmacol. 2000;393:179–195. doi: 10.1016/s0014-2999(00)00009-1. [DOI] [PubMed] [Google Scholar]
- 75.Sharma G, Grybko M, Vijayaraghavan S. Action Potential-Independent and Nicotinic Receptor-Mediated Concerted Release of Multiple Quanta at Hippocampal CA3-Mossy Fiber Synapses. J. Neurosci. 2008;28:2563–2575. doi: 10.1523/JNEUROSCI.5407-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Castro NG, Albuquerque EX. alpha-Bungarotoxin-sensitive hippocampal nicotinic receptor channel has a high calcium permeability. Biophys. J. 1995;68:516–524. doi: 10.1016/S0006-3495(95)80213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Roberts WM. Spatial calcium buffering in saccular hair cells. Nature. 1993;363:74–76. doi: 10.1038/363074a0. [DOI] [PubMed] [Google Scholar]
- 78.Dajas-Bailador FA, Mogg AJ, Wonnacott S. Intracellular Ca2+ signals evoked by stimulation of nicotinic acetylcholine receptors in SH-SY5Y cells: contribution of voltage-operated Ca2+ channels and Ca2+ stores. J. Neurochem. 2002;81:606–614. doi: 10.1046/j.1471-4159.2002.00846.x. [DOI] [PubMed] [Google Scholar]
- 79.Lu FM, Hawkins RD. Presynaptic and postsynaptic Ca(2+) and CamKII contribute to long-term potentiation at synapses between individual CA3 neurons. Proc. Natl. Acad. Sci. USA. 2006;103:4264–4269. doi: 10.1073/pnas.0508162103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thiagarajan TC, Piedras-Renteria ES, Tsien RW. alpha- and betaCaMKII. Inverse regulation by neuronal activity and opposing effects on synaptic strength. Neuron. 2002;36:1103–1114. doi: 10.1016/s0896-6273(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 81.Cammarota M, Bevilaqua LR, Viola H, Kerr DS, Reichmann B, Teixeira V, Bulla M, Izquierdo I, Medina JH. Participation of CaMKII in neuronal plasticity and memory formation. Cell Mol. Neurobiol. 2002;22:259–267. doi: 10.1023/A:1020763716886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lisman J, Schulman H, Cline H. The molecular basis of CaM-KII function in synaptic and behavioural memory. Nat. Rev. Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- 83.Kawamura Y, Manita S, Nakamura T, Inoue M, Kudo Y, Miyakawa H. Glutamate release increases during mossy-CA3 LTP but not during Schaffer-CA1 LTP. Eur. J. Neurosci. 2004;19:1591–1600. doi: 10.1111/j.1460-9568.2004.03258.x. [DOI] [PubMed] [Google Scholar]
- 84.Lauri SE, Bortolotto ZA, Nistico R, Bleakman D, Ornstein PL, Lodge D, Isaac JT, Collingridge GL. A role for Ca2+ stores in kainate receptor-dependent synaptic facilitation and LTP at mossy fiber synapses in the hippocampus. Neuron. 2003;39:327–341. doi: 10.1016/s0896-6273(03)00369-6. [DOI] [PubMed] [Google Scholar]
- 85.Mellor J, Nicoll RA. Hippocampal mossy fiber LTP is independent of postsynaptic calcium. Nat. Neurosci. 2001;4:125–126. doi: 10.1038/83941. [DOI] [PubMed] [Google Scholar]
- 86.Kwon HB, Castillo PE. Long-term potentiation selectively expressed by NMDA receptors at hippocampal mossy fiber synapses. Neuron. 2008;57:108–120. doi: 10.1016/j.neuron.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Luu P, Malenka RC. Spike Timing-Dependent Long-Term Potentiation in Ventral Tegmental Area Dopamine Cells Requires PKC. J. Neurophysiol. 2008 doi: 10.1152/jn.01384.2007. (Epub a head of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mechawar N, Saghatelyan A, Grailhe R, Scoriels L, Gheusi G, Gabellec MM, Lledo PM, Changeux JP. Nicotinic receptors regulate the survival of newborn neurons in the adult olfactory bulb. Proc. Natl. Acad. Sci. USA. 2004;101:9822–9826. doi: 10.1073/pnas.0403361101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Berger F, Gage FH, Vijayaraghavan S. Nicotinic receptor-induced apoptotic cell death of hippocampal progenitor cells. J. Neurosci. 1998;18:6871–6881. doi: 10.1523/JNEUROSCI.18-17-06871.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Roy TS, Andrews JE, Seidler FJ, Slotkin TA. Nicotine evokes cell death in embryonic rat brain during neurulation. J. Pharmacol. Exp. Ther. 1998;287:1136–1144. [PubMed] [Google Scholar]
