Abstract
T cell recognition typically involves both the engagement of a specific T cell receptor with a peptide/major histocompatibility complex (MHC) and a number of accessory interactions. One of the most important interactions is between the integrin lymphocyte function-associated antigen 1 (LFA-1) on the T cell and intracellular adhesion molecule 1 (ICAM-1) on an antigen-presenting cell. By using fluorescence video microscopy and an ICAM-1 fused to a green fluorescent protein, we find that the elevation of intracellular calcium in the T cell that is characteristic of activation is followed almost immediately by the rapid accumulation of ICAM-1 on a B cell at a tight interface between the two cells. This increased density of ICAM-1 correlates with the sustained elevation of intracellular calcium in the T cell, known to be critical for activation. The use of peptide/MHC complexes and ICAM-1 on a supported lipid bilayer to stimulate T cells also indicates a major role for ICAM-1/LFA-1 in T cell activation but, surprisingly, not for adhesion, as even in the absence of ICAM-1 the morphological changes and adhesive characteristics of an activated T cell are seen in this system. We suggest that T cell antigen receptor-mediated recognition of a very small number of MHC/peptide complexes could trigger LFA-1/ICAM-1 clustering and avidity regulation, thus amplifying and stabilizing the production of second messengers.
T cell recognition of a cell bearing a particular peptide/major histocompatibility complex (MHC) complex follows a well-described series of molecular and cellular events. The T cell flattens against the antigen-presenting cell (APC), T cell migration stops (1), and a tight interface forms (2, 3). A signaling cascade is also initiated (4) that may ultimately lead to T cell proliferation and/or the activation of T cell effector pathways such as cytokine secretion or cell killing. One of the early indicators of T cell activation is the elevation of intracellular calcium, (5, 6), which many studies have shown is a critical component of this process.
Although the binding of an αβ T cell antigen receptor (TCR) to its cognate peptide/MHC complex confers specificity to this process, a number of additional molecular interactions are known to be important [as recently reviewed by Croft and Dubey (7)]. Among the most significant of these is the binding of the integrin lymphocyte function-associated antigen 1 (LFA-1) on a T cell to intracellular adhesion molecule 1 (ICAM-1) on the APC (8–10). LFA-1 is markedly affected by T cell signaling (11, 12), in that its affinity for ICAM-1 is increased transiently following T cell activation (8, 9, 13) by an unknown mechanism. After T cell activation, ICAM-1 and LFA-1 are enriched in the interface, as determined by three-dimensional microscopy of fixed T cell/B cell pairs (A. Kupfer, personal communication). The presence of ICAM-1 on the APC is crucial for T cell activation in a mixed lymphocyte reaction and in the septic shock response to Staphylococcus aureus enterotoxin B, as revealed by mice deficient in ICAM-1 (14, 15).
One intriguing feature of T cell recognition is that only a small number of specific peptide/MHC ligands (n ≤ 200) are required for activation (16–19). This finding suggests that signaling through the TCR itself may be quite weak when antigen is limiting and that some type of amplification mechanism or mechanisms may exist. Valitutti et al. (20) have shown that one such mechanism is likely to be the serial engagement of each peptide/MHC complex with many TCR molecules.
Here we present an experimental system that allows one to simultaneously follow morphological changes, the intracellular calcium concentration, and the distribution of cell surface molecules on live cells in real time. We have used this approach to study the role of the ICAM-1/LFA-1 interaction during T cell recognition of a B lymphocyte bearing the appropriate peptide/MHC complex. By using a green fluorescent protein (GFP) fusion construct, we are able to follow the distribution of ICAM-1 and find that it accumulates in the T/B cell interface within seconds after the onset of the calcium signal. CHO cells can present antigen to T cells but do not exhibit this clustering phenomenon. When CHO cells expressing densities of ICAM-1/GFP equivalent to the clusters observed in B cells are used as APCs, significantly more of the responding T cells sustain their intracellular calcium levels versus ICAM-1low or null expressors. This result suggests that the increased density brought about by this clustering may stabilize the calcium signal at low peptide/MHC concentrations. These data suggest a model of T cell activation in which a specific but perhaps minimal signal through the TCR can commit the T cell to an activation program that can be maintained without having to rely exclusively on TCR/MHC interactions.
EXPERIMENTAL PROCEDURES
Cells.
