Abstract
The semantic variant of primary progressive aphasia (PPA) is characterized by the combination of word comprehension deficits, fluent aphasia and a particularly severe anomia. In this study, two novel tasks were used to explore the factors contributing to the anomia. The single most common factor was a blurring of distinctions among members of a semantic category, leading to errors of overgeneralization in word–object matching tasks as well as in word definitions and object descriptions. This factor was more pronounced for natural kinds than artifacts. In patients with the more severe anomias, conceptual maps were more extensively disrupted so that inter-category distinctions were as impaired as intra-category distinctions. Many objects that could not be named aloud could be matched to the correct word in patients with mild but not severe anomia, reflecting a gradual intensification of the semantic factor as the naming disorder becomes more severe. Accurate object descriptions were more frequent than accurate word definitions and all patients experienced prominent word comprehension deficits that interfered with everyday activities but no consequential impairment of object usage or face recognition. Magnetic resonance imaging revealed three characteristics: greater atrophy of the left hemisphere; atrophy of anterior components of the perisylvian language network in the superior and middle temporal gyri; and atrophy of anterior components of the face and object recognition network in the inferior and medial temporal lobes. The left sided asymmetry and perisylvian extension of the atrophy explains the more profound impairment of word than object usage and provides the anatomical basis for distinguishing the semantic variant of primary progressive aphasia from the partially overlapping group of patients that fulfil the widely accepted diagnostic criteria for semantic dementia.
Keywords: aphasia, frontotemporal lobar degeneration, language processing, progressive aphasia, semantic categorization
Introduction
The naming of objects, effortless as it may feel in the course of everyday life, is an immensely complex and uniquely human faculty that engages multiple cortical systems (Levelt et al., 1999; Damasio et al., 2004; DeLeon et al., 2007). Although object naming can proceed along any sensory modality, most of the research has focused on the verbal naming of visually presented objects. Naming can fail because of impairments in object recognition (as in patients with associative agnosia), in word comprehension (as in the case of Wernicke's aphasia), in lexical retrieval (as in the case of the tip-of-the-tongue phenomenon) in phonological encoding (as manifested by phonemic paraphasias) or in linking the object to its lexical representations (as in the case of optic aphasia) (Goodglass et al., 1976; Beauvois, 1982; Farah and Wallace, 1992; Goodglass, 1998; Levelt et al., 1999; Riddoch et al., 1999; Jefferies and Lambon Ralph, 2006). It is not possible to name an object if is not recognized, if the word that represents its name is not comprehended, or if the linkage of object to word becomes ineffective. Conversely, neither comprehension of the word nor recognition of the object is sufficient to support naming. Both must function optimally, simultaneously and interactively for naming to succeed.
There is a rich and growing literature on anomia, including articles that outline computational models of object classification, the category-specificity of naming disorders and the anatomical substrates of the underlying neural processes (Martin et al., 1996; Levelt et al., 1999; Capitani et al., 2003; Damasio et al., 2004; Bright et al., 2005; Martin, 2007; Rogers and Patterson, 2007). Many of the pioneering studies on anomia were based on patients with focal cerebrovascular lesions (Geschwind, 1967; Goodglass et al., 1976). Following a stroke, neuronal activity is completely lost within a circumscribed core lesion site, leading to an abrupt functional decline. The resultant naming impairments can be investigated in the acute phase by delineating the boundaries of impaired perfusion and linking them to the characteristics of the anomia (DeLeon et al., 2007). More chronically, the deficits tend to stabilize within a few months and lend themselves to meticulous investigations of clinico-anatomical correlations.
Recently, emphasis has also been directed to patients who develop progressive anomias as a consequence of neurodegenerative diseases (Grossman et al., 2004; Hillis et al., 2004; Masterson et al., 2007; Rohrer et al., 2008; Rogers and Friedman, 2008). Neurodegenerative diseases tend to have selective predilections for specific neuronal types, cortical layers and even neural systems. Moreover, the damage is almost always confined to grey matter and does not spread to white matter, as frequently happens in cerebrovascular accidents. No area undergoing neurodegeneration sustains a complete cessation of activity. Instead, the progressive neuronal loss leads to a gradual dissolution of function at the same time that the affected neural circuits undergo some degree of compensatory reorganization (Sonty et al., 2003; Vandenbulcke et al., 2005).
Clinico–anatomical correlations are more challenging in patients with neurodegenerative disease and the symptomatology can be more colourful, complex and fluid. If cerebrovascular lesions can be said to simulate the pulling of the plug from an electrical device, degenerative disease can be said to induce erratic perturbations and shorts superimposed on an indolently progressive malfunction. Anatomical delineations of the disease face serious obstacles since the boundaries of neuronal and synaptic loss are never sharp and since only peak areas of atrophy and hypometabolism can be detected by available imaging modalities. However, investigations of these patients are uniquely interesting for exploring the cognitive architecture of naming, especially since the partial perturbations induce subtle dissociations that are less frequently seen in patients with cerebrovascular disease. One neurodegenerative syndrome that has lent itself to particularly fruitful investigations of this type is primary progressive aphasia (PPA).
PPA is diagnosed when history and clinical investigations determine that the cause is neurodegenerative, and that the language impairment (defined by a dissolution of word-finding, word comprehension or grammar) is initially the principal obstacle to the customary conduct of daily living activities (Mesulam, 1982; Weintraub et al., 1990; Mesulam, 2003; Rogalski and Mesulam, 2007). Individual differences in the nature of the language impairment have led to the identification of several variants of PPA. According to a classification we use in clinical practice, the semantic variant (PPA-S) is characterized by poor single word comprehension and fluent aphasia but relatively preserved syntax, the agrammatic variant (PPA-G) by poor syntax and fluency but relatively preserved word comprehension and the logopenic variant (PPA-L) by word-finding hesitations, poor repetition and variable fluency on a background of preserved syntax and comprehension (Gorno-Tempini et al., 2008; Mesulam and Weintraub, 2008). The logopenic and agrammatic subtypes also fit diagnostic criteria for progressive non-fluent aphasia (PNFA), and the semantic subtype partially overlaps with the syndrome of semantic dementia (SD) (Neary et al., 1998; Adlam et al., 2006).
Anomia is one of the most common manifestations of PPA and may emerge as the major presenting deficit in patients with the logopenic subtype. However, patients with the semantic variants of this syndrome have the most consistent and profound deficits. They cannot name objects they are shown and in many instances cannot select the correct object placed among foils when its name is provided. This second component of the anomia, indicative of word comprehension deficits, is a characteristic feature of PPA-S. Patients with the logopenic and agrammatic variants of PPA may also fail to name objects. However, in contrast to PPA-S, patients with PPA-G and PPA-L are more successful in pointing to the correct object upon hearing its name, suggesting that their anomia reflects a processing bottleneck more closely related to word retrieval and phonological encoding than word knowledge.
The anomias of PPA, and especially in PPA-S, have been characterized extensively with respect to semantic categories, relationship to object knowledge and nature of naming errors (Weintraub et al., 1990; Hillis, 2002; Hillis et al., 2004; Rogers et al., 2004; Jefferies and Lambon Ralph, 2006; Rohrer et al., 2008). The current study was performed to further explore the cognitive mechanisms and neuroanatomical substrates of anomia in PPA-S with two novel tasks specifically designed to address hypotheses concerning the causes of the naming deficits. The two tasks enabled a principled and comprehensive evaluation of 40 object-name pairs from the vantage points of word definition, object description, reciprocal word–object matching and confrontation naming. All seven consecutively encountered patients who fulfilled the criteria of PPA-S and consented to participate were included in order to avoid a selection bias. The results are reported by individual patient as well as by group in order to combine the rich detail of single case investigations with the generalizability of group studies. This study also provides the first delineation of PPA-S as a progressive aphasic syndrome closely related to semantic dementia.
Methods
Subjects
All patients were right handed as determined by the Edinburgh scale (Oldfield, 1971), three were male and four female (Table 1). Age of symptom onset was in their fifties. Duration of disease at the time of testing varied from 3 to 8 years. The diagnosis of PPA was made on the basis of a progressive language disturbance (i.e. aphasia) that was initially the most salient feature of the clinical picture (i.e. primary) and that was caused by neurodegeneration (i.e. progressive). The presence of an aphasia was ascertained by an abnormal aphasia quotient (AQ) on the Western Aphasia Battery (WAB) (Kertesz, 1982). The progressive nature of the problem was documented by history taken from the patient, medical records and from at least one additional informant who lived in the same household. The diagnosis of semantic variant was based on the presence of single word comprehension deficits as tested by a subset consisting of 36 of the difficult items (items 157–92) of the Peabody Picture Vocabulary Test (PPVT-IV) (Dunn and Dunn, 2006) and the preservation of fluency as measured on the Western Aphasia Battery. Although articulatory ease, phrase length and rate of word production were preserved, the output contained word-finding hesitations, circumlocutions and simplifications characteristic of fluent aphasias (Mesulam, 2001). Further confirmation of the PPA-S diagnosis was obtained from abnormal performance on the Pyramids and Palm Trees (PPT) test (Howard and Patterson, 1992) and severe impairments on the Boston Naming Test (BNT) (Kaplan et al., 1983).