- 91.Pugh PC, Margiotta JF. Nicotinic acetylcholine receptor agonists promote survival and reduce apoptosis of chick ciliary ganglion neurons. Mol. Cell Neurosci. 2000;15:113–122. doi: 10.1006/mcne.1999.0810. [DOI] [PubMed] [Google Scholar]
- 92.Dajas-Bailador FA, Lima PA, Wonnacott S. The alpha7 nicotinic acetylcholine receptor subtype mediates nicotine protection against NMDA excitotoxicity in primary hippocampal cultures through a Ca(2+) dependent mechanism. Neuropharmacology. 2000;39:2799–2807. doi: 10.1016/s0028-3908(00)00127-1. [DOI] [PubMed] [Google Scholar]
- 93.Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani JA. Hippocampal synaptic transmission enhanced by low concentrations of nicotine [see comments] Nature. 1996;383:713–716. doi: 10.1038/383713a0. [DOI] [PubMed] [Google Scholar]
- 94.Zhang J, Berg DK. Reversible inhibition of GABAA receptors by alpha7-containing nicotinic receptors on the vertebrate postsynaptic neurons. J. Physiol. 2007;579:753–763. doi: 10.1113/jphysiol.2006.124578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li DP, Pan YZ, Pan HL. Acetylcholine attenuates synaptic GABA release to supraoptic neurons through presynaptic nicotinic receptors. Brain Res. 2001;920:151–158. doi: 10.1016/s0006-8993(01)03055-4. [DOI] [PubMed] [Google Scholar]
- 96.Lu Y, Marks MJ, Collins AC. Desensitization of nicotinic agonist-induced [3H]gamma-aminobutyric acid release from mouse brain synaptosomes is produced by subactivating concentrations of agonists. J. Pharmacol. Exp. Ther. 1999;291:1127–1134. [PubMed] [Google Scholar]
- 97.Alkondon M, Pereira EF, Barbosa CT, Albuquerque EX. Neuronal nicotinic acetylcholine receptor activation modulates gamma- aminobutyric acid release from CA1 neurons of rat hippocampal slices. J. Pharmacol. Exp. Ther. 1997;283:1396–1411. [PubMed] [Google Scholar]
- 98.Szabo SI, Zelles T, Lendvai B. Intracellular Ca2+ dynamics of hippocampal interneurons following nicotinic acetylcholine receptor activation. Neurochem. Int. 2008;52:135–141. doi: 10.1016/j.neuint.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 99.Rozsa B, Katona G, Kaszas A, Szipocs R, Vizi ES. Dendritic nicotinic receptors modulate backpropagating action potentials and long-term plasticity of interneurons. Eur. J. Neurosci. 2008;27:364–377. doi: 10.1111/j.1460-9568.2007.05999.x. [DOI] [PubMed] [Google Scholar]
- 100.Sharma G, Vijayaraghavan S. Nicotinic receptor signaling in nonexcitable cells. J. Neurobiol. 2002;53:524–534. doi: 10.1002/neu.10114. [DOI] [PubMed] [Google Scholar]
- 101.Singh SP, Kalra R, Puttfarcken P, Kozak A, Tesfaigzi J, Sopori ML. Acute and chronic nicotine exposures modulate the immune system through different pathways. Toxicol. Appl. Pharmacol. 2000;164:65–72. doi: 10.1006/taap.2000.8897. [DOI] [PubMed] [Google Scholar]
- 102.Villablanca AC. Nicotine stimulates DNA synthesis and proliferation in vascular endothelial cells in vitro. J. Appl. Physiol. 1998;84:2089–2098. doi: 10.1152/jappl.1998.84.6.2089. [DOI] [PubMed] [Google Scholar]
