Abstract
Insulin-like growth factor-1 receptor (IGF-1R) is an important mediator of tumor-cell survival and demonstrates prognostic significance in sarcoma. To explore potential therapeutic strategies for interrupting signaling through this pathway, we assessed the ability of cyclolignan picropodophyllin (PPP), a member of the cyclolignan family, to selectively inhibit the receptor tyrosine kinase (RTK) activity of IGF-1R in several sarcoma cell line model systems. Of the diverse sarcoma subtypes studied, osteosarcoma cell lines were found to be particularly sensitive to IGF-1R inhibition, including several multidrug resistant osteosarcoma cell lines with documented resistance to various conventional anticancer drugs. PPP shows relatively little toxicity in human osteoblast cell lines when compared to osteosarcoma cell lines. These studies demonstrate that PPP significantly inhibits IGF-1R expression and activation in both chemotherapy sensitive and resistant osteosarcoma cell lines. This inhibition of the IGF1-R pathway correlates with suppression of proliferation of osteosarcoma cell lines and with apoptosis induction as measured by monitoring PARP and its cleavage product and by quantitative measurement of apoptosis-associated CK18Asp396. Importantly, PPP increases the cytotoxic effects of doxorubicin in doxorubicin-resistant osteosarcoma cell lines U-2OSMR and KHOSMR. Furthermore, siRNA down-regulation of IGF-1R expression in drug resistant cell lines also caused re-sensitization to doxorubicin. Our data suggests that inhibition of IGF-1R with PPP offers a novel and selective therapeutic strategy for ostosarcoma, and at the same time, PPP is effective at reversing the drug-resistance phenotype in osteosarcoma cell lines.
Keywords: IGF-1R, PPP, osteosarcoma, drug resistance
Introduction
Primary bone tumors account for 5% to 10% of all new pediatric cancer diagnoses in the United States (1). Osteosarcoma is the most common malignant bone tumor in children and adolescents and accounts for about 60% of malignant bone tumors diagnosed in the first two decades of life (2). Standard treatment for osteosarcoma is surgery and chemotherapy. Chemotherapy treatment has significantly improved survival rates from 11% with surgery alone to approximately 70% when combined with chemotherapy (3, 4). Unfortunately, for the 40% of patients with progression after front-line therapy, further therapy with additional chemotherapy is palliative and too often toxic. It is estimated that less than 30% of patients with recurrent metastasis will be cured. The development of chemoresistance is associated with many events, such as defective apoptotic signaling in response to chemotherapy, overexpression of antiapoptotic proteins, and overexpression of multidrug resistance (MDR) gene protein Pgp (5–7). Successful management of osteosarcoma would be greatly aided by novel agents that interfere with both intrinsic and acquired mechanisms of drug resistance mechanisms.
The insulin-like growth factor (IGF) signaling pathway has been found to be critical for tumor cell proliferation, differentiation, and apoptosis (8–10). In vitro and in vivo studies have implicated IGF in the pathogenesis of breast, prostate, lung, and colon cancers (11–14). A member of the IGF family, the insulin-like growth factor-1 receptor (IGF-1R), is a ubiquitously expressed type 1 transmembrane- heterotetrameric receptor composed of two -subunits (Mr 130,000) and two β-subunits (Mr 90,000 each), with intrinsic tyrosine kinase activity (10, 15). Upon binding specific ligands (IGF-1 and IGF-2), IGF-1R undergoes autophosphorylation of tyrosine residues, leading to subsequent tyrosine phosphorylation of insulin receptor substrate 1, Src, and collagen homology proteins (Shc), resulting in the activation of PI3-kinase/Akt, RAS/MAP kinase, and Jak/Stat pathways (10, 15). These activated pathways regulate the function and expression of proteins involved in cell proliferation and survival (10, 16). IGF-1R thereby plays an important role in malignant transformation. Expression of functional IGF-1R is required for malignant cell transformation by known cellular and viral oncogenes (17). Studies on breast cancer have suggested that overexpression of IGF-1R is associated with local tumor recurrence and circulating concentrations of IGF-1 or its major binding protein IGF-BP3 is associated with increased risk of common cancers (18, 19). IGF-1R activation protects cells from a variety of apoptosis-inducing agents, including osmotic stress and anti-cancer drugs (20–22). Expression of IGF-1R in synovial sarcoma is associated with an aggressive phenotype and with a high incidence of lung metastases (23). In vitro, overexpression of the IGF-1R reduces growth factor requirement for tumor cell growth and cellular susceptibility to apoptosis (24). Pediatric tumors such as Ewing’s sarcoma, neuroblastoma, rhabdomyosarcoma and Wilm’s tumor express high levels of IGF-1R and, in the presence of IGF, increases proliferation and decreases apopotosis (10). Conversely, impairment of the IGF-1R function by antisense strategies, antibodies, or dominant negative mutants causes large-scale apoptosis of tumor cells, abrogation of tumor growth, and metastasis.(10, 15, 20) In particular, Ewing’s sarcoma xenografts are sensitive to anti-IGF-1R treatment (25). Therefore, modulating the IGF-1R pathway is now an attractive anti-cancer treatment strategy.
IGF-1 levels in humans are highest during the adolescent growth spurt, which coincides with the peak incidence of osteosarcoma, supporting the hypothesis that IGF-1 may contribute to the pathogenesis of osteosarcoma (26). In vitro studies have shown that osteosarcoma cell lines express IGF-1R, depend on IGF-1 ligand for proliferation and anti-apoptosis, and are growth inhibited with IGF-1R blockade (27). Finally, a recent study observed in a human osteosarcoma cell line, HOS 58, that proliferative activity was associated with high mRNA levels of IGF-1R, and the rate of proliferation decreased with a decline in IGF-1R expression (28).