Table 1.
Demographic and neuropsychological information on the six patients
| P1 | P2 | P3 | P4 | P5 | P6 | P7 | |
|---|---|---|---|---|---|---|---|
| Age at testing/gender | 57/F | 65/M | 56/F | 63/M | 53/F | 65/M | 54/F |
| Education | 16 | 20 | 18 | 18 | 16 | 16 | 12 |
| Handedness—Edinburgh | R(+65) | R(+80) | R(+100) | R(+100) | R(+100) | R(+100) | R(+100) |
| Symptom duration (years) | 3 | 7 | 7 | 5 | 3 | 8 | 3 |
| Autobiographical memory failures | NO | NO | NO | NO | NO | NO | NO |
| Object use/face recognition failures | 1 | NO | NO | NO | NO | NO | NO |
| Judgment of line orientation (30) | 21 | 20 | 30 | – | 28 | 25 | 17 |
| PPT pictures (52) | 40 | 38 | 42 | 42 | 28 | 29 | 42 |
| PPVT (36) | 16 | NA | 14 | 17 | 8 | NA | 10 |
| Boston Naming Test | 3/30 | 6/60 | 3/60 | 14/60 | 4/60 | 3/60 | 6/60 |
| Western Aphasia Battery AQ (100) | 77.5 | 81.2 | 75.5 | 88.2 | 65.9 | 73.2 | 83.2 |
| WAB Subtests | |||||||
| Information content (10) | 7 | 9 | 8 | 10 | 9 | 8 | 9 |
| Fluency (10) | 9 | 9 | 9 | 9 | 9 | 9 | 9 |
| Yes/no questions (60) | 57 | 51 | 51 | 60 | 51 | 54 | 60 |
| Auditory word recognition (60) | 45 | 49 | 46 | 59 | 42 | 56 | 54 |
| Sequential commands (80) | 75 | 80 | 64 | 71 | 28 | 70 | 80 |
| Repetition (100) | 98 | 95 | 84 | 97 | 74 | 85 | 91 |
| Object naming (60) | 20 | 19 | 21 | 33 | 6 | 12 | 31 |
| Word fluency (animals) (20) | 5 | 6 | 6 | 7 | 3 | 3 | 4 |
| Sentence completion (10) | 8 | 6 | 6 | 9 | 0 | 2 | 7 |
| Responsive speech (10) | 8 | 10 | 10 | 10 | 6 | 4 | 6 |
Handedness was evaluated with the Edinburgh scale. Autobiographical memory for recent events was assessed during the clinical visit in the presence of a reliable witness to the events. Caregivers were questioned about evidence for failures in object usage or face recognition. Abnormal values in the PPT, PPVT-IV, BNT and the Western Aphasia Battery Aphasia Quotient (WAB AQ) are shown in bold. Numbers in parentheses next to neuropsychological tests indicate maximum possible scores.
Informants were also questioned about object misuse and failures to identify familiar faces. Such occurrences were explicitly denied for P2–P7. The only positive response came from P1's husband who remembered one instance when she had tried to ‘turn on’ a regular toothbrush as if it had been an electric toothbrush. To pursue this incident further, one of us (E.R.) visited P1 at home and found no evidence of object or appliance misuse. In fact, P1 had just baked a cake and insisted that E.R. have a slice, serving it with no difficulty. The relative preservation of visuospatial skills was shown by performance on the Judgment of Line Orientation Test (Benton et al., 1978). Only P7 performed in the mildly impaired range. Autobiographical memory, tested in the presence of an informant, revealed no major impairment of declarative memory for recent events. Reliable informants reported that the initial problems were confined to language and that behavioural problems were not noticed for at least the first 2 years. Independence in activities of daily living was largely preserved, especially in the initial 2–3 years and many patients were still driving, shopping and taking care of their finances at the time of testing. In addition to the seven PPA patients (P1–P7), five normal control subjects (C1–C5) participated in Experiment 2. The control group did not significantly differ from the patient group in age (62.8 ± 6.7 versus 59 ± 5.2) or education (15 ± 1.7 versus 16.6 ± 2.5). Our patients did not fully meet the criteria of Neary et al. (1998) for semantic dementia because they did not have prosopagnosia or associative agnosia. However, they did fulfil the revised criteria proposed by Adlam et al. (2006), which defines semantic dementia as a subtype of PPA. The study was approved by the Northwestern University Institutional Review Board.
Imaging
Each subject had a structural MRI scan. The results were used to rule out other lesions that may have caused the aphasia and to delineate the areas of major atrophy. For two of the subjects (P2 and P6), only outside scans were available and areas of atrophy were identified by visual inspection of coronal and axial sections. For the other five patients, the scans were acquired with a Siemens Trio 3-Tesla scanner (Erlangen, Germany), using a 12-channel birdcage head coil, with a T1-weighted 3D MP-RAGE sequence (TR = 2300 ms, TE = 2.86 ms, flip angle = 9, FoV = 256 mm), recording 160 slices at a slice thickness of 1.2 mm according to the Alzheimer's Disease Neuroimaging Initiative (ADNI) protocol (http://www.loni.ucla.edu/ADNI/Research/Cores/documents/ADNI_Siemens_3.0T_Trio_VB12T.pdf). Cortical reconstruction was performed on each subject with the FreeSurfer image analysis suite version 4.1.0, which is available for download online (http://surfer.nmr.mgh.harvard.edu/). Spherical maps for each subject were morphed into a common spherical atlas using a non-linear surface-registration procedure. This allows for high-registration, surface-based averaging and comparison of cortical measurements across subjects (Fischl et al., 1999). The cortical thickness map of the PPA subject group was statistically contrasted to a map derived from a separate control group of 11 right-handed subjects (five male, six female, average age 63.6, average BNT score 58.7).
Experiment 1
There were two stimulus sets: 40 line drawings of common objects and 40 written words corresponding to the object names. They were evenly divided into four categories, two of artefacts (tools and clothes) and two of natural kinds (animals and fruits/vegetables). Test forms A and B each contained line drawings of 20 objects, 5 of each category, intermingled as to category but regularly spaced into 4 rows and 5 columns on an 8.5 × 11 sheet of laminated paper. Test forms C and D each contained the names of the objects in forms A and B, respectively, written on a sheet of the same size and spatially arranged in the same fashion as the drawings. Each of the drawings and each of the words were also placed on a small card for individual presentation.
There were 5 principal conditions using the same set of the 40 entities: (i) confrontation naming of the pictured object; (ii) description of the pictured object; (iii) definition of the printed word denoting the object; (iv) word-to-picture matching; and (v) picture-to-word matching. The patient was first shown object drawings, one at a time, and asked to name them and also describe their use or nature. They were also asked to read the words aloud and define them verbally. They were then administered the four forms of the matching tests, in an ACDB order. In forms A and B (word–picture matching) the subject was given a card with a single word on it and asked to point to the matching object on the test from. In forms C and D (picture–word matching) the subject was shown a single object picture and had to point to the matching word on the test form.
In 5 of the 7 patients, the verbal definitions of the 40 words and descriptions of the 40 objects were recorded, transcribed and scored into four categories as follows: (i) Clearly wrong, irrelevant or ‘don’t know’; (ii) Too general at the category level (e.g. ‘you eat it’, ‘you wear it’, ‘animal’ or ‘you use it’.); (iii) Too general at the sub-category level (e.g. ‘dessert’, ‘guys wear it’, ‘large animal in Africa’ or ‘use it for cooking’,); and (iv) Names the picture correctly or provides a description that is relatively unambiguous (e.g. ‘It is sweet, red and I put it in my cereal’, ‘guys wear it around the neck’, ‘cute animal around the house that is self absorbed’ or ‘use it to sweep the floor’. The responses were scored independently by two raters (M.M. and C.W.). The two sets of scores were then compared and the few discrepancies (<20% of the total) reconciled by consensus.