- 103.Tonnessen BH, Severson SR, Hurt RD, Miller VM. Modulation of nitric-oxide synthase by nicotine. J. Pharmacol. Exp. Ther. 2000;295:601–606. [PubMed] [Google Scholar]
- 104.Heeschen C, Jang JJ, Weis M, Pathak A, Kaji S, Hu RS, Tsao PS, Johnson FL, Cooke JP. Nicotine stimulates angiogenesis and promotes tumor growth and atherosclerosis. Nat. Med. 2001;7:833–839. doi: 10.1038/89961. [DOI] [PubMed] [Google Scholar]
- 105.Gahring LC, Persiyanov K, Rogers SW. Mouse strain-specific changes in nicotinic receptor expression with age. Neurobiol. Aging. 2005;26:973–980. doi: 10.1016/j.neurobiolaging.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 106.Gahring LC, Persiyanov K, Dunn D, Weiss R, Meyer EL, Rogers SW. Mouse strain-specific nicotinic acetylcholine receptor expression by inhibitory interneurons and astrocytes in the dorsal hippocampus. J. Comp. Neurol. 2004;468:334–346. doi: 10.1002/cne.10943. [DOI] [PubMed] [Google Scholar]
- 107.OLDS J. Effects of hunger and male sex hormone on self-stimulation of the brain. J. Comp. Physiol. Psychol. 1958;51:320–324. doi: 10.1037/h0040783. [DOI] [PubMed] [Google Scholar]
- 108.OLDS J. Self-stimulation of the brain; its use to study local effects of hunger, sex, and drugs. Science. 1958;127:315–324. doi: 10.1126/science.127.3294.315. [DOI] [PubMed] [Google Scholar]
- 109.Goeders NE, Smith JE. Cortical dopaminergic involvement in cocaine reinforcement. Science. 1983;221:773–775. doi: 10.1126/science.6879176. [DOI] [PubMed] [Google Scholar]
- 110.Franken IH. Drug craving and addiction: integrating psychological and neuropsychopharmacological approaches. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2003;27:563–579. doi: 10.1016/S0278-5846(03)00081-2. [DOI] [PubMed] [Google Scholar]
- 111.Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- 112.Benowitz NL, Henningfield JE. Establishing a nicotine threshold for addiction. The implications for tobacco regulation. N. Engl. J. Med. 1994;331:123–125. doi: 10.1056/NEJM199407143310212. [DOI] [PubMed] [Google Scholar]
- 113.Jones IW, Wonnacott S. Precise localization of alpha7 nicotinic acetylcholine receptors on glutamatergic axon terminals in the rat ventral tegmental area. J. Neurosci. 2004;24:11244–11252. doi: 10.1523/JNEUROSCI.3009-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fagen ZM, Mansvelder HD, Keath JR, McGehee DS. Short- and long-term modulation of synaptic inputs to brain reward areas by nicotine. Ann. N. Y. Acad. Sci. 2003;1003:185–195. doi: 10.1196/annals.1300.011. [DOI] [PubMed] [Google Scholar]
- 115.Mansvelder HD, De Rover M, McGehee DS, Brussaard AB. Cholinergic modulation of dopaminergic reward areas: upstream and downstream targets of nicotine addiction. Eur. J. Pharmacol. 2003;480:117–123. doi: 10.1016/j.ejphar.2003.08.099. [DOI] [PubMed] [Google Scholar]
- 116.Grady SR, Salminen O, Laverty DC, Whiteaker P, McIntosh JM, Collins AC, Marks MJ. The subtypes of nicotinic acetylcholine receptors on dopaminergic terminals of mouse striatum. Biochem. Pharmacol. 2007;74:1235–1246. doi: 10.1016/j.bcp.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhou FM, Liang Y, Dani JA. Endogenous nicotinic cholinergic activity regulates dopamine release in the striatum. Nat. Neurosci. 2001;4:1224–1229. doi: 10.1038/nn769. [DOI] [PubMed] [Google Scholar]
- 118.Marshall DL, Redfern PH, Wonnacott S. Presynaptic nicotinic modulation of dopamine release in the three ascending pathways studied by in vivo microdialysis: comparison of naive and chronic nicotine-treated rats. J. Neurochem. 1997;68:1511–1519. doi: 10.1046/j.1471-4159.1997.68041511.x. [DOI] [PubMed] [Google Scholar]
