Summary
The establishment of sexual identity is a crucial step of germ cell development in sexually reproducing organisms. Sex determination in the germline is controlled differently than in the soma, and often depends on communication from the soma. To investigate how sexual identity is established in the Drosophila germline, we first conducted a molecular screen for genes expressed in a sex-specific manner in embryonic germ cells. Sex-specific expression of these genes is initiated at the time of gonad formation (stage 15), indicating that sexual identity in the germline is established by this time. Experiments where the sex of the soma was altered relative to that of the germline (by manipulating transformer) reveal a dominant role for the soma in regulating initial germline sexual identity. Germ cells largely take on the sex of the surrounding soma, although the sex chromosome constitution of the germ cells still plays some role at this time. The male soma signals to the germline through the JAK/STAT pathway, while the nature of the signal from the female soma remains unknown. We also find that the genes ovo and ovarian tumor (otu) are expressed in a female-specific manner in embryonic germ cells, consistent with their role in promoting female germline identity. However, removing the function of ovo and otu, or reducing germline function of Sex lethal, had little effect on establishment of germline sexual identity. This is consistent with our findings that signals from the soma are dominant over germline autonomous cues at the initial stage of germline sex determination.
Keywords: Germ cells, Sex determination, Drosophila, transformer, ovarian tumor, JAK/STAT
INTRODUCTION
Sexual reproduction in animals requires proper differentiation of the germline into male or female gametes. Little is known about how sex determination is established in the germline, apart from the fact that it is often regulated differently than in the soma and is dependent on cues from the soma. In some species, such as Drosophila and mice, the sex chromosome constitution of the germline is also crucial for germline sex determination. Here, we study the establishment of sexual identity in the Drosophila germline, and how this is regulated by germline autonomous cues and signals from the soma.
In Drosophila, XX individuals are female and XY individuals are male, and this difference is determined by the number of X chromosomes (Bridges, 1914; Erickson and Quintero, 2007). In the soma, a two X-chromosome dose activates the female-determining gene Sex lethal (Sxl), which in turn activates transformer (tra). tra acts together with transformer 2 to regulate the alternative splicing of two key transcription factors, doublesex (dsx) and fruitless (fru). dsx controls male versus female morphology, while dsx and fru act together to control sex differences in the nervous system [reviewed by (Camara et al., 2008; Siwicki and Kravitz, 2009)].
In the germline, sexual identity is also regulated by the number of X chromosomes (Schüpbach, 1985), but X chromosome dosage is `counted' differently than in the soma (Granadino et al., 1993; Oliver et al., 1993; Schüpbach, 1985; Steinmann-Zwicky, 1993). Genes such as tra and dsx that determine sex in the soma are not required in the germ cells (Marsh and Wieschaus, 1978; Schupbach, 1982). Sxl is required in the germline and is thought to promote female identity, but it is activated differently than in the soma (Granadino et al., 1993; Schüpbach, 1985; Steinmann-Zwicky, 1993). In addition, Sxl expression is not sufficient to feminize XY germ cells, indicating that it does not act as a master switch in the germline, as it does in the soma (Hager and Cline, 1997). How X-chromosome number determines sexual identity in the germline is still not known, although the genes ovo and ovarian tumor (otu) are thought to act to promote female germline identity (King et al., 1978; Oliver et al., 1987; Pauli et al., 1993). Adult females, mutant for these genes, exhibit ovarian germline tumors similar to those observed following the transplantation of male germ cells into a female soma, indicating that the germ cells have been masculinized (reviewed by Casper and Van Doren, 2006; Hempel et al., 2008).
In addition to relying on their own sex chromosome constitution in an autonomous manner, germ cells also require signals from the soma for proper sexual development. These somatic signals are regulated by the somatic sex-determination pathway, and require genes such as tra and dsx (Nöthiger et al., 1989; Steinmann-Zwicky, 1994a; Van Deusen, 1977). Because tra acts in the soma but not in the germline, manipulating tra expression results in an incompatibility between the sex of the soma and the sex of the germline. In XX tra mutants, the normally female soma is masculinized (Sturtevant, 1945), resulting in `female' germ cells in a male soma. By contrast, forced expression of tra in XY animals feminizes the soma (McKeown et al., 1988), resulting in male germ cells in a female soma. Such manipulations represent a useful way to dissect the autonomous versus non-autonomous inputs that influence germline sex determination. Recently, it was demonstrated that one way in which the soma signals to regulate germline sexual identity acts through the Janus Kinase Signal Transducer and Activator of Transcription (JAK/STAT) pathway (Wawersik et al., 2005). Germ cells activate the JAK/STAT pathway when in contact with a male somatic gonad, and this influences male germ cell characteristics and male germ cell gene expression (Wawersik et al., 2005).
Germline sexual phenotypes have largely been studied in adults, and much less is known about when germline sex is initially established and how this initial process is regulated. Male-specific activation of the JAK/STAT pathway begins just prior to gonad coalescence (stage 13), soon after the germ cells make contact with the somatic gonad (Wawersik et al., 2005). Just after gonad coalescence (stage 15), male germ cells express male germline marker-1 (mgm-1), which appears to be an enhancer trap in the escargot locus (Staab et al., 1996; Streit et al., 2002). XX germ cells can express this male marker when present in a male soma (Janzer and Steinmann-Zwicky, 2001; Staab et al., 1996) or when not in contact with the somatic gonad (Heller and Steinmann-Zwicky, 1998), indicating that the female gonad represses mgm-1 expression in XX germ cells. However, mgm-1 expression is still observed in XY germ cells present in a female soma, at least initially, indicating that the female soma is unable to repress this aspect of male identity (Janzer and Steinmann-Zwicky, 2001). Male germ cells begin to proliferate soon after gonad formation, whereas female germ cells remain quiescent (Kerkis, 1931; Steinmann-Zwicky, 1994a), a sex-specific characteristic that is regulated by the soma through the JAK/STAT pathway (Wawersik et al., 2005).
To further our understanding of germline sex determination, we conducted a screen to identify molecular markers that are expressed in a sex-specific manner in the embryonic germ cells. These genes were then used as indicators to study how and when germline sexual identity is established through both germ cell autonomous mechanisms and signals from the surrounding soma. We find that the soma plays a dominant role in influencing sex-specific gene expression in the germline, with a minor role for germline autonomous cues. In addition, our data indicate that both the male and female somatic gonad signal to the germ cells to regulate their sex determination in the embryo.
MATERIALS AND METHODS
Fly stocks
The following fly stocks were used (unspecified fly stocks are from the Bloomington Stock Center): Oregon-R or w1118 (as wild type), P{Dfd-lacZ-HZ2.7} on X (W. McGinnis, University of California San Diego, CA, USA), Stat92E06346, Stat92EF (Baksa et al., 2002), UAS-upd (M. Zeidler, The University of Sheffield, UK), Df(3L) st-j7 (Δ tra), tra1, tre1 (R. Lehmann, Skirball Institute, New York Medical Center, NY, USA), otu1, otu17 and ovoD1rv23 (B. Oliver, NIDDK, NIH, Bethesda, MD, USA) srp3, Df(2R) stil-B and stil4 (D. Pauli, University of Geneva, Switzerland), UAS-traF-20J7, tubulin-GAL4-LL7, nanos-GAL4-VP16, osk301and oskCE4 (Lehmann and Nusslein-Volhard, 1986). STAT92E mutants: Stat92E06346/Stat92EF females were mated at permissive temperature (18°C) to Stat92E06346/TM3(UAS-GFP, kruppel-GAL4) males. The progeny were collected for 0-4 hours at 18°C then switched to a non-permissive temperature 29°C. The resulting embryos did not show STAT92E protein via antibody staining.