Each word and each picture was presented only once during the word-to-picture and picture-to-word matching tasks. The set of stimuli in test forms A (drawings) and C (written words) were as follows: socks, belt, tie, vest, glove, brush, stapler, spatula, funnel, broom, onion, corn, pepper, radish, celery, mouse, squirrel, frog, snake and cat. In forms B (drawings) and D (written words) the stimuli were: dress, coat, shirt, hat, shoe, hammer, pliers, saw, scissors, screwdriver, apple, grapes, strawberry, pear, pumpkin, elephant, hippopotamus, zebra, lion and camel. The stimuli were taken from the Northwestern Naming Battery (NNB), a comprehensive naming test that is being standardized (Thompson and Weintraub). There were no significant differences of word length, familiarity or frequency when names of living objects (animals and fruits/vegetables) were compared to names of non-living objects (clothing, tools) (5.65 ± 1.66 versus 5.60 ± 2.19 for length; 3.27 ± 0.87 versus 3.73 ± 0.55 for familiarity; 14.45 ± 15.9 versus 26.17 ± 30.7 for frequency using the Celex database). Six of the seven subjects underwent all phases of this experiment while subject P2 was only tested with the word-to-picture forms. The testing in P6 included all phases but was limited to 20 of the 40 items (forms A and C) since he could not tolerate more extensive testing. In P1, the test forms were re-administered 8 months following the initial testing to explore the stability of the impairments at the single item level. Neuropsychological investigations did not reveal significant impairments of reading, repetition, spatial attention, visual search or the ability to match identical drawings in any of the patients so that elementary visuospatial or word-form processing impairments could not account for the results.
Experiment 2
The stimuli were the same 40 objects and words used in Experiment 1. The patient heard a word and 400 ms later saw side-by-side pictures of two different objects on a computer screen. One picture (target) depicted the object designated by the spoken word; the other (distracter) did not. Targets and distracters were equally placed on the right and left side of the screen. There were 40 pairs, 20 with living objects as targets and 20 with non-living objects as targets. Each word was presented only once. Each drawing was presented twice, once as the target and once as the distracter. In half the trials, the distracter and target belonged to the same one of the four categories (i.e. clothing, tools, animals, fruits/vegetables). The subject was asked to press the computer key on the side of the object matching the stimulus word as soon as possible and reaction times (RTs) were recorded. There were no significant differences of length, familiarity or frequency in the target words used for trials of semantically related versus unrelated pictures (5.65 ± 1.92 versus 5.55 ± 1.92 for length; 3.54 ± 0.08 versus 3.50 ± 0.08 for familiarity; 20 versus 20 for frequency in the Celex database).
Statistical methods
Word–picture and picture–word matching errors were compared using paired t-test. The percentage of semantically related matching errors was compared to the chance rate of 25% using a one-sample z-test for proportions. Confidence intervals were calculated using exact binomial methods. Reaction time data are reported as mean ± SD. RTs were compared between conditions (related/unrelated, living/non-living) within person using the independent sample t-test. RTs were also analysed using two-factor (group, condition) repeated measures ANOVA with the interaction test indicating differential responses for patient and control groups. Mean RTs combined across patients were compared between conditions using an ANOVA post hoc t-test. Statistical significance was indicated when P < 0.05. For cortical thickness data, statistical surface maps were generated using a general linear model that displayed differences in cortical thickness between groups for each vertex. False Discovery Rate (FDR), which controls for the expected proportion of false positives in a statistical test, was applied to adjust for multiple comparisons (Genovese et al., 2002). A stringent threshold of P = 0.00001 was used to detect areas of peak cortical thinning (i.e. atrophy) in PPA compared to controls.
Results
Experiment 1
Confrontation naming
In agreement with the BNT scores, confrontation naming of the pictured objects used in this experiment was severely impaired (Table 2). Failure rates ranged from a low of 55% in P4 to a high of 98% in P1. Semantic or phonemic paraphasias were rare. If an object could be named by confrontation, it was always also correctly linked to the corresponding word in both matching conditions. The converse was not true. Thus, error rates were substantially lower in the matching than in the confrontation naming tasks (Table 2). The number of errors in the picture-to-word matching task was divided by the number of confrontation naming failures to determine the proportion of unnamed objects that also failed to be matched to the correct word (Table 2). This proportion represented an anomic subset where names were neither produced nor recognized. There were no significant differences in the average number of errors per patient when the word-to-picture task was compared to the picture-to-word task (P = 0.16, 16.7 versus 16 per patient).
Table 2.
Errors and error types in naming the 40 object pictures and in the word-to-picture (W–P) and picture-to-word (P–W) matching tasks (Experiment 1)
| Failures of confrontation naming | Errors in W–P matching | Percentage of W–P matching errors that are semantically related | Errors in P–W matching | Percentage of P–W matching errors that are semantically related | Percentage of object names that were neither produced nor recognized | |
|---|---|---|---|---|---|---|
| P1 | 39/40 | 22 | 91 | 19 | 100 | 49 |
| P2 | NA | 26 | 77 | NA | NA | NA |
| P3 | 30/40 | 14 | 64 | 13 | 85 | 43 |
| P4 | 22/40 | 2 | 100 | 5 | 100 | 22 |
| P5 | 37/40 | 30 | 63 | 33 | 55 | 89 |
| P6 | 18/20 | 9 | 22 | 15 | 20 | 83 |
| P7 | 28/40 | 14 | 93 | 11 | 100 | 39 |
The last column on the right lists the percentage of unnamed objects that also failed to be matched correctly in the P–W task. This number indicates the proportion of naming failures where the name was neither produced nor recognized.
Semantic determinants of matching errors
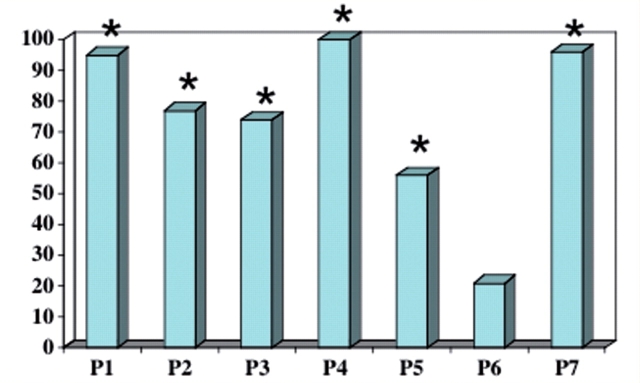
Forms A–D elicited errors in the form of mispointing to an incorrect item. The majority of mispointing errors in subjects P1–P5 and P7 were directed to items of the same semantic category as the target (Table 2, Fig. 1). This tendency for semantically related errors was statistically significant at the individual level for P1–P5 and P7, being weakest in P5 and strongest in P4. The phenomenon of mispointing to semantically related items was not observed in P6 who had the longest disease duration at testing and whose incorrect choices targeted semantically related items as frequently as items that belonged to another category.
Figure 1.
Percentage of pointing errors in the word–picture and picture–word matching tasks that consisted of semantically related items. The asterisks indicate percentages that were significantly greater than chance at the individual patient level (Experiment 1).
Reciprocity of word–object matching impairments and temporal consistency
P1 was tested at her initial visit and 8 months later. Table 3 shows her responses in the initial word-to-picture matching condition as well as the responses in the word-to-picture and picture-to-word conditions at retesting. Accuracy at the retesting session was identical (14 of 40 items) for both matching tasks. An item-by-item analysis of responses at the retesting session was conducted to compute concordance, defined as the number of instances where a correct response in either matching task was associated with a correct response in the other. This analysis showed a lack of concordance in 78% (95% CI 61–90%) of the 23 items that had been matched correctly in either the word-to-picture or picture-to-word format at the retesting session (Table 3). In nine instances, P1 gave correct answers at the initial and also at the second testing 8 months later to the same items in the word-to-picture matching test (asterisks in Table 3). Assuming that this repeated success represented a stable comprehension of the word, it is interesting that the reciprocal ability to match the picture of the object to the same word in the picture-to-word task at retest occurred in two (22%) of the nine instances (95% confidence interval 13–49%). The other patients did not have longitudinal testing. In the single test session that was available, the lack of item-level concordance of correct responses in the two types of matching tasks (defined as described above), was 34% for P3, 13% for P4, 58% for P5, 67% for P6 and 17% for P7.
Table 3.
