Abstract
We have discovered that intracellular redox state appears to be a necessary and sufficient modulator of the balance between self-renewal and differentiation in dividing oligodendrocyte-type-2 astrocyte progenitor cells. The intracellular redox state of freshly isolated progenitors allows prospective isolation of cells with different self-renewal characteristics. Redox state is itself modulated by cell-extrinsic signaling molecules that alter the balance between self-renewal and differentiation: growth factors that promote self-renewal cause progenitors to become more reduced, while signaling molecules that promote differentiation cause progenitors to become more oxidized. Moreover, pharmacological antagonists of the redox effects of these cell-extrinsic signaling molecules antagonize their effects on self-renewal and differentiation, indicating that cell-extrinsic signaling molecules that modulate this balance converge on redox modulation as a critical component of their effector mechanism.
Modulation of the balance between self-renewing divisions and differentiation is at the heart of precursor cell function in development, tissue repair, and tissue homeostasis; yet relatively little is known about physiological mechanisms central to such modulation. For example, on a biochemical level, one of the only properties reported to be predictive of self-renewal characteristics in precursor cells of different lineages [thus far, in hematopoietic stem cells and hepatic precursor cells (e.g., refs. 1–3)] is that cells with a greater tendency to undergo self-renewing divisions show a lesser extent of labeling with such mitochondrial dyes as rhodamine-123. This reduced labeling was originally thought to be due to expression of P-glycoprotein by cells with a greater self-renewal potential, but more recent studies on hematopoietic stem cells have suggested that it may instead be reflective of mitochondrial activity and intracellular redox state (4). The correlation of such an important physiological state with self-renewal characteristics is potentially of great interest. It is not known, however, whether this correlation is coincidental or whether it provides an important clue to understanding this critical feature of precursor cell function.
To examine the possibility that intracellular redox regulation plays an important role in modulating the self-renewal characteristics of precursor cells, we studied the correlation between redox state and the balance between self-renewal and differentiation in dividing oligodendrocyte-type-2 astrocyte (O-2A) progenitor cells. This population, which gives rise to the myelin-forming oligodendrocytes of the central nervous system (e.g., refs. 5–7), provides one of the most extensively characterized and tractable precursor cell systems available for such studies. Pure populations of O-2A progenitor cells can be induced to undergo division and differentiation by growth in simple chemically defined medium supplemented with platelet-derived growth factor (PDGF, the best characterized O-2A progenitor cell mitogen; e.g., refs. 8 and 9). Cell-extrinsic signaling molecules such as basic fibroblast growth factor (bFGF) and neurotrophin-3 (NT-3) promote more extensive progenitor cell self-renewal (10, 11), whereas signaling molecules like thyroid hormone (TH) and bone morphogenetic protein-4 induce differentiation along oligodendrocyte and astrocyte pathways, respectively (11–13). The extent to which such signaling molecules alter the balance between self-renewal and differentiation is extensive: it is possible to induce nearly synchronous differentiation of all clonally related cells into oligodendrocytes when cells are exposed to TH and type-1 astrocytes (14, 15); varying degrees of asymmetric division and differentiation are seen in the presence of other factors (11), and almost complete suppression of differentiation with continuous promotion of self-renewal occurs when cells are grown in the presence of both PDGF and bFGF (10). At least some of these signaling molecules also are known to be of importance in vivo. For example, hypothyroid animals show reduced oligodendrocyte generation (11, 16), and animals in which NT-3 levels are artificially increased show increased O-2A progenitor cell proliferation (17).
Methods
Preparation of Cell Cultures.
Purified O-2A progenitor cells were prepared by immunopurification from optic nerves of 7-day-old rats (e.g., ref. 11). Contaminating cells [type-1 astrocytes and oligodendrocytes, which did not label with the A2B5 mAb used to define O-2A progenitors (21, 43)] represented <0.5% of the total cells whereas O-2A progenitors were present at >99.5%. Cells were grown in poly-L-lysine-coated 80-cm2 flasks (Sigma; Mr = 175,000; 20 μg/ml) in modified chemically defined medium lacking thyroid hormone (11) supplemented with 10 ng/ml PDGF-AA (“minimal division medium”). For clonal analysis, cells were plated at 250 cells/flask, a density that yielded single colonies well-separated from each other at all time points under investigation (<1 colony/15 mm2). Ten-point dose-response curves were constructed for tert-butyldhroxide (t-BuOOH) and buthionine sulfoximine (BSO). The highest dose in which no cell death above control levels occurred over a 24- to 72-h period (as seen with 3-(4,5-dimethyl-2-thiazolyl)-2–5-diphenyl-2H-tetrazolium bromide (MTT)+ staining and/or Trypan Blue exclusion analysis) was then used as the midpoint of a low-multiple seven-point dose-response curve. We then used the highest final dose that showed no toxicity, which coincided with the lowest dose that causes a statistically significant change in redox state (as measured by Rosamine fluoresence). As expected, lower doses of redox-active compounds exhibited lesser effect. Bone morphogenetic protein-4 (BMP-4) (10 ng/ml) was a kind gift of Genetics Institute (Cambridge, MA). The 2(R,S)-D-ribo-(1′,2′,3′,4′-tetrahydroxybutyl) thiazolidine-4(R)-carboxylic acid (RibCys) and 2(R,S)-D-gluco-(1′,2′,3′,4′-tetrahydroxybutyl) thiazolidine-4(R)-carboxylic acid (GlcCys) were a kind gift of Jeanette Roberts (University of Utah). All factors were added 24 h after plating and every 48 h after that.
Immunocytochemistry.
