Abstract
(S)-5-[123I]iodo-3-(2-azetidinylmethoxy)pyridine (5-[123I]IA), a novel potent radioligand for high-affinity α4β2* neuronal nicotinic acetylcholine receptors (nAChRs), provides a means to evaluate the density and the distribution of nAChRs in the living human brain. We sought in healthy adult smokers and nonsmokers to (1) evaluate the safety, tolerability, and efficacy of 5-[123I]IA in an open nonblind trial and (2) to estimate the density and distribution of α4β2* nAChRs in the brain. Single photon emission computed tomography (SPECT) was performed for five hours after the intravenous administration of approximately 0.001 μg/kg (approximately 10 mCi) 5-[123I]IA. Blood pressure, heart rate, and neurobehavioral status were monitored before, during, and after the administration of 5-[123I]IA to twelve healthy adults (8 men and 4 women) (6 smokers and 6 nonsmokers) ranging in age from 19 to 46 years (mean = 28.25, standard deviation = 8.20). High plasma nicotine level was significantly associated with low 5-[123I]IA binding in (1) the caudate head, the cerebellum, the cortex, and the putamen, utilizing both the Sign and Mann-Whitney U tests, (2) the fusiform gyrus, the hippocampus, the parahippocampus, and the pons utilizing the Mann-Whitney U test, and (3)the thalamus utilizing the Sign test. We conclude that 5-[123I]IA is a safe, well-tolerated, and effective pharmacologic agent for human subjects to estimate high-affinity α4/β2 nAChRs in the living human brain.
Keywords: binding, infusions, density, distribution, treatment efficacy, safety, tomography
INTRODUCTION
Neuronal nicotinic acetylcholine receptors (nAChRs), excitatory ligand-gated ion channels (Hui et al., 2005; Kassiou et al., 2001) composed of five parts (Mogg et al., 2004; Rueter et al., 2006; Tuzun, 2006; Zaniewska et al., 2006) with at least twelve subtypes (α2 to α10 and β2 to β4) (Kassiou et al., 2001; Smith et al., 2007), form a pore through which pass Na+, K+, and Ca++ (Hui et al., 2005). In the brain there exist two forms of nAChRs, homomeric and heteromeric. Homomeric neuronal nAChRs containing five α7 units exhibit high affinity for α-bungarotoxin, an antagonist of nAChRs (Huang and Winzer-Serhan, 2006; Smith et al., 2007). Heteromeric neuronal nAChRs containing mainly α4 and β2 units exhibit high affinity for nicotinic receptors (Huang and Winzer-Serhan, 2006; Mogg et al., 2004; Smith et al., 2007). The stoichiometry of two α4 and three β2 subunits is two orders of magnitude more sensitive to acetylcholine than the stoichiometry of three α4 and two β2 subunits (Zwart et al., 2006).
The pharmacological effects of nicotine occur through the action of nicotine on α4β2* nAChRs (Smith et al., 2007). The principle pharmacological sites of action of nicotine appear to be the high-affinity α4β2* nAChRs. In rats, exposure to nicotine in the neonatal period (Huang and Winzer-Serhan, 2006) and in adolescence is associated with upregulation of nAChRs (Trauth et al., 1999). Nicotine up-regulates high specificity α4β2* nAChRs likely through promoting the assembly of receptors (Moroni et al., 2006). Upregulation of nAChRs, but not muscarinic acetylcholine receptors, has been demonstrated in response to nicotine. Chronic exposure to low doses of nicotine may upregulate nAChRs, while high doses may downregulate them (Wonnacott, 1990). In particular, chronic nicotine exposure produces functional upregulation of α4β2* nAChRs in baboons (Kassiou et al., 2001) and humans (Buisson and Bertrand, 2001). Thus, dysfunction of nAChRs likely follows nicotine exposure.
In the human brain the α4β2* nAChR subtype is distributed densely in the thalamus, the basal ganglia, the dentate gyrus, and moderately densely in the cortex, the hippocampal pyramidal layer, and the limbic system (Gotti et al., 1997; Léna and Changeux, 1998; Paterson and Nordberg, 2000). α4β2* nAChRs are sensitive to micromolar amounts of acetylcholine and nicotine (Leonard and Bertrand, 2001). The actions of nAChRs include the promotion of serotonin release in portions of the central nervous system (Zaniewska et al., 2007).
High-affinity α4β2* neuronal nAChRs bind [3H]nicotine and [3H]cytisine. Exposure to nicotine may precipitate dysfunction of high-affinity α4β2* nAChRs. While increased stimulation by agonists typically reduces the number of cell surface receptors, chronic exposure to nicotine through cigarette smoking increases the density (Bmax) of high-affinity α4β2* nAChRs in the cortex, the hippocampus, the cerebellum, and the striatum (Breese et al., 2000; Court et al., 1998; Gotti et al., 1997; Perry et al., 1999). Furthermore, human cigarette smokers exhibit a dose-dependent increase in high-affinity α4β2* nAChRs ([3H]nicotine) binding in the hippocampus and the thalamus (Breese et al., 1997) due to increased receptor density (Bmax) without altered affinity (Kd) (Benwell et al., 1988; Wonnacott, 1990).
nAChRs are implicated in the development and maintenance of multiple neuropsychiatric disorders including alcohol-related (Wong et al., 2003). and nicotine-related disorders, schizophrenia, Alzheimer’s disease (O’Brien et al., 2007; Yoshida et al., 2002; Zamani and Allen, 2001), Parkinson’s disease (Bordia et al., 2006; Fujita et al., 2006; Leonard and Bertrand, 2001; O’Brien et al., 2007; Valette et al., 1999), dementia with Lewy body disease (O’Brien et al., 2007); and tic disorders (American Psychiatric Association, 2000). In particular in many brain regions, people with schizophrenia who smoke cigarettes demonstrate markedly less binding of high affinity nAChRs than people without schizophrenia (Breese et al., 2000; Voineskos et al., 2007). The development of in vivo techniques to visualize particular nAChRs is likely to facilitate establishing the diagnosis, to determine how changes in nAChRs may reflect alterations in clinical status, to develop interventions targeted for the involved nAChRs, and to monitor the effects of treatments.
A highly specific probe for high affinity α4β2* and α6β2* nAChRs (Mogg et al., 2004) without the toxic limitations of epibatidine, 3-(2(S)-azetidinylmethoxy)pyridine (A-85380) offers promise as a ligand for human studies (Rueter et al., 2006). A-85380 can be utilized as a radiotracer for single photon emission computed tomography (SPECT) in the form of 5-[123I]iodo-3-(2(S)-azetidinylmethoxy)pyridine (5-[123I]IA) (Rueter et al., 2006). The wide availability of SPECT favors the usefulness of 5-[123I]IA brain imaging as a diagnostic tool for the general population.
In rats the binding affinity of 5-[123I]IA is comparable to A-85380 and seven times greater than (−)-nicotine (Saji et al., 2002). In rats 5-[123I]IA binds selectively to α4β2* nAChRs with high uptake in the thalamus, moderate uptake in the cortex, and low uptake in the cerebellum (Saji et al., 2002). In rats the accumulation of 5-[123I]IA was blocked in all brain regions by nicotine agonists, (−)-cysteine and (−)-nicotine (Saji et al., 2002). In monkeys chronic oral administration of nicotine produced upregulation of α4β2*, but not α3α6β2*, nAChRs (McCallum et al., 2006). Ninety minutes after the intravenous administration of 5-[123I]IA to baboons, most of the tracer remained unchanged in brain tissue after 90 minutes (Baldwin et al., 2006). The concentration of 5-[123I]IA is 5.8 times greater in the thalamus than in the cerebellum ninety minutes after the intravenous administration of 5-[123I]IA to baboons (Baldwin et al., 2006). In humans, intravenous administration of 5-[123I]IA produced radioactivity, highest in the thalamus (Fujita et al., 2002;Ueda et al., 2004), high in the brainstem, moderate in the cerebellum (Ueda et al., 2004), the brainstem (Fujita et al., 2002), and basal ganglia (Mamede et al., 2004), and low in cortical regions (Mamede et al., 2004; Ueda et al., 2004).
The many advantages of a radiotracer for single photon emission computed tomography (SPECT), specifically 123I, include the general wide availability of equipment to conduct the SPECT protocols and the longer half life of the radionuclide, e. g., 13.2 h for 123I (Paterson and Nordberg, 2000; Wong and Brašić, 2001). Moreover, of all the newly developed radiolabeled 3-pyridyl ethers, 5-[123I]IA has been extensively studied in rodents (Mukhin et al., 2000; Musachio et al., 1998; Vaupel et al., 1998; Ueda et al., 2004) and primates (Zoghbi et al., 1999) including pigs (Deuther-Conrad et al., 2006), baboons (Fujita et al., 2000; Kassiou et al., 2001; Musachio et al., 1999; Zoghbi et al., 2001), Rhesus monkeys (Chefer et al., 1998), and humans (Musachio et al., 2001; Ueda et al., 2004). This agent with a ki of approximately 11 pM (Koren et al., 1998) seems the most specific ligand for the α4β2* nAChR system (Gozzi et al., 2006) and has been the first to visualize nAChRs in human subjects.
Although considerable research has been performed utilizing 5-[123I]IA SPECT in humans, several questions remain unanswered. Table I summarizes the key points of prior studies. Table I differentiates prior research from the current study. We note the wide variability of the key scientific aspects of prior research. There exists no established protocol for diagnostic procedures for the in vivo visualization of the α4β2* nAChR system in primates and man. In particular we have developed an optimal method to model 5-[123I]IA SPECT data (Zhou et al., 2001). The safety, tolerability, pharmacology, and efficacy of 5-[123I]IA SPECT in humans, including smokers and nonsmokers must be established. For these reasons we share with our colleagues our unique experiences in to assess the safety, tolerability, pharmacology, quantitative modeling, and efficacy of 5-[123I]IA SPECT in humans. We incidentally had the opportunity to examine 5-[123I]IA SPECT in a couple of healthy nonsmokers who experienced considerable secondhand smoke exposure. Therefore, we seek to determine whether 5-[123I]IA can safely be administered to human beings to estimate the density and the distribution of high-affinity α4β2* nAChRs in the living human brain of light smokers and nonsmokers. We hypothesize that 5-[123I]IA SPECT is a safe efficacious agent to estimate the density and the distribution of high-affinity α4β2* nAChRs in the living human brain of smokers and nonsmokers utilizing an optimal quantitative modeling method (Zhou et al., 2001).
TABLE I.
Characteristics of nuclear neuroimaging of high-affinity α4β2* neuronal nicotinic acetylcholine receptors (nAChRs)
| Study | Species of subjects |
Sample size | Radiotracer | Duration in minutes of scan after start of radiotracer administration |
Form of radiotracer administration |
Method of plasma sampling for modeling |
Method of modeling |
Conclusion |
|---|---|---|---|---|---|---|---|---|
| Current (presented in abstract by Brasic et al., 2001; Musachio et al., 2001; Wong et al., 2001, 2002a) | Humans (Homo sapiens) | 6 smokers, 6 nonsmokers including 2 nonsmokers with secondhand smoke exposure | 5-[123I]IA | 300 | 7 B 5 B + CI | Column-switch HPLC (Hilton et al., 2000) | DV estimation by kinetic analysis (Zhou et al., 2001) |
|
| Baldwin et al., 2006 | Baboons (Papio anubis) | 1 without nicotine exposure | 5-[123I]IA | 90 | 1 B | HPLC | DV estimation by kinetic analysis |
|
| Bottlaender et al., 2003 | Humans (Homo sapiens) | 3 nonsmokers | 2-[18F]FA | 120 | 3 B | Radioactivity measurements corrected for physical decay | Regions of interest on PET scans |
|
| Brody et al., 2006 | Humans (Homo sapiens) | 11 smokers with tobacco dependence | 2-[18F]FA | 500 | 11 B + CI before and after cigarette smoking | Gas chromatography with nitrogen-phosphorus detection (Jacob et al., 1981) | Maximal fractional displacement of radioactivity | α4 β2* nAChRs are occupied almost fully in cigarette smokers with tobacco dependence. |
| Brust et al., 2008 | Pigs (mixed German landrace and Pietrain) | 24 | (−)-[18F]NCFHEB, (+)- [18F]NCFHEB, 2- [18F]FA | 420 | 24 CI | HPLC | Kinetic analysis with BioMedical Image Quantification Software PMOD Version 2.75 (PMOD Technologies, Zurich, Switzerland) |
|
| Chefer et al., 1998 | Rhesus monkeys (Macaca mulatta) | 1 | 5-[123I]IA | 270 | B | HPLC | Kinetic analysis with cerebellum as the reference tissue | 5-[123 I]IA is safe and efficacious for brain imaging in monkeys. |
| Chefer et al., 1999 | Rhesus monkeys (Macaca mulatta) | 2 | 2-[18F]FA | 240 | 2 B+CI | HPLC | Kinetic analysis with cerebellum as the reference tissue |
|
| Chefer et al., 2008 | Rhesus monkeys (Macaca mulatta) | 3 | [18F]NIDA522131 and 2-[18F]FA | 540 | 3 B | HPLC | Kinetic analysis with BioMedical Image Quantification Software PMOD Version 2.75 (PMOD Technologies, Zurich, Switzerland) |
|
| Colloby et al., 2008 | Humans (Homo sapiens) | 9 (4 AD, 5 DLB) | 5-[123I]IA before and after administration of donepezil | 120 | B | none | SPM2 |
|
| Cosgrove et al., 2007 | Humans (Homo sapiens) | 29 nonsmokers (10 men, 19 women) | 5-[123I]IA during early follicular and mid-luteal phases in women | 480 | B + CI | HPLC | MEDx software (Medical Numerics, Inc.) |
|
| Ding et al., 2000 | Baboons (Papio anubis) | 1 | 2-[18F]FA and 6-[18F]FA | 180 | B | HPLC | Kinetic analysis |
|
| Dollé et al., 1999 | Baboons (Papio papio) and Rhesus monkey (Macaca mulatta) | 1 baboon and 1 monkey | 2-[18F]FA; 5-[123I]IA; [18F]fluoronorchloroepibatidine | 90 | B | HPLC | Kinetic analysis |
|
| Fujita et al., 2006 | Humans (Homo sapiens) | 15 healthy; 10 PD | 5-[123I]IA | 230 | B | HPLC | Kinetic analysis |
|
| Fujita et al., 2002 | Humans (Homo sapiens) | 10 | 5-[123I]IA | 1440 | B | HPLC | Kinetic analysis |
|
| Fujita et al., 2000 | Baboons | 3 | 5-[123I]IA | 289–495 | B, B + CI | HPLC | Kinetic analysis | 5-[123I]IA is safe, efficacious in baboons. |
| Gallezot et al., 2005 | Humans (Homo sapiens) | 7 nonsmokers | 2-[18F]FA | 210 | B | HPLC | Kinetic analysis |
|