Immunostaining and in situ hybridization
Ninety-five genes with expression in embryonic gonads were selected from the BDGP Gene Expression Patterns database, which at the time included ≈40% of known genes. Embryos were fixed and devitellinized as previously described (Patel, 1994). Whole-mount in situ hybridization was performed as previously described (Lehmann and Tautz, 1994), followed by immunostaining as described by Patel (Patel, 1994) with modifications as described by DeFalco et al. (DeFalco et al., 2003). Embryos were genotyped using transgenic balancer chromosomes (UAS-GFP; kruppel-GAL4), and sex was determined using an X-chromosome transgene introduced through the fathers (P{Dfd-lacZ-HZ2.7}) (Bergson and McGinnis, 1990).
Antibodies used: AP-conjugated sheep anti-digoxigenin (1:2000, Roche), rabbit anti-GFP (1:2000, Torrey Pines Biolabs), rabbit anti-VASA (1:10,000, R. Lehmann), rabbit anti-STAT92E (1:1000, S. Hou, NCI, Frederick, MD, USA), AP-conjugated sheep anti-mouse (1:500; Jackson ImmunoResearch Laboratories). Fluorescently conjugated secondary antibodies were used at 1:500 (Molecular Probes, Rockland Scientific and Amersham Pharmacia Biotech).
The following clone numbers (gene name) were used for RNA probes: RE04051 (dpa), RE67590 (Mcm5), RE27528 (CG9253), LD21021 (CG6751), LD15481 (Rs1), LD16356 (RanGap), LD05521 (CG6693), LD15641 (Klp61f), RE70955 (CG9925), RH03206 (Jheh2), LD15196 (CG5149), pGEM-lacZ, RE64188 (otu), LD47350 (ovo), GH06596 (stil).
The percentage of embryos exhibiting strong, medium, low or no expression in the gonad was assessed. To combine data from different germ cell-specific probes, the percentage of embryos exhibiting any level of expression in the XY control was set as 100% and was used to normalize all other genotypes for that probe. The normalized data were then combined into a single average, and the values compared using Student's t-test, assuming equal variance.
RESULTS
Establishment of germ cell sexual identity
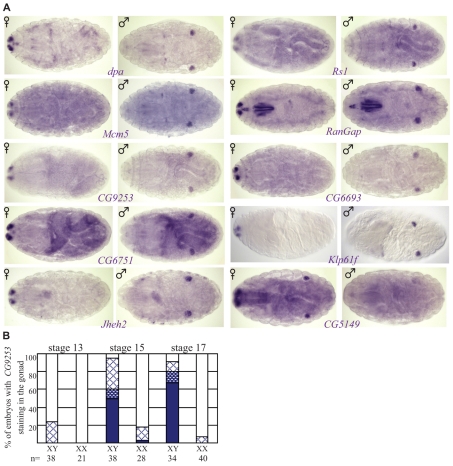
Since few tools exist with which to study germline sex determination, we conducted a molecular screen for genes expressed in a sex-specific manner in embryonic germ cells. We utilized information from the Berkeley Drosophila Genome Project Gene Expression database to identify genes expressed in embryonic gonads (Tomancak et al., 2007) and re-screened these genes by in situ hybridization using embryos whose sex could be unambiguously determined (see Materials and methods). Although most genes were expressed in the embryonic gonads of both sexes, our initial screen identified nine genes that exhibited male-biased expression in the gonad and one gene that was expressed at higher levels in female gonads (Fig. 1A). To determine whether these genes are expressed in the germline or the soma, we analyzed expression in embryos that lack germ cells (hypomorphic oskar mutants, see Table S1 in the supplementary material) and also examined whether the cells expressing the genes were co-labeled for the germ cell-specific VASA protein (data not shown). These tests indicated that the male-biased gene Juvenile hormone epoxide hydrolase 2 (Jheh2) and the female-biased gene CG5149 were expressed in the somatic gonad. In addition, disc proliferation abnormal (dpa) and mini-chromosome maintenance 5 exhibited low-level expression in the soma, but also appeared to be expressed in the germline. The remaining six genes were specific for the germline. RT-PCR analysis on adult gonads revealed that all genes except Klp61F were expressed in both ovaries and testes, although it is unknown whether this expression was in the soma or the germline (data not shown). Regardless, the expression of these genes in the embryonic germ cells makes them useful markers for the establishment of sex-specific gene expression in the germline. Although the sex-specific germ cell genes identified are all male-specific in the embryonic gonad, this is not simply because female germ cells are transcriptionally quiescent. Many of the genes we screened were expressed in the embryonic germ cells in both sexes (data not shown) and other genes, such as ovo and otu are preferentially expressed in female embryonic germ cells (see below). Therefore, the sex-specific pattern of gene expression in embryonic germ cells is likely to reflect the establishment of germline sexual identity.
Fig. 1.
Identification of genes expressed in a sex-specific manner in embryonic Drosophila gonads. (A) Stage 17 embryos labeled by in situ hybridization with probes for genes as indicated. Anterior is to the left. Embryos have also been hybridized with a probe for a Dfd-lacZ transgene (two dots in anterior) carried on the paternal X chromosome, which identifies XX embryos. (B) Graph indicating the percentage of XY or XX embryos with CG9253 expression in the gonads at stages 13, 15 and 17. In situ hybridization signals of varying intensity are shown; solid bars indicate strong signal, shaded bars, medium signal and hatched bars, weak signal.
Next, these molecular markers were used to determine when in development a sex-specific program of gene expression is established in the germline. Although some expression of these genes could be detected as the gonad was forming (stages 13 and 14), robust and sex-specific expression was only observed shortly after gonad formation (stage 15; Fig. 1B, see also Fig. S1 in the supplementary material). (Note: CG9925 is a control expressed in both male and female germ cells.) In addition, the difference in male versus female gonad expression was even clearer toward the end of embryogenesis (stage 17). We conclude that sex-specific gene expression in the germ cells, and therefore germline sexual identity, is established shortly after gonad formation.