Matching performance of P1 during the initial testing and at retest 8 months later (Experiment 1)
| Initial test | Retest |
||
|---|---|---|---|
| Stimulus | W–P | W–P | P–W |
| PUMPKIN | pumpkin | apple | pumpkin |
| HAT* | hat | hat | shirt |
| ELEPHANT | hippopotamus | zebra | hippopotamus |
| DRESS* | dress | dress | shirt |
| PLIERS | scissors | pliers | pliers |
| SHOE* | shoe | shoe | shoe |
| HAMMER* | hammer | hammer | scissors |
| ZEBRA | hippopotamus | elephant | elephant |
| SHIRT | coat | shirt | coat |
| STRAWBERRY | grapes | grapes | pear |
| APPLE | grapes | pumpkin | grapes |
| GRAPES* | grapes | grapes | grapes |
| SCREWDRIVER | hammer | scissors | pliers |
| PEAR | apple | hammer | pear |
| HIPPOPOTAMUS | hippopotamus | lion | hippopotamus |
| LION | lion | hippopotamus | elephant |
| SCISSORS* | scissors | scissors | grapes |
| SAW | hammer | hammer | screwdriver |
| COAT* | coat | coat | dress |
| CAMEL | camel | lion | hippopotamus |
| TIE | vest | vest | tie |
| BELT | tie | belt | vest |
| ONION | celery | onion | onion |
| SQUIRREL | squirrel | mouse | squirrel |
| SNAKE | cat | squirrel | squirrel |
| CORN | corn | pepper | corn |
| PAINTBRUSH | broom | stapler | paintbrush |
| SOCKS | socks | onion | socks |
| SPATULA | stapler | paintbrush | paintbrush |
| CAT | cat | mouse | squirrel |
| GLOVE | spatula | socks | belt |
| PEPPER* | pepper | pepper | radish |
| MOUSE | squirrel | mouse | mouse |
| FUNNEL | radish | stapler | stapler |
| STAPLER | paintbrush | celery | stapler |
| BROOM* | broom | broom | paintbrush |
| RADISH | corn | onion | corn |
| FROG | cat | cat | stapler |
| CELERY | onion | pepper | onion |
| VEST | vest | tie | belt |
| Correct | 18 | 14 | 14 |
The column on the left lists the test item. The test item was a word in the word–picture matching test (W–P) and an object in the picture–word matching test (P–W). Correct matches are underlined and the W–P matches that are correct at both testing sessions are indicated with an asterisk.
Verbal definition of objects and their names
Tables 4 and 5 show the distribution of the different kinds of answers in the five patients for whom recordings were available. The most common errors consisted of overgeneralizations, especially at the sub-category level. In the group as a whole, the pattern of overgeneralization was nearly as prominent for words as it was for objects. However, words elicited far fewer correct responses than objects. In some cases, the generic descriptors tended to be idiosyncratic or to reflect personal experience as in the case of P1 who called nearly all small animals ‘squirrels’, P3 who had been on safari and who defined the name and drawing of the larger animals as being ‘in Africa’, P5 who identified many of the small animals as ‘bugs’ and P7 who defined many fruits as ‘dessert’. There were also individual differences. Patient 5, for example, stood out with the high number of complete failures in word definitions. There were instances where picture descriptions were much more accurate than the definitions of the corresponding word. Striking examples of these discrepancies are given in Table 6. The converse dissociation of distinctly worse object descriptions was rarely encountered although in most of the items the picture definitions were just as overgeneralized as the definitions of the corresponding word.
Table 4.
Distribution of various types of responses when patients were asked to verbally define the 40 words and describe the 40 objects
| Individual patients | Wrong or ‘don’t know’ |
Too general at the category level |
Too general at the subcategory level |
Accurate |
||||
|---|---|---|---|---|---|---|---|---|
| Word (%) | Object (%) | Word (%) | Object (%) | Word (%) | Object (%) | Word (%) | Object (%) | |
| P1 | 13 | 7.7 | 49 | 28 | 33 | 56 | 5.1 | 7.7 |
| P3 | 5.1 | 5.1 | 23 | 20 | 54 | 39 | 18 | 42 |
| P4 | 7.5 | 0 | 12 | 2.5 | 58 | 40 | 22 | 58 |
| P5 | 42 | 10 | 40 | 45 | 12 | 32 | 5.0 | 12 |
| P7 | 7.5 | 0 | 22 | 5.0 | 52 | 52 | 18 | 42 |
Percentages may not add up to exactly 100% because of rounding to two digits.
Table 5.
Group means for the four types of responses
| Group means | Wrong or ‘don’t know’ (%) | Too general at the category level (%) | Too general at the subcategory level (%) | Accurate (%) |
|---|---|---|---|---|
| WORD | 15.1 | 29.4 | 41.9 | 13.6 |
| OBJECT | 4.6 | 20.2 | 44.1 | 32.4 |
Table 6.
Samples from individual patients showing superiority of picture description in comparison to word definition
| Item (patient) | Description of the picture | Definition of the word |
|---|---|---|
| Tie (P1) | This is what a man uses to wear | I don’t know |
| Camel (P3) | Another one that I was on. It was in Africa, too, I was a teenager when I first was on one. | It's used for something, maybe for pictures or something |
| Zebra (P7) | It's an animal and it's a big one. | The only thing I am remembering it might be, just something that could be put on top of something showing where something is. |
| Zebra (P4) | It's another fairly large animal that I think lives in India or Africa | A small animal that would be a flying type of animal. |
| Funnel (P4) | I use it at home to put things into a small space than runs through it. The water or oil whatever goes through it. | I can’t remember what a funnel is |
| Spatula (P4) | Used for food to move the food around particularly if you are cooking. | I don’t know what it is |
| Pumpkin (P4) | It's a vegetable that will be used … I am not sure what it is for. | It's an animal also. It's relatively, well, I can’t say if it is a small or big animal. |
Experiment 2
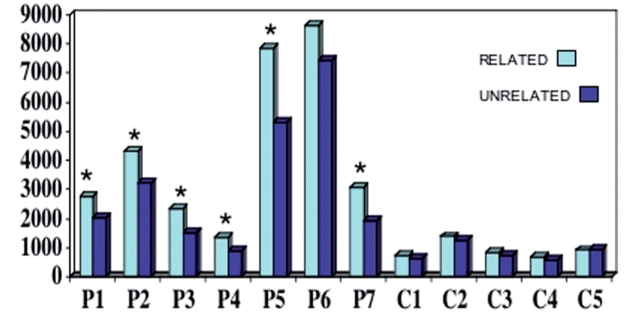
The preponderance of semantically related errors in the matching tasks of Experiment 1 suggested the presence of an inability to discriminate members of the same semantic class. Experiment 2 was designed to test this hypothesis. In this forced-choice RT experiment, accuracy was 75% or better in all patients except P2 whose accuracy was 45% (Table 7, Fig. 2). The normal controls had accuracy levels of 98–100%. In half of the 40 trials for each subject, the two objects on the screen belonged to the same category (tools, clothing, animals or vegetables/fruits) and were therefore semantically related whereas in the other half of the trials the two choices belonged to unrelated categories.
Table 7.
Accuracy and response speed in computerized word-to-picture trials where the two choices on the screen were semantically related or unrelated and where both were living or non-living objects (Experiment 2)
| Subject/accuracy (%) | Related RT | Unrelated RT | t-test | Living RT | Non-living RT | t-test |
|---|---|---|---|---|---|---|
| P1 (90) | 2781 ± 1252 | 2032 ± 501 | 0.03* | 3172 ± 1157 | 2346 ± 1271 | 0.156 |
| P2 (45) | 4323 ± 1153 | 3222 ± 1745 | 0.02* | 4976 ± 819 | 3736 ± 1119 | 0.014* |
| P3 (100) | 2350 ± 1352 | 1515 ± 781 | 0.022* | 2221 ± 1063 | 2479 ± 1641 | 0.681 |
| P4 (98) | 1366 ± 838 | 873 ± 313 | 0.021* | 1735 ± 1020 | 997 ± 372 | 0.045* |
| P5 (75) | 7849 ± 3398 | 5296 ± 1560 | 0.006* | 9518 ± 2913 | 6347 ± 3203 | 0.038* |
| P6 (85) | 8616 ± 3805 | 7444 ± 3684 | 0.335 | 10423 ± 3425 | 6989 ± 3509 | 0.046* |
| P7 (92) | 3053 ± 1735 | 1923 ± 924 | 0.014* | 3503 ± 2055 | 2604 ± 1303 | 0.257 |
| Patient mean | 4334 ± 2813 | 3186 ± 2368 | 0.0002* | 5078 ± 3505 | 3642 ± 2222 | 0.005* |
| C1 (100) | 708 ± 167 | 635 ± 107 | 0.117 | 718 ± 185 | 698 ± 157 | 0.801 |
| C2 (100) | 1379 ± 222 | 1257 ± 156 | 0.056 | 1391 ± 213 | 1367 ± 242 | 0.826 |
| C3 (100) | 830 ± 194 | 741 ± 156 | 0.117 | 759 ± 163 | 901 ± 204 | 0.101 |
| C4 (98) | 685 ± 185 | 591 ± 132 | 0.078 | 689 ± 152 | 681 ± 226 | 0.931 |
| C5 (100) | 910 ± 175 | 935 ± 204 | 0.689 | 918 ± 119 | 901 ± 224 | 0.829 |
| Control mean | 902 ± 282 | 831 ± 272 | 0.767 | 895 ± 291 | 909 ± 277 | 0.976 |
Significant differences are indicated by an asterisk.