| Horti et al., 2000 | Rhesus monkeys (Macaca mulatta) | 2 | 6-[18F]FA | 270 | B | HPLC | Kinetic analysis |
|
| Ishizu et al., 2004 | Humans (Homo sapiens) | 4 | 5-[123I]IA | 360 | B | Arterial blood sampling | Kinetic analysis |
|
| Kassiou et al., 2001 | Baboons (Papio hamadryas) | 2 | 5-[123I]IA | 180 | B | Plasma metabolite analysis | Kinetic analysis |
|
| Kassiou et al., 2002 | Baboons (Papio papio) | 3 | [76Br]BrPH | 132 to 240 | B | Plasma metabolite analysis | Kinetic analysis | [76Br]BrPH is safe and efficacious in baboons. |
| Kimes et al., 2003 | Humans (Homo sapiens) and Rhesus monkeys (Macaca mulatta) | 6 humans and 2 monkeys | 2-[18F]FA | 420 to 480 | B | Plasma metabolite analysis | Kinetic analysis |
|
| Mamede et al., 2004 | Humans (Homo sapiens) | 21 humans | 5-[123I]IA | 90 or 360 | B | TLC | Kinetic analysis |
|
| Mamede et al., 2007 | Humans (Homo sapiens) | 16 (6 nonsmokers and 10 smoders) | 5-[123I]IA | 360 | B | TLC | Kinetic analysis |
|
| Mitkovski et al., 2005 | Humans (Homo sapiens) | 10 | 2-[18F]FA | 120 | B | Arterial blood sampling | Kinetic analysis |
|
| Mitsis et al., 2007 | Humans (Homo sapiens) | unknown | 5-[123I]IA | 480 | B + CI | Venous sampling | Kinetic analysis |
|
| O’Brien et al., 2007 | Humans (Homo sapiens) | 32 (16 healthy and 16 AD) | 5-[123I]IA | 150 | B | HPLC | Kinetic analysis |
|
| Obrzut et al., 2005 | Humans (Homo sapiens) | 2 | 2-[18F]FA | 180 | B | plasma sampling | Kinetic analysis |
|
| Oishi et al., 2006, 2007 | Humans (Homo sapiens) | 10 healthy and 10 PD | 5-[123I]IA | 240 | B | Arterial blood sampling | Kinetic analysis |
|
| Picard et al., 2006 | Humans (Homo sapiens) | 7 healthy and 8 ADNFLE | 2-[18F]FA | 240 | B | Venous plasma sampling | SPM2 |
|
| Saji et al., 2002 | Common marmoset (Callithrix jacchus)I | 1 | 5-[123I]IA | 240 | B | Venous plasma sampling | Kinetic analysis |
|
| Sorger et al., 2007 | Humans (Homo sapiens) | 12 healthy and 97 patients with neurological disorders | 2-[18F]FA | 420 | B | HPLC or TLC | Kinetic analysis |
|
| Staley et al., 2005 | Humans (Homo sapiens) | 10 | 5-[123I]IA | 420 | B + CI | Plasma sampling | SPM99 |
|
| Staley et al., 2006 | Humans (Homo sapiens) and Rhesus monkeys (Macaca mulatta) | 32 humans (16 nonsmokers and 16 smokers) and two monkeys | 5-[123I]IA | 480 | B + CI | Plasma sampling | Kinetic analysis |
|
| Ueda et al., 2004 | Humans (Homo sapiens) | 4 | 5-[123I]IA | 1440 | B | Plasma sampling | Kinetic analysis |
|
| Valette et al., 1999 | Baboons (Papio papio) | 3 | 2-[18F]FA | 180 | B | Plasma sampling | Kinetic analysis |
|
| Wüllner et al., 2007 | Humans (Homo sapiens) | 14 (7 nonsmokers and 7 smokers) | 2-[18F]FA | 364 | CI | HPLC | SPM2 |
|
| Yoshida et al., 2002 | Humans (Homo sapiens) | 6 healthy and 5 AD | 5-[123I]IA | 60 | CI | Arterial blood sampling | Kinetic analysis |
|
[76Br]BrPH = [76Br]norchlorobromoepibatidine; [18F]NCFHEB = [18F]norchloro-fluoro-homoepibatidine; [18F]NIDA522131 = chloro-3-((2-(S)-azetidinyl)methoxy)-5-(2-[18F]fluoropyridin-4-yl)pyridine; 2-[18F]FA = 2-[18F]fluoro-3-(2(S)-azetidinylmethoxy)pyridine; 5-[123I]IA = (S)-5-[123I]iodo-3-(2-azetidinylmethoxy)pyridine; 6-[18F]FA = 6-[18F]fluoro-3-(2(S)-azetidinylmethoxy)pyridine; AD = Alzheimer’s disease; ADNFLE = autosomal dominant nocturnal frontal lobe epilepsy; B = bolus; B + CI = bolus and continuous infusion; CI = continuous infusion; DLB = dementia with Lewy bodies; DV = distribution volume; HPLC = high performance lipid chromatography; nAChRs = nicotinic acetylcholine receptors; PD = Parkinson’s disease; PET = positron emission tomography; SPECT = single photon emission computed tomography; SPM2 = Statistical Parametric Mapping 2 (http://www.fil.ion.ucl.ac.uk/spm/); SPM99 = Statistical Parametric Mapping, version 99; TLC = thin layer chromatography.
MATERIALS AND METHODS
The safety, tolerability, pharmacology, and efficacy of (S)-5-[123I]iodo-3-(2-azetidinylmethoxy)pyridine (5-[123I]IA) (Musachio et al., 2001) was assessed on twelve healthy adults, 6 light smokers and 6 nonsmokers (Wong et al., 2001).
At baseline before the administration of 5-[123I]IA and at follow-up after the administration of 5-[123I]IA, complete blood counts, comprehensive metabolic profiles, hepatic function profiles, thyroid function profiles, routine and microscopic urinalyses, urine toxicologies, and electrocardiograms were normal (Wong et al., 2001). Serum for human chorionic gonadotropin (HCG) demonstrated the absence of pregnancy in female subjects before the administration of 5-[123I]IA (Wong et al., 2001). At baseline before the administration of 5-[123I]IA, magnetic resonance imaging (MRI) of the brain was obtained (Wong et al., 2001). Subjects with cerebral abnormalities detected on MRI were excluded from further participation. To facilitate the co-registration of the magnetic resonance imaging (MRI) and the single photon emission computed tomography (SPECT) scans, subjects were fitted with thermoplastic face masks. The thermoplastic face mask was worn during the MRI and SPECT scans to maintain the head in the same position throughout each scan. Three MRI sequences were obtained as outlined in Table II. Because movement artifacts occur when the subjects talked or performed physical motions during the scans, we restricted our serial assessments to the short breaks between the scans. Therefore, the evaluation protocol used hourly was a brief battery that can be administered in seven minutes. Because subjects had at least one line in each arm and electrocardiogram leads in place, we limited the assessment battery to procedures administered while the subject sat quietly in order to minimize the risk of dislodging the lines in each arm and the electrocardiogram leads. Therefore subjects were observed sitting in chairs with feet flat on the floor.
TABLE II.
Characterization of the magnetic resonance imaging (MRI) sequences of the brain performed on twelve (12) healthy adult volunteers
| Series number | Format | TR in milliseconds | TE in milliseconds | Thickness in millimeters | Number of slices |
|---|---|---|---|---|---|
| 1 | T1 sagittal | 500 | 8 | 5.0 | 21 |
| 2 | T1 spoiled gradient (SPGR) recalled acquisition in the steady state axial | 35 | 6 | 1.5 | 124 |
| 3 | T2 oblique | 5900 | 95 | 5.0 | 27 |
TR = time to repetition; TE = time to echo.
Before the administration of 5-[123I]IA and for the subsequent 24 hours, the vital signs and electrocardiograms of the subjects were monitored and were normal (Wong et al., 2001). Before the administration of 5-[123I]IA, the subjects were administered Lugol’s solution (potassium iodide) to block entry of 123I into the thyroid (Ueda et al., 2004).. Before the administration of 5-[123I]IA and for the subsequent 24 hours, subjects were regularly monitored for possible adverse events by open-ended questions about their subjective states, e. g., “How are you feeling?” On the day of the administration of 5-[123I]IA, subjects were assessed for the presence of behavioral aberrations and abnormal movements by the administration of the Abnormal Involuntary Movement Scale (AIMS) (Brašić, 2003; Brasic et al., 2001, 2007a; National Institute of Mental Health, 1988) and the Brief Psychiatric Rating Scale (BPRS) (Overall and Gorham, 1962) at baseline before the start of the administration of 5-[123I]IA and during breaks occurring approximately at one, two, and three hours after the start of the administration of 5-[123I]IA. The administration of the AIMS during the breaks after the initiation of the administration of 5-[123I]IA was limited by the short time of the break and the presence of lines in both arms. Although the AIMS was fully administered at baseline before the commencement of the administration of 5-[123I]IA, the hourly administrations of the AIMS after the start of the administration of 5-[123I]IA were limited to the items listed in Table III (Brašić, 2003; Brasic et al., 2001, 2007a; National Institute of Mental Health, 1988). At baseline before the administration of 5-[123I]IA, items of the BPRS listed in Table IV were preceded by the phrase, “During the past week….” Due to the short period of time in the breaks, the BPRS was administered by asking the screening questions indicated in Table IV preceded by the phrase, “Since I last asked you, ….”. If the response to any item in Table IV was abnormal, then questions were asked to probe for details to determine the appropriate score for each item of the BPRS (Brasic et al., 2001; Overall and Gorham, 1962). The items of the BPRS omitted from Table IV were scored by observation only (Brasic et al., 2001; Overall and Gorham, 1962). All subjects were monitored for electrocardiogram, blood pressure, and pulse during each study. Subjects 1 through 5 and 11 and 12 received the dose of 5-[123I]IA as a rapid intravenous bolus injection. Subjects 6 through 10 received the 5-[123I]IA as a rapid intravenous bolus injection followed by a continuous infusion to maintain a constant plasma concentration of 5-[123I]IA throughout the course of the study (Table V). To obtain dynamic measurements of plasma radioactivity, 1.5mL aliquots of arterial blood were sampled as rapidly as possible during the initial 2 min following the commencement of the 5IA injection, then at less frequent intervals during the scans. Arterial blood was obtained frequently for radioactivity determination and metabolite analysis. Arterial blood (5mL) was sampled at 0, 5, 10, 15, 30, 60, 90, 120, 180, 240, and 300 min following the bolus administration of 5-[123I]IA and added to heparinized tubes containing 15mg sodium azide, and, following centrifugation, 0.5mL plasma was counted in a well counter.
TABLE III.
Items of the Abnormal Involuntary Movement Scale (AIMS)1 assessed at one, two, and three hours after the commencement of the administration of (S)-5-[123I]iodo-3-(2-azetidinylmethoxy)pyridine (5-[123I]IA) to twelve healthy adult volunteers
| Item number | Examiner’s statement to subject |
|---|---|
| 2. | “Is there anything like gum or candy in your mouth?” (“Please remove it.”)2 |
| 3. | “What is the current condition of your teeth?” “Do your teeth (or dentures) bother you now?” |
| 4. | “Do you notice any movements in your mouth, face, hands, or feet?” (“Describe them to me.”)2 (“How do they currently bother you?”)2 (“How do they currently interfere with your activities?”)2 |
| 7.3 | “Open your mouth.” |
| 8.3,4 | “Stick out your tongue. Keep it out.” |
Modified from Brašić, 2003.
The statements in parentheses were spoken only if applicable.
Items 7 and 8 were performed twice.
For item 8 the subject was told to keep the tongue out for at least ten seconds.
TABLE IV.
Items of the Brief Psychiatric Rating Scale (BPRS)1 administered at baseline before and at one, two, and three hours after the start of the administration of (S)-5-[123I]iodo-3-(2-azetidinylmethoxy)pyridine (5-[123I]IA) to twelve healthy adult volunteers
| Item number | Concluding phrase of question |
|---|---|
| 1. | “… have you been concerned about your present bodily health?” |
| 5. | “… have you been overly concerned or remorseful for past behavior?” |
| 8. | “… have you felt that you have unusual powers or abilities?” |
| 9. | “… have you been depressed, low, blue, or down in the dumps?” |
| 11. | “… have you felt that other people are out to hurt you?” |
| 12. | “… have you heard voices talking to you that other people who were present did not hear?” “… have you seen things that other people who were present did not see?” |
| 18. | “I need to check your memory. What is your full name?” “What is the name of the place where we are right now?” “What year are we in?” “What month are we in?” “What is today’s date?” |
Modified from Overall and Gorham, 1962.
TABLE V.
Injected activity, specific activity, and mass of (S)-5-[123I]iodo-3-(2-azetidinylmethoxy)pyridine (5-[123I]IA) administered to twelve healthy adult volunteers
| Subject number | Method of administration | Activity injected in mCi | Specific activity in mCi/micromol | Mass in micrograms |
|---|---|---|---|---|
| 1 | Bolus | 8.1 | 7, 522 | 0.39 |
| 2 | Bolus | 9.1 | >33, 600 | <0.10 |
| 3 | Bolus | 9.3 | >27, 064 | <0.13 |
| 4 | Bolus | 8.8 | >29, 340 | <0.12 |
| 5 | Bolus | 8.6 | 43, 160 | 0.07 |
| 6 | Bolus followed by continuous infusion | 7.8 | 20, 815 | 0.11 |
| 7 | Bolus followed by continuous infusion | 7.6 | 16, 207 | 0.13 |
| 8 | Bolus followed by continuous infusion | 5.2 | 21, 141 | 0.07 |
| 9 | Bolus followed by continuous infusion | 8.1 | 25,948 | 0.09 |
| 10 | Bolus followed by continuous infusion | 7.7 | 10,070 | 0.003 |
| 11 | Bolus | 2.1 | 16,117 | 0.0006 |
| 12 | Bolus | 7.35 | 8400 | 0.275 |
In several studies blood was sampled up to 22 hours after 5-[123I]IA administration. In those studies where 5-[123I]IA was delivered by bolus followed by continuous infusion (Table V), blood samples were taken at 30 min intervals between 3 and 5 hours after the beginning of the infusion. Plasma prepared by centrifugation was immediately analyzed for 5-[123I]IA and its metabolites by column-switch high performance lipid chromatography (HPLC) (Hilton et al., 2000). The plasma, diluted to 4mL with water, was passed through a small capture column packed with Oasis sorbent (Waters Corporation, Milford, Massachusetts) which was then washed with 1% acetonitrile. The eluate and wash, which contain metabolites too polar to be captured by the Oasis column, flowed through dual bismuth germanate (BGO) detector and the amount of radioactivity recorded by computer software (Laura, Bioscan Inc., Washington, DC)). More lipophilic metabolites and unchanged 5-[123I]IA were swept from the capture column onto an analytical column (Zorbax Extend C18, Agilent Technologies, Palo Alto, CA) by 50% acetonitrile: 0.25M triethylamine-HCl buffer pH 11.0. Effluent from the analytical column was also passed through the dual BGO detector (Hilton et al., 2000).