The following antibodies were used to analyze differentiation and for cell sorting: A2B5 mouse IgM mAb (43), which labels O-2A progenitor cells (21); mouse IgG3 anti-galactocerebroside (44), which labels oligodendrocytes; Dakopatts rabbit anti-glial fibrillary acidic protein, which labels astrocytes (45); and Ran-2 mAb, which labels type-1 astrocytes and meningeal cells (46). All dilutions, staining procedures, and fluorophore-coupled second layers were as described previously (e.g., ref. 11).
Analysis of Division and Differentiation of Purified O-2A Progenitor Cells.
Twenty-four hours after plating, we identified single cells growing in sufficient isolation so as not to compromise clonal analyses (e.g., as in ref. 11). Clones were marked and numbered on the plastic to allow daily identification and were analyzed on days 3–7. At the end of each experiment, flasks were immunolabeled to confirm morphological cell-type identification. Both analytical methods gave essentially identical results. All conditions were monitored for cell death. The ability to visualize all cells and the lack of macrophages meant that dead cells were readily observable. As expected in cultures exposed to progesterone, insulin and platelet-derived growth factor (PDGF) (along with, in some experiments, other survival promoting factors), cell death was rarely observed (47–49). No instances of O-2A progenitor cell death were observed, and oligodendrocyte death was observed extremely rarely. By 3 days, differences between various conditions were apparent. By 4–6 days after plating, differences were more pronounced; therefore, the majority of data presented represents 5-day time points. After 7 days, these patterns were retained, but the large clone sizes obtained led to difficulty in following clones out to this time point.
Cell Sorting.
Fluorescence-activated cell sorting was carried out on a Becton Dickinson Vantage FACSorter. Freshly dissociated immunopurified A2B5+/Ran-2−GalC− O-2A progenitor cells were incubated with 50 μg/ml chloromethyl tetramethyl (CMTM)-H2-Rosamine in DMEM-BS/TH− for 10 min, washed, and resuspended in HBSS with the viability dye Topro (Molecular Probes). Excitation at 488 nm was achieved by using an Argon-ion laser, and emission was read at 585 nm. Excitation at 633 nm was achieved by using a Helium–Neon ion laser, and emission was read at 665 nm to assess cell viability according to negative Topro staining. Topro-negative cells were gated and sorted according to fluorescence at 585 nm. The upper quintile of Rosamine-labeled viable cells were labeled Rosaminehigh and the bottom fifth were designated Rosaminelow. Cells were plated at clonal density and cultured as described.
Intracellular Redox Assay.
Immunopurified O-2A progenitor cells were plated at 104 cells/30-mm dish in minimal division medium. Cultures were re-fed after 24 h with 10 ng/ml PDGF-AA ± other factors, as indicated in the text. After 3, 12, or 18 h in these conditions, cells were washed with DMEM and 50 μg/ml CMTM-H2-Rosamine (MitoTracker Orange CM-HM2 TMRos7511) was added and cells were incubated for 10 min. Cells were washed three times in Ca2+/Mg2+-free HBSS. Then, 500 μl of HBSS and para-formaldehyde (1% final concentration) was added to increase dye retention, and cells were detached with a COSTAR cell lifter. Cells were analyzed on a Becton Dickinson FACScan excited by an Argon-ion laser. Unstained cells were on average 10–20 times less fluorescent than cells exposed to Rosamine. Conditions used were determined to maximize read-out of cytosolic reactive oxidative intermediate levels, as typically used for such analysis (e.g., ref. 50). The various conditions studied within each series were examined in randomized order in each experiment. Each condition was examined in triplicate, and each triplicate series was repeated a minimum of twice, and usually four times. Dye-loading procedures and dye concentrations were identical for JC-1 and rhodamine-123 as for Rosamine.
Results
Redox-State Analysis Allows Prospective Isolation of Cells with Different Self-Renewal Characteristics.
To determine whether intracellular redox state might be associated with the self-renewal potential of O-2A progenitor cells developing in vivo, we determined whether low levels of labeling with redox-sensitive dyes were indicative of enhanced self-renewal capacity in freshly isolated O-2A progenitors. Different dyes provide information on different aspects of redox state, which ultimately reflects the balance between reducing and oxidative equivalents within the cell. Redox state, however, is influenced in many ways, including by the state of mitochondrial activation, levels of reduced glutathione and other thiols, levels of NADH and NADPH, thioredoxin levels, and other factors. In our experiments, cells were labeled with JC-1, rhodamine-123, or dihydrotetramethylrosamine (reduced Rosamine) (18, 19), and FACS sorting was used to provide enriched populations of the upper and lower quintiles of labeled cells. Cells from each group were then plated at clonal density in the presence of PDGF, and basal self-renewal characteristics were determined. All dyes gave similar results, but Rosamine was associated with the best postsort viability; thus almost all data are presented only for Rosamine (see Methods); this dye appears to provide a broad measure of redox state (18) as described in Methods.