Autonomous versus non-autonomous control of germline sexual identity
Autonomous sex determination
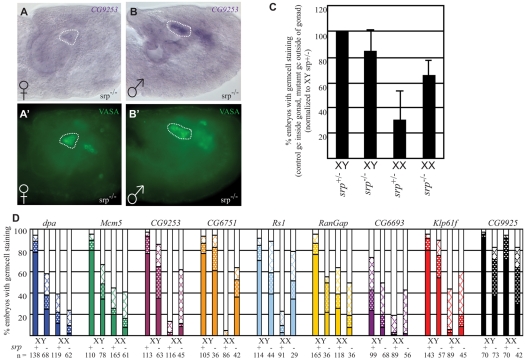
It has been demonstrated that proper sexual development of the germline is regulated by both germline autonomous inputs, based on the sex chromosome constitution of the germ cells (XX versus XY), and non-autonomous signals from the soma, but the details of these interactions are poorly understood (reviewed by Casper and Van Doren, 2006; Hempel et al., 2008). We used the newly identified sex-specific molecular markers to determine how these two components contribute to the establishment of germline sexual identity in the embryo. Previous studies have shown that mgm-1 is expressed in both XX and XY germ cells that are not in contact with the somatic gonad, suggesting that male identity in the germline is the `default' in the absence of the somatic gonad (Heller and Steinmann-Zwicky, 1998). We conducted a similar analysis using our larger panel of sex-specific markers. In srp mutants the endoderm is not properly specified (Reuter, 1994), and many germ cells fail to migrate out of the gut and never contact the somatic gonad (Warrior, 1994) (Fig. 2A′,B′). Sex-specific gene expression of germ cells not in contact with the somatic gonad was analyzed and compared to expression in germ cells present in a wild-type gonad. The data was combined into a single, normalized average for the six sex-specific genes that are exclusively expressed in the germline (Fig. 2C). Data for each of the individual genes are shown in Fig. 2D. We found that male-specific gene expression was increased in XX germ cells when not in contact with the somatic gonad, indicating that a female somatic gonad normally acts to repress male gene expression in XX germ cells. It was also consistently observed that male gene expression was more robust in XY germ cells than in XX germ cells outside of the gonad, indicating that there is already an autonomous component to germ cell sexual identity at this time. Lastly, XY germ cells outside of the gonad consistently exhibited less robust expression of male markers than did XY germ cells in the gonad, indicating that the male gonad environment is required for full male germ cell identity. Similar conclusions were drawn when gene expression in germ cells outside of the gonad was analyzed using an independent genetic background (tre1 mutants, see Fig. S2 in the supplementary material). Thus, although differences can be detected between XX and XY germ cells outside of the gonad, indicating that there is an autonomous contribution to germline sex determination at this time, there is also a strong non-autonomous contribution from both the male and female soma.
Fig. 2.
Sexual identity in germ cells outside of the somatic gonad. (A-B′) Stage 17 srp mutant (A,A′) XX and (B,B′) XY embryos labeled by in situ hybridization for (A,B) CG9253 and (A′,B′) VASA antibody. (C) Graph of normalized germ cell gene expression (see Materials and methods). (D) Graph of in situ hybridization signals in XY and XX germ cells inside gonadal soma (srp+/-) or outside of gonadal soma (srp-/-). Solid bars indicate strong signal, shaded bars, medium signal and hatched bars, weak signal.
The role of the male soma
We next wanted to investigate further how the soma regulates gene expression in the germline. First, the fate of female germ cells in a male somatic gonad was examined. tra is required for female identity in the soma, and tra mutant females are transformed to have a male phenotype. However, tra is not required in germ cells (Marsh and Wieschaus, 1978), so any changes observed in the germ cells must be due to effects on the soma. To confirm that the somatic gonad is transformed from female to male in tra mutants, we examined expression of a male-specific somatic gene, Jheh2, and a female-specific somatic gene, CG5149, which both exhibited the male pattern of expression in XX tra mutants (data not shown). We have also previously observed that other sexually dimorphic characteristics of the embryonic gonad are fully transformed from female to male in XX tra mutants (DeFalco et al., 2008; DeFalco et al., 2003; Le Bras and Van Doren, 2006).
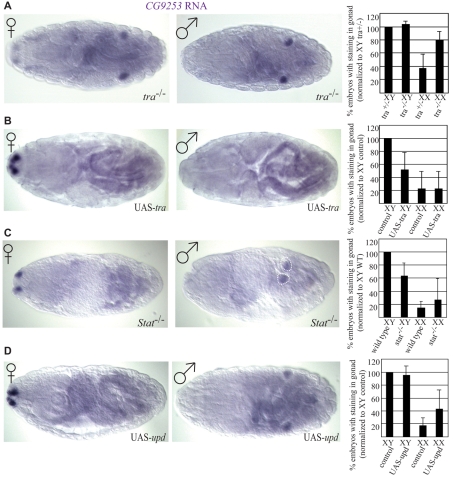
Masculinizing the soma using tra null mutants increased male gene expression in XX germ cells (Fig. 3A). The percentage of embryos with masculinized germ cells varied slightly from gene to gene, but all of the male-specific genes showed an increased percentage of expression in germ cells of XX tra mutant embryos (see Fig. S3A in the supplementary material). The number of male-specific genes that increased expression in XX tra mutants was greater than that observed in XX germ cells outside of the gonad (Fig. 2). This shows that the male soma was sufficient to allow germ cells to express a male program of sexual identity regardless of the sex chromosome constitution of the germline. Male gene expression in masculinized XX embryos was slightly, but consistently, lower than the expression in XY controls (Fig. 3A, see also Fig. S3A in the supplementary material), which might reflect the autonomous contribution of the germline control (XX versus XY).
Fig. 3.
Non-autonomous control of sex-specific germ cell gene expression. Expression of CG9253 assayed by in situ hybridization. Female embryos are identified by Dfd-lacZ expression from the paternal X chromosome. Graphs display combined average percentages of male gene expression normalized to the XY sibling controls. (A) In tra mutants (tra1/Δtra), XX germ cells exhibit male-specific gene expression. (B) Ectopic expression of tra in XY soma causes loss of male-specific germ cell gene expression. (C) Stat92E mutant germ cells have reduced male-specific gene expression. (D) Ectopic upd expression in XX germ cells does not induce all of the male-specific genes.
The role of the female soma
In the srp and tre1 experiments discussed above, XX germ cells exhibited an increased level of male-specific gene expression when not in contact with the female somatic gonad. This indicates that the female soma is able to repress male-specific gene expression (Heller and Steinmann-Zwicky, 1998). To examine this further, we analyzed gene expression in XY germ cells in contact with a female soma. Feminization of the XY soma was achieved by ectopically expressing tra under the UAS promoter using tubulin-GAL4. This was sufficient to feminize the male soma, as judged by activation of the female somatic gene CG5149 and repression of the male somatic gene Jheh2 (data not shown). We have previously used this approach to feminize all aspects of somatic gonad development that we have studied (DeFalco et al., 2008; DeFalco et al., 2003; Le Bras and Van Doren, 2006). Because tubulin-Gal4 driven gene expression is not detectable in the embryonic germline (our unpublished observations), and tra does not play a role in the germline (Marsh and Wieschaus, 1978), any changes observed in the germline are due to effects of tra expression in the soma.
We found that a female soma was indeed able to repress male gene expression in XY germ cells (Fig. 3B, see also Fig. S3B in the supplementary material). The percentage of XY embryos that exhibited male-specific gene expression in the germline was dramatically reduced when tra was expressed, compared with wild-type males. For most genes analyzed, the expression in feminized XY embryos was similar to that observed in control female embryos (see Fig. S3B in the supplementary material). These data indicate that the female soma is actively regulating the establishment of germline sexual identity during embryogenesis by repressing the male program of gene expression.