Figure 2.
Reaction times in the computerized word-to-picture matching task in trials where the two choices on the screen were semantically related or unrelated. Asterisks indicate significant differences at the individual level (Experiment 2).
The controls were significantly faster than the patients in trials based on related as well as unrelated pairs. The patient group was significantly slower in trials based on related pairs whereas the control group did not display such a difference (interaction P = 0.005). Combining all patients, there was a significant difference between RTs to related and unrelated pairs (P = 0.0002, Table 7). Individual t-tests showed that P1–P5 and P7 were each significantly slower in trials where the two choices were semantically related. P6, who also failed to show the tendency to mispoint to categorically related items in Experiment 1, was equally slow in responding to semantically related and unrelated foils, presumably because he was also insensitive to overall category boundaries.
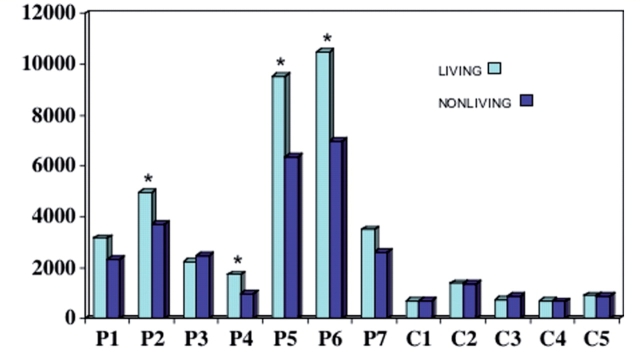
Greater semantic relatedness effect for natural kinds
The RTs were further analysed to see if the semantic relatedness effect favoured one category over another. We compared trials where both choices were of the same category of natural kinds (pairs of animals or of vegetables/fruits) to trials where both choices were artefacts (clothing or tools). As a group, the PPA-S patients were slower for pairs of living objects, indicating greater difficulty in making a choice (P = 0.005, Table 7). Normal controls did not show significant differences (interaction P = 0.04). On an individual basis, this category effect was significant in P2 and P4–P6 (Fig. 3). It is interesting that P6, who did not have a semantic relatedness effect in the overall analysis, did show greater slowing of responses for natural kinds, illustrating the selectivity of disease-related distortions of semantic maps. The category-specific difference of the relatedness effect could not be attributed to differences in accuracy since the patients were no more accurate in trials where both choices were living than in trials where they both were artefacts.
Figure 3.
Reaction times in trials on the computerized task where both choices were living or non-living objects. Asterisks indicate significant differences at the individual level (Experiment 2).
Imaging
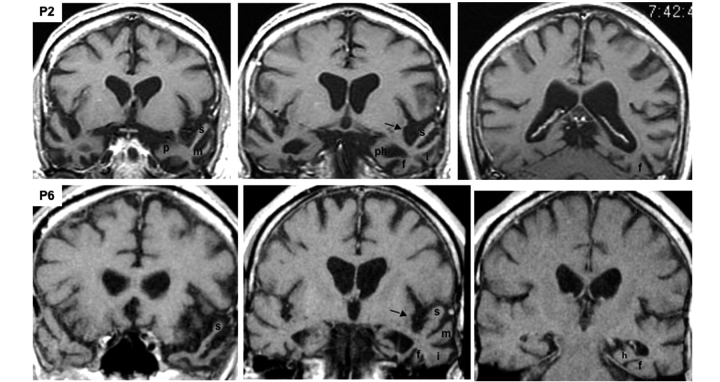
The cortical thickness maps revealed highly significant cortical thinning (e.g. atrophy) when the PPA group was compared to controls (Fig. 4). The atrophy was much more extensive in the left hemisphere and encompassed all parts of the anterior temporal lobe, including the anterior two thirds of the superior temporal (s), middle temporal (m), inferior temporal, and fusiform gyri as well the anterior parahippocampal gyrus (ph) and the temporal pole (p). In the right hemisphere areas of peak atrophy had a much more limited distribution, encompassing mostly the most anterior parts of the inferior temporal gyrus. Perisylvian atrophy, reflecting the thinning of the superior temporal gyrus was prominent only on the left. Only outside scans were available for P2 and P6 (Fig. 5). Qualitative analysis of their scans revealed nearly identical features to the scans of the other five patients, including the asymmetrical left perisylvian atrophy, and involvement of inferomedial temporal areas.
Figure 4.
Distribution of cortical thinning in group of five PPA-S patients (P1, P3–P5 and P6) compared to a control group. f = fusiform (occipitotemporal) gyrus; i = inferior temporal gyrus; m = middle temporal gyrus; p = temporal polar cortex; ph = parahippocampal gyrus; s = superior temporal gyrus. Significance is displayed as a log(10) P-value.
Figure 5.
T1-weighted MRI scans of P2 and P6. The sections are in the coronal plane and are displayed according to the radiological convention so that the left hemisphere is on the right side of each section. The arrows point to asymmetric widening of the left perisylvian region. For each patient, the section on the left is most anterior, at the level of the temporal pole and amygdala, the middle section is at the level of the anterior hippocampus and the section on the right is at the level of the mid-to-posterior fusiform gyrus. a = amygdala; f = fusiform (occipitotemporal) gyrus; h = hippocampus; i = inferior temporal gyrus; m = middle temporal gyrus; p = temporal polar cortex; ph = parahippocampal gyrus; s = superior temporal gyrus.
Discussion
The purpose of this study was to explore the nature of the anomia in seven patients with PPA-S. The first of two experiments probed the patients’ knowledge of 40 objects and their names from five vantage points: verbal naming of the object, verbal definition of the word, verbal description of the object, matching the object to a word and the reciprocal matching of the word to an object. A second experiment allowed a quantitative determination of word–object matching speed within the same set of 40 items in a setting that required the rapid selection of a target paired with a foil. The following discussion of the results is divided into sections addressing the cognitive characteristics of the anomia and some of the relevant neuroanatomical correlates.
Anomia with and without word recognition
The patients were profoundly anomic, even when asked to name highly familiar objects. In some instances, however, the name that could not be produced was successfully recognized in the matching tasks. In others, the name was neither produced nor recognized. It is reasonable to assume that the latter pattern of anomia reflects a more profound ‘semantic’ impairment of word or object concepts whereas the first pattern may reflect a greater contribution of lexical access or retrieval impairments. These two patterns were differentially distributed in individual patients. In P4, for example, only 22% of naming failures were also associated with word recognition (i.e. picture–word matching) failures whereas this percentage was higher than 80% for P5 and P6 (Table 2). In general, the least severely anomic patients (P3, P4 and P7), displayed the lowest proportion of naming errors associated with word recognition failures. The failure to name an object even when its name can be recognized, a pattern that appears to be particularly prominent at the early stages of PPA-S, is also the most common pattern of anomia in non-semantic PPA variants (Mesulam, 2001).
Distortion of semantic maps
Although the patients made many errors in the matching tasks, their responses did not reflect random choices. An item-by-item analysis showed that the majority of erroneous choices were semantically related to the stimulus. This phenomenon of coordinate errors implies a selective distortion of intra- but not inter-category distinctions. In the word-to-picture matching task of P1 (Table 3), for example, the word ‘snake’ appears to have evoked the general set of animal representations but without further differentiation of its individual members. Consequently, erroneous matches to pictures of ‘cat’ or ‘squirrel’ became more likely than a match to perceptually more similar but categorically different items such as ‘tie’ or ‘belt’. The two patients whose anomia displayed the highest component of word recognition impairments, P5 and P6 (Table 2, last column on the right), were also the two with the least tendency for such coordinate errors, revealing a semantic deficit severe enough to blur inter-category boundaries as well. The high number of coordinate errors and their gradual decline with disease progression had been observed using a different set of tasks in patients with the diagnosis of semantic dementia, many of whom would have satisfied the diagnostic criteria for PPA-S (Hodges et al., 1995; Rogers et al., 2004; Jefferies and Lambon Ralph, 2006; Rogers and Patterson, 2007).