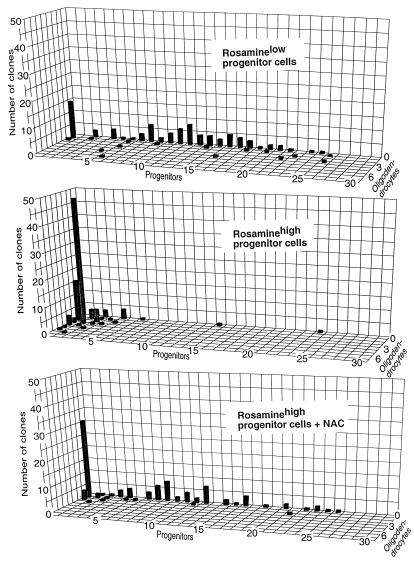
Rosaminelow cells grown in the presence of 10 ng/ml PDGF generated clones in which self-renewing divisions were prevalent (Fig. 1). Five days after plating, 70 ± 4.3% of these consisted only of dividing progenitors, and the average number of cells per clone was 11.1 ± 0.9. In contrast, Rosaminehigh cells, which would have had a higher intracellular level of oxidizing equivalents in vivo, underwent little division in these same conditions. With rare exceptions, the largest clones consisted of four cells (mean = 2.5 ± 0.4 cells/clone). Rosaminehigh cells also demonstrated a greatly increased tendency to generate oligodendrocytes. At day 5, 47 ± 4.6% of clones derived from Rosaminehigh cells consisted of one oligodendrocyte and no progenitors, and only 29 ± 5.4% of clones contained any progenitors at all. Critically, viability of all populations was identical. Rosaminelow and Rosaminehigh cells both underwent division and differentiation without notable cell death for at least 1 week after isolation. Cell morphology and antigenic phenotype was as seen in multiple previous publications (e.g., refs. 14, 20, and 21).
Figure 1.
Intracellular redox state of freshly isolated cells predicts self-renewal and differentiation in vitro. Analysis of clones was performed on freshly isolated immunopurified O-2A progenitors after cell sorting according to redox-sensitive Rosamine fluorescence. Rosaminehigh cells represent a population with greater oxidative load than Rosaminelow cells. After 5 days in basal division conditions, Rosaminelow progenitors had a greater tendency to undergo self-renewing divisions than Rosaminehigh cells, which in all but a few clones showed signs of rapid differentiation. Rosaminehigh cells grown in medium supplemented with NAC, however, divided extensively and thus had not irreversibly committed to differentiation at the time of isolation.
Despite their low frequency of division, Rosaminehigh O-2A progenitor cells had not already irreversibly committed to differentiation in vivo. Self-renewal of these cells was markedly enhanced by altering intracellular redox state through exposure to N-acetyl-L-cysteine (NAC; a potent anti-oxidant and a cysteine pro-drug that enhances production of glutathione, the most prevalent reduced thiol within cells and a crucial component of maintaining a reduced intracellular environment; refs. 22 and 23). When also exposed to NAC, the percentage of progenitor cell-containing clones at day 5 increased from 29 ± 5.4% to 65 ± 3.4%, and the average clone size increased almost threefold, from 2.5 ± 0.4 cells to 7.0 ± 1.2 cells/clone. These results suggest that alteration of intracellular redox state is a dynamic modulator of the balance between self-renewal and differentiation rather than merely being a secondary consequence of an irrevocable fate determination.
Anti-Oxidant and Pro-Oxidant Drugs Modulate Self-Renewal and Differentiation Characteristics in Opposite Directions.
To further examine effects of redox manipulations on self-renewal and differentiation, clonal O-2A progenitor cells were grown in the presence of PDGF ± redox-active compounds. To increase oxidative levels we used the pro-oxidant t-BuOOH (24), and BSO, which inhibits synthesis of glutathione (25). To render cells more reduced, medium was supplemented with NAC or with GlcCys, ProCys, or RibCys, three polyhydroxyalkyl thiazolidine carboxylic acid compounds. These pro-cysteine drugs contribute to glutathione biosynthesis when taken up by cells and their thialozolidine ring is opened enzymatically (26). Thus, the activity of GlcCys, ProCys, and RibCys is more specifically targeted to intracellular thiol pools than is NAC.
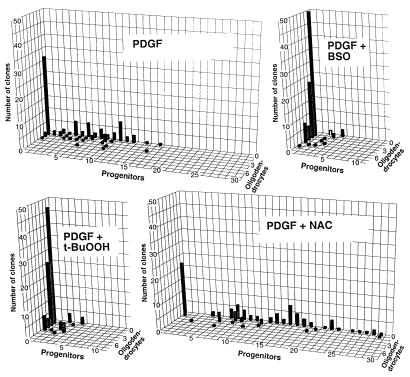
Treatment of dividing O-2A progenitor cells with PDGF + 1 μM BSO or 50 nM t-BuOOH was associated with diminished progenitor division and increased oligodendrocyte generation (Fig. 2; summarized in Table 1). In the presence of either BSO or t-BuOOH, approximately one-half of the founder cells differentiated without dividing, with the rest undergoing one to three divisions and yielding clones of two to seven cells after 5 days. Average clone sizes at this time point, in either condition, were 2.1 ± 0.4 cells/clone, less than one-half that seen in basal conditions (5.7 ± 0.8 cells/clone). Exposure to BSO or t-BuOOH also was associated with reductions in the number of clones containing only progenitors and increases in the proportion of clones containing only oligodendrocytes. For example, when clones were grown in the presence of PDGF for 5 days, 43 ± 3.0% of clones consisted wholly of progenitors. In contrast, in cultures exposed to PDGF + BSO or t-BuOOH for 5 days, only 5 ± 2.4% and 7 ± 3.7% of clones consisted solely of progenitor cells, respectively. Complementary changes were seen in the proportion of clones consisting solely of oligodendrocytes. After 5 days, 88 ± 8.3% of clones exposed to BSO or 83 ± 6.0% of clones exposed to t-BuOOH consisted wholly of oligodendrocytes, as compared to complete differentiation of 35 ± 6.6% of clones in basal division conditions. These outcomes are similar to those seen with exposure to TH (see Fig. 3 and 4).
Figure 2.