The JAK/STAT pathway is required for wild-type levels of male gene expression
Previously, we demonstrated that the male somatic gonad non-autonomously regulates male germ cell development by activating the JAK/STAT pathway in male germ cells (Wawersik et al., 2005). Blocking the JAK/STAT pathway blocked male-specific germ cell proliferation, and inhibited male-specific expression of mgm-1 in larval, but not embryonic germ cells (Wawersik et al., 2005). The fact that embryonic germ cells could still exhibit male-specific mgm-1 expression when the JAK/STAT pathway was blocked could indicate that other factors, such as germ cell-autonomous cues or repression by the female soma, are sufficient to allow male-specific gene expression in the germline. Alternatively, it could indicate that the JAK/STAT pathway was incompletely blocked in these previous experiments (e.g. due to maternal contribution of STAT when using zygotic mutants). To ensure a complete block of JAK/STAT pathway activity, we generated maternal/zygotic mutants for the only Drosophila homolog of STAT (Stat92E). Because the JAK/STAT pathway is required for fertility and early germ cell behavior (Hou et al., 1996; Li et al., 2003), a temperature-sensitive Stat92E mutant was used that has been demonstrated to create a strong loss-of-function condition at the restrictive temperature (Baksa et al., 2002). Embryos were collected at the permissive temperature, and then shifted to the restrictive temperature early in embryogenesis, before gonad formation, and we analyzed expression of the six male-specific germ cell genes. We found that the expression of these genes was still male-specific in maternal/zygotic Stat92E mutants (Fig. 3C, see also Fig. S3C in the supplementary material). The slight reduction in male gene expression observed in maternal/zygotic Stat92E mutants was similar to that observed in germ cells found outside of the somatic gonad in srp or tre1 mutants. This confirms that the JAK/STAT pathway is not required for initial induction of male-specific gene expression in the germline, and supports a role for this pathway in enhancing and maintaining male gene expression in the germline.
Our earlier work also showed that upregulation of the JAK/STAT pathway was sufficient to induce male-like germ cell proliferation and expression of the male germ cell genes mgm-1 and, to a lesser extent, dpa and Mcm5 in XX germ cells (Wawersik et al., 2005). We examined whether the JAK/STAT pathway was sufficient to induce expression of all of our male-specific germ cell genes by similarly activating the JAK/STAT pathway through the overexpression of a ligand for this pathway, UPD. As we observed previously, we saw a limited activation of the male germ cell genes dpa and Mcm5 in XX germ cells (see Fig. S3D in the supplementary material). However, activation of the other male germ cell genes was variable (Fig. 3D, see also Fig. S3D in the supplementary material), and expression of upd was not able to induce the expression of male germ cell genes to levels seen in control male embryos. This is in contrast to what was observed when XX germ cells were present in a male soma in tra mutants (Fig. 3A, Fig. S3A in the supplementary material). Thus, ectopic JAK/STAT signaling might not be able to override the ability of the female soma to repress male germline gene expression.
Role of female germline sex determination genes
Embryonic expression of the ovarian tumor loci
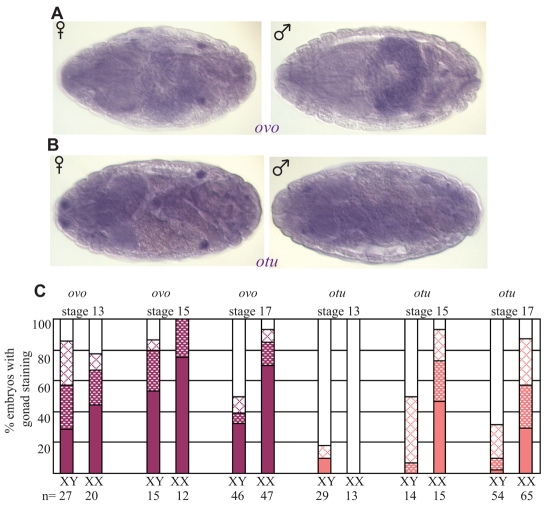
ovo and otu are known to be required during larval and adult stages for proper female germ cell sexual identity (King et al., 1978; Oliver et al., 1987; Pauli et al., 1993), and ovo is also thought to regulate otu expression (Lu et al., 1998). To determine whether these genes also play a role when germline sexual identity is established in the embryo, in situ hybridizations were preformed for these genes on sexed embryos. The RNAs of the two isoforms of ovo that are regulated in a sex-specific manner (ovo-A and ovo-B) are present maternally, and zygotic expression is initiated at embryonic stage 16 (Mével-Ninio et al., 1995; Yatsu et al., 2008). We analyzed ovo RNA expression with a probe that recognizes both isoforms and found ovo in both male and female gonads. However, at late stage 17, ovo expression increased in female germ cells compared with male germ cells (Fig. 4A,C). Whether this difference reflects female germ cell identity, or the fact that there are two copies of this X-linked gene in females, is not clear. Expression of otu was even more clearly biased toward female germ cells, and female embryos showed much more robust expression of otu at stages 15 and 17 (Fig. 4B,C). These data indicate that zygotic expression of otu may contribute to germline sex determination in the embryo, in addition to any role for the maternal proteins. In addition, these data support our previous conclusion that germline sex determination is established by stage 15 of embryogenesis, and indicate that this includes a female-specific, as well as a male-specific, pattern of gene expression in the germline.
Fig. 4.
ovo and otu show increased expression in female embryonic germ cells. RNA in situ hybridizations of stage 17 embryos. (A,B) ovo (A) and otu (B) both show strong expression in females and weak expression in males. (C) Graph indicates percentage of embryos with ovo and otu expression at stages 13, 15 and 17. Intensity of expression was classified as strong (solid bars), medium (shaded bars) or weak (hatched bars).
The ovarian tumor loci are not required for germline sexual identity in the embryo
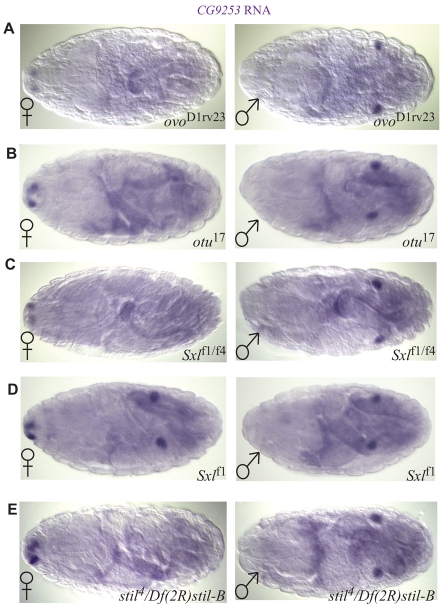
We next examined whether sex-specific gene expression in the germline was affected in ovo, otu or Sxl mutants. As these genes are thought to be important for female germ cell identity, we expected to see expression of male-specific genes in XX germ cells in these mutants. However, null alleles of ovo and otu caused no significant change in the expression of the male-specific germ cell genes we tested (Fig. 5A,B, see also Fig. S4A,B in the supplementary material). An allelic combination of Sxl that is thought to affect germline sex determination but not somatic sex determination (Sxlf1/f4) (Steinmann-Zwicky, 1994b) similarly had no effect on male-specific gene expression in the germline (Fig. 5C, Fig. S4C in the supplementary material). XX embryos with null alleles of Sxl, which affect both the soma and the germline, exhibited an increase in male germline gene expression (Fig. 5D, Fig. S4D in the supplementary material). However, this result was similar to that observed in tra mutants (Fig. 3A, Fig. S3A in the supplementary material), which affect the soma but not the germline, suggesting that the changes observed in null Sxl mutants are due to effects on the soma. We also examined male germline gene expression in mutants for stand still (stil), a gene that has also been implicated in germline sex determination (Mulligan et al., 1996; Pennetta and Pauli, 1997). Again, no change in expression was observed. Thus, we failed to observe a role for the ovarian tumor loci in regulating sex-specific gene expression in the embryonic germline. This is consistent with our findings that non-autonomous signals from the soma have a dominant role over the autonomous cues from the germline in the embryo.
