Abstract
Objective
Plaque with dense inflammatory cells, including macrophages, thin fibrous cap and superficial necrotic/lipid core is thought to be prone-to-rupture. We report a time-resolved laser-induced fluorescence spectroscopy (TR-LIFS) technique for detection of such markers of plaque vulnerability in human plaques.
Methods
The autofluorescence of carotid plaques (65 endarterectomy patients) induced by a pulsed laser (337 nm, 0.7 ns) was measured from 831 distinct areas. The emission was resolved spectrally- (360–550 nm range) and temporally- (0.3 ns resolution) using a prototype fiber-optic TR-LIFS apparatus. Lesions were evaluated microscopically and quantified as to the % of different components (fibrous cap, necrotic core, inflammatory cells, foam cells, mature and degraded collagen, elastic fibers, calcification, and smooth muscle cell of the vessel wall).
Results
We determined that the spectral intensities and time-dependent parameters at discrete emission wavelengths 1) allow for discrimination (sensitivity >81%, specificity >94%) of various compositional and pathological features associated with plaque vulnerability including infiltration of macrophages into intima and necrotic/lipid core under a thin fibrous cap, and 2) show a linear correlation with plaque biochemical content: elastin (P<0.008), collagen (P<0.02), inflammatory cells (P<0.003), necrosis (P<0.004).
Conclusion
Our results demonstrate the feasibility of TR-LIFS as a method for the identification of markers of plaque vulnerability. Current findings enable future development of TR-LIFS based clinical devices for rapid investigation of atherosclerotic plaques and detection of those at high-risk.
Keywords: atherosclerosis, diagnosis, fluorescence spectroscopy
1. INTRODUCTION
Numerous studies 1 have suggested that acute cardiovascular events are most commonly triggered by the structural weakening and disruption of the thin fibrotic cap of fibroatheroma followed by thrombus formation. Generally, this process occurs in atherosclerotic lesions known as rupture-prone or vulnerable plaques. Plaque “vulnerability” has been associated with several pathologic features including a thin fibrous cap, a large lipid pool, and increased macrophage and other inflammatory cell infiltration within the cap. Early detection and treatment of such plaques before rupture depend upon the availability of tools able to characterize in vivo plaque composition through minimally-invasive and nondestructive ways. Most clinical techniques identify luminal diameter (stenosis), wall thickness, and plaque volume, but are inefficient in identifying the rupture-prone plaque 1–6. New diagnostic techniques (including catheter-based) to localize and characterize vulnerable plaques are needed. Potential intravascular diagnostic techniques for assessment of plaque vulnerability include: magnetic resonance (MR) spectroscopy 6–8, intravascular ultrasound (IVUS – including high-frequency, elastography) 6,9, optical coherence tomography (OCT) 10–13, thermography 14,15, and optical spectroscopy methods (Raman, near-infrared (NIR), diffuse reflectance, and steady-state fluorescence) 5,16,17. We report here an innovative time-resolved fluorescence spectroscopy approach that has potential to detect distinct markers of plaque vulnerability as a stand alone technique or to complement the other imaging or spectroscopy modalities in detection of vulnerable plaques.
This study describes a fiber-optic based time-resolved laser induced fluorescence spectroscopy (TR-LIFS) technique that can be used in vivo to recognize the biochemical makeup of tissues including structural proteins, enzyme cofactors and lipid components. In previous studies, we validated this technique on fluorescent biomolecules constituent of normal and diseased arteries (elastin, collagens, free cholesterol, cholesteryl oleate and cholesteryl linoleate, LDL) 18–21, in human coronary and aortic postmortem specimens 21,22, and in vivo in an atherosclerotic rabbit model 23. Depending upon the light excitation wavelength used and fiber optic excitation-collection geometry, TR-LIFS facilitates evaluation of tissue composition within small tissues volume (∼0.6–1.5 mm diameter × 150–250 µm penetration depth), thus feasible for assessing the intimal composition of atherosclerotic plaques. Overall, TR-LIFS has potential for direct evaluation of relative changes of the elastin/collagen and collagen/lipid contents; and indirect assessment of cap thickness and infiltration of macrophage and other inflammatory cells affecting the collagen/lipid content within the excitation-collection tissue volume.
Carotid plaque represents an optimal study model for the validation of new optical devices for detection of plaque composition patients. This allows for direct access of plaque in vivo during carotid endarterectomy (CEA) using fiber optic probes (with or without distal rigidity) and without the need of intravascular procedures. The routine removal of the plaque during CEAs permits extensive TR-LIFS studies ex vivo in fresh plaque specimens that account for the plaque composition heterogeneity. Furthermore, it facilitates a direct validation of optical results against histopathological analysis. Consequently, we conducted this study in human carotid atherosclerotic plaque both in vivo in patients undergoing endarterectomy and ex vivo in the explanted plaques.
The goals of this study were 1) to use TR-LIFS to evaluate the composition of human carotid atherosclerotic plaques and to determine whether a TR-LIFS technique can distinguish fibrotic caps rich in macrophages and inflammatory cells and plaques with a necrotic/lipid core under a thin cap (rupture-prone) from plaques with caps rich in collagen (stable); and 2) to determine the TR-LIFS derived spectroscopic parameters that can be correlated to features of plaque vulnerability.
2. METHODS and MATERIALS
2.1. Patients and plaque samples
A total of 65 patients were included in this study. The patients were scheduled for CEA and underwent the planned operation. When clinical conditions of the patient allowed (14 patients), during the operation the TR-LIFS fiber optic probe was introduced through the common carotid arterotomy and the plaque was spectroscopically investigated (total: 28 locations, 1–3 measurements per plaque, blood was. During in-vivo investigations the blood was evacuated from a cross-clamped artery. The plaque was then removed surgically and immediately spectroscopically re-evaluated in detail ex-vivo. When investigations in vivo were not possible, the TR-LIFS studies were conducted only in ex vivo specimens. To account for the heterogeneity within plaque composition and structure, TR-LIFS data were acquired from several areas of the plaque (total: 813 locations, approximately 10 measurements per plaque). The time between plaque resection and TR-LIFS examination was less than 2 hours. The specimens were kept moist with saline (intermittently delivered drops) prior to and during TR-LIFS investigation. The location of each spectroscopic measurement was marked using India ink and the plaque specimen sent for histopathologic analysis. This study was approved by the institutional review board and all patients provided informed consent.