- 119.Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- 120.Koob GF. Drug addiction: the yin and yang of hedonic homeostasis. Neuron. 1996;16:893–896. doi: 10.1016/s0896-6273(00)80109-9. [DOI] [PubMed] [Google Scholar]
- 121.Besson M, David V, Suarez S, Cormier A, Cazala P, Changeux JP, Granon S. Genetic dissociation of two behaviors associated with nicotine addiction: beta-2 containing nicotinic receptors are involved in nicotine reinforcement but not in withdrawal syndrome. Psychopharmacol. (Berl.) 2006;187:189–199. doi: 10.1007/s00213-006-0418-z. [DOI] [PubMed] [Google Scholar]
- 122.Salas R, Main A, Gangitano D, De Biasi M. Decreased withdrawal symptoms but normal tolerance to nicotine in mice null for the alpha7 nicotinic acetylcholine receptor subunit. Neuropharmacology. 2007;53:863–869. doi: 10.1016/j.neuropharm.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jackson KJ, Martin BR, Changeux JP, Damaj MI. Differential role of nicotinic acetylcholine receptor subunits in physical and affective nicotine withdrawal signs. J. Pharmacol. Exp. Ther. 2008;325:302–312. doi: 10.1124/jpet.107.132977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Levin ED, Rezvani AH. Nicotinic treatment for cognitive dysfunction. Curr. Drug Target CNS Neurol. Disord. 2002;1:423–431. doi: 10.2174/1568007023339102. [DOI] [PubMed] [Google Scholar]
- 125.Levin ED. Nicotinic receptor subtypes and cognitive function. J. Neurobiol. 2002;53:633–640. doi: 10.1002/neu.10151. [DOI] [PubMed] [Google Scholar]
- 126.Rezvani AH, Levin ED. Cognitive effects of nicotine. Biol. Psychiatry. 2001;49:258–267. doi: 10.1016/s0006-3223(00)01094-5. [DOI] [PubMed] [Google Scholar]
- 127.Levin ED, Simon BB. Nicotinic acetylcholine involvement in cognitive function in animals. Psychopharmacol. (Berl.) 1998;138:217–230. doi: 10.1007/s002130050667. [DOI] [PubMed] [Google Scholar]
- 128.Warburton DM. Nicotine as a cognitive enhancer. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1992;16:181–191. doi: 10.1016/0278-5846(92)90069-q. [DOI] [PubMed] [Google Scholar]
- 129.Perry E, Walker M, Grace J, Perry R. Acetylcholine in mind: a neurotransmitter correlate of consciousness? Trends Neurosci. 1999;22:273–280. doi: 10.1016/s0166-2236(98)01361-7. [DOI] [PubMed] [Google Scholar]
- 130.Bartus RT, Dean RL, III, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–414. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- 131.Dineley KT, Westerman M, Bui D, Bell K, Ashe KH, Sweatt JD. Beta-amyloid activates the mitogen-activated protein kinase cascade via hippocampal alpha7 nicotinic acetylcholine receptors: In vitro and in vivo mechanisms related to Alzheimer's disease. J. Neurosci. 2001;21:4125–4133. doi: 10.1523/JNEUROSCI.21-12-04125.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Oddo S, LaFerla FM. The role of nicotinic acetylcholine receptors in Alzheimer's disease. J. Physiol. Paris. 2006;99:172–179. doi: 10.1016/j.jphysparis.2005.12.080. [DOI] [PubMed] [Google Scholar]
- 133.Oddo S, Caccamo A, Green KN, Liang K, Tran L, Chen Y, Leslie FM, LaFerla FM. Chronic nicotine administration exacerbates tau pathology in a transgenic model of Alzheimer's disease. Proc. Natl. Acad. Sci. USA. 2005;102:3046–3051. doi: 10.1073/pnas.0408500102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jellinger KA. Recent developments in the pathology of Parkinson's disease. J. Neural. Transm. Suppl. 2002:347–376. doi: 10.1007/978-3-7091-6139-5_33. [DOI] [PubMed] [Google Scholar]