PPP (picropodophyllin), a member of the cyclolignan family, is a new inhibitor of IGF-1R (29). The inhibitory effect of PPP on IGF-1R did not co-inhibit insulin receptor (IR) or competewith ATP in in vitro kinase assays, suggesting that it may inhibitIGF-1R autophosphorylation at the substrate level (30). PPP inhibits tyrosinephosphorylation of Y1136 in the activation loop of the IGF-1Rkinase domain. This agent has been shown to induce tumor regression and inhibitionof metastasis in several models of human cancer, and in vitro studies suggest development of only limited resistance in tumor cells after long-term PPP exposure (29–32). Recent studies showed that oral PPP is well tolerated in vivo and inhibits IGF-1R expression and growth of melanoma (33). To date, however, the effect of PPP on osteosarcoma and especially multidrug resistant osteosarcoma cells is undefined.
In this study, we determined whether the IGF-1 signaling pathway is of functional importance in osteosarcoma. We further investigate the effect of PPP on constitutive expression of IGF-1R, and whether a combination of minimally or non-toxic doses of PPP induces apoptosis, overcomes drug resistance, or enhances drug sensitivity in drug resistant osteosarcoma cell lines.
Materials and Methods
Cell Lines, Patient Tumor Samples and Antibodies
Human osteoblast cell line HOB-c (hipbone derived) was purchased from PromoCell GmbH (Heidelberg, Germany). The human osteosarcoma cell line U-2OS, KHOS, human uterine sarcoma cell line MES-SA and its doxorubicin selected drug resistant cell line MES-SA/Dx5, were purchased from the American Type Tissue Collection (Rockville, MD). The multidrug resistant U-2OSMR, was established as previously reported.(6, 34) Briefly, the doxorubicin resistant cell lines were selected over a period of six to ten months by continuous culture in media containing step-wise increases in doxorubicin. Dr. Efstathios Gonos (Institute of Biological Research & Biotechnology, Athens, Greece) provided the multidrug (selected with doxorubicin) resistant KHOS R2 (referred in the text below as KHOSMR) cell line (35). Dr. Katia. Scotlandi (Institute Orthopedics Rizzoli, Italy) provided ET-743 resistant TC-ET 6nM and TC-ET 12nM cell lines (36). Eight cases of osteosarcoma samples (1 to 8) were analyzed. Samples 1–4 were tissues from patients without chemotherapy and samples 5–8 were tissues from patients with chemotherapy. The Pgp1 monoclonal antibody C219 was purchased from Signet (Dedham, MA). The Goat anti-rabbit-HRP and goat anti-mouse-HRP were purchased from Bio-Rad (Hercules, CA). SuperSignal® West Pico Chemiluminescent Substrate was purchased from PIERCE (Rockford, IL). The rabbit polyclonal antibodies to human IGF-1R, AKT, pAKT and PARP were purchased from Cell Signaling Technologies (Cambridge, MA). The rabbit polyclonal antibody to human phosphor-IGF-1R (1158/1162/1163) was purchased from Invitrogen (Carlsbad, CA). The mouse monoclonal antibody to actin and MTT were purchased from Sigma-Aldrich (St. Louis, MO).
Drugs
The IGF-1R inhibitor picropodophyllin(PPP) was purchased from Calbiochem (La Jolla, CA). Stock solutions were prepared in DMSOand stored at −20 °C; they were diluted to the final concentration in fresh media before each experiment. In allexperiments, the final DMSO concentration was <0.1%. Doxorubicin and paclitaxel was obtained through unused residual clinical material provided by the pharmacy at the Massachusetts General Hospital. The stock solution of drugs were prepared according to the drug specifications and stored at −20°C.
Cell Culture
Human osteoblast cells HOB-c were cultured in osteoblast growth medium (PromoCell) with 10% fetal bovine serum (FBS). All other cell lines were cultured in RPMI 1640 (Invitrogen,) supplemented with 10% FBS, 100-units/ml penicillin and 100μg/ml streptomycin (Invitrogen). Cells were incubated at 37°C in 5% CO2-95% air atmosphere andpassaged when near confluent monolayers were achieved using trypsin-EDTA solution. Drug-resistant cell lines were periodically cultured in the respective drug to confirm their drug resistance characteristics. Cells were free on mycoplasma contamination as tested by MycoAlert(R) Mycoplasma Detection Kit from Cambrex (Rockland, ME).
Western Blotting
IGF-1R, phosphor-IGF-1R, AKT and Pgp1 proteins were analyzed in total cell lysates. Total cell lysates were prepared, and Western blot analysis was performed as previously described. Briefly, the cells were lysed in 1X RIPA lysis buffer (Upstate Biotechnology, Charlottesville, VA) and protein concentration was determined by the DC Protein Assay (Bio-Rad). Twenty-five micrograms of total protein were resolved on NuPage™ 4–12% Bis-Tris Gels (Invitrogen) and immunoblotted with specific antibodies. Primary antibodies were incubated in TBS (pH 7.4) with 0.1% Tween-20 with gentle agitation overnight at 4°C. Horseradish peroxidase (HRP)-conjugated secondary antibodies (Bio-Rad) were incubated in TBS (pH 7.4) with 5% nonfat milk (Bio-Rad) and 0.1% Tween-20, at a 1:2000 dilution for one hour at room temperature with gentle agitation. Positive immunoreactions were detected by using SuperSingal® West Pico Chemiluminescent Substrate. Densitometric analysis of Western blot results was performed with Adobe Photoshop 7.0 (San Jose, CA) as described in the User Guide and at: http://www.lukemiller.org/journal/2007/08/quantifying-western-blots-without.html.
Immunofluorescence
For immunostainings of cultured cells, osteosarcoma cells were grow in 8-well chamber (Nalge Nunc International, Rochester, NY ) for 48 hours and fixed in in 3.7% buffered paraformaldehyde. Immunostainings were performed using antibodies against the phosphorylated IGF-1 receptor (Tyr1131)/(1: 200), IGF-1R (1: 200) for 1 hour. After washing, the cells were incubated with Alexa Fluorsecondary antibodies (Invitrogen). To counter stain nuclei, the cells were incubated with PBS containing 1 μg/ml Hoechst 33342 (Invitrogen, Carlsbad, CA) for 1 min. Stained cells were then visualized on a Nikon Eclipse Ti-U fluorescence microscope (Nikon Corp.) equipped with a SPOT RT digital camera (Diagnostic Instruments, Inc., Sterling Heights, MI).