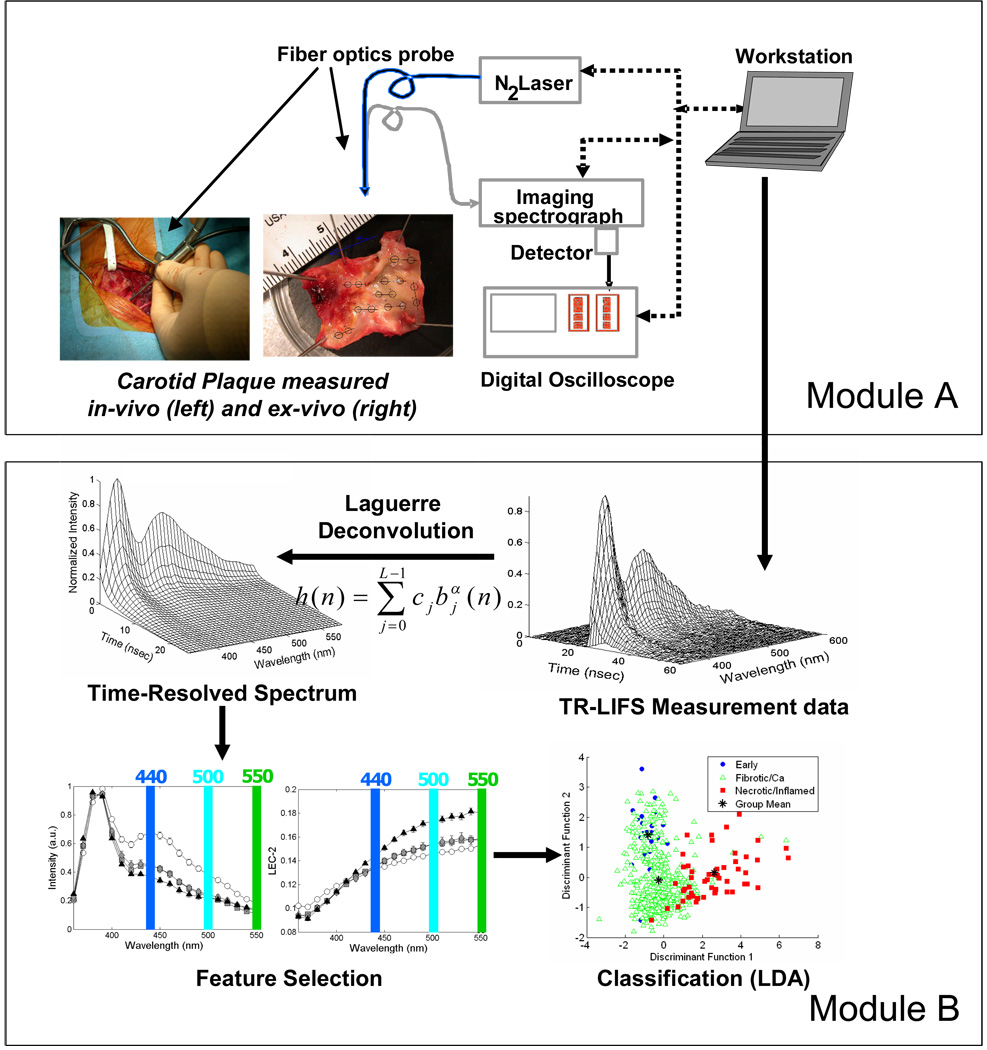
2.2. TR-LIFS Instrumentation, Measurements and Data Analysis (Figure 1)
Figure 1.
Schematic of the TR-LIFS system used to induce, collect, record and analyze the autofluorescence emission of the carotid plaques.
The detailed description of this methods section (TR-LIFS apparatus, experimental data acquisition protocols and analysis of the TR-LIFS data) is provided in the Appendix of this paper and in our earlier publications. 18,23, 24, 26,27 To characterize the fluorescence emission a set of parameters were used. This includes discrete spectral intensity values (Iλ) and two sets of parameters time-resolved parameters: the average lifetime (τf) values and the normalized value of the corresponding Laguerre expansion coefficients (LEC) Cj. Thus, a complete description of each sample fluorescence as a function of emission wavelength, λE, was given by the variation of a set of spectroscopic parameters (Iλ, τf, and LECs).
2.3. Histopathological Analysis
The processing and analysis of the histopathological samples including hematoxylin and eosin (H&E), trichrome/elastin, CD68 (for macrophages) and CD45 (for leukocytes) are detailed in the Appendix of this paper. The histopathologic sections were categorized based on intima thickness (TH), cap thickness (CTH), the presence of calcification, necrosis, and inflammation (lymphocytes and/or macrophage infiltration). The following six groups were defined following the nomenclature similar with that reported by Virmani et al 25: a) intimal thickening (IT) with TH < 200 µm and no necrosis, inflammation, or calcification; b) fibrous plaque/thick-cap (FP) with TH/CTH > 200 µm and no necrosis, inflammation or calcification; c) fibrocalcific plaque/thick-cap (FC) with TH/CTH >200 µm and significant calcification (≥20%), but no necrosis or inflammation; d) low-inflamed plaque/thick-cap (low-INF) with TH/CTH >200 µm and some necrosis/inflammation (<20%); e) inflamed plaque/thick-cap (INF) with TH/CTH >200 µm, some necrosis (<30%), and significant inflammation (≥20%); and f) superficial necrotic core covered by a thin fibrotic cap (NEC) with TH >200, CTH <200 µm and significant necrosis (≥30%).