- 135.Bordia T, Grady SR, McIntosh JM, Quik M. Nigrostriatal damage preferentially decreases a subpopulation of alpha6beta2* nAChRs in mouse, monkey, and Parkinson's disease striatum. Mol. Pharmacol. 2007;72:52–61. doi: 10.1124/mol.107.035998. [DOI] [PubMed] [Google Scholar]
- 136.Janhunen S, Ahtee L. Differential nicotinic regulation of the nigrostriatal and mesolimbic dopaminergic pathways: implications for drug development. Neurosci. Biobehav. Rev. 2007;31:287–314. doi: 10.1016/j.neubiorev.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 137.Shytle RD, Mori T, Townsend K, Vendrame M, Sun N, Zeng J, Ehrhart J, Silver AA, Sanberg PR, Tan J. Cholinergic modulation of microglial activation by alpha 7 nicotinic receptors. J. Neurochem. 2004;89:337–343. doi: 10.1046/j.1471-4159.2004.02347.x. [DOI] [PubMed] [Google Scholar]
- 138.Liu Q, Zhang J, Zhu H, Qin C, Chen Q, Zhao B. Dissecting the signaling pathway of nicotine-mediated neuroprotection in a mouse Alzheimer disease model. FAS EB J. 2007;21:61–73. doi: 10.1096/fj.06-5841com. [DOI] [PubMed] [Google Scholar]
- 139.Linert W, Bridge MH, Huber M, Bjugstad KB, Grossman S, Arendash GW. In vitro and in vivo studies investigating possible antioxidant actions of nicotine: relevance to Parkinson's and Alzheimer's diseases. Biochim. Biophys. Acta. 1999;1454:143–152. doi: 10.1016/s0925-4439(99)00029-0. [DOI] [PubMed] [Google Scholar]
- 140.Gopalaswamy AK, Morgan R. Smoking in chronic schizophrenia. Br. J. Psychiatry. 1986;149:523. [PubMed] [Google Scholar]
- 141.Bickford PC, Luntz-Leybman V, Freedman R. Auditory sensory gating in the rat hippocampus: modulation by brainstem activity. Brain Res. 1993;607:33–38. doi: 10.1016/0006-8993(93)91486-c. [DOI] [PubMed] [Google Scholar]
- 142.Cullum CM, Harris JG, Waldo MC, Smernoff E, Madison A, Nagamoto HT, Griffith J, Adler LE, Freedman R. Neurophysiological and neuropsychological evidence for attentional dysfunction in schizophrenia. Schizophr. Res. 1993;10:131–141. doi: 10.1016/0920-9964(93)90048-n. [DOI] [PubMed] [Google Scholar]
- 143.Adler LE, Hoffer LD, Wiser A, Freedman R. Normalization of auditory physiology by cigarette smoking in schizophrenic patients. Am. J. Psychiatry. 1993;150:1856–1861. doi: 10.1176/ajp.150.12.1856. [DOI] [PubMed] [Google Scholar]
- 144.Adler LE, Olincy A, Waldo M, Harris JG, Griffith J, Stevens K, Flach K, Nagamoto H, Bickford P, Leonard S, Freedman R. Schizophrenia, sensory gating, and nicotinic receptors. Schizophr. Bull. 1998;24:189–202. doi: 10.1093/oxfordjournals.schbul.a033320. [DOI] [PubMed] [Google Scholar]
- 145.Simosky JK, Stevens KE, Adler LE, Freedman R. Clozapine improves deficient inhibitory auditory processing in DBA/2 mice, via a nicotinic cholinergic mechanism. Psychopharmacol. (Berl.) 2003;165:386–396. doi: 10.1007/s00213-002-1285-x. [DOI] [PubMed] [Google Scholar]
- 146.Leonard S, Breese C, Adams C, Benhammou K, Gault J, Stevens K, Lee M, Adler L, Olincy A, Ross R, Freedman R. Smoking and schizophrenia: abnormal nicotinic receptor expression. Eur. J. Pharmacol. 2000;393:237–242. doi: 10.1016/s0014-2999(00)00035-2. [DOI] [PubMed] [Google Scholar]
- 147.Freedman R, Coon H, Myles-Worsley M, Orr-Urtreger A, Olincy A, Davis A, Polymeropoulos M, Holik J, Hopkins J, Hoff M, Rosenthal J, Waldo MC, Reimherr F, Wender P, Yaw J, Young DA, Breese CR, Adams C, Patterson D, Adler LE, Kruglyak L, Leonard S, Byerley W. Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc. Natl. Acad. Sci. USA. 1997;94:587–592. doi: 10.1073/pnas.94.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Huang X, Zheng F, Chen X, Crooks PA, Dwoskin LP, Zhan CG. Modeling subtype-selective agonists binding with alpha4beta2 and alpha7 nicotinic acetylcholine receptors: effects of local binding and long-range electrostatic interactions. J. Med. Chem. 2006;49:7661–7674. doi: 10.1021/jm0606701. [DOI] [PubMed] [Google Scholar]