MTT Cell Proliferation Assay
Drug cytotoxicity was assessed in vitro using the MTT assay as previously described (37). Briefly, 2×103 cells per well were plated in 96-well plates in culture medium (RPMI 1640 supplemented with 10% fetal bovine serum and penicillin/streptomycin) containing increasing concentrations of PPP, doxorubicin or both. After 96 hours of culture, 10 μl MTT (5 mg/ml in PBS, obtained from Sigma) were added to each well and the plates were incubated for 4 h. The resulting formazan product was dissolved with acid-isopropanol and the absorbance at a wavelength of 490 nm (A490) was read on a SPECTRAmaxR Microplate Spectrophotometer (Molecular Devices, Sunnyvale, CA). The absorbance values were normalized by assigning the value of the control line in the medium without drug to 1.0 and the value of the no cell control to 0. Experiments were performed in triplicate. Dose response curves were fitted with use of GraphPad PRISMR 4 software (GraphPad Software, San Diego, CA).
IGF-1R siRNA assay
The human IGF-1R On-Targetplus SMARTpool siRNA was purchased from Dharmacon, Inc. (Chicago, IL) and was used according to the manufacturer’s instructions. For transfection, cells were either plated on 96 well plates for MTT assays or plated on dishes for Western blot protein detection. Transfections were performed with siPORT™ NeoFX™ siRNA transfection reagents (Ambion. Inc, Austin, TX) as directed by the manufacturer. The Silencer™ EGFP siRNA (Ambion) and siRNA Control® reagent (Dharmacon) were used as positive and negative controls in all experiments. For IGF-1R inhibition, the final concentration of siRNA was 100 nM. Media was replaced with RPMI1640 supplemented with 10% FBS 24 hours after transfection. Total protein was isolated after 72 hours of IGF-1R siRNA transfection.
Apoptosis Assay
Whole-cell lysates were immunoblotted with specific antibodies to PARP (Cell Signaling Technologies) and its cleavage products. Positive immunoreactions were detected by using Super Signal® West Pico Chemiluminescent Substrate. Quantification of apoptosis was also evaluated using the M30-Apoptosense ELISA assay kit, as per manufacturer’s instructions (Peviva AB, Bromma, Sweden). U-2OSMR and KHOSMR cells were seeded at 8000 cells/per well in a 96-well plate for 24 hours before the addition of doxorubicin and PPP. The cells were then treated with 0.5 μM doxorubicin, 1 μM doxorubicin, 0.01 μM PPP alone or a combination of doxorubicin and PPP for an additional 48 hours. The cells were then lysed by adding 10 μl 10% NP-40 per well, and the manufacturer’s instructions for the apoptosis assay were then followed.
Results
Expression of IGF-1R in Osteosarcoma
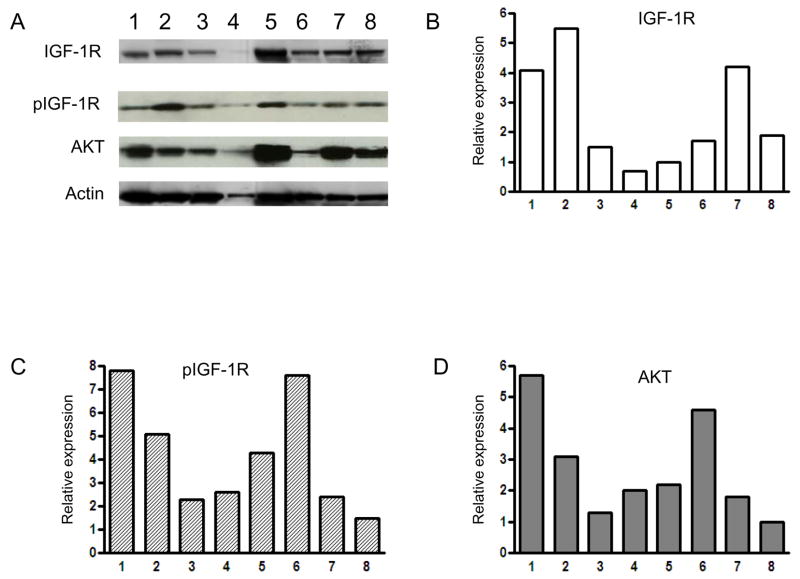
We observed the expression of IGF-1R in four pairs of multi-drug resistant sarcoma cell lines (two osteosarcoma cell lines, a Ewing’s sarcoma cell line, and a uterine sarcoma cell line). IGF-1R and phophorylated IGF1-R (pIGF-1R) is expressed in these cell lines as measured by Western blot (Fig. 1A). For confirmation, we measured the prevalence and activation of the IGF-1R in osteosarcoma cells by immunoflourescence, staining with antibody against IGF-1R and pIGF-1R. We also observed that osteosarcoma cell lines, including multidrug resistant cell lines, exhibited high levels of IGF-1R and pIGF-1R when compared to normal osteoblast cell lines (Fig. 1B). Furthermore, multidrug resistant cell lines KHOSMR, TC-ET and MES-5A/Dx5 demonstrated high expression of Pgp1 (multidrug resistance protein 1). To preclude the possibility that IGF-1R expression is an artifact induced by in vitro propagation, we also examined 8 freshly isolated primary osteosarcoma specimens, including samples from patients with resistance to conventional chemotherapy. Expression of IGF-1R and pIGF-1R was also universally observed in these primary patient samples (Fig. 2). Since the phosphatidylinositol 3-kinase (PI3K)–AKT pathway is known to be involved in the transmission of IGF-1R signals, we assayed for expression of AKT and observed that all osteosarcoma cell lines and primary tumor samples which express IGF-1R also express AKT (Fig. 1A, Fig. 2A and Fig. 2D).
Figure 1.