2.4. Statistical Analysis and Classification
A set of spectroscopic parameters (Iλ, τf and LECs at specific λE’s) likely to provide means of discrimination among different compositional features of the carotid plaque were identified by following methods we previously reported23,27,28 and reviewed in the Appendix. In addition, in order to investigate if these spectroscopic parameters reflect plaque composition, a correlation analysis was performed between the selected parameters and the relative content of the main plaque components (elastin, collagen/SMC, inflammatory cells and necrotic areas). For this correlation analysis, the mean value of the spectroscopic parameter was computed for each concentration value from 0–100%, in steps of 10% (Fig. 4). A linear correlation analysis was then applied between the spectroscopic parameter mean values and the plaque component concentrations. Correlation coefficients “r” and P-values were reported for each parameter-concentration correlation analysis. A P-value of <0.05 was assumed to indicate a statistically significant correlation. All statistical analysis was conducted using SPSS version 12.0. A stepwise linear discriminant analysis (SLDA) approach was adopted to generate a classification model (discriminant functions) and for sample classification. This approach (also described in the Appendix) was applied previously by our group to the classification of arterial plaque composition in a rabbit athereosclerotic model.23,28, 29
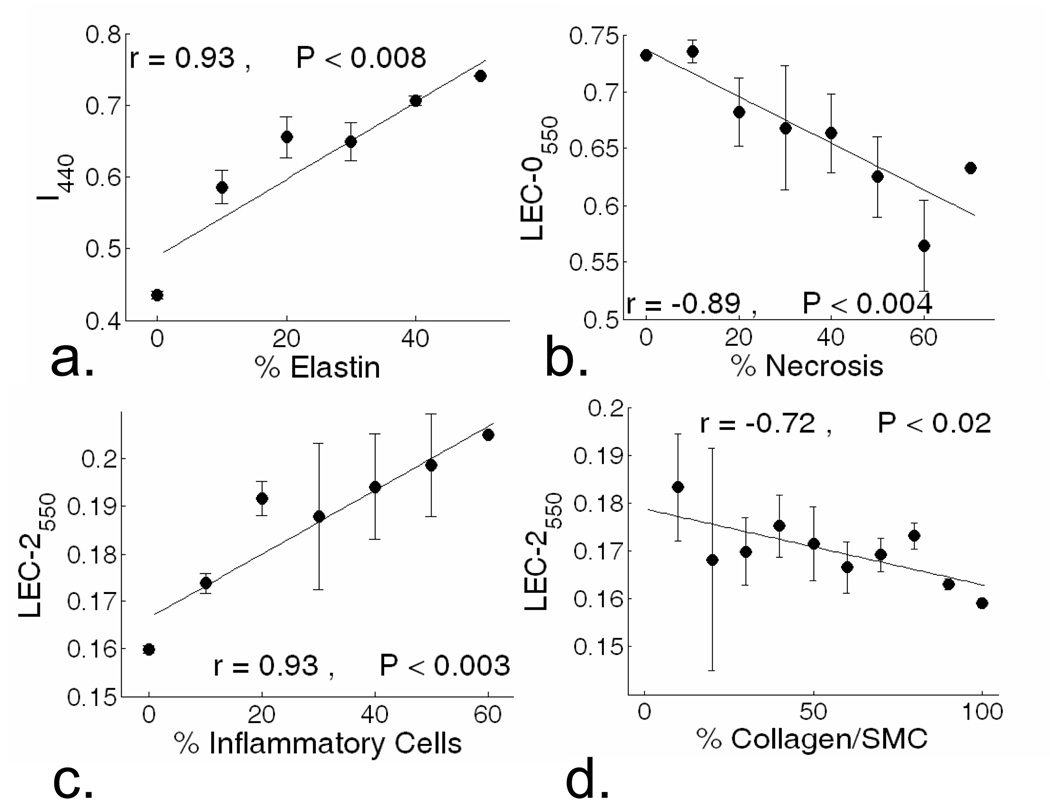
Figure 4.
Correlation analysis between spectroscopic parameters and plaque biochemical constituents. a) I440 positively correlated with elastin content. b) LEC-0550 negative correlated with necrosis content. c) LEC-2550 positively correlated with inflammation, and d) negatively correlated with collagen/SMC content.
3.RESULTS
3.1.Histolopathology
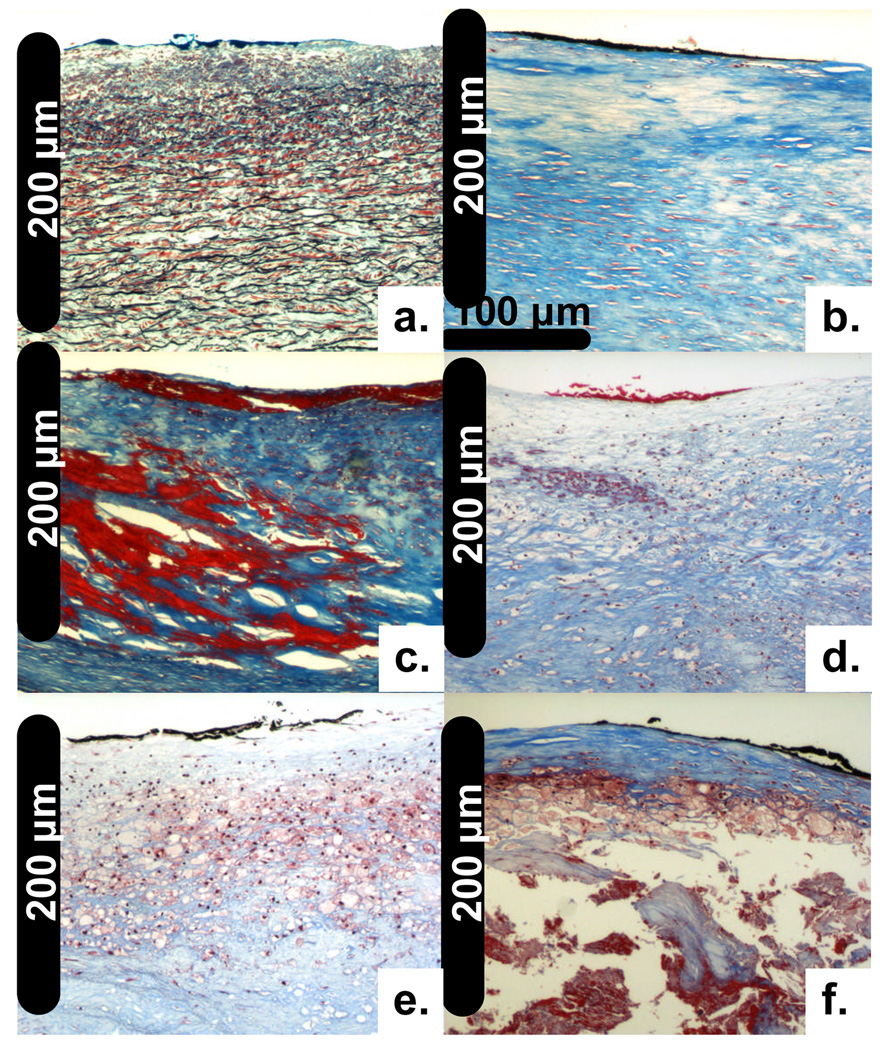
The plaques TR-LIFS investigated ex-vivo were histopathologicaly classified as follows: IT (n=28), FP (n=420), FC (n=38), low-INF (n=273), INF (n=34), and NEC (n=20). Representative features/sections for each histopathological group are shown in Figure 2. The IT samples were characterized by a thin intima (88±39 µm) overlaying a normal media rich in elastin fibers and SMC (Figure 2a). The composition of the FP samples was dominated by collagen fibers with various degrees of SMC accumulation (Fig. 2b). The FC samples presented various degrees of calcification (Figure 2c). The Low-INF samples were characterized by collagen fibers with scattered infiltration of lymphocytes and/or macrophages (Figure 2d). The INF samples presented a significant amount of inflammatory cells, mainly macrophages (Figure 2e). The NEC samples were characterized by a necrotic core with a thin fibrous cap (70±37 µm) with or without inflammatory cell infiltration (Figure 2f). Adjacent CD45 and CD68 stained sections (not shown) confirmed the presence of lymphocytes and macrophages, respectively. Out of the 28 TR-LIFS measurement conducted in vivo only 10 of them could be correlated with the histopathological analysis of plaques and classified as follows: IT (n=3) and FP (n=7). This was due to difficulties on accurately marking in vivo the location from where the TR-LIFS measurement was taken.
Figure 2.
Representative carotid plaque histological sections (elastic trichrome stain, 20x magnification). a) Intima thickening (IT); b) Fibrous plaque (FP); c) Fibro-calcified (FC); d) Low-Inflammation (Low-INF); e) Inflammation (INF); f) Necrotic (NEC).