The nature of the errors in the matching tasks of Experiment 1 led to the implication that the semantic map had been distorted specifically through a blurring of distinctions among members of the same category. This hypothesis was tested and confirmed by Experiment 2 where patients as a group were significantly slower in matching a word to one of two objects when both alternatives on the screen belonged to the same semantic category. Even in patients with >75% accuracy in this task (P1, P3, P4, P5 and P7), semantically related foils required a significantly longer time to reject. This slowing of responses indicated that members of the same category were less easy to differentiate from one another than from members of another category. Control subjects did not display this semantic competition (or interference) effect. The only subject who did not individually show a significant semantic relatedness effect was P6 who also displayed an absence of coordinate mispointing tendency in Experiment 1, and a very high rate of word recognition deficits underlying his anomia. This finding is consistent with the gradual progression of the anomia from a stage where only intra-category boundaries are distorted to one where inter-category boundaries are also compromised. Experiment 2 also showed that the blurred distinction among members of a category was more severe for natural kinds (animals, vegetables/fruits) than artefacts (tools, clothing). This preponderance of semantic impairment for natural kinds has been reported in semantic dementia and has led to several explanations, including the dominance of sensory (as opposed to functional) experiences associated with living things and the greater confusability of category members due to the greater number of shared attributes (Warrington and Shallice, 1984; Humphreys and Forde, 2001; Cree and McRae, 2003; Bright et al., 2005; Zannino et al., 2006).
Overload of processing routes
The equivalent severity of impairments in the word–picture and picture–word tasks of Experiment 1 could reflect the presence of fixed semantic lacunes that consistently fail to evoke associations regardless of the modality of the probe. However, an item-by-item analysis of errors revealed a more complex situation. Thus, when the picture-to-word matching task was compared to the reciprocal word-to-picture matching task, consistency (defined as the frequency with which correct responses in one task predicted correct responses in the other) was <50% in three of the six patients (P3, P4 and P7) for whom this information was available. Subject P1, was also tested longitudinally. In nine items of the word–picture task, P1 gave correct answers at the initial testing and also at retesting 8 months later. Assuming that the persistence of accurate responses at both testing sessions reflected a stable comprehension of the word rather than coincidence, it is interesting that the reciprocal ability to associate the picture of the same object to its name at the time of retesting occurred in only two of the nine instances.
These results suggest that the equivalent severity of impairments in the two matching tasks does not necessarily reflect damage to convergent name-object representations for specific entities. Rather, it appears as if some of the naming failures can also reflect an impediment to the free flow of information between word and object representations. Such a putative ‘traffic jam’ would cause a bottleneck that compromises the efficiency and breadth of the associations, without necessarily leading to an obliteration of specific items in semantic knowledge stores. An alternative possibility is that semantic representations in PPA-S are underspecified so that the patient may randomly select correct or related items. These observations on the lack of task and item consistency is somewhat at odds with previous reports on semantic dementia (Jefferies and Lambon Ralph, 2006) and may reflect differences in the methods of testing, patient population or disease severity. It does appear, however, that the obstruction to the flow of information between an object and its name is more severe for the category of natural kinds than the category of artifacts.
Overgeneralization of word and object concepts
The failures in the matching tasks of Experiments 1 and 2 could reflect distortions at the level of either word or object concepts. Asking the patient to define the words and describe the pictures should have provided the most direct differentiation of these two possibilities. In associative agnosia, for example, the mechanism of the anomia can be clarified by demonstrating that the patient cannot describe the object but can define the word. With this goal in mind, the patients were asked to define the 40 words and describe the 40 objects used in Experiments 1 and 2. The analysis of the transcribed answers showed that the vast majority of incorrect responses represented excessive generalizations at the category and especially at the sub-category level. The frequency of precise responses was greater for objects than words, especially in patients with the milder anomias (P3, P4 and P7 in Table 2). However, even the mildest anomic patient, P4, could describe with precision only slightly more than half of the objects.
These results need to be interpreted in conjunction with two caveats. First, our patients also displayed word finding impairments, simplifications and circumlocutions as part of their fluent aphasia. What appears as a vague verbal definition or description may therefore reflect a word finding impairment as much as an impairment of word or object knowledge. However, this aphasic component should have had an equivalent effect on object definitions and word definitions. Moreover, the very frequent errors of overgeneralization are consistent with the results of Experiments 1 and 2, neither of which depended on verbal output, and both of which revealed a selective blurring of intra-category distinctions, a phenomenon that would be expected to generate the over-generalizations observed in the patients’ definitions and descriptions. One way to circumvent this potential problem would be to probe object knowledge separately with non-verbal picture association tasks where the patient might be asked to determine which two of three objects are contextually more congruent.
The second caveat is more problematic. The picture of an object provides perceptual as well as associative clues about its nature whereas the name of an object provides clues that are strictly symbolic. Surface clues, such as the shape of a garment or the legs of an animal, could thus account for the apparently greater preservation of object identification, complicating the interpretation of the results in Tables 4 and 5. However, it should be noted that our subjects displayed frequent word comprehension lapses, even for familiar words, whereas there was no evidence of consequential impairments of object usage. By such a practical criterion, our patients had relatively preserved non-verbal object knowledge at least for familiar items, a conclusion consistent with some but not all reports of patients with progressive semantic aphasia (Bozeat et al., 2002; Negri et al., 2007). A cautious interpretation of the available information would lead to the conclusion that neither word nor object concepts are intact in PPA-S and that the distorted concepts lead to excessive generalizations reflecting a failure to differentiate a specific item from its congeners. It also appears, however, that the impairment is more severe for words, especially at the early stages.
Atrophy of the language and object networks
The cognitive locus of the object naming deficit in P1–P7 defies the sort of unitary account that can be proposed for the naming deficits seen in other syndromes such as optic aphasia, visual agnosia or Wernicke's aphasia. The analysis thus far indicates that the anomia in the present sample of patients reflects a complex and variable combination of impairments in word concepts, object concepts and perhaps also lexical access and retrieval. The distribution of neuronal loss is consistent with this heterogeneity of factors. In all cases, the atrophy encompassed anterior perisylvian components of the left hemisphere language network as well as anterior components of the inferotemporal face and object recognition network (Figs 4 and 5).
Each of these two networks has been shown to display a posterior-to-anterior synaptic hierarchy. In the left hemisphere language network, the hierarchy leads from the recognition of auditory word-forms, to the identification of intelligible words, the decoding of general word meaning and the association of the word to its unique referent, a process that extends from the temporoparietal junction into the most anterior parts of the superior and middle temporal gyri (Price et al., 1996; Scott et al., 2000; Grabowski et al., 2001; Gitelman et al., 2005; Lau et al., 2008; Mainy et al., 2008). The anterior parts of this left hemisphere language network were severely atrophied in the superior and middle temporal gyri of our patients. In specific, the region of severe atrophy overlapped with an area of the superior temporal gyrus selectively activated by tasks of synonym identification (Gitelman et al., 2005).
The face and object identification network has a more bilateral distribution but displays an analogous antero-posterior processing hierarchy. Thus, progressively more anterior components of the fusiform and inferior temporal gyri have been shown to mediate levels of identification that progress from the general to the increasingly more specific levels of representation (Gorno Tempini and Price, 2001; Damasio et al., 2004; Bright et al., 2005). The anterior components of this network, located in the anterior fusiform, parahippocampal, inferotemporal and temporopolar areas, were severely atrophied in our patients, but more so on the left side. The distribution of atrophy was remarkably uniform within the set of our seven patients and, with the possible exception of the consistent leftward asymmetry and clear involvement of perisylvian cortex, is in agreement with what has been reported in groups of semantic dementia patients (Price et al., 1996; Gorno-Tempini et al., 2004; Mummery et al., 1999; Bright et al., 2005).
The neurodegeneration in our patients triggered a reversion to a shallower mode of associative encoding whereby words and objects elicited overly general, insufficiently differentiated concepts. Only distinctions among major categories survived, each centered around prototypical exemplars. The severe atrophy in the anterior temporal lobe, where the more downstream components of both the language and object processing hierarchies are located, provides a plausible neuronal substrate for this pattern of impairment. Since the language network displays a strong left hemisphere lateralization whereas the object network does not, the asymmetrical atrophy helps to explain why language disturbances in this sample emerged as more consequential components of the clinical picture than impairments of object usage.
Neuropathology and the distinction of PPA-S from semantic dementia
The most common neurodegenerative disease underlying semantic dementia and PPA-S is a subtype of frontotemporal lobar degeneration with ubiquitin and TDP-43 inclusions (Knibb et al., 2006; Neumann et al., 2006). This neuropathological entity, now known as FTLD-TDP, can display a broad range of anatomical distributions (Josephs, 2008). The neuronal loss may be strikingly asymmetrical in some cases but not in others, and can selectively target the temporal lobes in some patients and the frontal lobes in others. The resultant heterogeneity of phenotypes helps to clarify the apparent controversy concerning the relationship of the semantic dementia syndrome to PPA-S. If one were to use relatively inclusive diagnostic criteria for semantic dementia, according to which aphasia and associative agnosia are equally important and necessary core components (Neary et al., 1998), some of the patients with the semantic dementia diagnosis will turn out to have early bilateral atrophy of the ventral temporal lobe with deficits that start within the face and object recognition system and then spread to the language system, while others will display a different temporal evolution and an asymmetrical onset of atrophy within the language-dominant hemisphere (Senaha et al., 2007; Bright et al., 2008; Czarnecki et al., 2008).