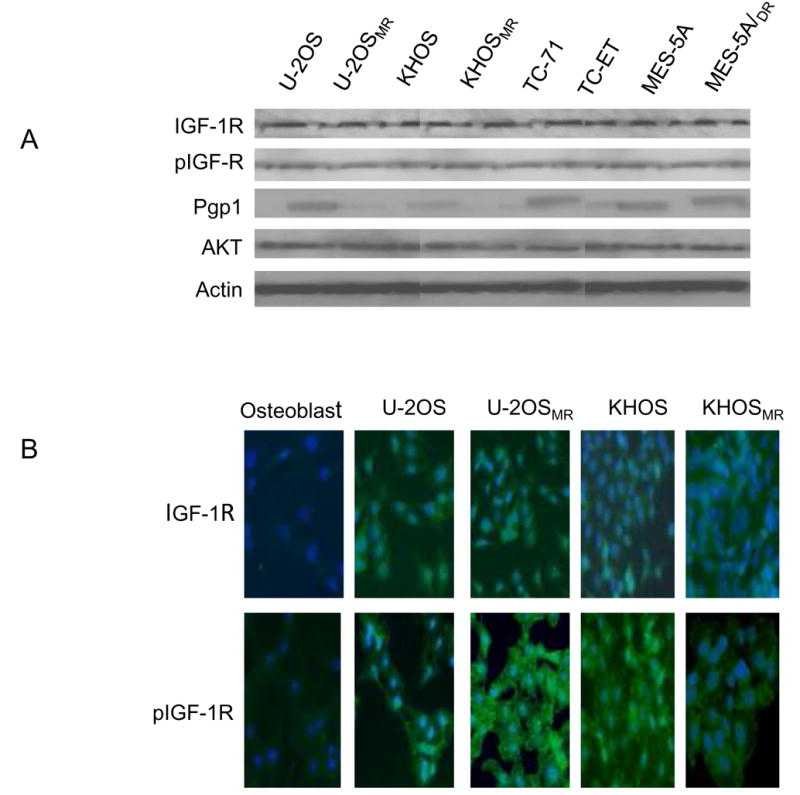
Expression of IGF-1R, phospho-IGF-1R (pIGF-1R) in the sarcoma cell lines as detected by Western blotting (A), immunofluorescence (B). A: Expression of IGF-1R, pIGF-1R in sarcoma drug sensitive and resistant cell line pairs determined by Western blot. Total cellular protein isolated from the indicated cell lines and immunoblotted with specific antibodies as described in Materials and Methods. The blots were also probed with an anti-actin monoclonal antibody to assess relative protein levels in the sample lanes. B: Confirmation of expression of IGF-1R, pIGF-R in osteosarcoma drug sensitive and resistant cell lines by immunofluroescence. Stained cells were then visualized on a Nikon Eclipse Ti-U fluorescence microscope.
Figure 2.
Expression of IGF-1R, pIGF-1R and AKT in osteosarcoma tissues determined by Western blot. Total cellular protein isolated from the osteosarcoma tissues and immunoblotted with specific antibodies as described in Materials and Methods. A: Expression of IGF-1R, pIGF-1R and AKT. Numbers 1 to 8 represent 8 different tissue samples. B, C and D: Western blots from A were analyzed using densitometry which was carried out in Photoshop and normalized to β-actin expression. Quantitative results for each protein were presented as a relative expression. B: IGF-1R, C: pIGF-1R and D: AKT
Effects of PPP on Cellular Proliferation in Osteosarcoma Cell Lines
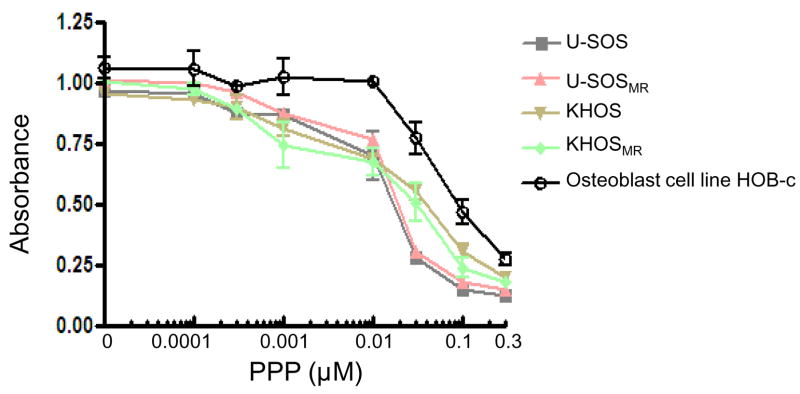
We examined the effect of PPP on the proliferation of osteosarcoma cell lines. After exposing the cell lines to PPP in complete cell culture media for 96 hours, the relative numbers of viable cells were determined by MTT assay. The osteosarcoma cell lines were growth inhibited by PPP (Fig. 3). Incubation of both drug sensitive and resistant osteosarcoma cell lines with increasing concentrations of PPP was found to decrease the amount of MTT absorbance in a dose-dependent manner. U-2OS and U-2OSMR were more sensitive to the PPP compared to normal osteoblast cells. A slightly higher resistance to PPP was observed in normal osteoblast cell lines. In addition, osteosarcoma cell lines and its drug-resistant subclones showed similar responses to PPP.
Figure 3.
Effects of PPP on the proliferation of osteosarcoma and normal osteoblast cells. Cells were treated with the PPP in RPMI1640 complete media at the indicated concentrations. The relative sensitivity of each line to PPP was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) analysis 96 hours posttreatment.