Oxidizing agents promote differentiation of dividing O-2A progenitor cells, whereas NAC promotes self-renewal. Purified O-2A progenitor cells were grown at clonal density with PDGF ± redox-active drugs. After 5 days, 100 randomly chosen clones were analyzed for each condition. Histograms show the number of oligodendrocytes per clone along the x axis, the number of progenitors per clone (y axis), and the number of clones of each composition (z axis). Here, and in Fig. 4, a representative experiment is shown; each experiment was repeated at least three times with similar results. Progenitor cells treated with NAC exhibited more self-renewal as evidenced by larger clone sizes and a greater representation of progenitors per clone. In contrast, cells exposed to the pro-oxidants BSO or t-BuOOH exhibited extensive differentiation and less self-renewal.
Table 1.
Summary of clonal culture analysis
| Condition | Reducing | Oxidizing | Proportion of clones consisting of only one oligodendrocyte | Proportion of clones containing only oligodendrocytes | Proportion of clones with both oligodendrocytes/ progenitors | Proportion of clones containing only progenitors | Average clone size |
|---|---|---|---|---|---|---|---|
| Control | 32 ± 6.3 | 35 ± 6.6 | 22 ± 5.8 | 43 ± 3.0 | 5.7 ± 0.8 | ||
| TH | + | ↑ 47 ± 3.9 | ↑ 74 ± 8.9 | 16 ± 3.4 | ↓ 10 ± 1.4 | ↓ 2.1 ± 0.4 | |
| NT-3 | + | ↓ 21 ± 2.4 | ↓ 21 ± 2.4 | 15 ± 2.6 | ↑ 64 ± 2.6 | ↑ 9.7 ± 1.2 | |
| BSO | + | ↑ 50 ± 4.3 | ↑ 88 ± 8.3 | ↓ 7 ± 2.9 | ↓ 5 ± 2.4 | ↓ 2.1 ± 0.4 | |
| t-BuOOH | + | ↑ 47 ± 5.5 | ↑ 83 ± 6.0 | ↓ 10 ± 4.5 | ↓ 7 ± 3.7 | ↓ 2.1 ± 0.4 | |
| NAC | + | ↓ 22 ± 4.1 | ↓ 22 ± 4.1 | 18 ± 5.9 | ↑ 60 ± 2.8 | ↑ 11.3 ± 2.2 | |
| ProCys | + | 25 ± 3.8 | ↓ 28 ± 4.2 | 15 ± 5.6 | ↑ 57 ± 8.1 | ↑ 10.3 ± 1.9 | |
| NT-3, BSO | + | + | ↑ 48 ± 8.2 | ↑ 63 ± 9.5 | 11 ± 7.3 | ↓ 26 ± 5.7 | ↓ 2.2 ± 0.4 |
| NAC, TH | + | + | 28 ± 7.2 | ↓ 30 ± 4.0 | ↓ 10 ± 5.3 | ↑ 60 ± 4.8 | 4.7 ± 1.1 |
The averaged data ± SEM are shown, with arrows indicating significant changes (P ≤ 0.05; Student's t test) and direction of change relative to control cultures. “Reducing” or “Oxidizing” columns indicate the effects of the pharmacological agents and signaling molecules examined on intracellular redox state. In cases where a signaling molecule and a pharmacological agent with opposing effects were examined together, both columns are marked. This table does not present data on clones in which there was both division and generation of at least one oligodendrocyte; for these values refer to text and Figs. 2 and 4.
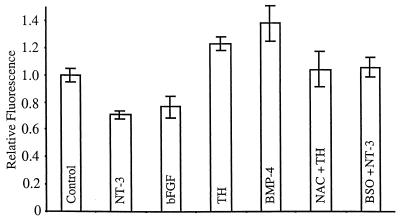
Figure 3.
Cell-signaling molecules that enhance self-renewal alter intracellular redox to a more reduced state, whereas those that promote differentiation alter intracellular redox to a more oxidized state. Cells were treated with various factors as indicated for 18 h, labeled with Rosamine, and analyzed by flow cytometry. Cells exposed to NT-3 or bFGF displayed less aggregate fluorescence, indicating a more reduced intracellular environment. Cells treated with TH or BMP-4 were on average more fluorescent, indicating increased oxidizing environments. Effects of NT-3 and TH on redox state were antagonized by treatment with BSO or NAC, respectively. Data are presented as the geometric means of triplicate analyses of multiple experiments, with data normalized to values obtained in the presence of PDGF alone.
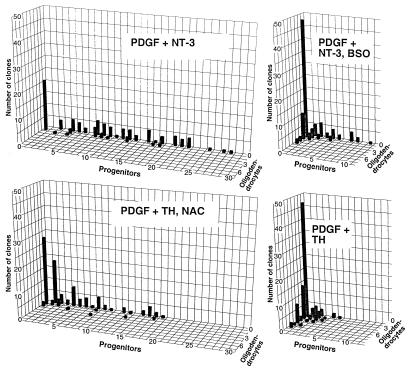
Figure 4.
NAC inhibits TH-mediated induction of differentiation, whereas BSO inhibits NT-3-mediated promotion of self-renewal. The ability of NT-3 to promote self-renewing divisions was blocked in the presence of BSO. After 5 days, the pattern of differentiation and self-renewal of clones treated with BSO + NT-3 resembled the profile exhibited in basal division conditions (compare with Fig. 2). Conversely, cells exposed to TH + NAC showed a profile of self-renewal similar to that seen in basal division conditions (see Fig. 2), in contrast with cultures supplemented with TH alone.