Cross calibration between the SPECT images and the well counter used for counting the time radioactivity plasma samples was carried out by counting a series of samples from the phantom which contains 5-[123I]IA solution and relating those values to SPECT images of the phantom acquired under conditions used for the imaging of human subjects.
Inter-frame head motion was corrected through a detailed process. First, brain outlines were defined automatically on each SPECT frame after smoothed with a gaussian kernel (full width half maximum = 8 mm). For this purpose, a threshold was defined to maximize the difference in mean radioactivity between the two shells one layer inside and outside the volumes of voxels equal to or above the threshold. Then the threshold was increased stepwise by 5% increments until the volume of voxels greater than the revised threshold attained a target brain volume of 1450 ml after the elimination of non-brain portions. Then, between-frame head movement was estimated by minimizing the square sum of differences between the radioactivities in outline voxels in the original frame and those given by interpolation and normalization by equating the means in the alignment frame using three linear and three rotational parameters. Inter-frame alignment was performed on successive frames because of similar radioactivity distributions between them. The distribution volume (DV) was estimated by kinetic analysis for all subjects save three (Zhou et al., 2001). The distribution volume (DV) was estimated by the ratio of tracer activity in the brain regions and the plasma around 300 minutes for subjects 6, 7, and 9 due to missing data (Zhou et al., 2001).
Regional radioactivity time-profiles were obtained by applying volumes of interest (VOIs) to head-movement corrected SPECT frames. VOIs of regions of particular interest, namely the head of the caudate nucleus, the cerebellum, the cingulate gyrus, the cortex, the frontal lobe, the fusiform gyrus, the hippocampus, the occipital lobe, the parahippocampus, the parietal lobe, the pons, the putamen, the temporal lobe, and the thalamus, were defined using a locally developed three-dimensional region-defining tool. Remaining VOIs were obtained by individual local standard VOI template. For this purpose, the volumes of the MRIs of individual subject were spatially normalized to a standard brain. The parameters for the spatial normalization were inversely applied to transform standard VOIs to the MRI space of each subject (Zhou et al., 2001).
RESULTS
Table VI records the sex, age, lateral preferences (Brasic et al., 2007b; Denckla, 1985), smoking status by self-report, nicotine exposure by self-report, and self-rated scores on the Fagerstrom Test for Nicotine Dependence (FTND) (Balfour and Fagerstrom, 1966) and the Tobacco Dependence Questionnaire (TDQ) (Kawakami et al., 1999) of the twelve subjects of this study.
TABLE VI.
Demographic characteristics, lateral preferences, and self-reported measures of nicotine exposure in twelve (12) healthy adult volunteers
| Subject number | Sex | Age in years | LP | Nicotine use by self report | Smoking status by self report | FTND | TDQ |
|---|---|---|---|---|---|---|---|
| 1 | male | 35 | All items right | none | nonsmoker | 0 | . |
| 2 | male | 28 | All items right | none | nonsmoker | 0 | 0 |
| 3 | male | 22 | All items right | smokes houka pipe, chews tobacco | light smoker | 0 | 4 |
| 4 | male | 32 | All items right | no direct use, lives with cigarette smoker | nonsmoker | 0 | 0 |
| 5 | male | 33 | All items right | smokes cigarettes | light smoker | 0 | 0 |
| 6 | female | 22 | All items right | smokes cigarettes | light smoker | 0 | 3 |
| 7 | female | 36 | Eye left, foot right, and hand right | smokes cigarettes | light smoker | 2 | 5 |
| 8 | male | 21 | All items right | none | nonsmoker | 0 | 0 |
| 9 | female | 22 | . | smokes cigarettes | light smoker | . | . |
| 10 | male | 19 | All items right | No smoking, no smoking roommates, frequently inhales second hand cigarette smoke in bars | nonsmoker | 0 | 0 |
| 11 | female | 46 | Eye right, hand left, and foot left | none | nonsmoker | 0 | 0 |
| 12 | male | 23 | . | none | > one pack cigarettes per day | . | . |
. (period) = missing data
FTND = Fagerström Test for Nicotine Dependence (Balfour and Fagerström, 1996)
LP = Lateral Preferences (Brasic et al., 2007b; Denckla, 1985)
TDQ = Tobacco Dependence Questionnaire (Kawakami et al., 1999)
Sex
There were 8 males and 4 females (Table VI).
Age
Subjects ranged in age from 19 to 46 years (mean = 28.25, standard deviation = 8.20) (Table VI).
Lateral preferences
Eight subjects displayed a preference for the right eye, right hand, and right foot. One subject displayed the preference for the right eye, the left hand, and the left foot. Another subject displayed the preference for the left eye, the right hand, and the right foot. Laterality was not assessed in two subjects (Brasic et al., 2007b; Denckla, 1985) (Table VI).
Cigarette smoking
Each subject was interviewed about current and past use of cigarettes, cigars, pipes, and other forms of tobacco. Six subjects were non-smokers. Of the non-smokers, four denied secondhand smoke exposure and incidentally two reported extensive secondhand smoke exposure. Subject 4, a non-smoker with a smoking roommate, and Subject 10, a non-smoker without smoking roommates who inhaled second-hand smoke frequently in restaurants where he worked as a waiter, are classified as experiencing secondhand smoke nicotine exposure. Six subjects were light smokers (Table VI). All subjects were asked to refrain from nicotine use on the day of the study. Subject 7 last smoked a cigarette at 2:30 am on the day of the study. Other subjects refrained from the use of nicotine on the day of the study. Table V summarizes the characteristics of the radiotracer dose of 5-[123I]IA administered to each subject. Table VII summarizes the plasma, salivary, and urinary levels of nicotine, cotinine, and caffeine obtained on each subject before injection of 5-[123I]IA.
TABLE VII.
Nicotine (N), cotinine(CO), and caffeine (CA) levels in ng/ml in plasma (P), saliva (S), and urine (U) of twelve healthy adult volunteers at baseline before the administration of (S)-5-[123I]iodo-3-(2-azetidinylmethoxy)pyridine (5-[123I]IA) unless noted otherwise
| Subject number | P N | P CO | P CA | S N | S CO | S CA | U N | U CO | U CA |
|---|---|---|---|---|---|---|---|---|---|
| 1 eight days prior to injection of 5IA | 2.80 | <10.0 | 35.5 | 4.07 | <10.0 | 23.7 | <1.0 | <10.0 | 40.2 |
| 1 day of injection of 5IA | 55.3 | <10.0 | 187 | 0 | 0 | 107 | <1.0 | <10.0 | 75.7 |
| 2 | 1.63 | 19.4 | 22.4 | 0 | 0 | <20.0 | 0 | 0 | <20.0 |
| 3 | 14.1 | 39.9 | 576 | 224 | 213 | 685 | 1.72 | 42.6 | 804 |
| 4 | <1.0 | <10.0 | 268 | <1.0 | 18.5 | 559 | 0 | <10.0 | 370 |
| 5 | 0 | <10.0 | 119 | 0 | <10.0 | 91.8 | 1.32 | 69.6 | 151 |
| 6 | 0 | <10.0 | 354 | 0 | <10.0 | 299 | 3.88 | 85.9 | 475 |
| 7 | 2.92 | 103 | 216 | 2.86 | 140 | 207 | 391 | 1160 | 207 |
| 8 | <1.0 | <10.0 | 0 | <1.0 | <10.0 | 0 | <1.0 | <10.0 | 0 |
| 9 | <1.0 | 71.4 | <20.0 | <1.0 | 69.5 | 0 | 172 | 532 | 0 |
| 10 | 0 | <10.0 | 61.9 | <1.0 | <10.0 | 157 | 8.22 | <10.0 | 140 |
| 11 | <1.0 | 0 | <20.0 | <1.0 | <10.0 | <20.0 | 0 | <10.0 | 25.7 |
| 12 | 3.20 | 153.2 | 105.4 | 37 | 163.1 | <20.0 | 172.2 | 1145.8 | 176.9 |
Metabolism of 5-[123I]IA
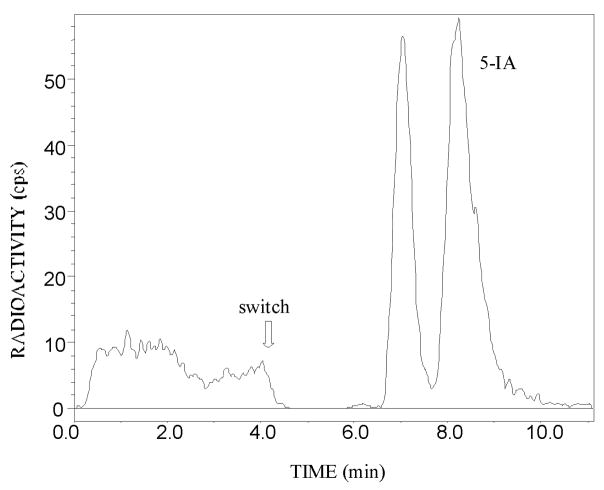
Analysis of plasma from arterial blood revealed that 5-[123I]IA was rapidly metabolized in human subjects. Unlike prior studies of 5-[123I]IA metabolism, we utilize a column switch high performance lipid chromatography(HPLC) method, which separates polar and lipophilic metabolites from the parent compound without concern for extraction efficiencies associated with solvent extraction, detected the presence of highly polar labeled species and a single lipophilic metabolite which elutes before the parent compound (Hilton et al., 2000) (Table I). For example, a graph of the plasma radioactivity in counts per second and time in minutes of the in vitro mixture of an aliquot of 5-[123I]IA and an aliquot of the plasma of a 32-year-old male nonsmoker (Subject 4) is presented in Figure 1.
Fig. 1.
Column-switch chromatography of (S)-5-[123I]iodo-3-(2-azetidinylmethoxy)pyridine (5-[123I]IA) added to human plasma of a 32-year-old male nonsmoker (Subject 4). In the first 4 minutes of the column-switch method (Hilton et al., 2000), 4mL of plasma is passed through a capture column (Oasis Sorbent, Waters Associates, 4.6 × 19 mm) at 2mL/min followed by a wash with 1% acetonitrile in water. The effluent containing polar species passes through the flow cell of a radiation detector. The parent compound and lipophilic metabolites (if present), which bind to the capture column, are eluted and transferred to the analytical column (Zorbax Extend C18 4.6 × 250 mm) in 50% acetonitrile in triethylamine-HCl buffer pH 11 at a flow rate of 1 mL/min. Radioactivity in this effluent is measured by passage through the radiation detector. The column-switching procedure (Hilton et al., 2000) permits the identification of 5IA after the switch.
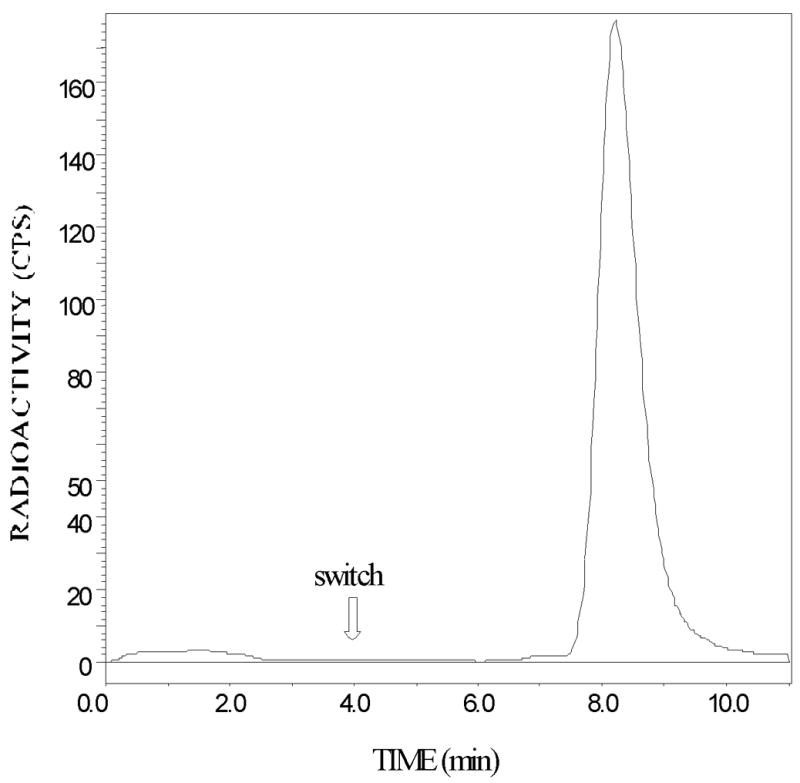
Additionally, a graph of the plasma radioactivity in counts per second and time in minutes of an aliquot of the plasma of a 32-year-old male nonsmoker (Subject 4) is presented in Figure 2.
Fig. 2.
Chromatography of an aliquot of the plasma of a 32-year-old male nonsmoker (Subject 4) obtained 60 min after the intravenous bolus administration of 8.8 mCi (S)-5-[123I]iodo-3-(2-azetidinylmethoxy)pyridine (5-[123I]IA). In the first 4 minutes of the column-switch method (Hilton et al., 2000), 4 mL of plasma passes through a capture column (Oasis Sorbent, Waters Associates, 4.6 × 19 mm) at 2 mL/min followed by a wash with 1% acetonitrile in water. The effluent containing polar species is passed through the flow cell of a radiation detector. The parent compound and lipophilic metabolites (if present), which bind to the capture column, are eluted and transferred to the analytical column (Zorbax Extend C18 4.6 × 250 mm) in 50% acetonitrile in triethylamine-HCl buffer pH 11 at a flow rate of 1 mL/min. Radioactivity in this effluent is measured by passage through the radiation detector. The column-switching procedure (Hilton et al., 2000) permits the identification of 5IA after the switch.
Thus, these finding are graphed for a 32-year-old male nonsmoker (Subject 4) (Figures 1 and 2).
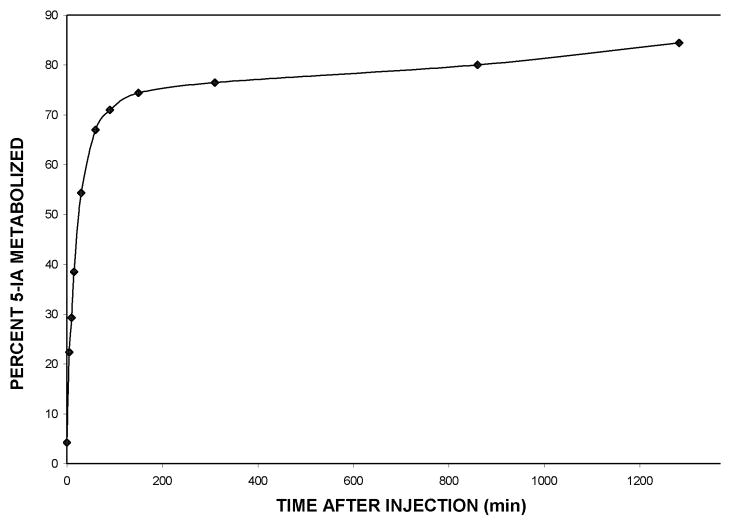
Figure 3 shows a typical time course of 5-[123I]IA metabolism. Figure 3 presents a graph of the radioactivity counts of the metabolites of 5-[123I]IA after the intravenous bolus administration of 9.3 mCi 5IA to a 22-year-old male light smoker (Subject 3). Within 30 minutes after the commencement of the administration of 5-[123I]IA, approximately half of the 5-[123I]IA has been metabolized with the extent of metabolism leveling to 75% after 2 hours with a very slow increase to 80% metabolized after 20 hours. In some studies both arterial and venous blood samples, drawn simultaneously 30 minutes or later after 5-[123I]IA administration, showed identical extents of metabolism.