Effect of PPP on IGF-1R Expression
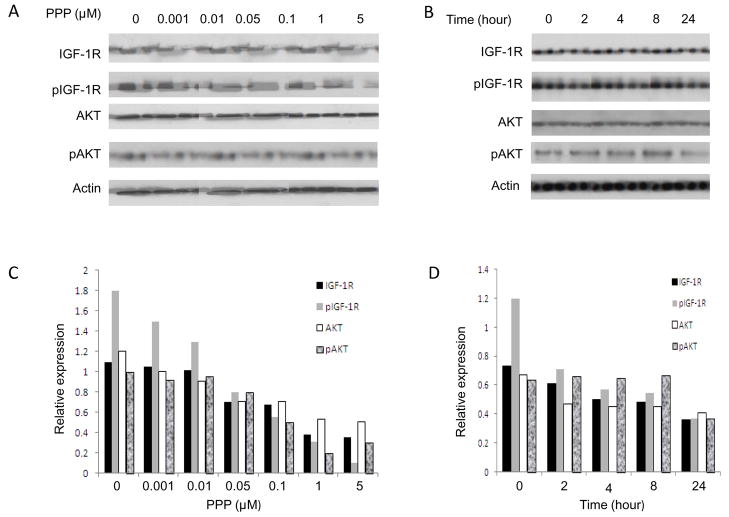
Because IGF-1R has been shown to be expressed to a high degree in drug sensitive and drug resistant osteosarcoma cell lines as compared with normal osteoblast cell line (Fig. 1), effects of PPP on the expression of IGF-1R, pIGF-R, and AKT in osteosarcoma cell line were analyzed by Western blot. The osteosarcoma cell lines were incubated either with a range of concentrations of PPP for 24 h, or with 1 μM PPP for 0, 2 h, 4 h, 8 h and 24 h. Figure 4 are representative the results from cell line U-2OSMR which has showed highly express IGF-1R (Fig. 1). Quantitative analyzed Western blot data demonstrated that PPP reduced both IGF-1R, pIGF-1R, AKT and pAKT expression in a dose- and time-dependent manner (Fig. 4). PPP-induced inhibition could be observed as early 2 h (1 μM), and with a concentration as low as 0.01 μM. These results are consistent with previous study of PPP in breast cancer and melanomacells (29, 38). Similar results were found in osteosarcoma cell lines U-2OS, KHOS as well as KHOSMR cell line (data not shown).
Figure 4.
Dose and time dependent of PPP down-regulated IGF-1R proteins in drug resistant osteosarcoma cells. U-2OSMR was treated with PPP in dose (A) or time (B) dependent manner in fresh complete medium. Total cellular proteins were subjected to immunoblotting with specific antibodies to IGF-1R, pIGF-1R, AKT and β-actin as described in “Materials and Methods”. A: PPP reduces IGF-1R proteins in U-2OSMR cells as dose dependent manner. U-2OSMR cells were cultured for 24 h in the presence of various concentrations of PPP as indicated. B: U-2OSMR cells were cultured for the time indicated (in hours) in the presence of 1 μM of PPP. C and D: Western blots from A and B were analyzed using densitometry as described in “Materials and Methods”.
PPP Enhances Apoptosis and Reduces Resistance in Drug Resistant Human Osteosarcoma Cell Lines
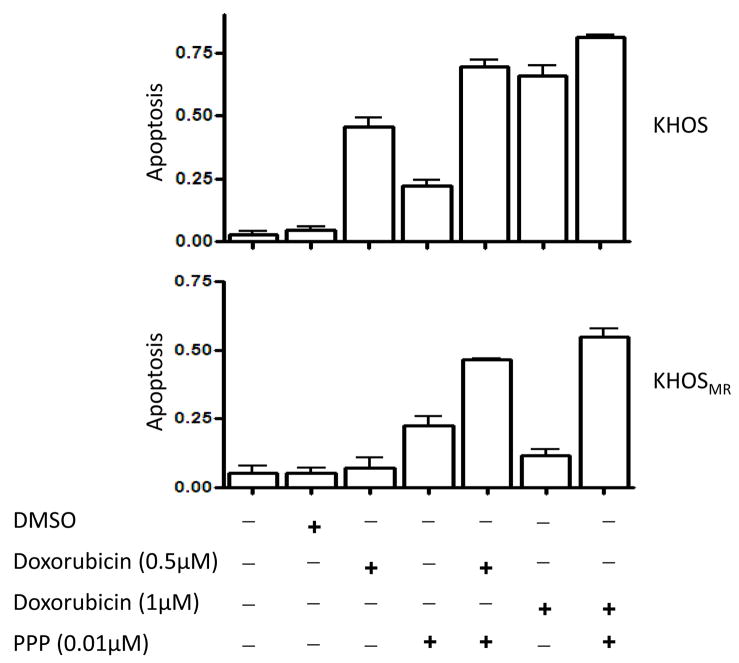
Inhibition of IGF-1R signaling in KHOS and KHOSMR may increase the levels of apoptosis induced by chemotherapy drugs. The addition of PPP to cells exposed to doxorubicin resulted in greater levels of apoptosis in both multidrug resistant osteosarcoma cell lines KHOS and KHOSMR (Fig. 5). Additionally, MTT assay that measures a combination of cellular proliferation and cytotoxicity also demonstrated that PPP has an additive effect on paclitaxel-induced cell death in U-2OS and U-2OSMR (Supplementary Fig. S1). These effects were also seen in KHOS and KHOSMR cells exposed to sublethal doses of doxorubicin and PPP (0.005 μM) (data not shown). Through these results, it is evident that PPP increased doxorubicin or paclitaxel induced cell death and partially overcame drug resistance.
Figure 5.
PPP enhances apoptosis induced by doxorubicin in drug resistant osteosarcoma cells. KHOS or KHOSMR cells were seeded at a density of 8,000 cells per well in a 96-well plate for 24 hours. Cells were then treated with different drugs for additional 48 hours. The cells were lysed with 10% NP40 and the M30-Apoptosense ELISA assay was done as described in Materials and Methods.
PPP Induced Apoptosis in Multidrug Resistant Osteosarcoma Cell Lines
The effectof PPP on the induction of apoptosis was further investigated byimmunoblotting for PARP cleavage. PARP cleavage was detectedafter the incubation of U-2OSMR or KHOSMR cells with PPP. A dose-response analysis revealed the appearanceof PARP cleavage products starting at a 0.005 μM PPP concentrationwhen cells were allowed to incubate for 24 h (Supplementary Fig. S2).