Clones of O-2A progenitors cultured in PDGF + 1 mM NAC, in contrast, exhibited enhanced self-renewal and a marked reduction in oligodendrocyte generation (Fig. 2). After 5 days, clones exposed to PDGF + NAC contained 11.3 ± 2.2 cells/clone, almost twice the value seen in clones exposed to PDGF alone, with many clones containing >15 cells. In cultures exposed to PDGF + NAC for 3 days, 80 ± 2.7% of clones contained only progenitors. Even after 5 days, 60 ± 2.8% of clones contained only progenitors in these conditions. In contrast, when grown in basal division conditions for 3 days, 47 ± 1.1% of clones consisted only of progenitor cells, with similar values (43 ± 3.0%) observed after 5 days. These outcomes are similar to those seen with exposure to NT-3 (see Figs. 3 and 4). Cells exposed to NAC alone (i.e., no PDGF present) differentiated into oligodendrocytes without dividing (not shown).
Enhanced self-renewal of O-2A progenitor cells grown in the presence of NAC continued for at least 7 days. Clones examined at this time were on average six times larger than clones exposed to PDGF alone. Moreover, in cultures exposed to PDGF + NAC for 7 days, 40 ± 5.9% of clones still contained only progenitors. In contrast, only 5 ± 2.6% of clones consisted solely of progenitors after 7 days exposure to PDGF alone. In those clones exposed to PDGF + NAC, however, and in which division occurred, 49 ± 8.5% contained one or more oligodendrocytes. Moreover, absolute numbers of oligodendrocytes in either condition were similar (151 ± 21 and 116 ± 38 for control and NAC-treated cultures, respectively), demonstrating that NAC did not preclude eventual differentiation. Supplementation of basal division medium with the cysteine pro-drugs GlcCys, ProCys, or RibCys had virtually identical effects to NAC, thus suggesting that the capacity of NAC to modify intracellular thiol balance was more important to its activity than its ability to function as an extracellular anti-oxidant (data not shown). All of the pro-oxidants and cysteine pro-drugs examined caused the expected direction of change in intracellular redox state, as determined by Rosamine fluorescence.
Signaling Molecules That Promote Differentiation Cause Greater Intracellular Oxidative Turnover, Whereas Signaling Molecules That Promote Self-Renewal Cause More Reduced Intracellular States.
Consistent with the suggestion that redox modulation is relevant to the effects of known extracellular signaling molecules that influence differentiation and self-renewal, Rosamine-labeled cells grown in the presence of PDGF supplemented with factors that promote self-renewal exhibited a more reduced intracellular redox state, whereas cells exposed to inducers of differentiation exhibited increased Rosamine fluorescence, indicating increased oxidative levels (Fig. 3). Such effects were seen within 18 h, well before differences are observed in progenitor or oligodendrocyte numbers. For example, after exposure to NT-3 + PDGF for 12 h, aggregate Rosamine fluorescence was 15 ± 6% lower than in the presence of PDGF alone. In comparison, treatment with either 1 mM NAC or 1 mM ProCys led to decreases in Rosamine fluorescence by 12 ± 4% and 18 ± 7%, respectively, within 6 h (P < 0.05; data not included). By 18 h after exposure to NT-3, relative fluorescence was 29 ± 3% lower than for control cells (P = 0.0075). Similarly, progenitors exposed to PDGF + bFGF exhibited a 23 ± 8% reduction in aggregate fluorescence values (P < 0.05; 18-h time point) as compared to cells exposed only to PDGF. The effect of NT-3 on redox levels was inhibited by simultaneous treatment with BSO. After exposure of cells to 1 μm of BSO + NT-3 + PDGF for 12 h, mean Rosamine fluorescence was increased by 6 ± 7% relative to growth in PDGF alone, a difference that was not significant.
Exposure to TH, which induces oligodendrocyte generation, had the opposite effect to NT-3 and was associated with greater Rosamine fluorescence. Cells exposed to TH showed an 11 ± 4% increase in fluorescence after 3 h and a statistically significant (23 ± 4%; P < 0.01) increase after 12 h. This effect was significantly diminished with simultaneous exposure to 1 mM NAC: After 12 h in the presence of NAC + TH + PDGF, the mean Rosamine fluorescence was only 4 ± 13% more than the control. Increased Rosamine fluorescence also was seen in cells exposed to BMP-4, even though this protein causes progenitors to differentiate into type-2 astrocytes (13) and acts through receptor and signaling systems very different from those relevant to TH action. The O-2A progenitor cells exposed to PDGF + BMP-4 exhibited a significant increase in fluorescence by 12 h (25 ± 11%; P < 0.05) and a highly significant increase (38 ± 13%; P = 0.004) after 18 h. Thus, exposure to inducers of differentiation caused an increase in Rosamine fluorescence, indicating an increase in mean oxidative levels, independent of whether oligodendrocyte or astrocyte differentiation was stimulated. As expected, analogous increases in oxidative levels were associated with exposure to 1 μM BSO or 50 nM t-BuOOH (data not shown).
Further demonstration of the effects on redox state of NT-3 and TH, the two signaling molecules for which the best evidence for an in vivo role has been provided (11, 16, 17), was obtained by analysis with the JC-1 dye (19), which provides information on mitochondrial inner membrance potential (ΔΨ). Treatment with TH for 18 h resulted in a 18 ± 11% decrease in ΔΨ (aggregate red:green fluorescence ratio; P < 0.05), an outcome associated with a more oxidative state within the cells (19). In contrast, treatment with NT-3 for 18 h was associated with a 16 ± 11% increase in ΔΨ (P < 0.05), consistent with a more reduced state within the cells.
Pharmacological Agents That Antagonize the Redox Effects of NT-3 and TH also Antagonize Their Effects on Self-Renewal and Differentiation.