3.2. Time-resolved fluorescence plaque characterization
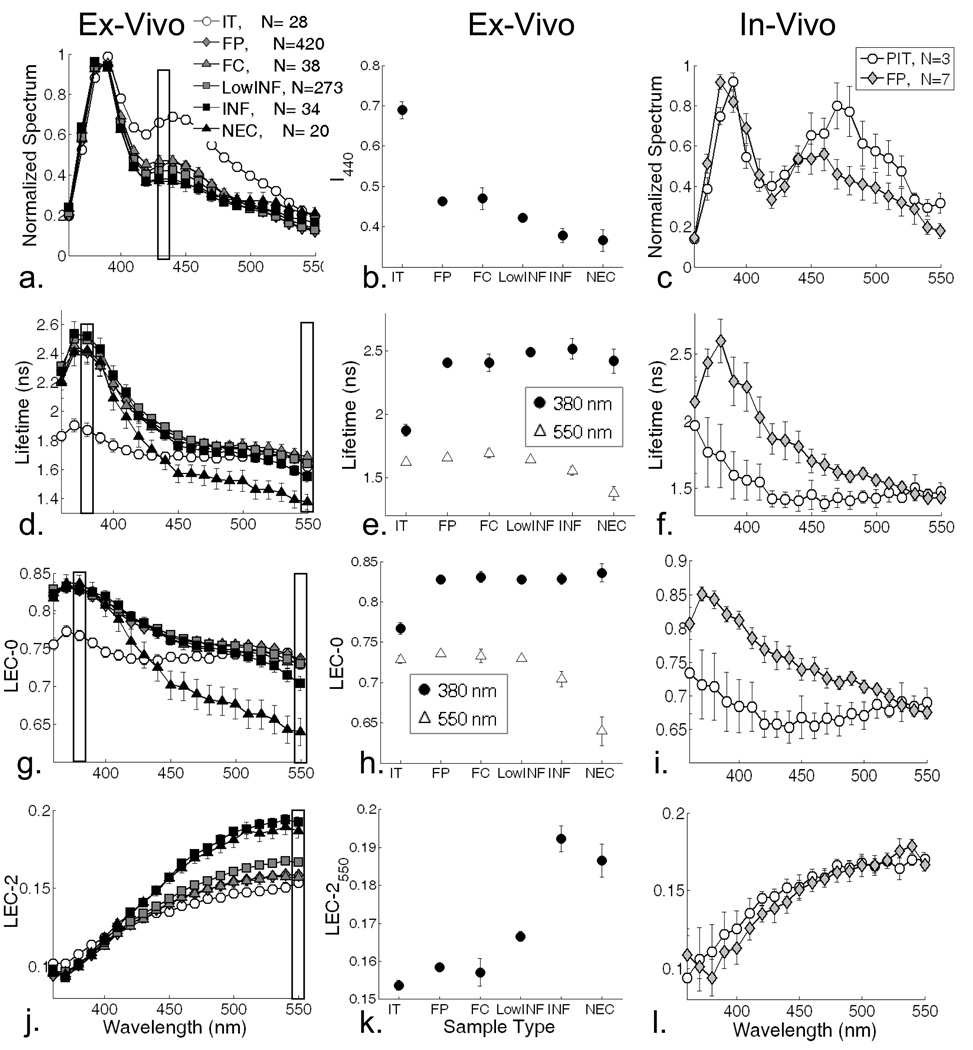
The time-resolved fluorescence spectrum of the atherosclerotic plaques can be fully described by the variation of the spectroscopic parameters Iλ, τf, and LECs as a function of λE. Figure 3 depicts these parameters for each lesion type/group investigated.
Figure 3.
Left panels: Spectroscopic parameters as a function of emission wavelength derived from the time-resolved spectra measured in ex vivo samples. Middle panel: statistical group comparisons (mean ± SE) for specific wavelengths – data corresponding to measurements ex vivo. Right panels: Spectroscopic parameters as a function of emission wavelength derived from the time-resolved spectra measured in vivo. Spectroscopic parameters: normalized intensity (a), (b), (c), average lifetime (d), (e), (f), LEC-0 (g), (h), (i), and LEC-2 (j), (k), (l).
Emission spectra and intensity values
The emission spectra of all histopathological groups measured ex vivo (Figure 3a) were largely overlapped, except for the IT group which presented a higher intensity in the 400–500 nm spectral range (Iλ= 0.69±.03 at 440 nm) when compared with all other groups (e.g. Iλ smaller than 0.5). The Iλ values at 440 nm (figure 3b) also showed also slightly higher values for the FP (0.46±.01) and FC (0.47±.03) groups when compared to the INF (0.38±.03) and NEC (0.37±.03) groups. Characteristics indicating that IT could be distinguished using intensities values in red-shifted wavelength range (Fig. 3a–b). The characteristics of the emission spectrum of the in vivo measurements for the IT and FP samples were similar to those measured ex vivo (Figure 3c). Nevertheless, the valley at ∼415 nm, well recognized to correspond to the Soret band of hemoglobin absorption 30, was accentuated in the in-vivo measurements.
Fluorescence average lifetimes
For ex vivo measurements, the analysis of average lifetime values along the emission wavelength (Figure 3d) showed that for the blue-shifted (below 420 nm) emission wavelengths IT samples were characterized by short lifetime values (τf=1.87±.09 ns at 380 nm) when compared with those of all other groups (τf longer than 2.4 ns at 380 nm). The IT’s lifetime values remained nearly constant along the emission spectrum while a decrease of lifetime with emission wavelength was observed for all other groups. The largest variation of the average lifetime with wavelength was observed for NEC samples. For example, the emission of NEC in the red-shifted (above 440 nm) wavelength range was characterized by the shortest lifetime values (τf=1.38±.04 ns at 550 nm) when compared with those of all other groups (τf longer than 1.55 ns at 550 nm). Characteristics indicating that NEC plaques could be distinguished using lifetime values in 500–550 nm range (Fig. 3d–e). The average lifetimes of IT and FP measured in vivo (Figure 3f) followed similar trends to those measured ex vivo. However, the averages lifetime values of IT were shorter above 400 nm (τf=1.3–1.5 ns ns) in vivo relative to ex vivo measurements.
