Abstract
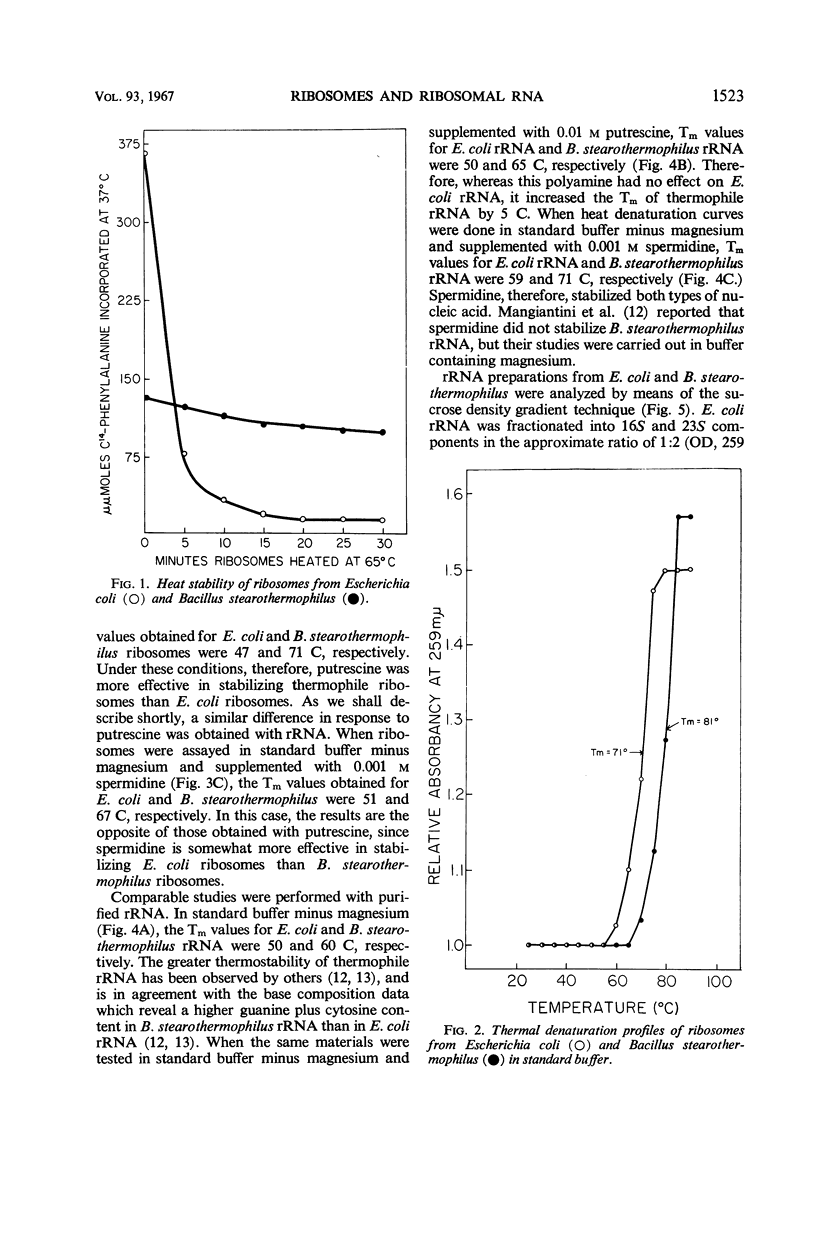
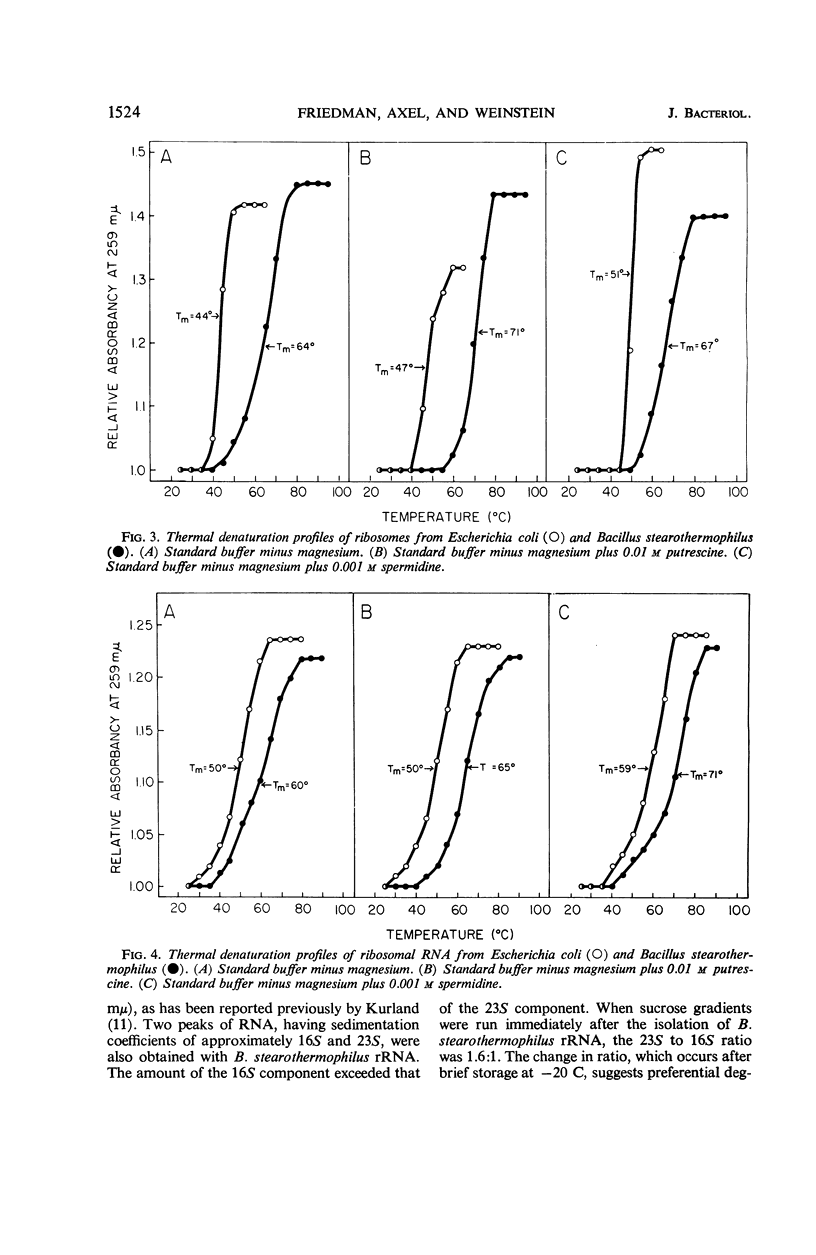
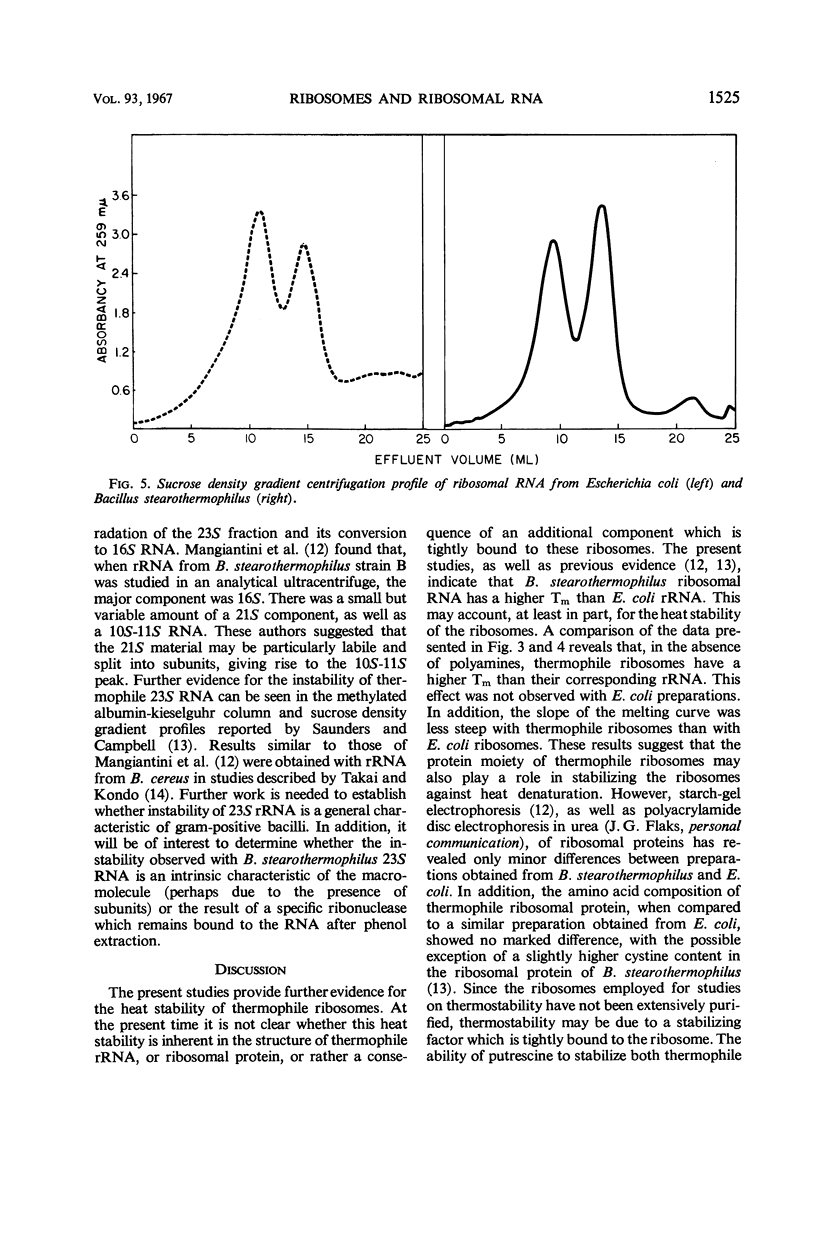
After heating at 65 C, ribosomes isolated from Bacillus stearothermophilus were strikingly more heat-stable than comparable preparations from Escherichia coli when tested for ability to support polyuridylic acid-directed phenylalanine incorporation at 37 C. The stability of ribosomes was also determined by measurements of hyperchromicity at 259 mμ while heating them from 25 to 90 C. In standard buffer containing 0.01 m Mg++, the Tm (temperature at the midpoint of total hyperchromicity) of E. coli and B. stearothermophilus ribosomes was 71 and 81 C, respectively. In a magnesium-free buffer, the Tm of E. coli and B. stearothermophilus ribosomes was 44 and 64 C, respectively. Putrescine (0.01 m) was more effective in stabilizing ribosomes from B. stearothermophilus than those from E. coli. Spermidine (0.001 m), on the other hand, was more effective in stabilizing ribosomes from E. coli than those from B. stearothermophilus. Melting curves of total ribosomal ribonucleic acid (rRNA) from E. coli and B. stearothermophilus revealed Tm values of 50 and 60 C, respectively. Putrescine stabilized thermophile rRNA, but had no effect on E. coli rRNA. Sucrose density gradients demonstrated that thermophile 23S ribonucleic acid was degraded during storage at −20 C; the 23S component from E. coli was stable under these conditions. The results are discussed in terms of the mechanism of ribosome heat stability and the role of the ribosome in governing the temperature limits for bacterial growth.