5C.C7 lymph-node cells were primed in vitro on irradiated B10.BR spleen loaded with 2 μM moth cytochrome C (MCC 88-103) peptide. Primed cells were cultured in the presence of interleukin 2 and used between day 8 and 12. CH27 cells were transfected with a construct expressing an ICAM-1/GFP fusion protein. CH27 that express the same amount or less of the ICAM-1/GFP fusion protein in comparison to the endogenous ICAM-1 were selected by fluorescence-activated cell sorting and cloned. The ICAM-1/GFP fusion consists of the full-length ICAM-1 protein (21) followed by a linker polypeptide with the amino acid sequence TSAAAGGGGSGGGGSGGGGS and then the GFP-Bex1 residues (22). It is expressed from the plasmid pBJ1Neo (23). A fusion protein containing the extracellular domain of ICAM-1, GFP, and a glycoprotein I (GPI) membrane anchor (24) was constructed as well. The extracellular domain of ICAM-1 (residues 1–482) was followed by a AAAGGGGS linker, GFP-Bex1, another GGGGSAAA linker, and the GPI tail. CHO cells were cotransfected with expression plasmids for I-Ek and the ICAM-1/GFP fusion protein. Transfected cells were sorted for high I-Ek expression and moderate and high (i.e., 4× moderate) ICAM-1/GFP expression. Moderate ICAM-1/GFP expressing cells were cloned and their GFP fluorescence was found to be comparable to CH27 cells that were transfected with the same ICAM-1/GFP expression construct and cloned as well. APCs were peptide loaded as described (25, 26).
Microscopy.
When CH27 B cells were used as APCs, CH27 cells and 5C.C7 T cells were mixed before the start of the experiment and immediately injected into a microscopy stage laminar flow chamber (see below). For cytochalasin D pretreatment, either B or T cells were kept at 2 μM cytochalasin D for 15 min before the start of the experiment and then diluted into the experiment 20-fold. To chelate intracellular calcium, bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate (BAPTA) was loaded at 20 μM concentration as an acetoxymethylester in parallel with the Fura-2. All other pharmacological reagents were added directly to the experiment at the indicated concentrations. For experiments with transfected CHO cells or supported lipid bilayers, T cells were added to an eight-well coverslip (Nunc) or the flow chamber to start the experiment. The temperature was controlled by air-heating with a custom designed heating box. In addition, the microscopy stage laminar flow chamber was heated with a Peltier element. Microscopy was performed by using two different systems. In most of the experiments, a Zeiss imaging system with a Zeiss Axiovert 135 microscope and the Attofluor extensions and software was used. Briefly, the system included infrared Nomarski optics, a beam splitter, an intensified charge-couple device (CCD) camera to record fluorescence and a CCD camera to capture the bright-field images. For quantitative measurements, a Zeiss Axiovert 100TV microscope with a cooled CCD camera (TEA/CCD512TKB/1, Princeton Instruments, Trenton, NJ) to collect all images and metamorph analysis software was used (Universal Imaging, West Chester, PA). In both setups Fura-2 was excited at 334 nm and 380 nm and GFP was excited at 488 nm. The emission was collected above 510 nm. No cross-excitation between the dyes could be detected. All movies were made by using Adobe (Mountain View, CA) premiere software, and stills were taken from these to create the figures. For confocal microscopy live cells couples were stained with a directly phycoerthrin-conjugated anti ICAM-1 antibody (PharMingen) and imaged immediately on a Molecular Dynamics MultiProbe 2010 CLSM confocal microscope.
Single Cell Adhesion Assays.
CHO cells transfected with I-Ek were grown to confluence and loaded with peptide on a 48 × 60-mm no. 1 glass coverslip that forms the bottom of a fluorescence microscope mounted laminar flow chamber with a flow channel of the dimensions: height, 0.26 mm; width, 5 mm; and length, 40 mm. 5C.C7 T cells interacted with the APCs for 16 min while the calcium signal and Nomarski images were recorded. Then the chamber was washed with a Syringe pump (Cavro, Sunnyvale, CA) at increasing flow rates. Adhesion was evaluated by imaging. The wall shear stress was calculated from a momentum balance on a Newtonian fluid, assuming a viscosity of 1.0 cP. For antibody blocking experiments, the antibodies YN1/1.7.4 [anti-ICAM-1, as an Fab-fragment (27)], M17/5.2 [anti-CD11a (28)], and 2E6 (anti CD18, ATCC no. HB226) were simultaneously present during the interaction of the T cells with the APCs at 10 μg/ml each. Activated T cells were found to adhere with an adhesion strength above 90 dynes/cm2 with or without these antibodies.
Supported Lipid Bilayers.