Fig. 5.
The role of female germ cell genes in the establishment of sexual identity. (A-E) RNA in situ hybridization for CG9253, with female embryos on the left and male embryos on the right. (A-C) Most XX (A) ovoD1rv23/D1rv23, (B) otu17/17 and (C) Sxlfl/f4 mutant germ cells show no masculinization compared with sibling control XX germ cells. This allele combination of Sxl affects the germ cells but not the soma. (D) In XX Sxlfl/f1, which affects both the germline and soma, germ cells are masculinized and show expression levels similar to those of XY germ cells. (E) In XX stil4/Df(2R)stil-B mutants, germ cells show no masculinization compared with sibling control XX germ cells.
Regulation of female germ cell gene expression
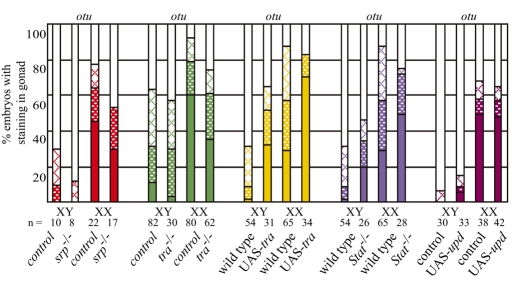
As otu exhibits clear female-biased expression in the embryonic germ cells, we used otu as an indicator of female identity in the germline, and investigated how autonomous and non-autonomous signals regulate otu expression (Fig. 6). Similar analyses were not done for ovo as female-specific expression is only apparent during a small time window. We found that germ cells located outside of the gonad in srp mutants were able to properly activate otu expression, which was still biased toward XX embryos. This indicates that otu expression is under germ cell autonomous control at this time. Furthermore, because we previously observed that XX germ cells outside of the gonad also initiate expression of male germ cell genes (Fig. 2), we conclude that these germ cells have a mixed identity when not in contact with the somatic gonad.
Fig. 6.
Autonomous and non-autonomous signals control otu expression in female germ cells. Percentage of stage 17 embryos exhibiting strong (solid bars) medium (shaded bars) or weak (hatched bars) otu in situ hybridization signal. Genotype of embryos is as indicated (see Materials and methods) and includes srp-, tra-, UAS-tra expressed with tubulin-Gal4, and UAS-upd expressed with nanos-Gal4.
Next, the role of the somatic gonad in regulating female-specific germ cell gene expression was examined. We observed that otu expression was decreased in XX germ cells exposed to a male soma (tra mutants) and increased in XY germ cells exposed to a female soma (UAS-tra, tubulin-Gal4, Fig. 6). Thus, non-autonomous signals from the soma have a clear contribution to female-specific germ cell gene expression, as was also observed for our male-specific genes. Little change was observed when the JAK/STAT pathway was inhibited or activated, indicating that other signals, such as those coming from the female soma, are important for controlling female germ cell identity.
DISCUSSION
The establishment of sexual identity in the germline
Our analysis demonstrates that a sex-specific program of gene expression is present in the germline soon after the time of gonad formation [stage 15, ∼12 hours after egg laying (AEL)]. We identified examples of both male-specific germline genes (Fig. 1, Fig. S1 in the supplementary material) and female-specific germline genes (Fig. 4), with other genes being expressed equally in the two sexes. Therefore, it is likely that the sex-specific pattern of gene expression in the germline represents the establishment of true sexual identity in the germline, as opposed to other differences that might be observed in the germline between the sexes, such as proliferation status or transcriptional competence. We conclude that sexual identity in the germline is established at least as early as stage 15. The observation that many genes examined initiate sex-specific germline expression at this time (see Fig. S1 in the supplementary material) might indicate that this is when germline sexual identity is first established. However, we have also found that the soma signals to the germline in a sex-specific manner just prior to gonad formation (stage 13, ∼10 hours AEL) (Wawersik et al., 2005), indicating that germ cells could determine their sex even earlier. Because the zygotic genome is only robustly activated in the germline during gastrulation (stage 9, ∼4 hours AEL) (Van Doren et al., 1998), this sets a narrow time window during which sexual identity is established in the germline.
It is significant that germline sexual identity appears to be first manifested as the germ cells contact the somatic gonad. This is also the time that sexual dimorphism is first observed in the somatic gonad and that it exhibits sex-specific patterns of gene expression (Fig. 1A, Jheh2 and CG5149) (Kitadate et al., 2007; Le Bras and Van Doren, 2006; Wawersik et al., 2005). The finding that germ cells might only establish their sexual identity as they contact the somatic gonad is consistent with the strong role of the soma in determining germline sexual identity. In addition, signaling from the germline back to the soma also occurs at this time (Kitadate et al., 2007).
The establishment of a sex-specific pattern of gene expression in the germline requires these cells to acquire germ cell identity, in addition to sexual identity. Previous work has established that germ cell-specific transcription is independent of the somatic environment, and is autonomously regulated in the germ cells (Van Doren et al., 1998). Indeed, we see that germ cell expression of the genes we report here can be independent of the somatic gonad (Fig. 2). Germ cell-specific gene expression is likely to be regulated by the germ plasm, and several maternally expressed germline transcription factors have recently been identified (Yatsu et al., 2008).
Autonomous versus non-autonomous control of germline sexual identity
Previously, it was known that sexual identity in the germline requires autonomous cues, along with non-autonomous cues from the surrounding soma, but little was known about how the signals work together to establish sexual identity (reviewed by Casper and Van Doren, 2006; Hempel et al., 2008). XX germ cells cannot develop normally in a male soma, and XY germ cells cannot develop normally in a female soma. Although we find evidence for both autonomous and non-autonomous regulation of germline sexual identity in the embryo, it appears that non-autonomous signals from the soma are dominant over germline autonomous cues. When XX germ cells are present in a male soma (tra mutants), they exhibit a clear increase in expression of male germ cell genes (Fig. 3A), and decreased expression of the female-specific gene otu (Fig. 6). Similarly, XY germ cells present in a female soma (UAS-tra) exhibit a strong repression of male germ cell genes (Fig. 3B), while otu expression is increased (Fig. 6). Thus, in each case the germ cells largely take on the sexual identity of the surrounding soma, independent of their own sex chromosome constitution. However, subtle differences in gene expression between XX and XY germ cells remain in these situations, indicating some germ cell autonomous control of sexual identity. In addition, when germ cells are present outside of the gonad (srp mutants), some differences are also observed between XX and XY germ cells. When outside of the gonad, XY germ cells are more likely than XX germ cells to express male-specific genes (Fig. 2), and XX germ cells maintain some otu expression, while otu is largely off in XY germ cells (Fig. 6). Thus, there is at least some autonomous contribution of the germ cell sex chromosome genotype to germline sexual identity at this early stage.