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN M. B. The thermophilic aerobic sporeforming bacteria. Bacteriol Rev. 1953 Jun;17(2):125–173. doi: 10.1128/br.17.2.125-173.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARCA M., CALVORI C., FRONTALI L., TECCE G. THE ENZYMIC SYNTHESIS OF AMINOACYL DERIVATIVES OF SOLUBLE RIBONUCLEIC ACID FROM BACILLUS STEAROTHERMOPHILUS. Biochim Biophys Acta. 1964 Jul 22;87:440–448. doi: 10.1016/0926-6550(64)90116-1. [DOI] [PubMed] [Google Scholar]
- Algranati I. D., Lengyel P. Polynucleotide-dependent incorporation of amino acids in a cell-free system from thermophilic bacteria. J Biol Chem. 1966 Apr 25;241(8):1778–1783. [PubMed] [Google Scholar]
- Arca M., Frontali L., Tecce G. Lack of specificity in the formation amino-acyl-sRNA as a possible source of coding errors. Biochim Biophys Acta. 1965 Oct 11;108(2):326–328. doi: 10.1016/0005-2787(65)90023-7. [DOI] [PubMed] [Google Scholar]
- DUBIN D. T., ROSENTHAL S. M. The acetylation of polyamines in Escherichia coli. J Biol Chem. 1960 Mar;235:776–782. [PubMed] [Google Scholar]
- FRIEDMAN S. M., WEINSTEIN I. B. LACK OF FIDELITY IN THE TRANSLATION OF SYNTHETIC POLYRIBONUCLEOTIDES. Proc Natl Acad Sci U S A. 1964 Oct;52:988–996. doi: 10.1073/pnas.52.4.