Fig. 3.
Graph of the time course of the percent of plasma radioactivity present as (S)-5-[123I]iodo-3-(2-azetidinylmethoxy)pyridine (5-[123I]IA) metabolites after the intravenous bolus administration of 9.3 mCi 5-[123I]IA to a 22-year-old male light smoker (Subject 3).
The activity of 5-[123I]IA injected ranged from 2.14 to 9.34 mCi (Table V). The specific activity of 5-[123I]IA injected ranged from 7,522 to 43,160 mCi/μmol (Table V). The mass of 5-[123I]IA injected ranged from 0.0006 to 0.39 μg (Table V). Dynamic SPECT (Trionix TriadXLT) collected images over 6 h with 20 acquisitions. A two-compartmental (plasma and brain tissue) model was used for the kinetic analysis and parametric imaging of VOIs drawn on co-registered MRI scans. The plasma radioactivity of centrifuged whole arterial blood obtained during the scan was measured with gamma counter. Plasma radiotracer metabolites were also assayed with high performance lipid chromatography (HPLC) The metabolite-corrected plasma input fraction was used for kinetic modeling. Binding potentials was calculated by mathematical modeling of μ utilizing the procedures employed for other radioligands of this series (Yokoi et al., 1999). Additionally, the concentration of nAChR binding sites was estimated utilizing kinetic modeling (Fujita et al., 2000).
Distribution of 5-[123I]IA in the brain
For each brain region of each each subject VOI kinetic analysis yielded distribution volumes (DVs) (mL/mL) as listed on Table VIII. To examine the DV as a function of recent nicotine exposure, we separated the subjects in high and low plasma nicotine groups utilizing the median value for plasma nicotine as the dividing point. For each brain region VOI kinetic analysis yielded distribution volumes (DVs) (mL/mL) (mean ± standard error of the mean (SEM)) for the six subjects with high and the six subjects with low plasma nicotine levels as listed on Table IX. Then we performed the Sign Test (Conover, 1980) and the Mann-Whitney U Test (Conover, 1980) on the VOIs and status as high or low nicotine plasma level. The effect size of the Mann-Whitney U statistic (Rosenthal, 1991) was also computed (SPSS Inc., 2007). Utilizing the one-tailed Sign Test, high plasma nicotine levels were associated with low receptor binding in all regions except the fusiform gyrus, the hippocampus, the parahippocampus, and the pons (SPSS Inc., 2007). Utilizing the Mann-Whitney U Test, high plasma nicotine levels were associated with low receptor binding in all regions except the thalamus (SPSS Inc., 2007). Please refer to Table IX. Peak thalamic binding occurred at 2.5 h and reversible binding with substantial dissociation by 6 h is demonstrated by time activity curves. Thus, 5-[123I]IA appears to label nAChRs in humans (Musachio et al., 2001; Wong et al., 2001).
TABLE VIII.
Distribution volumes (DVs) in mL/mL of (S)-5-[123I]iodo-3-(2-azetidinylmethoxy)pyridine (5-[123I]5IA) in the brain regions of twelve healthy adult volunteers
| Brain region\Subject number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cerebellum | 8.71 | 11.10 | 9.88 | 5.40 | 12.40 | 16.17 | 19.56 | 24.89 | 19.98 | 26.87 | 13.64 | 31.09 |
| Caudate head | 13.69 | 14.15 | 13.05 | 7.75 | 18.10 | 21.94 | 33.19 | 23.69 | 20.21 | 40.99 | 13.99 | 33.97 |
| Cingulate gyrus | 12.58 | 14.07 | 12.41 | 7.55 | 19.17 | 21.36 | 31.88 | 23.45 | 19.40 | 39.39 | 13.23 | 32.10 |
| Cortex | 12.80 | 15.30 | 13.01 | 7.62 | 18.26 | 20.87 | 29.15 | 21.84 | 18.71 | 37.44 | 13.89 | 35.41 |
| Frontal lobe | 9.60 | 10.62 | 8.56 | 5.46 | 12.83 | 16.24 | 21.57 | 19.94 | 17.99 | 27.91 | 12.07 | 29.13 |
| Fusiform gyrus | 9.34 | 11.08 | 9.58 | 5.68 | 12.70 | 15.72 | 24.07 | .1 | .1 | 29.46 | 11.19 | 27.46 |
| Hippocampus | 13.51 | 16.62 | 14.45 | 8.32 | 21.71 | 22.68 | 34.98 | .1 | .1 | 46.11 | 14.56 | 35.45 |
| Pons | 15.12 | 20.44 | 20.44 | 9.23 | 25.18 | 26.54 | 32.85 | .1 | .1 | 46.39 | 16.71 | 45.47 |
| Occiptal lobe | 7.00 | 7.99 | 7.15 | 4.17 | 8.61 | 11.37 | 19.46 | 17.13 | 14.25 | 23.82 | 9.66 | 21.92 |
| Parietal lobe | 9.78 | 10.88 | 8.71 | 5.17 | 11.80 | 15.81 | 23.94 | 19.24 | 17.04 | 30.48 | 10.84 | 28.20 |
| Parahippocampus | 10.44 | 13.05 | 11.21 | 6.61 | 16.41 | 18.26 | 26.10 | .1 | .1 | 33.92 | 13.04 | 30.45 |
| Temporal lobe | 8.20 | 9.41 | 8.39 | 4.48 | 11.36 | 12.20 | 20.13 | 19.32 | 14.97 | 22.26 | 10.41 | 27.89 |
| Thalamus | 27.50 | 29.24 | 23.21 | 15.04 | 31.57 | 42.25 | 53.84 | 62.14 | 35.80 | 118.47 | 23.39 | 60.88 |
| Putamen | 14.13 | 17.04 | 15.63 | 8.74 | 20.21 | 23.84 | 33.44 | 23.96 | 20.74 | 45.13 | 16.02 | 39.33 |
A period (.) indicates missing data.
TABLE IX.
Median distribution volumes (DVs) in mL/mL of (S)-5-[123I]iodo-3-(2-azetidinylmethoxy)pyridine (5-[123I]5IA) in the brain regions of six healthy adult volunteers with low plasma nicotine levels and six healthy adult volunteers with high plasma nicotine levels,, probability values of one-tailed exact Sign Tests of significance, Mann-Whitney U statistics, probability values of exact tests of significance of Mann-Whitney U statistics, and effect sizes of Mann-Whitney U statistics for volumes of interest (VOIs)
| Brain region | Median DV of low plasma nicotine group | Median DV of high plasma nicotine group | Exact one- tailed sign test of significance P value < (SPSS, 2007) | Mann- Whitney U statistic (SPSS, 2007) | Mann- Whitney U statistic exact test of significance P value < (SPSS, 2007) | Mann- Whitney U statistic effect size estimator (Rosenthal, 1991, page 19; SPSS, 2007)) |
|---|---|---|---|---|---|---|
| Caudate head | 21.94 | 13.69 | 0.031 | 5.00 | 0.048 | −0.16 |
| Cerebellum | 19.56 | 9.88 | 0.031 | 5.00 | 0.048 | −0.16 |
| Cingulate cortex | 21.36 | 12.58 | 0.031 | 5.00 | 0.048 | −0.16 |
| Cortex | 20.87 | 13.00 | 0.031 | 5.00 | 0.048 | −0.16 |
| Frontal cortex | 17.99 | 9.60 | 0.031 | 4.00 | 0.030 | −0.17 |
| Fusiform gyrus | 19.90 | 9.46 | 0.125 | 0.00 | 0.010 | −0.20 |
| Hippocampus | 28.83 | 13.98 | 0.125 | 1.00 | 0.019 | −0.18 |
| Occiptal cortex | 14.25 | 7.15 | 0.031 | 4.00 | 0.030 | −0.17 |
| Parahippocampus | 22.18 | 10.83 | 0.125 | 1.00 | 0.019 | −0.18 |
| Parietal cortex | 17.04 | 9.78 | 0.031 | 5.00 | 0.048 | −0.16 |
| Pons | 29.70 | 17.78 | 0.125 | 2.00 | 0.038 | −0.16 |
| Putamen | 23.84 | 15.63 | 0.031 | 5.00 | 0.048 | −0.16 |
| Temporal cortex | 14.97 | 8.39 | 0.031 | 4.00 | 0.030 | −0.17 |
| Thalamus | 42.25 | 27.50 | 0.031 | 8.00 | 0.149 | −0.17 |
The efficacy of 5-[123I]IA as a nAChR radioligand in the living human brain is indicated by good brain uptake and a distribution profile consistent with nAChR density. After the intravenous bolus administration of 5-[123I]IA to a healthy 35-year-old male nonsmoker (Subject 1), the SPECT images of the nAChRs are illustrated in the cerebral cortex and the thalamus in Figure 4 and in the temporal lobes and the cerebellum in Figure 5.
Fig. 4.
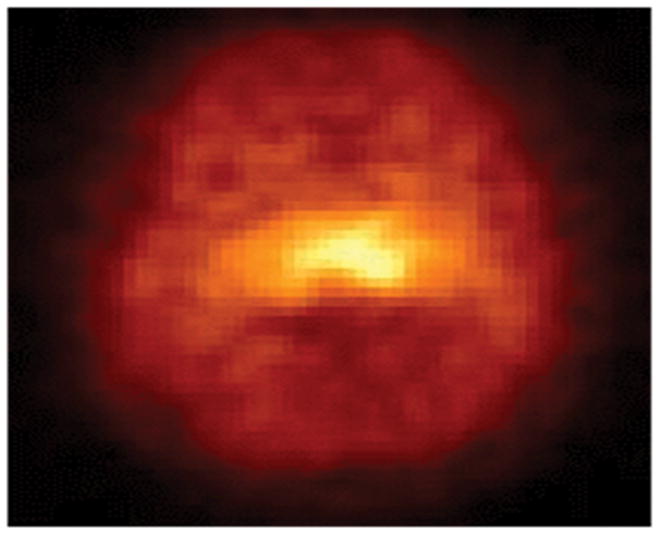
Single photon emission computed tomography (SPECT) images of the high-affinity α4β2* neuronal nicotinic acetylcholine receptors (nAChRs) in the cerebral cortex, thalamus, temporal lobes, and cerebellum on a transverse section through the thalamus after the intravenous bolus administration of 8.1 mCi (S)-5-[123I]iodo-3-(2-azetidinylmethoxy)pyridine (5-[123I]IA) to a healthy 35-year-old male nonsmoker (Subject 1). The anterior part of the brain is on top of the image. The right side of the brain is illustrated on the left side of the image.
Fig. 5.
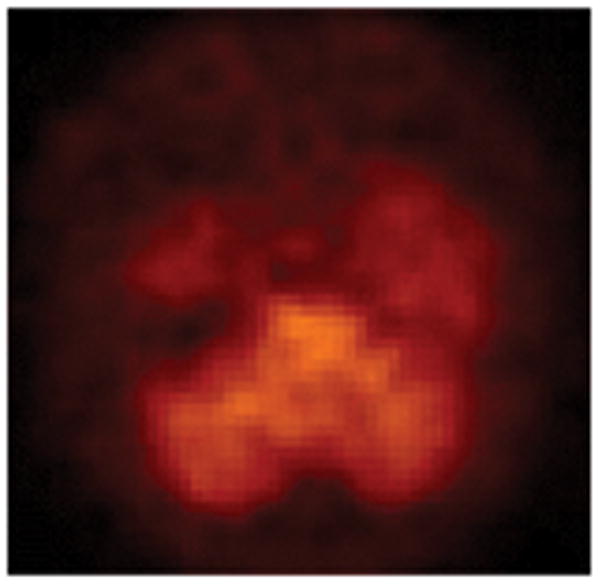
Single photon emission computed tomography (SPECT) images of the high-affinity α4β2* neuronal nicotinic acetylcholine receptors (nAChRs) in the cerebral cortex, the thalamus, the temporal lobes, and the cerebellum on a transverse section through the cerebellum after the intravenous bolus administration of 8.1 mCi (S)-5-[123I]iodo-3-(2-azetidinylmethoxy)pyridine (5-[123I]IA) to a healthy 35-year-old male nonsmoker (Subject 1). The anterior part of the brain is on top of the image. The right side of the brain is illustrated on the left side of the image.
Peak thalamic uptake occurred approximately 140 minutes after injection. Total volumes of distribution (DVs) were 28.23 in the thalamus and 13.06 in the cerebellum (Musachio et al., 2001). Maximal cerebellar uptake of 5-[123I]IA occurred 40 minutes after injection of the radiotracer. Clearance in the thalamus (t2 = 117 minutes) is slower than in cerebellum (t2 = 64 minutes). Figure 6 illustrates parametric images of the nAChRs at three time points after the administration of 5-[123I]IA to a 35-year-old male non-smoker (Subject 1).
Fig. 6.
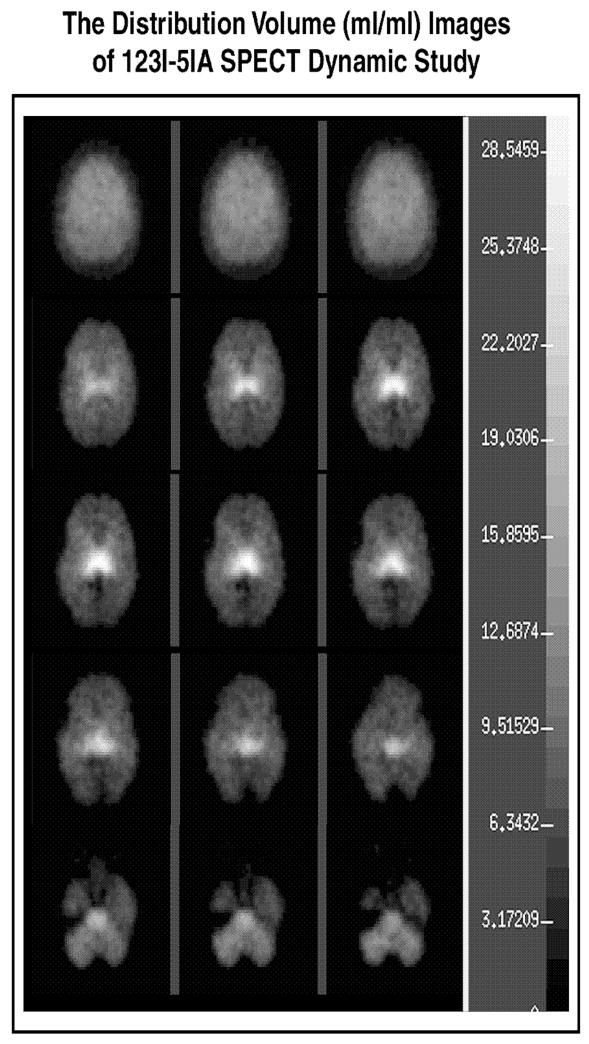
Parametric single photon emission computed tomography (SPECT) images of the high-affinity α4β2* neuronal nicotinic acetylcholine receptors (nAChRs) in the cerebral cortex, the thalamus, the temporal lobes, and the cerebellum on a transverse sections through the brain at three time points (columns) after the intravenous bolus administration of 8.1 mCi (S)-5-[123I]iodo-3-(2-azetidinylmethoxy)pyridine (5-[123I]IA) to a healthy 35-year-old male nonsmoker (Subject 1). The superior part of the brain is on top of the image. The right side of the brain is illustrated on the left side of the image.