We next asked whether the effects of NT-3 and TH [the cell-extrinsic signaling molecules known to modulate O-2A progenitor development in vivo (11, 16, 17)], could be blocked by pharmacological manipulation of redox state in the opposite direction to that induced by the signaling molecule itself. Cells grown in the presence of PDGF + TH + NAC exhibited a similar profile of self-renewal as cells grown in the presence of PDGF alone, indicating that NAC countered the effects of TH (compare Figs. 2 and 4). After 5 days, 60 ± 4.8% of clones contained only progenitors in cultures exposed to TH + NAC, a proportion even greater than that observed in clones exposed only to PDGF (i.e., 43 ± 3.0%). In contrast, only 10 ± 1.4% of clones consisted entirely of progenitors when cells were exposed to PDGF + TH alone. Progenitor cells exhibited more self-renewal when exposed to TH + NAC, and average clone size was 4.7 ± 1.1 cells after 5 days (as compared to 2.1 ± 0.4 cells/clone in cultures exposed to PDGF + TH). Just under one-half of the clones differentiated without division when TH was present, and 81 ± 10.8% of the clones in which at least one division occurred contained one or more oligodendrocytes. When NAC was also present, in contrast, only 30 ± 4.0% of clones consisted wholly of oligodendrocytes (and most of these clones consisted of a single oligodendrocyte) and only 17 ± 4.2% of clones in which at least one division occurred contained at least one oligodendrocyte.
In complementary experiments, exposure to PDGF + 1 μM BSO inhibited the ability of NT-3 to enhance self-renewal. Clones grown in the presence of PDGF + NT-3 showed an increase in self-renewing divisions as determined by average clonal size (9.7 ± 1.2 cells), a 70% increase over control values. In cultures treated also with 1 μM BSO, however, mean clone size after 5 days was 2.2 ± 0.4 cells, and one-half of the clones were single cells that differentiated into oligodendrocytes without dividing. NT-3 exposure also was associated with a lesser proportion of clones containing at least one oligodendrocyte (36 ± 2.6% by day 5, as compared to 57 ± 3.0% in basal division medium). BSO inhibited this effect, and 74 ± 5.7% of clones contained at least one oligodendrocyte in cultures treated with NT-3 + BSO.
Discussion
We have discovered that intracellular redox-state modulation appears to be a central biochemical/molecular regulator of the balance between self-renewal and differentiation. Redox-state modulation satisfies all of the following criteria required to support this conclusion: (i) The proposed regulator should be altered in its level and/or function by cell-extrinsic signaling molecules that modulate the balance between self-renewal and differentiation, with signaling molecules that have opposite effects on self-renewal and differentiation exerting opposite effects on the proposed regulator. (ii) Alterations like those caused by exposure to these signaling molecules should have the same effect as the signaling molecules themselves. (iii) Substances that antagonize the alterations in the regulator caused by the cell-extrinsic signaling molecules should block their effects on this balance. (iv) Progenitor cell populations isolated from developing animals on the basis of the state of the proposed regulator should exhibit predictably different self-renewal characteristics consistent with the outcome of the other analyses.
That the changes we see are likely to be relevant to normal development is indicated by the fact that we can prospectively enrich freshly isolated cells with differing self-renewal potential and that signaling molecules known to modify oligodendrocyte generation in vivo modify intracellular redox state. Likely in vivo relevance is also indicated by our findings that the redox alterations caused by NT-3 and TH appear to be necessary to the mechanism by which these cell signaling molecules modulate the balance between self-renewal and differentiation.
Identification of a biochemical/molecular regulator that meets all of the above criteria is a strikingly different outcome from past efforts to understand how the balance between self-renewal and differentiation is controlled. For example, the strongest evidence for a protein that might represent such a regulator has been reported for the cyclin-dependent kinase inhibitor p27Kip1. P27 levels progressively accumulate as O-2A progenitor cells proliferate (27) and O-2A progenitor cells isolated from p27-/- mice undergo more division than do wild-type cells (28, 29). Ectopic p27expression, however, causes O-2A progenitor cells to undergo cell cycle arrest in the absence of differentiation (30), a different outcome from that of rendering dividing O-2A progenitor cells slightly more oxidized by exposure to TH or pharmacological pro-oxidants. Moreover, no data has been provided that p27 levels are predictive of self-renewal potential or that experimental alterations in p27 levels yield the characteristic range of clonal behaviors caused by TH, NT-3, or redox manipulation. Thus, although p27 may be a part of the mechanism that modulates the balance between self-renewal and differentiation, the evidence for intracellular redox state as a central modulator appears to be more comprehensive.
Available data provides strong reason to believe that redox modulation functions as a central integrator of multiple processes related to self-renewal and differentiation, rather than as a controller of a single unique downstream effector pathway. Many different signaling pathways appear to converge on regulation of redox state, and redox alterations can in turn modulate several different pathways of possible relevance in modulation of self-renewal and differentiation. Multiple components of the redox regulatory network can be altered by exposure of cells to such cell-extrinsic signaling molecules as neurotrophins (31, 32), type 1 interferon (33), stem cell factor (34), transforming growth factor-β (35), inflammatory cytokines (e.g., ref. 36), and thyroid hormone (37), and also by ras activation (38). Increased levels of oxidative stress can in turn induce elevations in such proteins as the cyclin-dependent kinase inhibitor p21waf1/cip1 (39) and could potentially modify function of transcription complexes (such as AP-1; ref. 40), thus affecting cell division and/or differentiation. In addition, intracellular redox state can itself impinge on many aspects of cell signaling (e.g., see ref. 41 for review).