Laguerre expansion coefficients
The variation of the first four LECs (zero-order: LEC-0 through the 3rd -order: LEC-3) as a function of emission wavelength provided an additional means of characterizing and comparing each tissue group. The LEC-0 changes with emission wavelength followed similar trends as those described above for the average lifetimes (Figure 3g–h). For the blue-shifted (below 420 nm) wavelengths IT samples were characterized by smaller LEC-0 values (e.g. 0.77±.01 at 380 nm) when compared with those of all other groups (above 0.83 at 380 nm). For the red-shifted (above 440 nm) wavelengths NEC samples were characterized by small LEC-0 values (0.70±.01 at 550 nm) when compared with those of all other groups (larger than 0.73 at 550 nm). The changes of the 2nd-order LEC (LEC-2) with the wavelength of emission (Figure 3j–k) showed that for red-shifted wavelengths (above 450 nm) the INF and NEC plaque areas are characterized by higher LEC-2 values (0.192±.003 and 0.187±.004, respectively, at 550 nm) with respect to all other groups (smaller than 0.17 at 550 nm). In addition, higher LEC-2 values were observed for Low-INF (0.167±.001) when compared to the fibrotic groups FC (0.157±.003) and FP (0.159±.001); and the IT (0.154±.004) group. The variation of LECs of IT and FP measured in vivo (Fig. 3i and 3l) followed similar trends to those measured ex vivo. Nevertheless, in vivo relative to ex vivo investigations show that the LEC-0 values of IT samples were smaller in vivo (0.65–0.7).
3.3.Correlation between plaque composition and spectroscopic parameters
Figure 4 depicts the linear correlation between plaque biochemical content and spectroscopic parameters within the ROI measured ex vivo. Plaque elastin content, ranging from 0% (in the advanced FP, FC, INF and NEC) to 50% (in IT) showed a positive correlation with the I440 (Figure 4a). Necrotic area, ranging from 0% (in stable plaques) to 70% (in NEC), presented a negative correlation with the LEC-0550 nm (Figure 4b). A similar correlation was determined for the average lifetime values (data not shown). The inflammatory cell (lymphocyte/macrophage) content, ranging from 0% (in stable plaques) to 60 % (in INF and NEC), showed a positive correlation with the LEC-2550 (Figure 4c). In contrast, LEC2550 (figure 4d) was negatively correlated to the collagen/SMC content.
3.4.Feature Selection
The application of one-way ANOVA to multidimensional TR-LIFS data sets (Iλ, τf, and LECs values at each emission wavelength) to ex vivo data sets have resulted in a reduction of this set to 10 statistically significant features: I440, τ380, τ550, LEC0380, LEC0550, LEC1380, LEC1550, LEC2500, LEC2550, LEC3380. The wavelengths with the lowest P-value were chosen for each parameter to form the new feature space.
3.5.Classification
Two classification goals were defined aiming to discriminate: a) IT, FP/FC (as one group) and INF/NEC (as one group) lesions; and b) IT, FP/FC/low-INF (as one group) and INF/NEC (as one group) lesions. The FP and FC lesions showed significant overlap in all the spectroscopic parameters. This indicated that classification between these two groups was not feasible based on TR-LIFS alone. Therefore, for classification purpose, FP and FC lesions were grouped together. Also, inflammation and necrosis represent two key markers of vulnerability often present simultaneously in plaques, thus for classification purpose, INF and NEC were grouped together.
Results of the classification aiming to discriminate IT, FP/FC and INF/NEC lesions are shown in Table 1. The overall cross-validation classification performance was 74.3%. IT was discriminated with sensitivity (SE) and specificity (SP) larger than 80%. However, there was an appreciable overlap between IT and FP/FC. Also, the INF and NEC lesions were detected with high SE (larger than 80%) and high SP (larger than 90%). Results of the classification aiming to discriminate IT, FP/FC/Low-INF and INF/NEC lesions are given in the Appendix (Table 2).
Table 1.
Classification accuracy (sensitivity and specificity): discrimination of IT, FP/FC and INF/NEC.
| Fluorescence Spectroscopy | SE | SP | |||
|---|---|---|---|---|---|
| IT | FP/FC | INF/NEC | (%) | (%) | |
| IT (N=28) | 25 | 3 | 0 | 89.3 | 80.7 |
| FP/FC (N=458) | 99 | 332 | 27 | 72.5 | 84.2 |
| INF/NEC (N=54) | 0 | 10 | 44 | 81.5 | 94.4 |
4. DISCUSSION
This is the first study to demonstrate that a TR-LIFS technique allows for detection of markers of vulnerability in human atherosclerotic plaques, including infiltration of macrophage and other inflammatory cells into the intima and lipid/necrotic core under a thin fibrous cap. Also, we report here a rather comprehensive analysis of time-resolved fluorescence emission features that allows for discrimination of various compositional and pathological features of human atherosclerotic plaques. Current findings represent the ground base for further development of intravascular TR-LIFS diagnostic devices.
TR-LIFS features in plaque characterization and qualitative discrimination of rupture-prone plaques
Intrinsically, TR-LIFS measurements are rich in information content. The measurement allows recording of orthogonal features of the fluorescent emission from tissue that encompasses a detailed description of fluorescence intensity decay along the emission spectrum of each sample. Moreover, the application of the Laguerre deconvolution approach to TR-LIFS data, developed by our group and used in this study, generated not only conventional fluorescence lifetime value(s) but also a new set of spectroscopic-related parameters (set of Laguerre coefficients). Consequently, numerous fluorescence parameters are available for characterization of the fluorescence emission from atherosclerotic plaques and for identification of plaque type. As exemplified in Figure 3, upon 337 nm excitation the emission spectrum and the discrete intensity values within 420–500 nm wavelengths range can be used to delineate the early plaques. The average lifetime and LEC-0 values in two emission wavelength ranges, 360–400 nm and 450–550 nm, permit separation of early plaques and necrotic plaques, respectively. In addition, the LEC-2 coefficients in 450–550 nm range facilitate discrimination of both inflamed and necrotic plaques. Important to note is the use of LECs to describe the fluorescence intensity decay of the atherosclerotic plaques. These coefficients are key parameters in the discrimination of inflamed and necrotic plaques, the two most common forms of vulnerable plaque. Also, we identified that the trends of certain LECs values (e.g. LEC-0 and LEC-2 at 550 nm emission, Figure 3h) follow the plaque transition from stable characteristics (FP, FC) to features indicating weakening of the fibrotic cap (LowINF) and then to features associated with prone to rupture plaques (INF/NEC).
The large number of spectroscopic parameters generated by a TR-LIFS measurement provides a means for discrimination of distinct compositional features of atherosclerotic plaques. However, acquisition and analysis of a large database is time expensive and thus may preclude translation of TR-LIFS systems to practical applications. This study has identified pathways that can overcome such drawbacks and enable development of TR-LIFS systems with the near real time display of diagnostic information, thus leading to solutions that are clinically relevant. For example, we determined that a small subset of spectral and time-resolved parameters (10 parameters) can provide a means for delineating early from advanced lesions, and more important, the presence of necrosis and inflammatory cells (foam cells and lymphocytes) in the plaques. Moreover, our results indicate that these spectroscopic parameters are retrieved from four discrete emission wavelengths (380 nm, 440 nm, 500 nm and 550 nm). This finding is particularly important as it allows for the design of TR-LIFS systems that collect fluorescence decay at a limited number of wavelength bandwidth as recently reported30. Consequently, it will allow for construction of TR-LIFS devices with even shorter data acquisition time (less that 1 sec for each point measurement). Also, relevant to practical applications are the analytical methods employed in this study (Laguerre expansion of kernels combined with linear discriminant analysis) that can facilitate near real-time TR-LIFS data analysis. In a previous study, we demonstrated the ability of the Laguerre expansion technique for fast (less than 35 milliseconds per wavelength) deconvolution of the fluorescence impulse function and analysis of biological systems 27,28. Thus, a fast real-time classification algorithm can be developed by assigning a discriminant score for different types of tissue or plaques. The discriminant score can then be compared with the acquired fluorescence from the unknown tissue so as to classify the tissue and display the diagnostic information in near real-time.