Simultaneous arterial and venous blood samples of radiotracer and metabolites demonstrated that an equilibrium in the distribution volume of 5-[123I]IA was attained in arterial and venous samples at 300 minutes.
Safety of 5-[123I]5IA in humans
Electrocardiogram, blood pressure, and pulse did not change for any subject during any study. Therefore, no subject demonstrated any adverse physiological effect of 5-[123I]IA. Subjects displayed no abnormalities of behavior or movement before or after the administration of 5-[123I]IA (Brasic et al., 2001).
In summary, there were no physical, physiological, behavioral, emotional, psychological, or movement abnormalities (Brasic et al., 2001) before or after the administration of 5-[123I]5IA (Wong et al., 2001). Furthermore, high plasma nicotine level was significantly associated with low 5-[123I]IA binding in (1) the caudate head, the cerebellum, the cortex, and the putamen, utilizing both the Sign and the Mann-Whitney U tests, (2) the fusiform gyrus, the hippocampus, the parahippocampus, and the pons utilizing the Mann-Whitney U test, and (3) the thalamus utilizing the Sign test.
DISCUSSION
Twelve healthy adults, 6 smokers, 2 nonsmokers with secondhand smoke exposure, and 4 nonsmokers without secondhand smoke exposure, were studied with SPECT for five hours after the intravenous administration of 5-[123I]IA. No subject demonstrated adverse effects of 5-[123I]IA during the monitoring for the first 24 hours after the administration of 5-[123I]IA or on subsequent follow-up visits. Thus, we conclude that 5-[123I]IA is a safe, effective agent to administer to human subjects to estimate the high-affinity α4/β2 nAChRs in the living human brain and to discriminate humans with and without recent exposure to nicotine.
All subjects were categorized in low or high plasma nicotine level groups using the median plasma nicotine level obtained on the day of scan as the midpoint. High plasma nicotine level was associated with low 5-[123I]IA binding in (1) the caudate head, the cerebellum, the cingulate cortex, the cortex, the frontal cortex, the occipital cortex, the parietal cortex, the putamen, and the temporal cortex utilizing both the one-tailed Sign test and the Mann-Whitney U test, (2) the fusiform gyrus, the hippocampus, the parahippocampus, and the pons utilizing the Mann-Whitney U test, but not the one-tailed Sign test, and (3) the thalamus utilizing the one-tailed Sign test, but not the Mann-Whitney U test (SPSS Inc., 2007) (See Table IX). Since high plasma nicotine levels indicate recent exposure to nicotine, people with high plasma nicotine levels apparently have high intrasynaptic nicotine and high nAChR binding with nicotine so few nAChRs are unoccupied when the radiotracer is administered. However, up-regulation and down-regulation of nAChRs cannot be determined by this study because these subjects apparently had recent exposure to nicotine. People apparently must abstain from nicotine for at least seven days in order to estimate the density of unoccupied nicotinic receptors without the effect of residual nicotine (Staley et al., 2006). Thus, these findings confirm the efficacy of 5-[123I]IA to bind to nAChRs in the living human brain. Chronic exposure to nicotine is known to sensitize nicotinic AChRs (Buisson and Bertrand, 2001; Wonnacott, 1990) so this is an expected finding (Table VIII). The selective binding in particular parts of the brain in this study is consistent with animal findings. In rats chronic nicotinic exposure caused up-regulation of α4β2* nAChRs in many brain regions except habenulopeduncular structures, particular thalamic nuclei, and many brainstem areas (Nguyen et al., 2003). In a baboon, acute treatment with (−)-nicotine led to reductions in α4β2* nAChR uptake of 32% in the frontal cortex and of 52 % in the thalamus (Kassiou et al., 2001), while chronic treatment with (−)-nicotine 2.0 mg/kg//24h for 14 days produced increments in volume of distribution by 52% in the thalamus and 50% in the cerebellum seven days after the stop of the treatment (Kassiou et al., 2001). Therefore, in the baboon acute treatment with (−)-nicotine produces occupancy of α4β2* nAChRs and chronic treatment produces upregulation of the receptors (Kassiou et al., 2001). Thus, we conclude that 5-[123I]IA is an effective agent to identify the density and the distribution of α4β2* nAChRs in the living human brain of smokers and nonsmokers.
Therefore, we have demonstrated the safety of 5-[123I]IA to identify the density and the distribution of α4β2* nAChRs in the living human brain during a five-hour SPECT evaluation of healthy adults including smokers and nonsmokers.
These results are the basis to study the safety and the efficacy of 5-[123I]IA in other populations, including healthy adults who are heavy smokers, senior citizens, children, and adolescents. Additionally future investigations of subjects with neuropsychiatric disorders that are hypothesized to involve nicotinic acetylcholine dysfunction (Mihailescu and Drucker-Colin, 2000), including Parkinson’s disease (Durany et al., 2000; Fujita et al., 2006), schizophrenia (Durany et al., 2000; Leonard et al., 1998, 1999), Tourette’s disorder (Dursun and Reveley, 1996, 1997; Sanberg et al., 1997; Shytle et al., 2000; Silver et al., 1996.), Rett’s disorder, Alzheimer’s disease (O’Brien et al., 2007; Wong, et al., 2002a), and alcohol dependence (Wong, et al., 2003).
We note that incidentally two of the healthy controls actually had marked secondhand smoke exposure. This serendipitous occurrence provides the means to test 5-[123I]IA SPECT as a tool to estimate the density and the distribution of high-affinity α4β2* nAChRs in a small number of healthy adults who experience environmental exposure to cigarette smoke. Secondhand smoke exposure is a devastating public health problem resulting in marked pulmonary morbidity and cardiovascular mortality (Eisner et al., 2007). Reduction in secondhand smoke exposure results in reduction in the risk of acute myocardial infarction (Bruintjes and Krantz, 2007; Stranges et al., 2007). Additionally exposure to secondhand smoke is associated with reductions in the health-related quality of life of nonsmokers, particularly women (Bridevaux et al., 2007). Victims of secondhand smoking, particularly children, may be unaware of secondhand smoke exposure. Also victims of secondhand smoke exposure may be unaware of the extent of the alterations of the central nervous system induced by secondhand smoke exposure.
Multiple neuronal nAChRs and agonists for nAChRs (Schapira et al., 2002), including a variety developed by means of computer technology (Nicolotti et al., 2001), may merit clinical trials for the treatment of neuropsychiatric disorders associated with dysfunction of nAChRs (Bordia et al., 2006; Nicolotti et al., 2001). Therefore, estimation of the density and the distribution of α4/β2 nAChRs by means of SPECT after the administration of 5-[123I]IA before, during, and after the administration of potential therapeutic interventions for neuropsychiatric disorders will likely be a useful diagnostic and therapeutic guage.
Utilizing 2-[18F]FA, Brody and colleagues (2006) demonstrated that in human smokers inhalation of one or two mouthfuls of cigarette smoke led to uptake in half the α4β2* nAChRs and that uptake of half the α4β2* nAChRs occurred with plasma nicotine levels of 0.87 ng/mL (Brody et al., 2006). Additionally PET with 2-[18F]FA demonstrated greater regional uptake in the brainstem and the cerebellum in smokers than in nonsmokers (Wullner et al., 2007). PET with 2-[18F]FA is a promising agent to quantify the density and the distribution of α4β2* nAChRs in humans. However, the limited availability of PET restrict the usefulness 2-[18F]FA imaging in the general population.
Other pharmacological agents also offer promise estimate the density and the distribution of α4/β2 nAChRs in the living human brain. Although [11C](S)-(−)-nicotine has been used in the past in an attempt to visualize human nAChRs, this radiotracer suffers from a high degree of non-specific binding that makes it far from ideal as a specific nAChR probe (Scheffel et al., 2000; Villemagne et al., 1998). [79Br]Norchlorobromoepibatidine (Kassiou et al., 2002) and [18F]norchloro-fluoro-homoepibatidine are being evaluated as a specific high-affinity radioligands for nAChRs (Brust et al., 2008). However, the toxic effects of epibatidine and its congeners may prohibit its use in humans (Horti et al., 2000; Rueter et al., 2006; Scheffel et al., 2000). A-85380 in the form of 2-[18F]fluoro-3-(2(S)-azetidinylmethoxy)pyridine (2-[18F]FA) has been demonstrated to be a safe and efficacious agent to visualize high affinity β4β2* nAChRs in monkeys (Chefer et al., 1999; Vaupel et al., 2005), baboons (Valette et al., 1999), and humans (Bottlaender et al., 2003; Gallezot et al., 2005; Kimes et al., 2003; Mitkovski et al., 2005; Schildan et al., 2007; Sorger et al., 2007).
In nonsmokers uptake of 5-[123I]IA is comparable in men and women across the estrous cycle (Cosgrove et al., 2007). There are no differences in 5-[123I]IA uptake in women in follicular and luteal phases of their estrous cycles (Cosgrove et al., 2007). Uptake of 5-[123I]IA is inversely proportional to age in healthy humans with the most marked reductions in the thalamus, frontal cortex, parietal cortex, and anterior cingulate (Mitsis et al., 2007). In ten healthy nonsmokers the administration of 5-[123I]IA by bolus followed by continuous infusion produced reliable stability of 5-[123I]IA time-activity curves in brain and plasma at 5 hours after the beginning of the infusion (Staley et al., 2005).
5-[123I]IA has been a valuable agent to monitor the density and distribution of α4β2* nAChRs in people with a variety of neuropsychiatric disorders. Uptake of 5-[123I]IA is reduced in the pons and both frontal and both striatal region in addition to the right medial temporal region of people with Alzheimer’s disease (AD) (O’Brien et al., 2007). Furthermore uptake of 5-[123I]IA in people with Parkinson’s disease (PD) without dementia is reduced in cortical and subcortical regions (Fujita et al., 2006) and in the brainstem and frontal cortex (Oishi et al., 2006, 2007). Furthermore, upregulation of nAChRs occurred in smokers subsiding after 21 days of smoking abstinence by means of scans after the administration of 5-[123I]IA (Ishizu et al., 2004; Mamede et al., 2007).
Limitations of this study include the inclusion of subjects with variable nicotine exposure. Except for subject 7, all the smokers were apparently extremely light smokers so there were limited differences in nicotine exposure between the smokers and the nonsmokers. Additionally subjects 4 and 10 were nonsmokers with considerable environmental exposure to nicotine through second-hand cigarette smoke. Rating scales to determine nicotine dependence, i.e., the Fagerstrom Test for Nicotine Dependence (FTND) (Balfour and Fagerstrom, 1996) and the Tobacco Dependence Questionnaire (TDQ) (Kawakami et al., 1999), yielded low scores for most subjects (Table V) to confirm that none of the subjects exhibited nicotine dependence. The differentiation of subjects with and without nicotine exposure was difficult to determine. Likely all participants in the study had minimal to low nicotine exposure. Additional research with larger sample sizes of nonsmokers with and without secondhand smoke exposure and smokers, both light and heavy will facilitate the confirmation of the findings of this pilot study. We finally concluded that plasma nicotine level is the best indication of recent nicotine exposure regardless of the source of nicotine (Table VII). High plasma nicotine was associated with low binding for 5-[123I]5IA in all brain regions studied, particularly the caudate head, the cerebellum, the cortical regions, and the putamen (Table IX). These associations likely reflect acute recent exposure to nicotine. Utilization of heavy smokers and nonsmokers in future studies will likely produce more marked contrasts in nicotine exposures. Utilization of heavy smokers with long histories of cigarette smoking will likely provide the basis to study chronic nicotine exposure in the human brain. In sum, this study was limited by the small sample sizes and the variability in radiotracer administration and nicotine exposure. This preliminary study suggests the SPECT with 5-[123I]IA is a safe, effective tool to estimate the density and the distribution of α4β2* nAChRs in the brains of smokers as well as nonsmokers with and without secondhand smoke exposure.
Future research is needed to compare and contrast the safety and efficacy of 5-[123I]IA and other α4β2* nAChR radiotracers, including 2-[18F]fluoro-3-[2-((S)-3-pyrrolinyl)methoxy]pyridine, and [18F]6-chloro-3-((2-(S)-azetidinyl)methoxy)-5-(2-fluoropyridin-4-yl)pyridine (NIDA522131) (Chefer et al., 2008), and [18F]nifene (Easwaramoorthy et al., 2007).
Additionally, we hypothesize that the visualization of neuronal nAChRs utilizing nuclear neuroimaging techniques (Wong and Brašić, 2001) will facilitate the development of pharmacological interventions for Rett’s disorder, Alzheimer’s disease (Wong et al., 2002a), Tourette’s disorder, and other neuropsychiatric disorders (Wong et al., 2002b). Since nicotine increases α4β2* nAChRs in the striata of monkeys, 5-[123I]IA and 2-[18F]FA neuronuclear imaging may provide the tools to monitor beneficial effects of treatment of individuals with neuropsychiatric disorders with nicotine and nicotine agonists (Bordia et al., 2006). Since cognitive deficits in laboratory animals are associated with reductions in nAChRs in the hippocampus (Pocivavsek et al., 2006), experiments to look for reductions in nAChR density in the hippocampus and related limbic structures in schizophrenia and other neuropsychiatric disorders with cognitive deficits may be useful. Possible therapeutic agents include those with favorable effects for young monkeys such as nAChRs for α4β2* receptors, including ((R)-5-(2-azetidinylmethoxy)-2-chloropyridine (ABT-594), and for α7 receptors, including 2-methyl-5-(6-phenyl-pyridazin-3-yl)-octahydro-pyrrolo[3,4-c]pyrrole (A-582491) (Buccafusco et al., 2007).