In this context, one of the more intriguing aspects of our studies is the observation that what appear to be relatively small changes in intracellular redox state are associated with profound differences in outcome. Such observations are consistent with findings that as little as a 10% decrease in average glutathione levels significantly decreases calcium influx in peripheral blood lymphocytes stimulated with anti-CD3 antibody (42). In addition, we have noted that the changes in geometric means of Rosamine fluorescence values shown in Fig. 3 were associated with a redistribution of cells within the normal range of redox values, rather than an alteration of the range itself. A lower mean is associated with more cells exhibiting low levels of Rosamine fluorescence, whereas a higher value is associated with a redistribution of cells to a more oxidatively active portion of the range. Thus, exposure to NT-3, bFGF, TH, and BMP-4 appears to alter intracellular redox profiles within a distribution that is otherwise tightly regulated as to its upper and lower boundaries. An ability of relatively small changes in redox state over a relatively narrow range to modulate cell function would enable this fundamental component of cellular physiology to function as a highly sensitive central rheostat that integrates cell-intrinsic states with cell-extrinsic signals.
Acknowledgments
We are most grateful to our colleagues for helpful insights and criticism, and in particular to Chris Pröschel, Patrick Tresco, and Mahendra Rao. This research was supported by generous grants from the Huntsman Cancer Foundation, the Multiple Sclerosis Society, and the National Institutes of Health.
Abbreviations
- BSO
buthionine sulfoximine
- bFGF
basic fibroblast growth factor
- NAC
N-acetyl-l-cysteine
- NT-3
neurotrophin-3
- O-2A
oligodendrocyte-type-2 astrocyte
- PDGF
platelet-derived growth factor
- TH
thyroid hormone
- BMP-4
bone morphogenetic protein-4
- t-BuOOH
tert-butyldhroxide
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.170209797.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.170209797
References
- 1.Bertoncello I, Hodgson G, Bradley T. Exp Hematol. 1985;13:999–1006. [PubMed] [Google Scholar]
- 2.Mulder A, Visser J. Exp Hematol. 1987;15:99–104. [PubMed] [Google Scholar]
- 3.Reid L. In: Textbook of Tissue Engineering. Lanza R, Langer R, Chick W, editors. Austin, TX: Landes; 1996. [Google Scholar]
- 4.Kim M, Cooper D, Hayes S, Spangrude G. Blood. 1998;91:4106–4117. [PubMed] [Google Scholar]
- 5.Miller R H, French-Constant C, Raff M C. Annu Rev Neurosci. 1989;12:517–534. doi: 10.1146/annurev.ne.12.030189.002505. [DOI] [PubMed] [Google Scholar]
- 6.Raff M C, Miller R H, Noble M. Cold Spring Harbor Symp Quant Biol. 1983;48:569–572. doi: 10.1101/sqb.1983.048.01.061. [DOI] [PubMed] [Google Scholar]
- 7.Groves A K, Barnett S C, Franklin R J, Crang A J, Mayer M, Blakemore W F, Noble M. Nature (London) 1993;362:453–455. doi: 10.1038/362453a0. [DOI] [PubMed] [Google Scholar]
- 8.Noble M, Murray K, Stroobant P, Waterfield M D, Riddle P. Nature (London) 1988;333:560–562. doi: 10.1038/333560a0. [DOI] [PubMed] [Google Scholar]
- 9.Richardson W D, Pringle N, Mosley M J, Westermark B, Dubois Dalcq M. Cell. 1988;53:309–319. doi: 10.1016/0092-8674(88)90392-3. [DOI] [PubMed] [Google Scholar]
- 10.Bögler O, Wren D, Barnett S C, Land H, Noble M. Proc Natl Acad Sci USA. 1990;87:6368–6372. doi: 10.1073/pnas.87.16.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ibarrola N, Mayer-Pröschel M, Rodriguez-Pena A, Noble M. Dev Biol. 1996;180:1–21. doi: 10.1006/dbio.1996.0280. [DOI] [PubMed] [Google Scholar]
- 12.Barres B A, Lazar M A, Raff M C. Development (Cambridge, UK) 1994;120:1097–1108. doi: 10.1242/dev.120.5.1097. [DOI] [PubMed] [Google Scholar]
- 13.Mabie P, Mehler M, Marmur R, Papavasiliou A, Song Q, Kessler J. Neuroscience. 1997;17:4112–4120. doi: 10.1523/JNEUROSCI.17-11-04112.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Temple S, Raff M C. Cell. 1986;44:773–779. doi: 10.1016/0092-8674(86)90843-3. [DOI] [PubMed] [Google Scholar]
- 15.Raff M C, Lillien L E, Richardson W D, Burne J F, Noble M D. Nature (London) 1988;333:562–565. doi: 10.1038/333562a0. [DOI] [PubMed] [Google Scholar]
- 16.Ahlgren S, Wallace H, Bishop J, Neophytou C, Raff M. Mol Cell Neurosci. 1997;9:420–432. doi: 10.1006/mcne.1997.0631. [DOI] [PubMed] [Google Scholar]
- 17.Barres B A, Raff M C, Gaese F, Bartke I, Dechant G, Barde Y A. Nature (London) 1994;367:371–375. doi: 10.1038/367371a0. [DOI] [PubMed] [Google Scholar]
- 18.Whitaker J E, Moore P L, Haugland R P, Haugland R P. Biochem Biophys Res Commun. 1991;175:387–393. doi: 10.1016/0006-291x(91)91576-x. [DOI] [PubMed] [Google Scholar]