TR-LIFS features in the characterization of arterial wall composition and pathway for quantitative discrimination of rupture-prone plaques
The current TR-LIFS study of carotid plaques complements and is in agreement with previous work conducted in human aorta and coronary arteries that identified relationships between the fluorescence emission of normal and diseased arterial wall and the emission of the intrinsic fluorescent constituents in arterial tissue.18–21,31,32 Upon UV irradiation, the fluorescence emission of arteries originates from several intrinsic fluorescent constituents which we summarized previously.20,21 These include structural proteins (elastin and collagens) and lipid components (free cholesterol, cholesterol esters, lipoproteins). Nevertheless, structural proteins elastin and collagen types I and III are the primary fluorophores in normal and diseased arteries. Elastin (broad band: 70 nm full with at half maximum (FWHM) with peak at ∼ 400 nm; average lifetime: ∼2 ns), largely found in the media and internal elastic lamina, dominates the fluorescence emission of normal arterial wall and early plaques; while collagen type I (narrow band: ∼40 nm FWHM with peak ∼385 nm; average lifetime: 3–4 ns) is the main contributor to the fluorescence of fibrotic plaques.20,21 In current the study (Figure 3) the spectral signature (broad band: 80 nm FWHM) and the fluorescence lifetime (∼1.9 ns) values obtained for carotid IT samples demonstrate the significant contribution of elastin fluorescence to their overall fluorescence emission; whereas, the fluorescence emission signature (narrow band: ∼40 nm, lifetime ∼2.5 ns) of all other types of arterial plaques was dominated by collagen fluorescence.
The atherosclerotic stages between normal and advanced fibrotic are characterized by accumulation within the intima of various types and amounts of lipids localized either in macrophage foam cells or in the extracellular space. These lipids alter the intimal composition and consequently modulate the elastin-collagen fluorescence signature, in particular, the time-domain features. Lipids, typically, are characterized by a shorter-lived fluorescence when compared with structural proteins fluorescence 19–21. Accumulation of macrophage, smooth muscle cells, cellular debris and other plaque constituents can alter the intimal morphology, dislocate the structural proteins, and change the constituent relative content within a particular ROI. Thus, in turn these conditions can also alter the elastin-collagen fluorescence signature. It was shown that human coronary and aortic plaques with large lipid pools and thin fibrotic caps have a shorter fluorescence lifetime (∼2.7 ns) than that of fibrotic plaques (∼3.2 ns)20,21. Also, a shorter-lived fluorescence was reported for fatty streaks in human arteries (∼1 ns) when compared to that of normal arterial wall (∼2.2 ns) 21. Similar trends were observed in a rabbit atherosclerotic model, where macrophage-rich lesions presented a faster decay dynamic than the collagen-rich lesions or normal artery 23. In this study, the fluorescence lifetime of INF and NEC samples with relatively high lipid and macrophage vs collagen content showed a faster fluorescence intensity decay dynamic (at 550 nm emission ∼1.5 ns and ∼1.3 ns, respectively) than that of FP and FC with a relatively low lipid vs collagen content (∼1.7 ns). Albeit no direct comparison between current fluorescence lifetime values and the values previously reported can be made due to differences of experimental conditions and methods of data analysis, current results confirm that the presence of lipid components in plaque generally decreases the overall fluorescence lifetime of the atherosclerotic tissue.
In addition, this study shows that specific spectroscopic parameters are linearly correlated to the biochemical content of plaques; thus it opens a new pathway for discrimination of plaques based on their biochemical composition (quantitative classification). We determined that parameters derived from the red-shifted wavelength range such as I440 (Figure 3b) are linearly correlated (positive) to that of elastin content within the ROI. Thus, the analysis of intensity values has potential to identify normal arterial wall. LEC-2550 (Figure 4) was found positively correlated with the macrophage and inflammatory cells content (markers of plaque instability) and negatively correlated with collagen and smooth muscle cell content (markers of plaque stability). This is an important finding suggesting that LEC-2 values represent key parameters in the discrimination of rupture-prone plaques. Yet another Laguerre coefficient LEC-0550 was found linearly correlated (negative) with the necrotic area within the ROI, suggesting that analysis of LEC-0 values may allows for identification of necrotic areas under the fibrotic cap. As the penetration depth of irradiation at about 337 nm in the artery ranges between 150–250 µm21, detection of plaques with thin fibrotic caps and large lipid or necrotic core is possible.
Calcium deposits were histopathologically identified in numerous plaques investigated in this study. Calcium is known to exhibit sharp fluorescence emission peaks in the 350–660 nm range upon 308 nm excitation, 31 but calcium does not fluoresce upon 335 nm. The latter condition was also observed in the current study (excitation 337 nm). The fluorescence emission of plaques with calcification was dominated by the collagen fluorescence typically present in calcified plaques in the samples investigated here. Thus, calcified plaques can not be distinguished from fibrotic plaques using 337 nm. As a result, in the current study we were not able to distinguish fibrotic from fibrocalcified lesions. The use of excitation wavelengths within the calcium absorption bands, however, can overcome this limitation.
In vivo vs ex vivo TR-LIFS measurements
The TR-LIFS study of surgical removed plaques permitted acquisition of a large number of measurements that accounted for the heterogeneity of composition, capturing the most clinical relevant biochemical features (including inflammation), and enabling accurate correlation with the histopathological features at each measurement. While the main chemical components and morphological structures of atherosclerotic plaques are expected to undergo minimal changes after surgical removal of the plaque, morphometric effects such us shrinkage may affect the relative content of fluorescent constituents within a particular ROI. Current results showed that TR-LIFS features (spectral feature, average lifetimes, Laguerre coefficients) retrieved from in vivo measurements during endarterectomy resemble those obtained ex vivo. Nevertheless, some effects were identified, primary on the spectral profile. As expected, much stronger hemoglobin absorption was observed in-vivo when compared to ex-vivo measurements, demonstrated as an enhanced spectral valley at ∼415 nm. This effect was more enhanced on near normal areas (IT) rather than fibrotic/fibrous cap areas. However, the trends of the time decay parameters along emission wavelengths remained virtually unchanged. This demonstrates, as expected, that time-resolved measurements are minimally influenced by fluctuation in fluorescence intensity due to the presence of the endogenous absorbers such as blood; and thus a TR-LIFS approach is more robust for investigations in clinical environment. We also determined that the actual values of time decay parameters of IT appear to change more after plaque explantation than those of the fibrotic plaques. We plan to study this aspect further and to identify if such differences (in vivo vs ex vivo) reflect changes in the tissue thickness between ex vivo and in vivo samples due to lack of longitudinal stress in the ex vivo case. Challenges with accurate identification of the location of the spectroscopic measurement after plaque explantation and limited time to collect data during surgery have resulted in only a few point measurements per plaque.