The finding that uptake of 5-[123I]IA is reduced in the cerebellum and temporal, parietal and occipital cortices in people with Parkinson’s disease (PD) who receive high daily doses of dopamine agonists (Oishi et al., 2006, 2007) merits development in other patient populations treated with dopamine agonists including restless legs syndrome and migraine headaches. 5-[123I]IA can be administered at baseline, during, and after the course of treatment of people with PD and other neuropsychiatric disorders with dopamine agonists, dopamine antagonists, and related agents to determine the occupancy of α4/β2* nAChRs. Additionally, 5-[123I]IA SPECT offers promise as an objective measurement tool to monitor the progression of patients with PD for clinical purposes.
Since people with autosomal dominant nocturnal frontal lobe epilepsy (ADNFLE) exhibit greater uptake of 2-[18F]FA in mesencephalon, the pons, and the cerebellum than healthy adults (Picard et al., 2006), 2-[18F]FA PET and 5-[123I]IA SPECT will likely represent promising tools to identify progression and remission of the disorder during clinical trials.
Although treatment with donepezil, an anticholinesterase inhibitor, produced no change in α4β2* nAChR uptake with 5-[123I]IA SPECT in small samples of patients with Alzheimer’s disease (AD) and diffuse Lewy body (DLB) disease (Colloby et al., 2008), other acetylcholine modulators likely will demonstrate beneficial effects in clinical trials. Additionally, 5-[123I]IA SPECT offers promise as an objective measurement tool to monitor the progression of patients with AD and related dementias for clinical purposes.
Furthermore, since A-85380 is an analgesic, A-85380 may be developed for the treatment of pain conditions (Rueter et al., 2006). Then, 5-[123I]IA and other radiotracers for nAChRs may be useful to determine the possible dysfunction of α4β2* nAChRs in patients with chronic pain before, during, and after treatments with A-85380 and other analgesics.
The similarity of A-85380 to antidepressants in mice and rats suggests the possible therapeutic role of A-85380 for human depression (Rueter et al., 2006). Then, 5-[123I]IA and other radiotracers for nAChRs may be useful to determine the possible dysfunction of α4β2* nAChRs in patients with depression and related disorders before, during, and after treatments with A-85380 and other antidepressants..
Genetic studies provide evidence that genetic interaction between α4 and β2 subunits of the high affinity nicotinic receptor may be linked to schizophrenia. In combination CHRNA4 and CHRNB2 genes, monitors of the expression of neuronal high-affinity nicotinic receptors, may be linked to schizophrenia (De Luca et al., 2006). In people with schizophrenia heavy cigarette smoking daily was associated with the CHRNA4 rs3746372 allele 1 (Voineskos et al., 2007). Heavy cigarette smoking in people with schizophrenia is associated with the intragenic interaction between rs3787116 and rs3746372 (Voineskos et al., 2007). These findings suggest a genetic basis for heavy cigarette smoking in people with schizophrenia (Voineskos et al., 2007). Additionally utilization of 5-[123I]IA SPECT will likely constitute a useful tool to monitor the effects of interventions for schizophrenia and nicotine dependence. In contrast to other nuclear neuroimaging studies of α4β2* nAChRs, we have utilized an optimal method on quantitative modeling (Zhou et al., 2001) to demonstrate the safety, efficacy, and tolerability of 5-[123I]IA SPECT in smokers as well as in nonsmokers.
Acknowledgments
The preparation of this manuscript is supported by the Essel Foundation, the National Alliance for Research on Schizophrenia and Depression (NARSAD), the National Institutes of Health (NIH), and the Tourette Syndrome Association (TSA), Inc.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 2000. Text Revision (DSM-IV-TR™) [Google Scholar]
- Balfour DJK, Fagerström KO. Pharmacology of nicotine and its therapeutic use in smoking cessation and neurodegenerative disorders. Pharmacol Ther. 1996;72:51–81. doi: 10.1016/s0163-7258(96)00099-x. [DOI] [PubMed] [Google Scholar]
- Baldwin RM, Zoghbi SS, Staley JK, Brenner E, Al-Tikriti MS, Amici L, Fujita M, Innis RB, Tamagnan G. Chemical fate of the nicotinic acetylcholinergic radiotracer [123I]5-IA-85380 in baboon brain and plasma. Nucl Med Biol. 2006;33:549–554. doi: 10.1016/j.nucmedbio.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Benwell MEM, Balfour DJK, Anderson JM. Evidence that tobacco smoking increases the density of (−)-[3H]nicotine binding sites in human brain. J Neurochem. 1988;50:1243–1247. doi: 10.1111/j.1471-4159.1988.tb10600.x. [DOI] [PubMed] [Google Scholar]
- Bordia T, Parameswaran N, Fan H, Langston JW, McIntosh JM, Quik M. Partial recovery of striatal nicotinic receptors in 1-methy-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-lesioned monkeys with chronic oral nicotine. J Pharmacol Exp Ther. 2006;319:285–292. doi: 10.1124/jpet.106.106997. [DOI] [PubMed] [Google Scholar]
- Bottlaender M, Valette H, Roumenov D, Dolle F, Coulon C, Ottaviani M, Hinnen F, Ricard M. Biodistribution and radiation dosimetry of 18F-fluoro-A-85380 in healthy volunteers. J Nucl Med. 2003;44:596–601. [PubMed] [Google Scholar]
- Brašić JR. Treatment of movement disorders in autism spectrum disorders. In: Hollander E, editor. Autism Spectrum Disorders. Volume 24 of the Medical Psychiatry Series. New York: Marcel Dekker, Inc. ; 2003. pp. 273–346. http://www.dekker.com. [Google Scholar]
- Brasic JR, Bronson B, Chun TT. Tardive dyskinesia. eMedicine Journal. 2007a;8(3) http://www.emedicine.com/neuro/topic362.htm.
- Brasic JR, Musachio JL, Zhou Y, Cascella N, Nestadt G, Osman M, Gay O, Hilton J, Kuwabara H, Crabbe A, Rousset O, Fan H, Al-Humadi MA, Al-Humadi BA, Arkles JS, Thompson T, Kalaff A, Smith J, Reinhardt MJ, Dogan AS, Ford B, Wong DF. Neuropsychiatric assessment of [123I]iodo-3-(2 azetidinylmethoxy)pyridine. Mov Disord. 2001;16(supplement 1):S54. [abstract] [Google Scholar]
- Brasic JR, Wong D, Eroglu A. PET scanning in autism spectrum disorders. Emedicine Journal. 2007b;8(3) http://www.emedicine.com/neuro/topic440.htm.
- Breese CR, Lee MJ, Adams CE, Sullivan B, Logel J, Gillen KM, Marks MJ, Collins AC, Leonard S. Abnormal regulation of high affinity nicotinic receptors in subjects with schizophrenia. Neuropsychopharmacology. 2000;23:351–364. doi: 10.1016/S0893-133X(00)00121-4. [DOI] [PubMed] [Google Scholar]
- Breese CR, Marks MJ, Logel J, Adams CE, Sullivan B, Collins AC, Leonard S. Effect of smoking history on [3H]nicotine binding in human postmortem brain. J Pharmacol Exp Ther. 1997;282:7–13. [PubMed] [Google Scholar]
- Bridevaux P-O, Cornuz J, Gaspoz J-M, Burnand B, Ackermann-Liebrich U, Schindler C, Leuenberger P, Rochat T, Gerbase MW SAPALDIA Team. Secondhand smoke and health-related quality of life in never smokers: results from the SAPALDIA Cohort Study 2. Arch Intern Med. 2007;167:2516–2523. doi: 10.1001/archinte.167.22.2516. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Olmstead RE, Farahi J, Scheibal D, Jou J, Allen V, Tiongson E, Chefer SI, Koren AO, Mukhin AG. Cigarette smoking saturates brain α4β2 nicotinic acetylcholine receptors. Arch Gen Psychiatry. 2006;63:907–915. doi: 10.1001/archpsyc.63.8.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruintjes G, Krantz MJ. Acute vs chronic secondhand smoke exposure. Arch Intern Med. 2007;167:731. doi: 10.1001/archinte.167.7.731-a. [letter] [DOI] [PubMed] [Google Scholar]
- Brust P, Patt JT, Deuther-Conrad W, Becker G, Patt M, Schildan A, Sorger D, Kendziorra K, Meyer P, Steinbach J, Sabri O. In vivo measurement of nicotinic acetylcholine receptors with [18F]norchloro-fluoro-homoepibatidine. Synapse. 2008;62:205–218. doi: 10.1002/syn.20480. [DOI] [PubMed] [Google Scholar]
- Buccafusco JJ, Terry AV, Jr, Decker MW, Gopalakrishnan M. Profile of nicotinic acetylcholine receptor agonists ABT-594 and A-582941, with differential subtype selectivity, on delayed matching accuracy by young monkeys. Biochem Pharmacol. 2007;74:1202–1211. doi: 10.1016/j.bcp.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Buisson B, Bertrand D. Chronic exposure to nicotine upregulates the human α4β2 nicotinic acetylcholine receptor function. J Neurosci. 2001;21:1819–1829. doi: 10.1523/JNEUROSCI.21-06-01819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chefer SI, Horti AG, Koren AO, Gundisch D, Links JM, Kurian V, Dannals RF, Mukhin AG, London ED. 2-[18F]F-A-85380: a PET radioligand for α4β2 nicotinic acetylcholine receptors. NeuroReport. 1999;10:2715–2721. doi: 10.1097/00001756-199909090-00005. [DOI] [PubMed] [Google Scholar]
- Chefer SI, Horti AG, Lee KS, Koren AO, Jones DW, Gorey JG, Links JM, Mukhin AG, Weinberger DR, London ED. In vivo imaging of brain nicotinic acetylcholine receptors with 5-[123I]iodo-A-85380 using single photon emission computed tomography. Life Sci. 1998;63:PL355–PL360. doi: 10.1016/s0024-3205(98)00514-1. [DOI] [PubMed] [Google Scholar]
- Chefer SI, Pavlova OA, Zhang Y, Vaupel DB, Kimes AS, Horti AG, Stein E, Mukhin AG. NIDA522131, a new radioligand for imaging extrathalamic nicotinic acetylcholine receptors: in vitro and in vivo evaluation. J Neurochem. 2008;104:306–315. doi: 10.1111/j.1471-4159.2007.05009.x. [DOI] [PubMed] [Google Scholar]
- Colloby SJ, Pakrasi S, Perry EK, Pimlott SL, Wyper DJ, Williams ED, McKeith IG, O’Brien JT. The effects of donepezil on nicotinic receptor status in dementia: a 123I-5IA-85380 SPECT study. J Neurol Neurosurg Psychiatry. 2008;79:485–487. doi: 10.1136/jnnp.2006.108209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover WJ. Practical Nonparametric Statistics. 2. New York: John Wiley & Sons; 1980. pp. 122–129.pp. 216–223. [Google Scholar]
- Cosgrove KP, Mitsis EM, Bois F, Frohlich E, Tamagnan GD, Krantzler E, Perry E, Maciejewski PK, Epperson CN, Allen S, O’Malley S, Mazure CM, Seibyl JP, van Dyck CH, Staley JK. 123I-5-IA-85380 SPECT imaging of nicotinic acetylcholine receptor availability in nonsmokers: effects of sex and menstrual cycle. J Nucl Med. 2007;48:1633–1640. doi: 10.2967/jnumed.107.042317. [DOI] [PubMed] [Google Scholar]
- Court JA, Lloyd S, Thomas N, Piggott MA, Marshall EF, Morris CM, Lamb H, Perry RH, Johnson M, Perry EK. Dopamine and nicotinic receptor binding and levels of dopamine and homovanillic acid in human brain related to tobacco use. Neuroscience. 1998;87:63–78. doi: 10.1016/s0306-4522(98)00088-8. [DOI] [PubMed] [Google Scholar]
- De Luca V, Voineskos S, Wong G, Kennedy JL. Genetic interaction between α4 and β2 subunits of high affinity nicotinic receptor: analysis in schizophrenia. Exp Brain Res. 2006;174:292–296. doi: 10.1007/s00221-006-0458-y. [DOI] [PubMed] [Google Scholar]
- Denckla MB. Revised Neurological Examination for Subtle Signs (1985) Psychopharmacol Bull. 1985;21(4):773–800. [PubMed] [Google Scholar]
- Deuther-Conrad W, Wevers A, Becker G, Schildan A, Patt M, Sabri O, Steinbach J, Brust P. Autoradiography of 2-[18F]F-A-85380 on nicotinic acetylcholine receptors in the porcine brain in vitro. Synapse. 2006;59:201–210. doi: 10.1002/syn.20232. [DOI] [PubMed] [Google Scholar]
- Ding Y-S, Liu N, Wang T, Marecek J, Garza V, Ojima I, Fowler JS. Synthesis and evaluation of 6-[18F]fluoro-3-(2(S)-azetidinylmethoxy)pyridine as a PET tracer for nicotinic acetylcholine receptors. Nucl Med Biol. 2000;27:381–389. doi: 10.1016/s0969-8051(00)00094-9. [DOI] [PubMed] [Google Scholar]
- Dollé F, Dolci L, Valette H, Hinnen F, Vaufrey F, Guenther I, Fuseau C, Coulon C, Bottlaender M, Crouzel C. Synthesis and nicotinic acetylcholine receptor in vivo binding properties of 2-fluoro-3-[2(S)-2-azetidinylmethoxy]pyridine: a new positron emission tomography ligand for nicotinic receptors. J Med Chem. 1999;42:2251–2259. doi: 10.1021/jm9910223. [DOI] [PubMed] [Google Scholar]
- Durany N, Zöchling R, Boissl KW, Paulus W, Ransmayr G, Tatschner T, Danielczyk W, Jellinger K, Deckert J, Riederer P. Human post-mortem striatal α4β2 nicotinic acetylcholine receptor density in schizophrenia and Parkinson’s syndrome. Neurosci Lett. 2000;287:109–112. doi: 10.1016/s0304-3940(00)01144-7. [DOI] [PubMed] [Google Scholar]
- Dursun SM, Reveley MA. The efficacy of a dose-escalated application of transdermal nicotine plus sulpiride in Tourette’s syndrome. Eur Psychiatry. 1996;11:204–206. doi: 10.1016/0924-9338(96)88392-1. [DOI] [PubMed] [Google Scholar]