The genes of the ovarian tumor loci, ovo, otu, stil and Sxl, are thought to contribute to autonomous germline sexual identity by promoting female identity in XX germ cells. At this early stage, we did not observe a change in sex-specific germ cell gene expression in mutants for ovo, otu or stil, or in embryos with mutations that reduce the function of Sxl in the germline (Fig. 5). Although this could indicate that these genes do not play a role in the initial establishment of germline sexual identity, these observations could also be due to the dominant effects of the soma on sex-specific germ cell gene expression at this time. These mutations would be expected to masculinize XX germ cells, causing them to exhibit a more male-like pattern of gene expression. However, even fully male (XY) germ cells exhibit a female pattern of gene expression when in a female soma (UAS-tra, Fig. 2B, Fig. 6). Thus, the dominant effect of the female soma might mask any masculinizing effects from the removal of ovarian tumor locus genes at this time.
Both the male and female soma control germline sexual identity
We see evidence for at least two types of non-autonomous regulation of germline sexual identity by the soma, one coming from the male soma and the other from the female soma. Both XY and XX germ cells can activate male-specific gene expression when not in contact with the somatic gonad (Fig. 2), but expression of these genes does not appear to be as robust as when germ cells are in contact with a male soma (srp/+ XY, Fig. 2C,D; tra- XX, Fig. 3A, Fig. S3A in the supplementary material). Furthermore, when XX germ cells are in contact with a male soma, expression of the female germ cell gene otu is partially repressed (tra- XX, Fig. 6). Thus, the male soma is required for full levels of male gene expression in the germ cells, and for the repression of female genes.
The signal from the male soma to the germline appears to be primarily, and perhaps exclusively, acting through the JAK/STAT pathway. Previously, we have shown that the male soma activates the JAK/STAT pathway in the germ cells, and that this is required for proper male germ cell behavior (Wawersik et al., 2005). This pathway is necessary for the proper male-specific proliferation of embryonic germ cells, and for the maintenance, but not the initiation, of male-specific mgm-1 expression. The JAK/STAT ligand UPD is also sufficient for the induction of germ cell proliferation and mgm-1 expression, and for partial induction of mcm5 and dpa expression. Here we find that, when the JAK/STAT pathway is inactivated, sex-specific gene expression in the germline resembles that of germ cells that are not in contact with the somatic gonad (compare Fig. 2 with Fig. 3C). Ectopic expression of upd in females is able to induce some aspects of male-specific gene expression in XX germ cells (Fig. S3D), but not as robustly as when XX germ cells are present in a male soma. This is likely to be due to the fact that, when upd is expressed in an otherwise female soma, it is in competition with female signals that repress male gene expression (see below). We conclude that the JAK/STAT pathway is important for the regulation of germline sex determination by the male soma, and may be the primary or only signal from the male soma to the embryonic germ cells. It is essential for the male-specific pattern of germ cell proliferation, and for the robust initiation and maintenance of male-specific gene expression (Wawersik et al., 2005) (this work).
It is clear that the female soma also plays a key role in regulating the sexual identity of the germline. Both XX and XY germ cells exhibit some level of male-specific gene expression when outside of the gonad (Fig. 2), but this expression is dramatically repressed when in contact with a female soma (XX, wt Fig. 1; XY, UAS-tra Fig. 3B). This repression has also been observed with mgm-1 (Heller and Steinmann-Zwicky, 1998). In addition, XY germ cells exhibit some female-specific otu expression when in contact with a female soma (UAS-tra, Fig. 6). Therefore, the female soma is essential for the proper sex-specific regulation of germline gene expression, although the nature of the signal from the female soma to the germ cells remains unknown.
The source of both the male and female somatic signals to the germline is likely to be the somatic gonad. The germ cells only show signs of receiving the JAK/STAT signal when in contact with male somatic gonad (Wawersik et al., 2005), and germ cells outside of the gonad do not appear to receive the proper sex-specific signals (e.g. srp mutants). Furthermore, it is known that somatic regulation of germline sex is controlled by the sex determination cascade, acting through tra and dsx (Steinmann-Zwicky, 1994a). However, the only cells to express DSX within the embryo are part of the somatic gonad (DeFalco et al., 2008; Hempel and Oliver, 2007). Thus, the somatic control over germline sex determination is likely to represent local signaling within the gonad environment, rather than long-range signaling from other somatic cell types.
Germline sex determination in other species
Somatic control over germline sex determination is a common feature of germ cell sexual development. In the mouse, germ cells first manifest sex-specific behaviors as they contact the somatic gonad in the genital ridge. The initial behavior of the germ cells is dependent on the sex of the soma, as male germ cells exhibit female behavior (early meiosis) when contacting a female soma, and female germ cells exhibit male behavior when in contact with a male soma (reviewed by McLaren, 2003). At least some aspects of this sex-specific behavior are regulated by retinoic acid (RA) levels controlled by the soma; female germ cells receive a high level of RA signal, but the male soma degrades RA so that male germ cells receive less of this signal (Bowles et al., 2006; Koubova et al., 2006). Interestingly, we find that the somatic gonad in Drosophila expresses Jheh2 in a male-specific manner (Fig. 1A). JHEH2 hydrolyzes Juvenile Hormone (JH), which is structurally related to RA. JH analogs have also been observed to influence sex determination in crustaceans (Olmstead and Leblanc, 2002), which further supports this interesting parallel between vertebrates and invertebrates.
In mammals, the germline sex chromosome constitution is also important for germ cell development. As in Drosophila, mouse and human Y chromosome genes are crucial for spermatogenesis. However, the number of X chromosomes also appears to play an autonomous role in germline sexual development in mammals. XX germ cells in a male soma [e.g. in Sex reversed (Sxr) mice (McLaren and Monk, 1981)] appear initially male and do not enter meiosis, but eventually die. XO germ cells in a male soma survive and progress further in spermatogenesis (Sutcliffe et al., 1991). Similarly, having two X chromosomes promotes female germ cell identity. XX germ cells are predisposed to enter meiosis on the female timetable compared with XO germ cells under the same conditions (McLaren and Monk, 1981), and are biased towards a female pattern of imprinting (Durcova-Hills et al., 2004; Durcova-Hills et al., 2006). Thus, as in Drosophila, the germ cell genotype contributes to germ cell sex determination in the mouse and depends on the number of X chromosomes. Similarly, humans with altered sex chromosome constitutions, such as those with Turner's Syndrome (XO females) or Klinefelter's Syndrome (XXY males) have relatively normal somatic development but exhibit severe germline defects (Hjerrild et al., 2008; Paduch et al., 2009), indicating that the proper number of X chromosomes in the germline is essential for germline development.
However, in other species, the sex chromosome constitution of germ cells does not play an important role in germline sex determination. In the housefly, Musca domestica, transplanted germ cells develop normally according to the somatic sex of their host and produce fertile gametes (Hilfiker-Kleiner et al., 1994). Some species also exhibit dramatic sexual plasticity in the germline, with the same individual being able to produce both sperm and egg, such as in hermaphrodites [e.g. C. elegans (Ellis, 2008)] or in species that exhibit natural sex reversal [e.g. wrasses and gobies (Devlin and Nagahama, 2002)]. Given the diversity of mechanisms animals use for establishing sex determination in the soma, it is not surprising that there might be considerable diversity in the germline as well. However, it appears that an important role for the soma in controlling sexual identity in the germline is a common theme in germ cell development. Whether or not the sex chromosome constitution of the germline is also important for fertility in an organism places additional constraints on the evolution of sex determination mechanisms, sexual plasticity and the sex chromosomes.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/cgi/content/full/136/22/3821/DC1
Supplementary Material
The molecular screen described in this work was greatly facilitated by the Berkeley Drosophila Genome Project's Gene Expression Patterns Project. We are grateful to the numerous colleagues who have generously supplied us with fly stocks and reagents for this work, and we have specifically acknowledged them within the Materials and methods. We also acknowledge the Bloomington Stock Center (Indiana University) and the Developmental Studies Hybridoma Bank (University of Iowa) for providing reagents. We thank Brian Oliver and members of the Van Doren lab for helpful discussions and critical reading of the manuscript. This work was supported by the National Institutes of Health (HD46619 and GM084356). Deposited in PMC for release after 12 months.