TR-LIFS in atherosclerotic plaque diagnostics: clinical translation perspective and current study limitations
As a first step in the development of intravascular catheters to be used in characterization and diagnosis of atherosclerotic plaques, the present study demonstrated that TR-LIFS measurements can be easily conducted via a fiber-optic interface with biological tissue. As noted above, modalities for fast data acquisition/analysis were also identified which demonstrate the feasibility of TR-LIFS technique for near-real time analysis and display of diagnostic data. However, engineering of flexible catheters and methodologies for TR-LIFS intravascular application has to be further addressed. The relatively shallow UV light penetration of excitation light makes TR-LIFS a suitable method for detection of markers of plaque vulnerability in thin fibrotic caps, but also limits the ability of this technique 1) to assess plaque morphology associated with other markers of plaque vulnerability such as the size of the lipid pool or expansive (positive) remodeling or 2) to distinguish other important features involved in plaque pathology including pathologic intimal thickening25 vs fibrous thick-cap atheroma. Such limitations, however, can be further addressed though complementary imaging techniques. As earlier suggested earlier for other spectroscopic methods currently evaluated for clinical diagnostic of vulnerable plaques (e.g. NIR and Raman spectroscopy) an optimum approach to vulnerable plaque detection would need to incorporate structural definition of a high-resolution modality (e.g. OCT, intravascular MRI or high-frequency IVUS) with biochemical processes detected by TR-LIFS. Concurrent studies in our lab are investigating the potential of combining TR-LIFS and IVUS modalities for simultaneous discrimination of the morphological and compositional features of atherosclerotic plaques.
Conclusion
The results of this study have demonstrated the ability of TR-LIFS to detect biochemical features in atherosclerotic plaques which are clinically relevant. Due to TR-LIFS high specificity in the detection of inflammation in human atherosclerotic plaques, a potential application of this technique is clinical diagnosis of high-risk plaques through intravascular fiber-optic catheters. Moreover, future optimization of TR-LIFS data acquisition/analysis approaches, as identified here, will enable near-real time (less than 1 second) tissue diagnosis. Such catheters could work in conjunction with either conventional catheterization procedures (angioscopy and IVUS) or newly developed optical techniques currently evaluated in clinical settings such as OCT, NIR, and Raman 5,11,16. Due to its ability to detect the compositional makeup of the arterial wall, another potential application of TR-LIFS is the clinical management of stenting procedures. Knowledge of arterial wall composition at the time of percutaneous coronary interventions may allow the physician to “personalize” the selection of the type of stent (e.g. uncoated or drug-eluting), thus more effectively reducing the risk of restenosis and target-vessel revascularization. Yet, the sensitivity of TR-LIFS measurement to the arterial wall content in structural proteins suggests the potential application of this technique in assessing the effect of experimental drugs in plaque regression and stabilization. Thus, TR-LIFS may also play an important role in drug-discovery studies in vivo in animal models.
Supplementary Material
ACKNOWLEDGEMENT
This study was supported by the National Institute of Health Grant R01 HL 67377. We thank the vascular surgeons and nurses at the UCLA Vascular Center for their support with data collection from patients; and Dr. Pramod Butte for his contribution to data acquisition.
REFERENCES
- 1.Waksman R, Serruys PW, Dunitz M. Handbook of the vulnerable plaque. London, UK: Taylor & Francis Group; 2004. [Google Scholar]
- 2.Libby P, Aikawa M. Stabilization of atherosclerotic plaques: New mechanisms and clinical targets. Nat Med. 2002;8:1257–1262. doi: 10.1038/nm1102-1257. [DOI] [PubMed] [Google Scholar]
- 3.Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient-A call for new definitions and risk assessment strategies: Part I. Circulation. 2003;108:1664–1672. doi: 10.1161/01.CIR.0000087480.94275.97. [DOI] [PubMed] [Google Scholar]
- 4.MacNeill BD, Lowe HC, Takano M, Fuster V, Jang IK. Intravascular modalities for detection of vulnerable plaque: current status. Arterioscler Thromb Vasc Biol. 2003;23:1333–1342. doi: 10.1161/01.ATV.0000080948.08888.BF. [DOI] [PubMed] [Google Scholar]
- 5.Moreno PR, Muller JE. Identification of high-risk atherosclerotic plaques: a survey of spectroscopic methods. Curr Opin Cardiol. 2002;17:638–647. doi: 10.1097/00001573-200211000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Fayad ZA, Fuster V. Clinical imaging of the high-risk or vulnerable atherosclerotic plaque. Circ. Res. 2001;89:305–316. doi: 10.1161/hh1601.095596. [DOI] [PubMed] [Google Scholar]
- 7.Hatsukami TS, Ross R, Nayak L, Polissar Yuan C. Visualization of fibrous cap thickness and rupture in human atherosclerotic carotid plaque in vivo with high-resolution magnetic resonance imaging. Circulation. 2000;102:959–964. doi: 10.1161/01.cir.102.9.959. [DOI] [PubMed] [Google Scholar]
- 8.Zimmermann GG, Erhart P, Schneider J, vonSchulthess GK, Schmidt M, Debatin JF. Intravascular MR imaging of atherosclerotic plaque: Ex vivo analysis of human femoral arteries with histologic correlation. Radiology. 1997;204:769–774. doi: 10.1148/radiology.204.3.9280257. [DOI] [PubMed] [Google Scholar]
- 9.de Korte CL, Carlier SG, Mastik F, Doyley MM, van der Steen AFW, Serruys PW, Bom N. Morphological and mechanical information of coronary arteries obtained with intravascular elastography-Feasibility study in vivo. Eur.Heart J. 2002;23:405–413. doi: 10.1053/euhj.2001.2806. [DOI] [PubMed] [Google Scholar]
- 10.Pasterkamp G, Falk E, Woutman H, Borst C. Techniques characterizing the coronary atherosclerotic plaque: Influence on clinical decision making. J.Am.Coll.Cardiol. 2000;36:13–21. doi: 10.1016/s0735-1097(00)00677-x. [DOI] [PubMed] [Google Scholar]