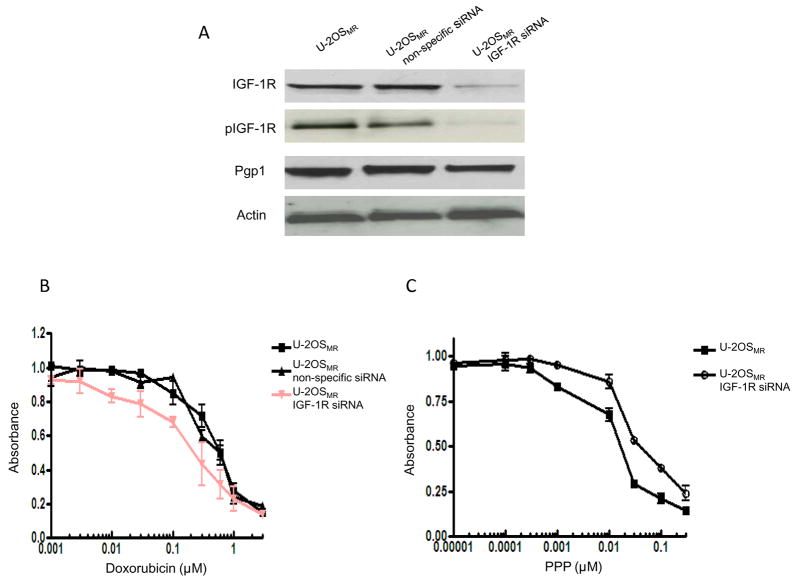
Effects of Inhibition of IGF-1R Expression by siRNA on Drug Sensitivities
The evaluation of IGF-1R mRNA expression levels’ contribution to multidrug resistance took two steps. First, the IGF-1R gene in U-2OSMR cells was downregulated with siRNA. Western blot showed that IGF-1R expression was significantly decreased after the cells treated with siRNA (Fig. 6A). Then, relative drug sensitivities were evaluated by comparison of IC50 values determinedby MTT in siRNA-treated and control multidrug resistant cell lines. Cytotoxicitywas measured 96 hours after treatment with IGF-1R siRNA (see Materials and Methods). The results showed that IGF-1R down-regulation by siRNA partially recovered sensitivity to doxorubicin(Fig. 6B). Treatment IGF-1R siRNA knockdown cells with PPP showed a modest higher resistance to PPP as compared with cells without IGF-1R knockdown (Fig. 6C). These data are consistent with the results showed PPP inhibit osteosarcoma cell growth and induce apopotosis (Fig 3). Moreover, while IGF-1R siRNA could significantly inhibit expression of IGF-1R, no significant changes in Pgp1 expression was observed in this IGF-1R knockdown cell line (Fig. 6A).
Figure 6.
Effect of IGF-1R inhibition on drug sensitivity in drug resistant cells. Multidrug resistant cell line U-2OSMR was transfected with On-Targetplus SMARTpool IGF-1R siRNA as well as non-specific siRNA. A: Confirmation of IGF-1R protein knockdown by Western blot. Total protein was isolated 72 hours post-transfection and IGF-1R, pIGF-1R and Pgp1 expression was analyzed by Western blotting. B: The relative sensitivity of each treatment to doxorubicin was determined by MTT analysis 96 hours post-transfection. C: Effects of IGF-1R knockdown by siRNA on PPP sensitivity inU-2OSMR cells.
Discussion
Identification of both novel therapeutic strategies and potent drugs which are effective against sarcomas is a high priority goal for sarcoma oncologists. The IGF-1R signaling pathway plays a significant role in the growth and proliferation of tumor cells. Several lines of evidence have demonstrated that IGF-1 production and IGF-1R expression may prevent cell death and lead to drug resistance through upregulation of survival proteins in human cancers (10, 20, 21, 39). Therefore, IGF-1R proteins emerged as important targets for cancer therapy. Previous studies with IGF-1R inhibitors such as NVP-AEW541, R1507, and SCH-717454 have demonstrated that these compounds have the capabilities of inhibiting tumor cell growth and inducing apoptosis in several human cancers (10, 15, 20). Cells with a high level of IGF-1R expression are sensitive to these inhibitors. In this study, we demonstrate that IGF-1R is highly expressed in chemotherapy resistant osteosarcoma cell lines as well as in primary osteosarcoma tissues. Our findings further demonstrated that PPP inhibits IGF-1R expression in osteosarcoma cell lines. Moreover, we found that the osteosarcoma cell lines were particularly sensitive to IGF-1R inhibition when compared with normal osteoblast cells or with cells that were genetically suppressed for IGF-1R expression by siRNA knockdown. The inhibition of the IGF-1R pathway in osteosarcoma cell lines correlated with suppression of proliferation and with induction of apoptosis.
The molecular mechanism of PPP action on different tumor cell is still unknown. PPP appears to block IGF-1R phosphorylation and promote IGF-1R degradation, whereas the homologous insulin receptor is not affected (29, 31). IFG-1R null fibroblasts have been shown to be resistant to PPP, whereas PPP reduces the viability of cancer cell lines and causes tumor regression in mouse xenografts of multiple myeloma and uveal melanoma (32, 38). We also showed that osteosarcoma cell lines that were induced to no longer express IGF-1R by treatment with siRNA become insensitive to PPP (Figure 6). PPP has been shown to associate with inhibition of the PI3K/Akt, VEGF and activation of ERK pathways (30, 31, 40). More recently, treatment of constitutively active oncogenic K-ras (mutant K-rasG12D) tumor-bearing animals by PPP resulted in a dramatic decrease in tumor mass of the main forms of basal-like breast cancer. PPP also was effective against xenografts of the human basal-like breast cancer cell line MDA-MB-231, which carries a K-rasG13D mutation (41).
The development of multidrug resistance has posed major obstacles to the efficacy of chemotherapy for cancer treatment (2, 5, 6). We therefore investigated the effects of PPP in combination with conventional therapeutic agents that are currently used in the treatment of osteosarcoma. We observed that the MDR1 (Pgp1) positive osteosarcoma cells are sensitive to PPP-induced cell death. At the same time, the synergistic activity of PPP with doxorubicin suggests that PPP could induce apoptosis in doxorubicin-resistant cells through mechanisms independent of inhibition of Pgp1. We found that PPP could increase the sensitivity of osteosarcoma drug resistant cells to various cytotoxic chemotherapeutic agents (doxorubicin, paclitaxel). These studies provided a proof of principle that PPP is active against osteosarcoma cell lines with known resistance to conventional (doxorubicin, paclitaxel, vincrinstine) anticancer agents, as well as primary tumor cells from osteosarcoma patients. These preclinical studies provide the framework for clinical evaluation of PPP, either as a monotherapy or in combination with doxorubicin, to treat osteosarcoma and overcome drug resistance.