References
- Baksa, K., Parke, T., Dobens, L. L. and Dearolf, C. R. (2002). The Drosophila STAT protein, stat92E, regulates follicle cell differentiation during oogenesis. Dev. Biol. 243, 166-175. [DOI] [PubMed] [Google Scholar]
- Bergson, C. and McGinnis, W. (1990). An autoregulatory enhancer element of the Drosophila homeotic gene Deformed. EMBO J. 9, 4287-4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles, J., Knight, D., Smith, C., Wilhelm, D., Richman, J., Mamiya, S., Yashiro, K., Chawengsaksophak, K., Wilson, M. J., Rossant, J. et al. (2006). Retinoid signaling determines germ cell fate in mice. Science 312, 596-600. [DOI] [PubMed] [Google Scholar]
- Bridges, C. B. (1914). Direct proof through non-disjunction that the sex-linked genes of Drosophila are borne by the X-chromosome. Science 40, 107-109. [DOI] [PubMed] [Google Scholar]
- Camara, N., Whitworth, C. and Van Doren, M. (2008). The creation of sexual dimorphism in the Drosophila soma. Curr. Top. Dev. Biol. 83, 65-107. [DOI] [PubMed] [Google Scholar]
- Casper, A. and Van Doren, M. (2006). The control of sexual identity in the Drosophila germline. Development 133, 2783-2791. [DOI] [PubMed] [Google Scholar]
- DeFalco, T. J., Verney, G., Jenkins, A. B., McCaffery, J. M., Russell, S. and Van Doren, M. (2003). Sex-specific apoptosis regulates sexual dimorphism in the Drosophila embryonic gonad. Dev. Cell 5, 205-216. [DOI] [PubMed] [Google Scholar]
- DeFalco, T., Camara, N., Le Bras, S. and Van Doren, M. (2008). Nonautonomous sex determination controls sexually dimorphic development of the Drosophila gonad. Dev. Cell 14, 275-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin, R. H. and Nagahama, Y. (2002). Sex determination and sex differentiation in fish: an overview of genetic, physiological, and environmental influences. Aquaculture 208, 191-364. [Google Scholar]
- Durcova-Hills, G., Burgoyne, P. and McLaren, A. (2004). Analysis of sex differences in EGC imprinting. Dev. Biol. 268, 105-110. [DOI] [PubMed] [Google Scholar]
- Durcova-Hills, G., Hajkova, P., Sullivan, S., Barton, S., Surani, M. A. and McLaren, A. (2006). Influence of sex chromosome constitution on the genomic imprinting of germ cells. Proc. Natl. Acad. Sci. USA 103, 11184-11188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis, R. E. (2008). Sex determination in the Caenorhabditis elegans germ line. Curr. Top. Dev. Biol. 83, 41-64. [DOI] [PubMed] [Google Scholar]
- Erickson, J. W. and Quintero, J. J. (2007). Indirect effects of ploidy suggest x chromosome dose, not the x:a ratio, signals sex in Drosophila. PLoS Biol. 5, e332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granadino, B., Santamaria, P. and Sánchez, L. (1993). Sex determination in the germ line of Drosophila melanogaster: activation of the gene Sex-lethal. Development 118, 813-816. [DOI] [PubMed] [Google Scholar]
- Hager, J. H. and Cline, T. W. (1997). Induction of female Sex-lethal RNA splicing in male germ cells: implications for Drosophila germline sex determination. Development 124, 5033-5048. [DOI] [PubMed] [Google Scholar]
- Heller, A. and Steinmann-Zwicky, M. (1998). In Drosophila, female gonadal cells repress male-specific gene expression in XX germ cells. Mech. Dev. 73, 203-209. [DOI] [PubMed] [Google Scholar]
- Hempel, L. U. and Oliver, B. (2007). Sex-specific DoublesexM expression in subsets of Drosophila somatic gonad cells. BMC Dev. Biol. 7, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempel, L. U., Kalamegham, R., Smith, J. E., 3rd and Oliver, B. (2008). Drosophila germline sex determination: integration of germline autonomous cues and somatic signals. Curr. Top. Dev. Biol. 83, 109-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilfiker-Kleiner, D., Dübendorfer, A., Hilfiker, A. and Nöthinger, R. (1994). Genetic control of sex determination in the germ line and soma of the housefly, Musca domestica. Development 120, 2531-2538. [DOI] [PubMed] [Google Scholar]
- Hjerrild, B. E., Mortensen, K. H. and Gravholt, C. H. (2008). Turner syndrome and clinical treatment. Br. Med. Bull. 86, 77-93. [DOI] [PubMed] [Google Scholar]
- Hou, X. S., Melnick, M. B. and Perrimon, N. (1996). marelle acts downstream of the Drosophila HOP/JAK kinase and encodes a protein similar to the mammalian STATs. Cell 84, 411-419. [DOI] [PubMed] [Google Scholar]
- Janzer, B. and Steinmann-Zwicky, M. (2001). Cell-autonomous and somatic signals control sex-specific gene expression in XY germ cells of Drosophila. Mech. Dev. 100, 3-13. [DOI] [PubMed] [Google Scholar]
- Kerkis, J. (1931). The growth of the gonads in Drosophila melanogaster. Genetics 16, 212-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, R. C., Bahns, M., Horowitz, R. and Larramendi, P. (1978). A mutation that affects female and male germ cells differentially in Drosophila melanogaster. J. Insect Morphol. Embryol. 7, 359-375. [Google Scholar]
- Kitadate, Y., Shigenobu, S., Arita, K. and Kobayashi, S. (2007). Boss/Sev signaling from germline to soma restricts germline-stem-cell-niche formation in the anterior region of Drosophila male gonads. Dev. Cell 13, 151-159. [DOI] [PubMed] [Google Scholar]
- Koubova, J., Menke, D. B., Zhou, Q., Capel, B., Griswold, M. D. and Page, D. C. (2006). Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc. Natl. Acad. Sci. USA 103, 2474-2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bras, S. and Van Doren, M. (2006). Development of the male germline stem cell niche in Drosophila. Dev. Biol. 294, 92-103. [DOI] [PubMed] [Google Scholar]
- Lehmann, R. and Nusslein-Volhard, C. (1986). Abdominal segmentation, pole cell formation, and embryonic polarity require the localized activity of oskar, a maternal gene in Drosophila. Cell 47, 141-152. [DOI] [PubMed] [Google Scholar]
- Lehmann, R. and Tautz, D. (1994). RNA in situ hybridization. Methods Cell Biol. 44, 567-598. [DOI] [PubMed] [Google Scholar]
- Li, J., Xia, F. and Li, W. X. (2003). Coactivation of STAT and Ras is required for germ cell proliferation and invasive migration in Drosophila. Dev. Cell 5, 787-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, J., Andrews, J., Pauli, D. and Oliver, B. (1998). Drosophila OVO zinc-finger protein regulates ovo and ovarian tumor target promoters. Dev. Genes Evol. 208, 213-222. [DOI] [PubMed] [Google Scholar]