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman S. M., Weinstein I. B. Fidelity in protein synthesis: proline miscoding in a thermophile system. Biochem Biophys Res Commun. 1965 Nov 22;21(4):339–345. doi: 10.1016/0006-291x(65)90199-3. [DOI] [PubMed] [Google Scholar]
- Friedman S. M., Weinstein I. B. Protein synthesis in a subcellular system from Bacillus stearothermophilus. Biochim Biophys Acta. 1966 Mar 21;114(3):593–605. doi: 10.1016/0005-2787(66)90107-9. [DOI] [PubMed] [Google Scholar]
- Gaughran E. R. THE THERMOPHILIC MICROORGANISMS. Bacteriol Rev. 1947 Sep;11(3):189–225. doi: 10.1128/br.11.3.189-225.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOFFLER H. Protoplasmic differences between mesophiles and thermophiles. Bacteriol Rev. 1957 Dec;21(4):227–240. doi: 10.1128/br.21.4.227-240.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiantini M. T., Tecce G., Toschi G., Trentalance A. A study of ribosomes and of ribonucleic acid from a thermorphilic organism. Biochim Biophys Acta. 1965 Jun 8;103(2):252–274. doi: 10.1016/0005-2787(65)90166-8. [DOI] [PubMed] [Google Scholar]
- Saunders G. F., Campbell L. L. Ribonucleic acid and ribosomes of Bacillus stearothermophilus. J Bacteriol. 1966 Jan;91(1):332–339. doi: 10.1128/jb.91.1.332-339.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKAI M., KONDO N. Studies on the ribosomal ribonucleic acid from Bacillus cereus. Biochim Biophys Acta. 1962 Jun 11;55:875–879. doi: 10.1016/0006-3002(62)90900-9. [DOI] [PubMed] [Google Scholar]