Supplementary Material
Acknowledgments
This project was supported by a grant from the Gattegno and Wechsler funds. Dr. Duan is supported, in part, through a grant from Ovarian Cancer Research Foundation (OCRF), and a grant from the National Cancer Institute, NIH (Nanotechnology Platform Partnership), R01-CA119617. Dr. Choy is supported by the Jennifer Hunter Yates Foundation.
Financial support: The Gattegno and Wechsler funds
Abbreviations
- IGF-1R
insulin-like growth factor-1 receptor
- PPP
cyclolignan picropodophyllin
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Chou AJ, Geller DS, Gorlick R. Therapy for osteosarcoma: where do we go from here? Paediatr Drugs. 2008;10:315–27. doi: 10.2165/00148581-200810050-00005. [DOI] [PubMed] [Google Scholar]
- 3.Trent JC. Rapid evolution of the biology and treatment of sarcoma. Curr Opin Oncol. 2008;20:393–4. doi: 10.1097/CCO.0b013e328303ba31. [DOI] [PubMed] [Google Scholar]
- 4.Siegel HJ, Pressey JG. Current concepts on the surgical and medical management of osteosarcoma. Expert Rev Anticancer Ther. 2008;8:1257–69. doi: 10.1586/14737140.8.8.1257. [DOI] [PubMed] [Google Scholar]
- 5.Chou AJ, Gorlick R. Chemotherapy resistance in osteosarcoma: current challenges and future directions. Expert Rev Anticancer Ther. 2006;6:1075–85. doi: 10.1586/14737140.6.7.1075. [DOI] [PubMed] [Google Scholar]
- 6.Yang C, Yang S, Wood KB, et al. Multidrug resistant osteosarcoma cell lines exhibit deficiency of GADD45alpha expression. Apoptosis. 2009;14:124–33. doi: 10.1007/s10495-008-0282-x. [DOI] [PubMed] [Google Scholar]
- 7.Pasello M, Michelacci F, Scionti I, et al. Overcoming glutathione S-transferase P1-related cisplatin resistance in osteosarcoma. Cancer Res. 2008;68:6661–8. doi: 10.1158/0008-5472.CAN-07-5840. [DOI] [PubMed] [Google Scholar]
- 8.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915–28. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 9.Ullrich A, Gray A, Tam AW, et al. Insulin-like growth factor I receptor primary structure: comparison with insulin receptor suggests structural determinants that define functional specificity. EMBO J. 1986;5:2503–12. doi: 10.1002/j.1460-2075.1986.tb04528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim SY, Toretsky JA, Scher D, Helman LJ. The Role of IGF-1R in Pediatric Malignancies. Oncologist. 2009;14:83–91. doi: 10.1634/theoncologist.2008-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lann D, LeRoith D. The role of endocrine insulin-like growth factor-I and insulin in breast cancer. J Mammary Gland Biol Neoplasia. 2008;13:371–9. doi: 10.1007/s10911-008-9100-x. [DOI] [PubMed] [Google Scholar]
- 12.Rowlands MA, Gunnell D, Harris R, Vatten LJ, Holly JM, Martin RM. Circulating insulin-like growth factor peptides and prostate cancer risk: A systematic review and meta-analysis. Int J Cancer. 2009;124:2416–29. doi: 10.1002/ijc.24202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LeRoith D, Roberts CT., Jr The insulin-like growth factor system and cancer. Cancer Lett. 2003;195:127–37. doi: 10.1016/s0304-3835(03)00159-9. [DOI] [PubMed] [Google Scholar]
- 14.Sachdev D, Yee D. Disrupting insulin-like growth factor signaling as a potential cancer therapy. Mol Cancer Ther. 2007;6:1–12. doi: 10.1158/1535-7163.MCT-06-0080. [DOI] [PubMed] [Google Scholar]
- 15.Chitnis MM, Yuen JS, Protheroe AS, Pollak M, Macaulay VM. The type 1 insulin-like growth factor receptor pathway. Clin Cancer Res. 2008;14:6364–70. doi: 10.1158/1078-0432.CCR-07-4879. [DOI] [PubMed] [Google Scholar]
- 16.Surmacz E. Growth factor receptors as therapeutic targets: strategies to inhibit the insulin-like growth factor I receptor. Oncogene. 2003;22:6589–97. doi: 10.1038/sj.onc.1206772. [DOI] [PubMed] [Google Scholar]
- 17.Baserga R. The insulin-like growth factor I receptor: a key to tumor growth? Cancer Res. 1995;55:249–52. [PubMed] [Google Scholar]
- 18.Renehan AG, Zwahlen M, Minder C, O’Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363:1346–53. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- 19.Cohen P. Clinical implications of the IGF-cancer connection. Growth Horm IGF Res. 2001;11:336–8. doi: 10.1054/ghir.2001.0255. [DOI] [PubMed] [Google Scholar]
- 20.Casa AJ, Dearth RK, Litzenburger BC, Lee AV, Cui X. The type I insulin-like growth factor receptor pathway: a key player in cancer therapeutic resistance. Front Biosci. 2008;13:3273–87. doi: 10.2741/2925. [DOI] [PubMed] [Google Scholar]
- 21.Oh SH, Jin Q, Kim ES, Khuri FR, Lee HY. Insulin-like growth factor-I receptor signaling pathway induces resistance to the apoptotic activities of SCH66336 (lonafarnib) through Akt/mammalian target of rapamycin-mediated increases in survivin expression. Clin Cancer Res. 2008;14:1581–9. doi: 10.1158/1078-0432.CCR-07-0952. [DOI] [PubMed] [Google Scholar]
- 22.Peretz S, Kim C, Rockwell S, Baserga R, Glazer PM. IGF1 receptor expression protects against microenvironmental stress found in the solid tumor. Radiat Res. 2002;158:174–80. doi: 10.1667/0033-7587(2002)158[0174:irepam]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 23.Xie Y, Skytting B, Nilsson G, Brodin B, Larsson O. Expression of insulin-like growth factor-1 receptor in synovial sarcoma: association with an aggressive phenotype. Cancer Res. 1999;59:3588–91. [PubMed] [Google Scholar]