- Marsh, J. L. and Wieschaus, E. (1978). Is sex determination in germ line and soma controlled by separate genetic mechanisms? Nature 272, 249-251. [DOI] [PubMed] [Google Scholar]
- McKeown, M., Belote, J. M. and Boggs, R. T. (1988). Ectopic expression of the female transformer gene product leads to female differentiation of chromosomally male Drosophila. Cell 53, 887-895. [DOI] [PubMed] [Google Scholar]
- McLaren, A. (2003). Primordial germ cells in the mouse. Dev. Biol. 262, 1-15. [DOI] [PubMed] [Google Scholar]
- McLaren, A. and Monk, M. (1981). X-chromosome activity in the germ cells of sex-reversed mouse embryos. J. Reprod. Fertil. 63, 533-537. [DOI] [PubMed] [Google Scholar]
- Mével-Ninio, M., Terracol, R., Salles, C., Vincent, A. and Payre, F. (1995). ovo, a Drosophila gene required for ovarian development, is specifically expressed in the germline and shares most of its coding sequences with shavenbaby, a gene involved in embryo patterning. Mech. Dev. 49, 83-95. [DOI] [PubMed] [Google Scholar]
- Mulligan, P. K., Campos, A. R. and Jacobs, J. R. (1996). Mutations in the gene stand still disrupt germ cell differentiation in Drosophila ovaries. Dev. Genet. 18, 316-324. [DOI] [PubMed] [Google Scholar]
- Nöthiger, R., Jonglez, M., Leuthold, M., Meier-Gerschwiler, P. and Weber, T. (1989). Sex determination in the germ line of Drosophila depends on genetic signals and inductive somatic factors. Development 107, 505-518. [DOI] [PubMed] [Google Scholar]
- Oliver, B., Perrimon, N. and Mahowald, A. (1987). The ovo locus is required for sex-specific germ line maintenance in Drosophila. Genes Dev. 1, 913-923. [DOI] [PubMed] [Google Scholar]
- Oliver, B., Kim, Y.-J. and Baker, B. S. (1993). Sex-lethal, master and slave: a hierarchy of germ-line sex determination in Drosophila. Development 119, 897-908. [DOI] [PubMed] [Google Scholar]
- Olmstead, A. W. and Leblanc, G. A. (2002). Juvenoid hormone methyl farnesoate is a sex determinant in the crustacean Daphnia magna. J. Exp. Zool. 293, 736-739. [DOI] [PubMed] [Google Scholar]
- Paduch, D. A., Bolyakov, A., Cohen, P. and Travis, A. (2009). Reproduction in men with Klinefelter syndrome: the past, the present, and the future. Semin. Reprod. Med. 27, 137-148. [DOI] [PubMed] [Google Scholar]
- Patel, N. (1994). Imaging neuronal subsets and other cell types in whole-mount Drosophila embryos and larvae using antibody probes. Methods Cell Biol. 44, 445-487. [DOI] [PubMed] [Google Scholar]
- Pauli, D., Oliver, B. and Mahowald, A. P. (1993). The role of the ovarian tumor locus in Drosophila melanogaster germ line sex determination. Development 119, 123-134. [DOI] [PubMed] [Google Scholar]
- Pennetta, G. and Pauli, D. (1997). stand still, a Drosophila gene involved in the female germline for proper survival, sex determination and differentiation. Genetics 145, 975-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter, R. (1994). The gene serpent has homeotic properties and specifies endoderm versus ectoderm within the Drosophila gut. Development 120, 1123-1135. [DOI] [PubMed] [Google Scholar]
- Schupbach, T. (1982). Autosomal mutations that interfere with sex determination in somatic cells of Drosophila have no direct effect on the germline. Dev. Biol. 89, 117-127. [DOI] [PubMed] [Google Scholar]
- Schüpbach, T. (1985). Normal female germ cell differentiation requires the female X chromosome to autosome ratio and expression of sex-lethal in Drosophlia melanogaster. Genetics 109, 529-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siwicki, K. K. and Kravitz, E. A. (2009). Fruitless, doublesex and the genetics of social behavior in Drosophila melanogaster. Curr. Opin. Neurobiol. 2, 200-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staab, S., Heller, A. and Steinmann-Zwicky, M. (1996). Somatic sex-determining signals act on XX germ cells in Drosophila embryos. Development 122, 4065-4071. [DOI] [PubMed] [Google Scholar]
- Steinmann-Zwicky, M. (1993). Sex determination in Drosophila: sis-b, a major numerator element of the X:A ratio in the soma, does not contribute to the X:A ratio in the germ line. Development 117, 763-767. [DOI] [PubMed] [Google Scholar]
- Steinmann-Zwicky, M. (1994a). Sex determination of the Drosophila germ line: tra and dsx control somatic inductive signals. Development 120, 707-716. [DOI] [PubMed] [Google Scholar]
- Steinmann-Zwicky, M. (1994b). Sxl in the germline of Drosophila: a target for somatic late induction. Dev Genet 15, 265-274. [DOI] [PubMed] [Google Scholar]
- Streit, A., Bernasconi, L., Sergeev, P., Cruz, A. and Steinmann-Zwicky, M. (2002). mgm 1, the earliest sex-specific germline marker in Drosophila, reflects expression of the gene esg in male stem cells. Int. J. Dev. Biol. 46, 159-166. [PubMed] [Google Scholar]
- Sturtevant, A. H. (1945). A gene in Drosophila Melanogaster that transforms females into males. Genetics 30, 297-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe, M. J., Darling, S. M. and Burgoyne, P. S. (1991). Spermatogenesis in XY, XYSxra and XOSxra mice: a quantitative analysis of spermatogenesis throughout puberty. Mol. Reprod. Dev. 30, 81-89. [DOI] [PubMed] [Google Scholar]
- Tomancak, P., Berman, B. P., Beaton, A., Weiszmann, R., Kwan, E., Hartenstein, V., Celniker, S. E. and Rubin, G. M. (2007). Global analysis of patterns of gene expression during Drosophila embryogenesis. Genome Biol. 8, R145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Deusen, E. B. (1977). Sex determination in germ line chimeras of Drosophila melanogaster. J. Embryol. Exp. Morphol. 37, 173-185. [PubMed] [Google Scholar]
- Van Doren, M., Williamson, A. and Lehmann, R. (1998). Regulation of zygotic gene expression in Drosophila primordial germ cells. Current Biol. 8, 243-246. [DOI] [PubMed] [Google Scholar]
- Warrior, R. (1994). Primordial germ cell migration and the assembly of the Drosophila embryonic gonad. Dev. Biol. 166, 180-194. [DOI] [PubMed] [Google Scholar]
- Wawersik, M., Milutinovich, A., Casper, A. L., Matunis, E., Williams, B. and Van Doren, M. (2005). Somatic control of germline sexual development is mediated by the JAK/STAT pathway. Nature 436, 563-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsu, J., Hayashi, M., Mukai, M., Arita, K., Shigenobu, S. and Kobayashi, S. (2008). Maternal RNAs encoding transcription factors for germline-specific gene expression in Drosophila embryos. Int. J. Dev. Biol. 52, 913-923. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.