The more restrictive criteria needed for the PPA-S diagnosis eliminates this variability by requiring that an impairment of language, manifested by word comprehension and aphasic deficits, be the most salient feature of early disease. It is not surprising, therefore, that P1–P7, each having met the PPA criteria, uniformly displayed prominent language impairments and leftward asymmetry of the atrophy (Figs 4 and 5). The PPA diagnosis therefore encompasses only a subset of semantic dementia patients who fit the Neary et al. criteria, namely those in whom a fluent aphasia and impaired single word comprehension constitute the most salient aspects of the initial disease (Adlam et al., 2006). Even in our subset of patients, however, the neuronal damage spread beyond the language network and into the object recognition network, reflecting the relatively common regional predilection of FTLD-TDP for the anterior temporal lobe as a whole. This anatomical distribution of atrophy is not necessarily unique to FTLD-TDP, explaining why an identical phenotype has also been reported in some patients who display the neuropathology of Alzheimer's disease at post-mortem examination (Knibb et al., 2006; Mesulam et al., 2008). It is the leftward asymmetry of atrophy, rather than the cellular nature of the neuropathology, that accounts for the salience of the language impairment in P1–P7 and that places PPA-S within the overall spectrum of PPA syndromes.
Conclusions
The defining feature of all PPA variants is a progressive language disorder (i.e. aphasia) that emerges as the principal feature of the initial clinical picture. The semantic variant of primary progressive aphasia (PPA-S) is uniquely characterized by word comprehension impairments. Patients with this syndrome also have a fluent aphasia and a remarkably severe object naming impairment. Our experiments show that the anomia in PPA-S is multifactorial and that its nature evolves as the disease becomes more severe. Loss of word comprehension accounts for only part of the naming failures since the patients can successfully recognize many of the words that they are unable to retrieve during confrontation naming. As the anomia becomes more severe, names that cannot be produced also fail to be recognized, indicating a gradual increase in the contribution of semantic mechanisms. Initially, the semantic impairment selectively distorts intra-category differentiations and leads to overgeneralized concepts. This inability to proceed from generic to specific representations may directly contribute to the anomia since naming in everyday life is dependent on the specific identification of an individual object and its differentiation from congeners. It is as if a hypothetical resolving power for semantic distances has been diminished, interfering first with intra- and eventually with inter-category discriminations. The major neuronal loss in PPA-S is confined to the anterior temporal lobe. It is also much more severe in the left hemisphere, and encompasses the language as well as object recognition networks. The clinical features and neuropathological substrates of this syndrome provide unique opportunities for exploring the cognitive mechanisms of object naming and the biological features that make the language-dominant hemisphere a preferential target for neurodegeneration.
Funding
National Institute on Deafness and Communication Disorders (DC008552); National Institute on Aging [AG13854 (Alzheimer's Disease Center)].
Glossary
Abbreviations
- PPA
primary progressive aphasia
- PNFA
progressive non-fluent aphasia
- SD
semantic dementia
References
- Adlam A-LR, Patterson K, Rogers TT, Nestor PJ, Salmond CH, Acosta-Cabronero J, et al. Semantic dementia and fluent primary progressive aphasia: two sides of the same coin? Brain. 2006;129:3066–80. doi: 10.1093/brain/awl285. [DOI] [PubMed] [Google Scholar]
- Beauvois M-F. Optic aphasia: a process of interaction between vision and language. Philos Trans R Soc Lond B Biol Sci. 1982;298:35–47. doi: 10.1098/rstb.1982.0070. [DOI] [PubMed] [Google Scholar]
- Benton AL, Varney NR, Hamsher KDS. Visuospatial judgement. Arch Neurol. 1978;35:364–7. doi: 10.1001/archneur.1978.00500300038006. [DOI] [PubMed] [Google Scholar]
- Bozeat S, Lambon Ralph MA, Patterson K, Hodges J. When objects lose their meaning: What happens to their use? Cogn Affect Behav Neurosci. 2002;2:236–51. doi: 10.3758/cabn.2.3.236. [DOI] [PubMed] [Google Scholar]
- Bright P, Moss HE, Stamatakis EA, Tyler LK. The anatomy of object processing: the role of anteromedial temporal cortex. Q J Exp Psychol. 2005;58B:361–77. doi: 10.1080/02724990544000013. [DOI] [PubMed] [Google Scholar]
- Bright P, Moss ME, Stamatakis EA, Tyler LK. Longitudinal studies of semantic dementia: the relationship between structural and functional changes over time. Neuropsychologia. 2008;46:2177–88. doi: 10.1016/j.neuropsychologia.2008.02.019. [DOI] [PubMed] [Google Scholar]
- Capitani E, Laiacona M, Mahon B, Caramazza A. What are the facts of category-specific semantic deficits? A critical review of the clinical literature. Cogn Neuropsychol. 2003;20:213–61. doi: 10.1080/02643290244000266. [DOI] [PubMed] [Google Scholar]
- Cree GS, McRae K. Analyzing the factors underlying the structure and computation of the meaning of chipmunk, cherry, chisel, cheese, and cello (and many other such concrete nouns) J Exp Psychol (General) 2003;132:163–201. doi: 10.1037/0096-3445.132.2.163. [DOI] [PubMed] [Google Scholar]
- Czarnecki K, Duffy JR, Nehl CR, Cross SA, Molano JR, Jack CR, et al. Very early semantic dementia with progressive temporal lobe atrophy. Arch Neurol. 2008;65:1659–63. doi: 10.1001/archneurol.2008.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio H, Tranel D, Grabowski T, Adolphs R, Damasio AR. Neural systems behind word and concept retrieval. Cognition. 2004;92:179–229. doi: 10.1016/j.cognition.2002.07.001. [DOI] [PubMed] [Google Scholar]
- DeLeon J, Gottesman RF, Kleinman JT, Newhart M, Davis C, Heidler-Gary J, et al. Neural regions assential for distinct cognitive processes underlying picture naming. Brain. 2007;130:1408–22. doi: 10.1093/brain/awm011. [DOI] [PubMed] [Google Scholar]
- Dunn LA, Dunn LM. Pearson: American Guidance Services Publishing; 2006. Peabody Picture Vocabulary Test-4. [Google Scholar]
- Farah MJ, Wallace MA. Semantically-bounded anomia: implications for the neural implementation of naming. Neuropsychologia. 1992;30:609–21. doi: 10.1016/0028-3932(92)90066-u. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Human Brain Mapping. 1999;8:272–84. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols TE. Thresholding of statistical maps in functional imaging using the false discovery rate. NeuroImage. 2002;15:870–8. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Geschwind N. The varieties of naming errors. Cortex. 1967;3:97–112. [Google Scholar]
- Gitelman DR, Nobre AC, Sonty S, Parrish TB, Mesulam M-M. Language network specializations: an analysis with parallel task design and functional magnetic resonance imaging. NeuroImage. 2005;26:975–85. doi: 10.1016/j.neuroimage.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Goodglass H. Stages of lexical retrieval. Aphasiology. 1998;12:287–98. [Google Scholar]
- Goodglass H, Kaplan E, WSeintraub S, Ackerman N. The ‘tip-of-the-tongue’ phenomenon in aphasia. Cortex. 1976;12:145–53. doi: 10.1016/s0010-9452(76)80018-4. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Brambati SM, Ginex V, Ogar J, Dronkers NF, Marcone A, et al. The logopenic/phonological variant of primary progressive aphasia. Neurology. 2008;71:1227–34. doi: 10.1212/01.wnl.0000320506.79811.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno Tempini ML, Price CJ. Identification of famous faces and buildings. Brain. 2001;124:2087–97. doi: 10.1093/brain/124.10.2087. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Donkers NF, Rankin RP, Ogar JM, Phengrasamil L, Rosen HJ, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol. 2004;55:335–46. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski TJ, Damasio H, Tranel D, Boles Ponto LL, Hichwa RD, Damasio AR. A role for left temporal pole in the retrieval of words for unique entities. Human Brain Mapping. 2001;13:199–212. doi: 10.1002/hbm.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, McMillan C, Moore P, Ding L, Glosser G, Work M, et al. What's in a name: voxel-based morphometric analyses of MRI and naming difficulty in Alzheimer's disease, frontotemporal dementia and corticobasal degeneration. Brain. 2004;127:628–49. doi: 10.1093/brain/awh075. [DOI] [PubMed] [Google Scholar]