- Dursun SM, Reveley MA. Differential effects of transdermal nicotine on microstructured analyses of tics in Tourette’s syndrome: an open study. Psychol Med. 1997;27:483–487. doi: 10.1017/s0033291796003984. [DOI] [PubMed] [Google Scholar]
- Easwaramoorthy B, Pichika R, Collins D, Potkin SG, Leslie FM, Mukherjee J. Effect of acetylcholinesterase inhibitors on the binding of nicotinic α4/β2 receptor PET tracer, 18F-nifene: a measure of acetylcholine competition. Synapse. 2007;61:29–36. doi: 10.1002/syn.20338. [DOI] [PubMed] [Google Scholar]
- Eisner MD, Wang Y, Haight TJ, Balmes J, Hammond SK, Tager IB. Secondhand smoke exposure, pulmonary function, and cardiovascular mortality. Ann Epidemiol. 2007;17:364–373. doi: 10.1016/j.annepidem.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Elazar Z, Paz M. Potentiation of haloperidol catalepsy by microinjections of nicotine into the striatum or pons in rats. Life Sci. 1999;64:1117–1125. doi: 10.1016/s0024-3205(99)00040-5. [DOI] [PubMed] [Google Scholar]
- Fujita M, Ichise M, Zoghbi SS, Liow J-S, Ghose S, Vines DC, Sangare J, Lu J-Q, Cropley VL, Iida H, Kim KM, Cohen RM, Bara-Jimenez W, Ravina B, Innis RB. Widespread decrease of nicotinic acetylcholine receptors in Parkinson’s disease. Ann Neurol. 2006;59:174–177. doi: 10.1002/ana.20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M, Seibyl JP, Vaupel DB, Tamagnan G, Early M, Zoghbi SS, Baldwin RM, Horti AG, Koren AO, Mukhin AG, Khan S, Bozkurt A, Kimes AS, London ED, Innis RB. Whole-body biodistribution, radiation absorbed dose, and brain SPET imaging with [123I]5-I-A-85380 in healthy human subjects. Eur J Nucl Med Mol Imaging. 2002;29:183–190. doi: 10.1007/s00259-001-0695-z. [DOI] [PubMed] [Google Scholar]
- Fujita M, Tamagnan G, Zoghbi SS, Al-Tikriti MS, Baldwin RM, Seibyl JP, Innis RB. Measurement of α4β2 nicotinic acetylcholine receptors with [123I]5-I-A-85380 SPECT. J Nucl Med. 2000;41:1552–1560. [PubMed] [Google Scholar]
- Gallezot J-D, Bottlaender M, Grégoire M-C, Roumenov D, Deverre J-R, Coulon C, Ottaviani M, Dolle F, Syrota A, Valette H. In vivo imaging of human cerebral nicotinic acetylcholine receptors with 2–18F-fluoro-A-85380 and PET. J Nucl Med. 2005;46:240–247. [PubMed] [Google Scholar]
- Gotti C, Fornasari D, Clementi F. Human neuronal nicotinic receptors. Prog Neurobiol. 1997;53:199–237. doi: 10.1016/s0301-0082(97)00034-8. [DOI] [PubMed] [Google Scholar]
- Gozzi A, Schwarz A, Reese T, Bertani S, Crestan V, Bifone A. Region-specific effects of nicotine on brain activity: a pharmacological MRI study in the drug-naive rat. Neuropsychopharmacology. 2006;31:1690–1703. doi: 10.1038/sj.npp.1300955. [DOI] [PubMed] [Google Scholar]
- Hilton J, Yokoi F, Dannals RF, Ravert HT, Szabo Z, Wong DF. Column-switching HPLC for the analysis of plasma in PET imaging studies. Nucl Med Biol. 2000;27:627–630. doi: 10.1016/s0969-8051(00)00125-6. [DOI] [PubMed] [Google Scholar]
- Horti AG, Chefer SI, Mukhin AG, Koren AO, Gundisch D, Links JM, Kurian V, Dannals RF, London ED. 6-[18F]fluoro-A-85380, a novel radioligand for in vivo imaging of central nicotinic acetylcholine receptors. Life Sci. 2000;67:463–469. doi: 10.1016/s0024-3205(00)00635-4. [DOI] [PubMed] [Google Scholar]
- Huang LZ, Winzer-Serhan UH. Chronic neonatal nicotine upregulates heteromeric nicotinic acetylcholine receptor binding without change in subunit mRNA expression. Brain Res. 2006;1113:94–109. doi: 10.1016/j.brainres.2006.06.084. [DOI] [PubMed] [Google Scholar]
- Hui X, Gao J, Xie X, Suto N, Ogiku T, Wang M-W. A robust homogeneous binding assay for α4β2 nicotinic acetylcholine receptor. Acta Pharmacol Sin. 2005;26:1175–1180. doi: 10.1111/j.1745-7254.2005.00202.x. [DOI] [PubMed] [Google Scholar]
- Ishizu K, Mamede M, Ueda M, Mukai T, Iida Y, Saji H, Saga T. Nicotinic acetylcholine receptors before and after cigarette withdrawal in smokers: quantitative 5IA-SPECT study. NeuroImage. 2004;22(supplement 2):T114. [abstract] [Google Scholar]
- Jacob P, III, Wilson M, Benowitz NL. Improved gas chromatographic method for the determination of nicotine and cotinine in biologic fluids. J Chromatogr. 1981;222:61–70. doi: 10.1016/s0378-4347(00)81033-6. [DOI] [PubMed] [Google Scholar]
- Kassiou M, Bottlaender M, Loc’h C, Dolle F, Musachio JL, Coulon C, Ottaviani M, Dannals RF, Maziere B. Pharmacological evaluation of a Br-76 analog of epibatidine: a potent ligand for studying brain nicotinic acetylcholine receptors. Synapse. 2002;45:95–104. doi: 10.1002/syn.10087. [DOI] [PubMed] [Google Scholar]
- Kassiou M, Eberl S, Meikle SR, Birrell A, Constable C, Fulham MJ, Wong DF, Musachio JL. In vivo imaging of nicotinic receptor upregulation following chronic (−)-nicotine treatment in baboon using SPECT. Nuc Med Biol. 2001;28:165–175. doi: 10.1016/s0969-8051(00)00206-7. [DOI] [PubMed] [Google Scholar]
- Kawakami N, Takatsuka N, Inaba S, Shimizu H. Developmental of a screening questionnaire for tobacco/nicotine dependence according to ICD-10, DSM-III-R, and DSM-IV. Addict Behav. 1999;24:155–166. doi: 10.1016/s0306-4603(98)00127-0. [DOI] [PubMed] [Google Scholar]
- Kimes AS, Horti AG, London ED, Chefer SI, Contoreggi C, Ernst M, Friello P, Koren AO, Kurian V, Matochik JA, Pavlova O, Vaupel DB, Mukhin AG. 2-[18F]F-A85380: PET imaging of brain nicotinic acetylcholine receptors and whole body distribution in humans. FASEB J. 2003;17:1331–1333. doi: 10.1096/fj.02–0492fje. Published online May 20, 2003. [DOI] [PubMed] [Google Scholar]
- Koren AO, Horti AG, Mukhin AG, Gündisch D, Kimes AS, Dannals RF, London ED. 2-, 5-, and 6-Halo-3-(2(S)-azetidinylmethoxy)pyridines: synthesis, affinity for nicotinic acetylcholine receptors, and molecular modeling. J Med Chem. 1998;41:3690–3698. doi: 10.1021/jm980170a. [DOI] [PubMed] [Google Scholar]
- Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996;4:153–158. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- Léna C, Changeux J-P. Allosteric nicotinic receptors, human pathologies. J Physiol Paris. 1998;92:63–74. doi: 10.1016/S0928-4257(98)80140-X. [DOI] [PubMed] [Google Scholar]
- Leonard S, Bertrand D. Neuronal nicotinic receptors: from structure to function. Nicotine Tob Res. 2001;3:203–223. doi: 10.1080/14622200110050213. [DOI] [PubMed] [Google Scholar]
- Leonard S, Breese CR, Lee MJ, Logel J, Adams CE, Freedman R. Abnormal regulation of high affinity nicotine receptors in schizophrenia. Schizophr Res. 1999;36(1–3):74. [abstract] [Google Scholar]
- Leonard S, Gault J, Adams C, Breese CR, Rollins Y, Adler LE, Olincy A, Freedman R. Nicotinic receptors, smoking and schizophrenia. Restor Neurol Neurosci. 1998;12:195–201. [PubMed] [Google Scholar]
- Mamede M, Ishizu K, Ueda M, Mukai T, Iida Y, Fukuyama H, Saga T, Saji H. Quantification of human nicotinic acetylcholine receptors with 123I-5IA SPECT. J Nucl Med. 2004;45:1458–1470. [PubMed] [Google Scholar]
- Mamede M, Ishizu K, Ueda M, Mukai T, Iida Y, Kawashima H, Fukuyama H, Togashi K, Saji H. Temporal change in human nicotinic acetylcholine receptor after smoking cessation: 5IA SPECT study. J Nucl Med. 2007;48:1829–1835. doi: 10.2967/jnumed.107.043471. [DOI] [PubMed] [Google Scholar]
- McCallum SE, Parameswaran N, Bordia T, Fan H, McIntosh JM, Quik M. Differential regulation of mesolimbic α3*/α6β2* and α4β2* nicotinic acetylcholine receptor sites and function after long-term oral nicotine to monkeys. J Pharmacol Exp Ther. 2006;318:381–388. doi: 10.1124/jpet.106.104414. [DOI] [PubMed] [Google Scholar]
- Mihailescu S, Drucker-Colín R. Nicotine, brain nicotinic receptors, and neuropsychiatric disorders. Arch Med Res. 2000;31:131–144. doi: 10.1016/s0188-4409(99)00087-9. [DOI] [PubMed] [Google Scholar]
- Mitkovski S, Villemagne VL, Novakovic KE, O’Keefe G, Tochon-Danguy H, Mulligan RS, Dickinson KL, Saunder T, Gregoire M-C, Bottlaender M, Dolle F, Rowe CC. Simplified quantification of nicotinic receptors with 2[18F]F-A-85380 PET. Nucl Med Biol. 2005;32:585–591. doi: 10.1016/j.nucmedbio.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Mitsis EM, Cosgrove KP, Staley JK, Frohlich EB, Bois F, Tamagnan GD, Estok KM, Seibyl JP, van Dyck CH. [123I]5-IA-85380 SPECT imaging of β2-nicotinic acetylcholine receptor availability in the aging human brain. Ann NY Acad Sci. 2007;1097:168–170. doi: 10.1196/annals.1379.015. [DOI] [PubMed] [Google Scholar]
- Mogg AJ, Jones FA, Pullar IA, Sharples CGV, Wonnacott S. Functional responses and subunit composition of presynaptic nicotinic receptor subtypes explored using the novel agonist 5-iodo-A-85380. Neuropharmacology. 2004;47:848–859. doi: 10.1016/j.neuropharm.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Moroni M, Zwart R, Sher E, Cassels BK, Bermudez I. α4β2 nicotinic receptors with high and low acetylcholine sensitivity: pharmacology, stoichiometry, and sensitivity to long-term exposure to nicotine. Mol Pharmacol. 2006;70:755–768. doi: 10.1124/mol.106.023044. [DOI] [PubMed] [Google Scholar]
- Mukhin AG, Gundisch D, Horti AG, Koren AO, Tamagnan G, Kimes AS, Chambers J, Vaupel DB, King SL, Picciotto MR, Innis RB, London ED. 5-Iodo-A-85380, an α4β2 subtype-selective ligand for nicotinic acetylcholine receptors. Mol Pharmacol. 2000;57:642–649. doi: 10.1124/mol.57.3.642. [DOI] [PubMed] [Google Scholar]
- Musachio JL, Brasic JR, Scheffel UA, Rauseo PA, Fan H, Osman M, Kellar KJ, Xiao Y, Hilton J, Zhou Y, Wong DF. Radiosynthesis, in vitro/in vivo pharmacologic characterization, and initial human SPECT imaging studies with [I-123] 5-Iodo-A-85380: a high affinity nicotinic acetylcholine receptor (nAChR) radioprobe. American Chemical Society Meeting. Abstracts of papers - American Chemical Society; Washington, District of Columbia: American Chemical Society; April 1, 2001. p. 183-NUCL. [abstract] [Google Scholar]
- Musachio JL, Scheffel U, Finley PA, Zhan Y, Mochizuki T, Wagner HN, Jr, Dannals RF. 5-[I-125/123]Iodo-3(2(S)-azetidinylmethoxy)pyridine, a radioiodinated analog of A-85380 for in vivo studies of central nicotinic acetylcholine receptors. Life Sci. 1998;62:PL351–PL357. doi: 10.1016/s0024-3205(98)00180-5. [DOI] [PubMed] [Google Scholar]
- Musachio JL, Villemagne VL, Scheffel UA, Dannals RF, Dogan AS, Yokoi F, Wong DF. Synthesis of an I-123 analog of A-85380 and preliminary SPECT imaging of nicotinic receptors in baboon. Nucl Med Biol. 1999;26:201–207. doi: 10.1016/s0969-8051(98)00101-2. [DOI] [PubMed] [Google Scholar]
- National Institute of Mental Health, Alcohol, Drug Abuse, and Mental Health Administration, Public Health Service, Department of Health, Education and Welfare. Abnormal Involuntary Movement Scale (AIMS) Psychopharmacol Bull. 1988;24(4):781–783. [PubMed] [Google Scholar]
- Newhouse PA, Kelton M. Nicotinic systems in central nervous systems disease: degenerative disorders and beyond. Pharm Acta Helv. 2000;74:91–101. doi: 10.1016/s0031-6865(99)00047-3. [DOI] [PubMed] [Google Scholar]
- Nguyen HN, Rasmussen BA, Perry DC. Subtype-selective up-regulation by chronic nicotine of high-affinity nicotinic receptors in rat brain demonstrated by receptor autoradiography. J Pharmacol Exp Ther. 2003;307:1090–1097. doi: 10.1124/jpet.103.056408. [DOI] [PubMed] [Google Scholar]
- Nicolotti O, Pellegrini-Calace M, Carrieri A, Altomare C, Centeno NB, Sanz F, Carotti A. Neuronal nicotinic receptor agonists: a multi-approach development of the pharmacophore. J Comput Aided Mol Des. 2001;15:859–872. doi: 10.1023/a:1013115717587. [DOI] [PubMed] [Google Scholar]
- O’Brien JT, Colloby SJ, Pakrasi S, Perry EK, Pimlott SL, Wyper DJ, McKeith IG, Williams ED. α4β2 nicotinic receptor status in Alzheimer’s disease using 123I-5IA-85380 single-photon-emission computed tomography. J Neurol Neurosurg Psychiatry. 2007;78:356–362. doi: 10.1136/jnnp.2006.108209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrzut SL, Koren AO, Mandelkern MA, Brody AL, Hoh CK, London ED. Whole-body radiation dosimetry of 2-[18F]fluoro-A-85380 in human PET imaging studies. Nucl Med Biol. 2005;32:869–874. doi: 10.1016/j.nucmedbio.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Oishi N, Hashikawa K, Yoshida H, Ishizu K, Ueda M, Kawashima H, Saji H, Fukuyama H. Quantification of nicotinic acetylcholine receptors in Parkinson disease with 123I-5IA SPECT. Mov Disord. 2006;21(supplement 15):S568. doi: 10.1016/j.jns.2007.02.014. [abstract] [DOI] [PubMed] [Google Scholar]