- 11.Tearney GJ, Yabushita H, Houser SL, Aretz HT, Jang IK, Schlendorf KH, Kauffman CR, Shishkov M, Halpern EF, Bouma BE. Quantification of macrophage content in atherosclerotic plaques by optical coherence tomography. Circulation. 2003;107:113–119. doi: 10.1161/01.cir.0000044384.41037.43. [DOI] [PubMed] [Google Scholar]
- 12.Jang IK, Tearney G, Bouma B. Visualization of tissue prolapse between coronary stent struts by optical coherence tomography-Comparison with intravascular ultrasound. Circulation. 2001;104:2754. doi: 10.1161/hc4701.098069. [DOI] [PubMed] [Google Scholar]
- 13.Bouma BE, Tearney GJ, Yabushita H, Shishkov M, Kauffman CR, Gauthier DD, MacNeill BD, Houser SL, Aretz HT, Halpern EF, Jang IK. Evaluation of intracoronary stenting by intravascular optical coherence tomography. Heart. 2003;89:317–320. doi: 10.1136/heart.89.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verheye S, De Meyer GR, Van Langenhove G, Knaapen MWM, Kockx MM. In vivo temperature heterogeneity of atherosclerotic plaques is determined by plaque composition. Circulation. 2002;105:1596–1601. doi: 10.1161/01.cir.0000012527.94843.bf. [DOI] [PubMed] [Google Scholar]
- 15.Stefanadis C, Toutouzas K, Vavuranakis M, Tsiamis E, Tousoulis D, Panagiotakos DB, Vaina S, Pitsavos C, Toutouzas R. Statin treatment is associated with reduced thermal heterogeneity in human atherosclerotic plaques. Eur Heart J. 2002;23:1664–1669. doi: 10.1053/euhj.2002.3174. [DOI] [PubMed] [Google Scholar]
- 16.Motz JT, Fitzmaurice M, Miller A, Gandhi SJ, Haka AS, Galindo LH, Dasari RR, Kramer JR, Feld MS. In vivo Raman spectral pathology of human atherosclerosis and vulnerable plaque. J Biomed Opt. 2006;11:021003. doi: 10.1117/1.2190967. [DOI] [PubMed] [Google Scholar]
- 17.Angheloiu GO, Arendt JT, Muller MG, Haka AS, Georgakoudi I, Motz JT, Scepanovic OR, Kuban BD, Myles J, Miller F, Podrez EA, Fitzmaurice M, Kramer JR, Feld MS. Intrinsic fluorescence and diffuse reflectance spectroscopy identify superficial foam cells in coronary plaques prone to erosion. Arterioscler Thromb Vasc Biol. 2006;26:1594–1600. doi: 10.1161/01.ATV.0000225699.36212.23. [DOI] [PubMed] [Google Scholar]
- 18.Marcu L, Grundfest WS, Maarek JMI. Photobleaching of arterial fluorescent compounds: Characterization of elastin, collagen and cholesterol time-resolved spectra during prolonged ultraviolet irradiation. Photochem Photobiol. 1999;69:713–721. [PubMed] [Google Scholar]
- 19.Maarek JMI, Marcu L, Snyder WJ, Grundfest WS. Time-resolved fluorescence spectra of arterial fluorescent compounds: Reconstruction with the Laguerre expansion technique. Photochem Photobiol. 2000;71:178–187. doi: 10.1562/0031-8655(2000)071<0178:trfsoa>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 20.Marcu L, Grundfest WS, Fishbein MC. “Time-resolved Laser-Induced Fluorescence Spectroscopy for Staging Atherosclerotic Lesions”. In: Mycek MA, Pogue B, editors. Fluorescence in Biomedicine. New York: Marcel Dekker; 2003. pp. 397–430. [Google Scholar]
- 21.Marcu L, Fishbein MC, Maarek JMI, Grundfest WS. Discrimination of human coronary artery atherosclerotic lipid-rich lesions by time-resolved laser-induced fluorescence spectroscopy. Arterioscler Thromb Vasc Biol. 2001;21:1244–1250. doi: 10.1161/hq0701.092091. [DOI] [PubMed] [Google Scholar]
- 22.Maarek JMI, Marcu L, Fishbein MC, Grundfest WS. “Time-resolved fluorescence of aortic wall: use for improved identification of atherosclerotic lesions”. Lasers Surg Med. 2000;27:241–254. doi: 10.1002/1096-9101(2000)27:3<241::aid-lsm6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 23.Marcu L, Jo JA, Fang Q, Papaioannou T, Dorafshar A, Reil T, Qiao JH, Baker JD, Fishbein MC, Freischlag JA. In-Vivo Detection of Macrophages in a Rabbit Atherosclerotic Model by Time-Resolved Laser-Induced Fluorescence Spectroscopy. Atherosclerosis. 2005;181:295–303. doi: 10.1016/j.atherosclerosis.2005.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fang Q, Papaioannou T, Jo JA, Vaitha R, Shastry K, Marcu L. Time-domain laser-induced fluorescence spectroscopy apparatus for clinical diagnostics. Rev Sci Instrum. 2004;75:151–162. doi: 10.1063/1.1634354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Virmani R, Burke A, Farb A, Kolodgie FD. Pathology of the Vulnerable Plaque. J Am Coll Cardiol. 2006;47:13–18. doi: 10.1016/j.jacc.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 26.Marmarelis VZ. Identification of nonlinear biological systems using laguerre expansions of kernels. Ann Biomed Eng. 1993;21:573–589. doi: 10.1007/BF02368639. [DOI] [PubMed] [Google Scholar]
- 27.Jo JA, Fang Q, Papaioannou T, Marcu L. Fast model-free deconvolution of fluorescence decay for analysis of biological systems”. J Biomed Opt. 2004;9(4):743–752. doi: 10.1117/1.1752919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jo JA, Fang Q, Papaioannou T, Baker JD, Dorafshar AH, Reil T, Qiao JH, Fishbein MC, Freischlag JA, Marcu L. Laguerre-based method for analysis of time-resolved fluorescence data: application to in-vivo characterization and diagnosis of atherosclerotic lesions. J Biomed Opt. 2006;11:021004-1–021004-13. doi: 10.1117/1.2186045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duda RO, Hart PE, Stork DG. Pattern Classification. New York: John Wiley and Sons; 2004. [Google Scholar]
- 30.Sun YH, Liu R, Elson DS, Hollars C, Jo JA, Park J, Sun Y, Marcu L. Simultaneous time-and wavelength-resolved fluorescence spectroscopy for near real-time tissue diagnosis. Opt Lett. 2008;33(6):630–632. doi: 10.1364/ol.33.000630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richards-Kortum R, Sevick-Muraca E. Quantitative optical spectroscopy for tissue diagnosis. Annual Rev Phys Chem. 1996;47:555–606. doi: 10.1146/annurev.physchem.47.1.555. [DOI] [PubMed] [Google Scholar]
- 32.Morguet AJ, Körber B, Abel B, Hippler H, Wiegand V, Kreuzer H. Autofluorescence spectroscopy using a simultaneous plaque ablation and fluorescence excitation. Lasers Surg Med. 1994;14:238–248. doi: 10.1002/lsm.1900140306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.