- 19.Smiley S T, Reers M, Mottola-Hartshorn C, Lin M, Chen A, Smith T W, Steele G D J, Chen L B. Proc Natl Acad Sci USA. 1991;88:3671–3675. doi: 10.1073/pnas.88.9.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noble M, Murray K. EMBO J. 1984;3:2243–2247. doi: 10.1002/j.1460-2075.1984.tb02122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raff M C, Miller R H, Noble M. Nature (London) 1983;303:390–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- 22.Meister A, Anderson M E, Hwang O. J Am Coll Nutr. 1986;5:137–151. doi: 10.1080/07315724.1986.10720121. [DOI] [PubMed] [Google Scholar]
- 23.Meister A, Anderson M E. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- 24.Ochi T. Arch Toxicol. 1993;67:401–410. doi: 10.1007/BF01977401. [DOI] [PubMed] [Google Scholar]
- 25.Martensson J, Jain A, Stole E, Frayer W, Auld P, Meister P. Proc Natl Acad Sci USA. 1991;88:9360–9364. doi: 10.1073/pnas.88.20.9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts J, Nagasawa H, Zera R, Fricke R, Goon D. J Med Chem. 1987;30:1891–1896. doi: 10.1021/jm00393a034. [DOI] [PubMed] [Google Scholar]
- 27.Durand B, Gao F, Raff M. EMBO J. 1997;16:306–317. doi: 10.1093/emboj/16.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Casaccia-Bonnefil P, Tikoo R, Kiyokawa H, Friedrich V, Jr, Chao M V, Koff A. Genes Dev. 1997;11:2335–2346. doi: 10.1101/gad.11.18.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Durand B, Fero M, Roberts J, Raff M. Curr Biol. 1998;6:431–440. doi: 10.1016/s0960-9822(98)70177-0. [DOI] [PubMed] [Google Scholar]
- 30.Tikoo R, Osterhout D, Casaccia-Bonnefil P, Seth P, Koff A, Chao M. J Neurobiol. 1998;36:431–440. [PubMed] [Google Scholar]
- 31.Sampath D, Perez-Polo R. Neurochem Res. 1997;22:351–362. doi: 10.1023/a:1027387105882. [DOI] [PubMed] [Google Scholar]
- 32.Gabaizadeh R, Staecker H, Liu W, Van De Water T. Brain Res Mol Brain Res. 1997;50:71–78. doi: 10.1016/s0169-328x(97)00173-3. [DOI] [PubMed] [Google Scholar]
- 33.Lewis J, Huq A, Najarro P. J Biol Chem. 1996;271:184–190. doi: 10.1074/jbc.271.22.13184. [DOI] [PubMed] [Google Scholar]
- 34.Lee J. Oncogene. 1998;17:1653–1662. doi: 10.1038/sj.onc.1202102. [DOI] [PubMed] [Google Scholar]
- 35.Islam K, Kayanoki Y, Kaneto H, Suzuki K, Asahi M, Fujii J, Taniguchi N. Free Radical Biol Med. 1997;22:1007–1017. doi: 10.1016/s0891-5849(96)00493-5. [DOI] [PubMed] [Google Scholar]
- 36.Hampton M, Fadeel B, Orrenius S. Ann NY Acad Sci. 1998;854:328–335. doi: 10.1111/j.1749-6632.1998.tb09913.x. [DOI] [PubMed] [Google Scholar]
- 37.Pillar T, Seitz H. Eur J Endocrinol. 1997;136:231–239. doi: 10.1530/eje.0.1360231. [DOI] [PubMed] [Google Scholar]
- 38.Lee A, Fenster B, Ito H, Takeda K, Bae N, Hirai T, Yu Z, Ferrans V, Howard B, Finkel T. J Biol Chem. 1999;274:7936–7940. doi: 10.1074/jbc.274.12.7936. [DOI] [PubMed] [Google Scholar]
- 39.Esposito F, Cuccovillo F, Vanoni M, Cimino F, Anderson C W, Appella E, Russo T. Eur J Biochem. 1997;245:730–737. doi: 10.1111/j.1432-1033.1997.00730.x. [DOI] [PubMed] [Google Scholar]
- 40.Xanthoudakis S, Curran T. EMBO J. 1992;11:653–665. doi: 10.1002/j.1460-2075.1992.tb05097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kamata H, Hirata H. Cell Signalling. 1999;11:1–14. doi: 10.1016/s0898-6568(98)00037-0. [DOI] [PubMed] [Google Scholar]
- 42.Staal F, Anderson M, Staal G, Herzenberg L, Gitler C, Herzenberg L. Proc Natl Acad Sci USA. 1994;91:3619–3622. doi: 10.1073/pnas.91.9.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eisenbarth G S, Walsh F S, Nirenberg M. Proc Natl Acad Sci USA. 1979;76:4913–4917. doi: 10.1073/pnas.76.10.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ranscht B, Clapshaw P A, Price J, Noble M, Seifert W. Proc Natl Acad Sci USA. 1982;79:2709–2713. doi: 10.1073/pnas.79.8.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bignami A, Eng L F, Dahl D, Uyeda C T. Brain Res. 1972;43:429–435. doi: 10.1016/0006-8993(72)90398-8. [DOI] [PubMed] [Google Scholar]
- 46.Bartlett P F, Noble M D, Pruss R M, Raff M C, Rattray S, Williams C A. Brain Res. 1981;204:339–351. doi: 10.1016/0006-8993(81)90593-x. [DOI] [PubMed] [Google Scholar]
- 47.Mayer M, Bhakoo K, Noble M. Development (Cambridge, UK) 1994;120:142–153. doi: 10.1242/dev.120.1.143. [DOI] [PubMed] [Google Scholar]
- 48.Mayer M, Noble M. Proc Natl Acad Sci USA. 1994;91:7496–7500. doi: 10.1073/pnas.91.16.7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barres B A, Schmidt R, Sendnter M, Raff M C. Development (Cambridge, UK) 1993;118:283–295. doi: 10.1242/dev.118.1.283. [DOI] [PubMed] [Google Scholar]
- 50.Shapiro H. Practical Flow Cytometry. New York: Wiley-Liss; 1990. pp. 39–342. [Google Scholar]