- Hillis AE. Modality-specific deterioration in naming verbs in nonfluent primary progressive aphasia. J Cog Neurosci. 2002;14:1099–1108. doi: 10.1162/089892902320474544. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Oh S, Ken L. Deterioration of naming nouns versus verbs in primary progressive aphasia. Ann Neurol. 2004;55:268–75. doi: 10.1002/ana.10812. [DOI] [PubMed] [Google Scholar]
- Hodges J, Graham N, Patterson K. Charting the progression in semantic dementia: implications for the organization of semantic memory. Memory. 1995;3:463–95. doi: 10.1080/09658219508253161. [DOI] [PubMed] [Google Scholar]
- Howard D, Patterson K. Bury St. Edmonds Suffolk. UK: Thames Valley Test Company; 1992. Pyramids and palm trees: a test of symantic access from pictures and words. [Google Scholar]
- Humphreys GW, Forde EME. Hierarchies, similarity, and interactivity in object recognition: “category-specific” neurospychological deficits. Behav Brain Sci. 2001;24:453–509. [PubMed] [Google Scholar]
- Jefferies E, Lambon Ralph MA. Semantic impairment in stroke aphasia versus semantic dementia: a case-series comparison. Brain. 2006;129:2132–47. doi: 10.1093/brain/awl153. [DOI] [PubMed] [Google Scholar]
- Josephs KA. Frontotemporal dementia and related disorders: deciphering the enigma. Ann Neurol. 2008;64:4–14. doi: 10.1002/ana.21426. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. Lea & Febiger. 1983. The Boston Naming Test. Philadelphia. [Google Scholar]
- Kertesz A. San Antonio. Texas: The Psychological Corporation; 1982. Western aphasia battery. [Google Scholar]
- Knibb JA, Xuereb JH, Patterson K, Hodges JR. Clinical and pathological characterization of progressive aphasia. Ann Neurol. 2006;59:156–65. doi: 10.1002/ana.20700. [DOI] [PubMed] [Google Scholar]
- Lau EF, Phillips C, Poeppel D. A cortical network for semantics: (de)constructing the N400. Nat Rev Neurosci. 2008;9:920–33. doi: 10.1038/nrn2532. [DOI] [PubMed] [Google Scholar]
- Levelt WJM, Roelofs A, Meyer AS. A theory of lexical access in speech production. Behav Brain Sci. 1999;22:1–38. doi: 10.1017/s0140525x99001776. [DOI] [PubMed] [Google Scholar]
- Mainy N, Jung J, Baciu M, Kahane P, Schoendorff B, Minotti L, et al. Cortical dynamics of word recognition. Human Brain Mapp. 2008;29:1215–30. doi: 10.1002/hbm.20457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A. The representation of object concepts in the brain. Ann Rev Psychol. 2007;58:25–45. doi: 10.1146/annurev.psych.57.102904.190143. [DOI] [PubMed] [Google Scholar]
- Martin A, Wiggs CL, Ungerleider LG, Haxby JV. Neural correlates of category-specific knowledge. Nature. 1996;379:649–52. doi: 10.1038/379649a0. [DOI] [PubMed] [Google Scholar]
- Masterson J, Druks J, Kopelman M, Clare L, Garley C, Hayes M. Selective naming (and comprehension) deficits in Alzheimer's disease? Cortex. 2007;43:921–34. doi: 10.1016/s0010-9452(08)70691-9. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Slowly progressive aphasia without generalized dementia. Ann Neurol. 1982;11:592–8. doi: 10.1002/ana.410110607. [DOI] [PubMed] [Google Scholar]
- Mesulam M-M. Primary progressive aphasia. Ann Neurol. 2001;49:425–32. [PubMed] [Google Scholar]
- Mesulam M-M. Primary progressive aphasia: a language-based dementia. N Engl J Med. 2003;348:1535–42. doi: 10.1056/NEJMra022435. [DOI] [PubMed] [Google Scholar]
- Mesulam M-M, Weintraub S. Primary progressive aphasia and kindred disorders. In: Duyckaerts C, Litvan I, editors. Handbook of clinical neurology. New York: Elsevier; 2008. pp. 573–87. [DOI] [PubMed] [Google Scholar]
- Mesulam M, Wicklund A, Johnson N, Rogalski E, Leger GC, Rademaker A, et al. Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Ann Neurol. 2008;63:709–19. doi: 10.1002/ana.21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Wise RJS, Vandenberghe R, Price CJ, Hodges JR. Disrupted temporal lobe connections in semantic dementia. Brain. 1999;122:61–73. doi: 10.1093/brain/122.1.61. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration. A consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–54. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Negri GA, Lunardelli A, Reverberi C, Gigli GL, Rumiati RI. Degraded semantic knowledge and accurate object use. Cortex. 2007;43:376–88. doi: 10.1016/s0010-9452(08)70463-5. [DOI] [PubMed] [Google Scholar]
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–3. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;87:256–9. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Price CJ, Moore CJ, Humphreys GW, Frackowiak RSJ, Friston KJ. The neural regions sustaining object recognition and naming. Proc R Soc London. 1996;263:1501–7. doi: 10.1098/rspb.1996.0219. [DOI] [PubMed] [Google Scholar]
- Riddoch MJ, Humphreys GW, Gannon T, Blott W, Jones V. Memories are made of this: the effects of time on stored visual knowledge in a case of visual agnosia. Brain. 1999;122:537–59. doi: 10.1093/brain/122.3.537. [DOI] [PubMed] [Google Scholar]
- Rogalski E, Mesulam M-M. An update on primary progressive aphasia. Curr Neurol Neurosci Rep. 2007;7:388–92. doi: 10.1007/s11910-007-0060-0. [DOI] [PubMed] [Google Scholar]
- Rogers SL, Friedman RB. The underlying mechanisms of semantic memory loss in Alzheimer's dosease and semantic dementia. Neuropsychologia. 2008;46:12–21. doi: 10.1016/j.neuropsychologia.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers TT, Lambon Ralph MA, Garrard P, Bozeat S, McClelland JL, Hodges JR, et al. Structure and deterioration of semantic memory: a neuropsychological and computational investigation. Psychol Rev. 2004;111:205–35. doi: 10.1037/0033-295X.111.1.205. [DOI] [PubMed] [Google Scholar]
- Rogers TT, Patterson K. Object categorization: reversals and explanations of the basic-level advantage. J Exp Psychol: General. 2007;136:451–69. doi: 10.1037/0096-3445.136.3.451. [DOI] [PubMed] [Google Scholar]
- Rohrer JD, Knight WD, Warren JE, Fox NC, Rossor MN, Warren JD. Word-finding difficulty: a clinical analysis of the progressive aphasias. Brain. 2008;131:8–38. doi: 10.1093/brain/awm251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SK, Blank CC, Rosen S, Wise RJ. Identification of a pathway for intelligible speech in the left temporal lobe. Brain. 2000;123:2400–6. doi: 10.1093/brain/123.12.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senaha MLH, Caramelli P, Porto CS, Nitrini R. Verbal and non-verbal semantic impairment from fluent primary progressive aphasia to semantic dementia. Dementia Neuropsychol. 2007;2:203–11. doi: 10.1590/s1980-57642008dn10200014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonty SP, Mesulam M-M, Thompson CK, Johnson NA, Weintraub S, Parrish TB, et al. Primary progressive aphasia: PPA and the language network. Ann Neurol. 2003;53:35–49. doi: 10.1002/ana.10390. [DOI] [PubMed] [Google Scholar]
- Thompson C, Weintraub S. Northwestern Naming Battery (unpublished experimental version) [Google Scholar]
- Vandenbulcke M, Peeters R, Van Hecke P, Vandenberghe R. Anterior temporal laterality in primary progressive aphasia shifts to the right. Ann Neurol. 2005;58:362–70. doi: 10.1002/ana.20588. [DOI] [PubMed] [Google Scholar]
- Warrington EK, Shallice T. Category specific semantic impairments. Brain. 1984;107:829–54. doi: 10.1093/brain/107.3.829. [DOI] [PubMed] [Google Scholar]
- Weintraub S, Rubin NP, Mesulam MM. Primary progressive aphasia. Longitudinal course, neuropsychological profile, and language features. Arch Neurol. 1990;47:1329–35. doi: 10.1001/archneur.1990.00530120075013. [DOI] [PubMed] [Google Scholar]
- Zannino GD, Perri R, Pasqualetti P, Di Paola M, Caltagirone C, Carlesimo GA. The role of semantic distance in category-specific impairments for living things: evidence from a case of semantic dementia. Neuropsychologia. 2006;44:1017–28. doi: 10.1016/j.neuropsychologia.2005.11.006. [DOI] [PubMed] [Google Scholar]