- 24.Kurmasheva RT, Houghton PJ. IGF-I mediated survival pathways in normal and malignant cells. Biochim Biophys Acta. 2006;1766:1–22. doi: 10.1016/j.bbcan.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Manara MC, Landuzzi L, Nanni P, et al. Preclinical in vivo study of new insulin-like growth factor-I receptor--specific inhibitor in Ewing’s sarcoma. Clin Cancer Res. 2007;13:1322–30. doi: 10.1158/1078-0432.CCR-06-1518. [DOI] [PubMed] [Google Scholar]
- 26.Henderson BE, Ross RK, Pike MC, Casagrande JT. Endogenous hormones as a major factor in human cancer. Cancer Res. 1982;42:3232–9. [PubMed] [Google Scholar]
- 27.Kappel CC, Velez-Yanguas MC, Hirschfeld S, Helman LJ. Human osteosarcoma cell lines are dependent on insulin-like growth factor I for in vitro growth. Cancer Res. 1994;54:2803–7. [PubMed] [Google Scholar]
- 28.Viereck V, Siggelkow H, Pannem R, Braulke T, Scharf JG, Kubler B. Alteration of the insulin-like growth factor axis during in vitro differentiation of the human osteosarcoma cell line HOS 58. J Cell Biochem. 2007;102:28–40. doi: 10.1002/jcb.21274. [DOI] [PubMed] [Google Scholar]
- 29.Girnita A, Girnita L, del Prete F, Bartolazzi A, Larsson O, Axelson M. Cyclolignans as inhibitors of the insulin-like growth factor-1 receptor and malignant cell growth. Cancer Res. 2004;64:236–42. doi: 10.1158/0008-5472.can-03-2522. [DOI] [PubMed] [Google Scholar]
- 30.Vasilcanu D, Girnita A, Girnita L, Vasilcanu R, Axelson M, Larsson O. The cyclolignan PPP induces activation loop-specific inhibition of tyrosine phosphorylation of the insulin-like growth factor-1 receptor. Link to the phosphatidyl inositol-3 kinase/Akt apoptotic pathway Oncogene. 2004;23:7854–62. doi: 10.1038/sj.onc.1208065. [DOI] [PubMed] [Google Scholar]
- 31.Vasilcanu R, Vasilcanu D, Rosengren L, et al. Picropodophyllin induces downregulation of the insulin-like growth factor 1 receptor: potential mechanistic involvement of Mdm2 and beta-arrestin1. Oncogene. 2008;27:1629–38. doi: 10.1038/sj.onc.1210797. [DOI] [PubMed] [Google Scholar]
- 32.Menu E, Jernberg-Wiklund H, De Raeve H, et al. Targeting the IGF-1R using picropodophyllin in the therapeutical 5T2MM mouse model of multiple myeloma: beneficial effects on tumor growth, angiogenesis, bone disease and survival. Int J Cancer. 2007;121:1857–61. doi: 10.1002/ijc.22845. [DOI] [PubMed] [Google Scholar]
- 33.Economou MA, Andersson S, Vasilcanu D, et al. Oral picropodophyllin (PPP) is well tolerated in vivo and inhibits IGF-1R expression and growth of uveal melanoma. Invest Ophthalmol Vis Sci. 2008;49:2337–42. doi: 10.1167/iovs.07-0819. [DOI] [PubMed] [Google Scholar]
- 34.Yang C, Hornicek FJ, Wood KB, Schwab JH, Mankin H, Duan Z. RAIDD expression is impaired in multidrug resistant osteosarcoma cell lines. Cancer Chemother Pharmacol. 2009 Jan 6; doi: 10.1007/s00280-008-0912-6. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 35.Lourda M, Trougakos IP, Gonos ES. Development of resistance to chemotherapeutic drugs in human osteosarcoma cell lines largely depends on up-regulation of Clusterin/Apolipoprotein J. Int J Cancer. 2007;120:611–22. doi: 10.1002/ijc.22327. [DOI] [PubMed] [Google Scholar]
- 36.Manara MC, Perdichizzi S, Serra M, et al. The molecular mechanisms responsible for resistance to ET-743 (Trabectidin; Yondelis) in the Ewing’s sarcoma cell line, TC-71. Int J Oncol. 2005;27:1605–16. [PubMed] [Google Scholar]
- 37.Duan Z, Foster R, Bell DA, et al. Signal transducers and activators of transcription 3 pathway activation in drug-resistant ovarian cancer. Clin Cancer Res. 2006;12:5055–63. doi: 10.1158/1078-0432.CCR-06-0861. [DOI] [PubMed] [Google Scholar]
- 38.Girnita A, All-Ericsson C, Economou MA, et al. The insulin-like growth factor-I receptor inhibitor picropodophyllin causes tumor regression and attenuates mechanisms involved in invasion of uveal melanoma cells. Clin Cancer Res. 2006;12:1383–91. doi: 10.1158/1078-0432.CCR-05-1106. [DOI] [PubMed] [Google Scholar]
- 39.Jones HE, Gee JM, Barrow D, Tonge D, Holloway B, Nicholson RI. Inhibition of insulin receptor isoform-A signalling restores sensitivity to gefitinib in previously de novo resistant colon cancer cells. Br J Cancer. 2006;95:172–80. doi: 10.1038/sj.bjc.6603237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Economou MA, Wu J, Vasilcanu D, et al. Inhibition of VEGF secretion and experimental choroidal neovascularization by picropodophyllin (PPP), an inhibitor of the insulin-like growth factor-1 receptor. Invest Ophthalmol Vis Sci. 2008;49:2620–6. doi: 10.1167/iovs.07-0742. [DOI] [PubMed] [Google Scholar]
- 41.Klinakis A, Szabolcs M, Chen G, Xuan S, Hibshoosh H, Efstratiadis A. Igf1r as a therapeutic target in a mouse model of basal-like breast cancer. Proc Natl Acad Sci U S A. 2009;106:2359–64. doi: 10.1073/pnas.0810221106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.