- Oishi N, Hashikawa K, Yoshida H, Ishizu K, Ueda M, Kawashima H, Saji H, Fukuyama H. Quantification of nicotinic acetylcholine receptors in Parkinson’s disease with 123I-5IA SPECT. J Neurol Sci. 2007;256:52–60. doi: 10.1016/j.jns.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- Paterson D, Nordberg A. Neuronal nicotinic receptors in the human brain. Prog Neurobiol. 2000;61:75–111. doi: 10.1016/s0301-0082(99)00045-3. [DOI] [PubMed] [Google Scholar]
- Perry DC, Dávila-Garcia MI, Stockmeier CA, Kellar KJ. Increased nicotinic receptors in brains from smokers: membrane binding and autoradiography studies. J Pharmacol Exp Ther. 1999;289:1545–1552. [PubMed] [Google Scholar]
- Picard F, Bruel D, Servent D, Saba W, Fruchart-Gaillard C, Schöllhorn-Peyronneau M-A, Roumenov D, Brodtkorb E, Zuberi S, Gambardella A, Steinborn B, Hufnagel A, Valette H, Bottlaender M. Alteration of the in vivo nicotinic receptor density in ADNFLE patients: a PET study. Brain. 2006;129:2047–2060. doi: 10.1093/brain/awl156. [DOI] [PubMed] [Google Scholar]
- Pocivavsek A, Icenogle L, Levin ED. Ventral hippocampal α7 and α4β2 nicotinic receptor blockade and clozapine effects on memory in female rats. Psychopharmacology (Berl) 2006;188:597–604. doi: 10.1007/s00213-006-0416-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R. Meta-analytic procedures for social research, revised. Newbury Park, California: Sage; 1991. p. 19. [Google Scholar]
- Rueter LE, Donnelly-Roberts DL, Curzon P, Briggs CA, Anderson DJ, Bitner RS. A-85380: a pharmacological probe for the preclinical and clinical investigation of the α4β2 nicotinic acetylcholine receptor. CNS Drug Rev. 2006;12:100–112. doi: 10.1111/j.1527-3458.2006.00100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saji H, Ogawa M, Ueda M, Iida Y, Magata Y, Tominaga A, Kawashima H, Kitamura Y, Nakagawa M, Kiyono Y, Mukai T. Evaluation of radioiodinated 5-iodo-3-(2(S)-azetidinylmethoxy)pyridine as a ligand for SPECT investigations of brain nicotinic acetylcholine receptors. Ann Nucl Med. 2002;16:189–200. doi: 10.1007/BF02996300. [DOI] [PubMed] [Google Scholar]
- Sanberg PR, Silver AA, Shytle RD, Philipp MK, Cahill DW, Fogelson HM, McConville BJ. Nicotine for the treatment of Tourette’s syndrome. Pharmacol Ther. 1997;74:21–25. doi: 10.1016/s0163-7258(96)00199-4. [DOI] [PubMed] [Google Scholar]
- Schapira M, Abagyan R, Totrov M. Structural model of nicotinic acetylcholine receptor isotypes bound to acetylcholine and nicotine. BMC Struct Biol. 2002;2:1. doi: 10.1186/1472-6807-2-1. http://www.biomedcentral.com/1472-6807/2/1. [DOI] [PMC free article] [PubMed]
- Scheffel U, Horti AG, Koren AO, Ravert HT, Banta JP, Finley PA, London ED, Dannals RF. 6-[18F]Fluoro-A-85380: an in vivo tracer for the nicotinic acetylcholine receptor. Nucl Med Biol. 2000;27:51–56. doi: 10.1016/s0969-8051(99)00082-7. [DOI] [PubMed] [Google Scholar]
- Schildan A, Patt M, Sabri O. Synthesis procedure for routine production of 2-[18F]fluoro-3-(2(S)-azetidinylmethoxy)pyridine (2-[18F]F-A-85380) Appl Radiat Isot. 2007;65:1244–1248. doi: 10.1016/j.apradiso.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Shytle RD, Silver AA, Sanberg PR. Comorbid bipolar disorder in Tourette’s syndrome responds to the nicotinic receptor antagonist mecamylamine (Inversine) Biol Psychiatry. 2000;48:1028–1031. doi: 10.1016/s0006-3223(00)00945-8. [DOI] [PubMed] [Google Scholar]
- Silver AA, Shytle RD, Philipp MK, Sanberg PR. Case study: long-term potentiation of neuroleptics with transdermal nicotine in Tourette’s syndrome. J Am Acad Child Adolesc Psychiatry. 1996;35:1631–1636. doi: 10.1097/00004583-199612000-00015. [DOI] [PubMed] [Google Scholar]
- Smith JW, Mogg A, Tafi E, Peacey E, Pullar IA, Szekeres P, Tricklebank M. Ligands selective for α4β2 but not α3β4 or α7 nicotinic receptors generalize to the nicotine discriminative stimulus in the rat. Psychopharmacology (Berl) 2007;190:157–170. doi: 10.1007/s00213-006-0596-8. [DOI] [PubMed] [Google Scholar]
- Sorger D, Becker GA, Patt M, Schildan A, Grossmann U, Schliebs R, Seese A, Kendziorra K, Kluge M, Brust P, Mukhin AG, Sabri O. Measurement of the α4β2* nicotinic acetylcholine receptor ligand 2-[18F]fluoro-A-85380 and its metabolites in human blood during PET investigation: a methodological study. Nucl Med Biol. 2007;34:331–342. doi: 10.1016/j.nucmedbio.2006.12.008. [DOI] [PubMed] [Google Scholar]
- SPSS Inc. SPSS Base 16.0 User’s Guide. Chicago: SPSS Inc; 2007. pp. 528–529.pp. 531–532. [Google Scholar]
- Staley JK, Krishnan-Sarin S, Cosgrove KP, Krantzler E, Frohlich E, Perry E, Dubin JA, Estok K, Brenner E, Baldwin RM, Tamagnan GD, Seibyl JP, Jatlow P, Picciotto MR, London ED, O’Malley S, van Dyck CH. Human tobacco smokers in early abstinence have higher levels of β2*nicotinic acetylcholine receptors than nonsmokers. J Neurosci. 2006;26:8707–8714. doi: 10.1523/JNEUROSCI.0546-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley JK, van Dyck CH, Weinzimmer D, Brenner E, Baldwin RM, Tamagnan GD, Riccardi P, Mitsis E, Seibyl JP. 123I-5-IA-85380 SPECT measurement of nicotinic acetylcholine receptors in human brain by the constant infusion paradigm: feasibility and reproducibility. J Nucl Med. 2005;46:1466–1472. [PubMed] [Google Scholar]
- Stranges S, Bonner MR, Fucci F, Cummings KM, Freudenheim JL, Dorn JM, Muti P, Giovino GA, Hyland A, Trevisan M. Acute vs chronic secondhand smoke exposure. Arch Intern Med. 2007;167:731–732. doi: 10.1001/archinte.166.18.1961. In reply. [letter] [DOI] [PubMed] [Google Scholar]
- Trauth JA, Seidler FJ, McCook EC, Slotkin TA. Adolescent nicotine exposure causes persistent upregulation of nicotinic cholinergic receptors in rat brain regions. Brain Res. 1999;851:9–19. doi: 10.1016/s0006-8993(99)01994-0. [DOI] [PubMed] [Google Scholar]
- Tüzün E. Neuronal acetylcholine receptor alpha 9-subunit: a possible central nervous system autoantigen. Med Hypotheses. 2006;67:561–565. doi: 10.1016/j.mehy.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Ueda M, Iida Y, Mukai T, Mamede M, Ishizu K, Ogawa M, Magata Y, Konishi J, Saji H. 5-[123I]Iodo-A-85380: assessment of pharmacological safety, radiation dosimetry and SPECT imaging of brain nicotinic receptors in healthy human subjects. Ann Nucl Med. 2004;18:337–344. doi: 10.1007/BF02984473. [DOI] [PubMed] [Google Scholar]
- Valette H, Bottlaender M, Dollé F, Guenther I, Fuseau C, Coulon C, Ottaviani M, Crouzel C. Imaging central nicotinic acetylcholine receptors in baboons with [18F]fluoro-A-85380. J Nucl Med. 1999;40:1374–1380. [PubMed] [Google Scholar]
- Vaupel DB, Tella SR, Huso DL, Wagner VO, III, Mukhin AG, Chefer SI, Horti AG, London ED, Koren AO, Kimes AS. Pharmacological and toxicological evaluation of 2-flouro-3-(2(S)-azetidinylmethoxy)pyridine (2-F-A-85380), a ligand for imaging cerebral nicotinic acetylcholine receptors with positron emission tomography. J Pharmacol Exp Ther. 2005;312:355–365. doi: 10.1124/jpet.104.073999. [DOI] [PubMed] [Google Scholar]
- Vaupel DB, Mukhin AG, Kimes AS, Horti AG, Koren AO, London ED. In vivo studies with [125I]5-I-A-85380, a nicotinic acetylcholine receptor radioligand. Neuroreport. 1998;13:2311–2317. doi: 10.1097/00001756-199807130-00030. [DOI] [PubMed] [Google Scholar]
- Villemagne VL, Musachio JL, Scheffel U. Nicotine and related compounds as PET and SPECT ligands. In: Arneric SP, Brioni JD, editors. Neuronal Nicotinic Receptors: Pharmacology and Therapeutic Opportunities. New York: Wiley-Liss; 1998. pp. 235–250. [Google Scholar]
- Voineskos S, De Luca V, Mensah A, Vincent JB, Potapova N, Kennedy JL. Association of alpha4beta2 nicotinic receptor and heavy smoking in schizophrenia. J Psychiatry Neurosci. 2007;32:412–416. [PMC free article] [PubMed] [Google Scholar]
- Wong DF, Brašić JR. In vivo imaging of neurotransmitter systems in neuropsychiatry. Clin Neurosci Res. 2001;1:35–45. [Google Scholar]
- Wong DF, Brasic JR, Zhou Y, Gay O, Crabb AH, Kuwabara H, Hilton J, Osman M, Scheffel UA, Rousset O, Fan H, Dannals RF, Musachio JL. Human imaging of α4β2 nicotinic acetylcholine receptors in vivo using 123I5-iodo-A-85380. J Nucl Med. 2001;42(5 supplement):142P. [abstract] [Google Scholar]
- Wong DF, Jr, Maini A, Alexander M, Zhou Y, Brasic J, Scheffel U, Fan H, Hilton J, Dannals R, Musachio J. Imaging nicotinic receptors in the development of allosteric potentiating ligands for cognitive enhancement in Alzheimers dementia. J Nucl Med. 2002a;43(5 Supplement):109P–110P. [abstract] [Google Scholar]
- Wong DF, Maini A, Rousset OG, Brašić JR. Positron emission tomography--a tool for identifying the effects of alcohol dependence on the brain. Alcohol Res Health. 2003;27:161–173. http://pubs.niaaa.nih.gov/publications/arh27-2/161-173.pdf. [PMC free article] [PubMed] [Google Scholar]
- Wong DF, Potter WZ, Brasic JR. Proof of concept: functional models for drug development in humans. In: Davis KL, Charney D, Coyle JT, Nemeroff C, editors. Neuropsychopharmacology: The Fifth Generation of Progress. Baltimore: The American College of Neuropsychopharmacology and Lippincott Williams & Wilkins; 2002b. pp. 457–473. [Google Scholar]
- Wonnacott S. The paradox of nicotinic acetylcholine receptor upregulation by nicotine. Trends Pharmacol Sci. 1990;11:216–219. doi: 10.1016/0165-6147(90)90242-z. [DOI] [PubMed] [Google Scholar]
- Wüllner U, Gündisch D, Herzog H, Minnerop M, Joe A, Warnecke M, Jessen F, Schutz C, Reinhardt M, Eschner W, Klockgether T, Schmaljohann J. Smoking upregulates α4β2* nicotinic acetylcholine receptors in the human brain. Neurosci Lett. 2008;430:34–37. doi: 10.1016/j.neulet.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Yokoi F, Musachio J, Hilton J, Kassiou M, Ravert HT, Mathews WB, Dannals RF, Stephane M, Wong DF. Kinetic modeling of nicotinic acetylcholine receptor ligand, [C11]A-84543 in a baboon PET study. J Nucl Med. 1999;40(5 supplement):263P. [abstract] [Google Scholar]
- Yoshida H, Inoue M, Oyanagi C, Katsumi Y, Mukai T, Ishizu K, Hashikawa K, Fukuyama H. Nicotinic acetylcholine receptors in Alzheimer’s disease: 5IA-SPECT study. Neuroimage. 2002;16(3):S103. Part 2 of 2 parts. [abstract] [Google Scholar]
- Zamani MR, Allen YS. Nicotine and its interaction with β-amyloid protein: a short review. Biol Psychiatry. 2001;49:221–232. doi: 10.1016/s0006-3223(00)01108-2. [DOI] [PubMed] [Google Scholar]
- Zaniewska M, McCreary AC, Przegaliński E, Filip M. Effects of the serotonin 5-HT2A and 5-HT2C receptor ligands on the discriminative stimulus effects of nicotine in rats. Eur J Pharmacol. 2007;571:156–165. doi: 10.1016/j.ejphar.2007.05.067. [DOI] [PubMed] [Google Scholar]
- Zaniewska M, McCreary AC, Przegaliński E, Filip M. Evaluation of the role of nicotinic acetylcholine receptor subtypes and cannabinoid system in the discriminative stimulus effects of nicotine in rats. Eur J Pharmacol. 2006;540:96–106. doi: 10.1016/j.ejphar.2006.04.034. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Brasic JR, Musachio JL, Zukin SR, Kuwabara H, Crabb AH, Endres CJ, Hilton J, Fan H, Wong DF. Human [123I]5-I-A-85380 dynamic SPECT studies in normals: kinetic analysis and parametric imaging. Nuclear Science Symposium Conference Record, 2001 Institute of Electrical and Electronics Engineers (IEEE), Incorporated; 2001. pp. 1335–1340. [Google Scholar]
- Zoghbi SS, Tamagnan G, Fujita M, Baldwin RM, Al-Tikriti MS, Amici L, Seibyl JP, Innis RB. Measurement of plasma metabolites of (S)-5-[123I]iodo-3-(2-azetidinylmethoxy)pyridine (5-IA-85380), a nicotinic acetylcholine receptor imaging agent, in nonhuman primates. Nucl Med Biol. 2001;28:91–96. doi: 10.1016/S0969-8051(00)00188-8. [DOI] [PubMed] [Google Scholar]
- Zoghbi SS, Tamagnan G, Fujita M, Baldwin RM, Amici L, Seibyl JP, Innis RB. Metabolism of 5-I-123-Iodo-3-(2(3)-azetidinylmethoxy)pyridine, a nicotinic acetylcholine receptor imaging agent, in non-human primates. J Labelled Comp Radiopharm. 1999;42(supplement 1):S666–S668. [Google Scholar]
- Zwart R, Broad LM, Xi Q, Lee M, Moroni M, Bermudez I, Sher E. 5-I A-85380 and TC-2559 differentially activate heterologously expressed α4β2 nicotinic receptors. Eur J Pharmacol. 2006;539:10–17. doi: 10.1016/j.ejphar.2006.03.077. [DOI] [PubMed] [Google Scholar]