- 149.van Haaren F, Anderson KG, Haworth SC, Kem WR. GTS-21, a mixed nicotinic receptor agonist/antagonist, does not affect the nicotine cue. Pharmacol. Biochem. Behav. 1999;64:439–444. doi: 10.1016/s0091-3057(99)00054-4. [DOI] [PubMed] [Google Scholar]
- 150.Uteshev VV, Meyer EM, Papke RL. Regulation of neuronal function by choline and 4OH-GTS-21 through alpha 7 nicotinic receptors. J. Neurophysiol. 2003;89:1797–1806. doi: 10.1152/jn.00943.2002. [DOI] [PubMed] [Google Scholar]
- 151.Hunter BE, de Fiebre CM, Papke RL, Kem WR, Meyer EM. A novel nicotinic agonist facilitates induction of long-term potentiation in the rat hippocampus. Neurosci. Lett. 1994;168:130–134. doi: 10.1016/0304-3940(94)90433-2. [DOI] [PubMed] [Google Scholar]
- 152.Freedman R, Olincy A, Buchanan RW, Harris JG, Gold JM, Johnson L, Allensworth D, Guzman-Bonilla A, Clement B, Ball MP, Kutnick J, Pender V, Martin LF, Stevens KE, Wagner BD, Zerbe GO, Soti F, Kem WR. Initial Phase 2 Trial of a Nicotinic Agonist in Schizophrenia. Am. J. Psychiatry. 2008 doi: 10.1176/appi.ajp.2008.07071135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Koike K, Hashimoto K, Takai N, Shimizu E, Komatsu N, Watanabe H, Nakazato M, Okamura N, Stevens KE, Freedman R, Iyo M. Tropisetron improves deficits in auditory P50 suppression in schizophrenia. Schizophr. Res. 2005;76:67–72. doi: 10.1016/j.schres.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 154.Broad LM, Felthouse C, Zwart R, McPhie GI, Pearson KH, Craig PJ, Wallace L, Broadmore RJ, Boot JR, Keenan M, Baker SR, Sher E. PSAB-OFP, a selective alpha 7 nicotinic receptor agonist, is also a potent agonist of the 5-HT3 receptor. Eur. J. Pharmacol. 2002;452:137–144. doi: 10.1016/s0014-2999(02)02273-2. [DOI] [PubMed] [Google Scholar]
- 155.Astles PC, Baker SR, Boot JR, Broad LM, Dell CP, Keenan M. Recent progress in the development of subtype selective nicotinic acetylcholine receptor ligands. Curr. Drug Targets CNS. Neurol. Disord. 2002;1:337–348. doi: 10.2174/1568007023339256. [DOI] [PubMed] [Google Scholar]
- 156.Marrero MB, Papke RL, Bhatti BS, Shaw S, Bencherif M. The neuroprotective effect of 2-(3-pyridyl)-1-azabicyclo [3.2.2]nonane (TC-1698), a novel alpha7 ligand, is prevented through angiotensin II activation of a tyrosine phosphatase. J. Pharmacol. Exp. Ther. 2004;309:16–27. doi: 10.1124/jpet.103.061655. [DOI] [PubMed] [Google Scholar]
- 157.Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Mol. Pharmacol. 2006;70:801–805. doi: 10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- 158.Hurst RS, Hajos M, Raggenbass M, Wall TM, Higdon NR, Lawson JA, Rutherford-Root KL, Berkenpas MB, Hoffmann WE, Piotrowski DW, Groppi VE, Allaman G, Ogier R, Bertrand S, Bertrand D, Arneric SP. A novel positive allosteric modulator of the alpha7 neuronal nicotinic acetylcholine receptor: in vitro and in vivo characterization. J. Neurosci. 2005;25:4396–4405. doi: 10.1523/JNEUROSCI.5269-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jensen AA, Frolund B, Liljefors T, Krogsgaard-Larsen P. Neuronal nicotinic acetylcholine receptors: structural revelations, target identifications, and therapeutic inspirations. J. Med. Chem. 2005;48:4705–4745. doi: 10.1021/jm040219e. [DOI] [PubMed] [Google Scholar]