Supported lipid bilayers containing proteins were prepared and handled as described (29). Briefly, genetically engineered versions of I-Ek and ICAM-1 (see below) in which the transmembrane domains have been substituted by GPI tails were purified by affinity chromatography in the presence of detergent, incorporated into small unilammelar vesicles by dialysis, and spread on cleaned glass coverslips. The extracellular domain of ICAM-1 was linked to a GPI tail (24) at residue 482 and purified on a YN1 affinity column by base elution. Membrane incorporated I-Ek was quantified by ELISA by using two different protocols as described (29). Both methods gave similar results: a 100 nM solution of the GPI-linked I-Ek in the detergent dialysis produced a supported bilayer with a protein density in the range of 100–1,000/μm2. I-Ek in supported lipid bilayers was peptide loaded overnight at 25 μM peptide concentration in 5% FCS in PBS adjusted to pH 6.5. Lower concentrations of activating peptide were achieved by dilution into a neutral peptide (25, 26).
RESULTS
Multicolor Video Microscopy.
To observe early events in the interaction of a T cell with an APC in real-time and under physiological conditions, we used video imaging systems that allow us to capture both fluorescence and differential interference contrast images. The later allows the characterization of cell position and behavior, whereas fluorescence detection allows us to monitor T cell activation by using the ratiometric dye Fura-2 (30) as well as a variant of GFP (GFP-Bex1) (21), which has increased, red-shifted fluorescence. All experiments described below use primed T cells from the lymph nodes of 5C.C7 TCR transgenic mice that recognize the moth cytochrome c peptide 88–103 presented by the class II MHC molecule, I-Ek (31, 32). A variety of APCs or surrogates were used as stimulants.
ICAM-1 Rapidly Accumulates in the B/T Cell Interface After T Cell Activation.
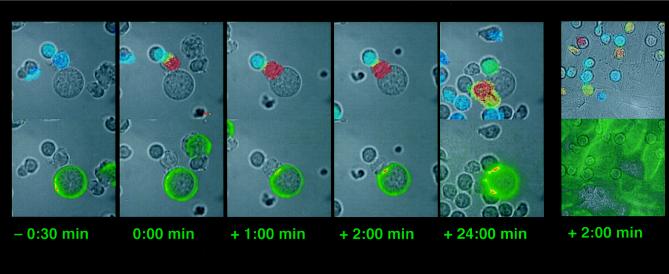
In Fig. 1 we show an example of the typical sequence of events that occurs when 5C.C7 T cells are mixed with B cell lymphoma cells stablely transfected with ICAM-1/GFP and which also express the I-Ek molecule and have been loaded with peptide. GFP is fused to the ICAM-1 C terminus with a flexible 17-aa linker. The ICAM-1/GFP expression level has been adjusted to match the endogenous ICAM-1 expression level. After a variable period of cell–cell contact the T cell activates as measured by a rapid rise in intracellular calcium and concomitantly forms a tight B/T cell interface. Interestingly, the initial rise in T cell calcium levels is followed very quickly (first detectable within 20 ± 4 sec) by the movement of ICAM-1 into this interface. Even though the first accumulation is detectable within seconds, ICAM-1 keeps accumulating over several minutes before reaching a plateau that is maintained for at least 0.5 h (data not shown). The average gain in ICAM-1 after 10 min is 1.8-fold (± 0.35, n = 22) as determined by measuring the GFP intensity with a cooled CCD camera over the whole interface. This corresponds to 3.5 times of the preactivation level of the endogenous ICAM-1. In cases where the calcium level then returns close to baseline, the accumulation of ICAM-1 persists (Fig. 1). No ICAM-1 redistribution occurs before calcium becomes elevated in the T cell. When more than one T cell is activated by the same B cell, each interface shows an accumulation of ICAM-1 (Fig. 1). However, in several hundred observed T cell activations, we have yet to find a case where one T cell forms interfaces with two different B cells. This result suggests that cell polarity is crucial for the T cell but not for the B cell in the accumulation of ICAM-1 at the interface and is consistent with the findings of Negulescu et al. (2), who found a preferred recognition interface on the T cell.
Figure 1.
Mobilization of ICAM-1/GFP during T cell recognition of B cells and MHC II-transfected CHO cells. Each panel consists of two identical bright-field images. The upper one is overlaid with a transparent color scale of the ratio of the fluorescence emissions at 340 nm and 380 nm of the calcium sensitive dye Fura-2. This ratio is correlated with the intracellular calcium concentration. Blue indicates low, resting calcium; and red, high calcium. The bottom bright-field image is overlaid with a transparent color scale of the GFP fluorescence. Green indicates low ICAM-GFP concentration, yellow medium, and red high. The five left panels show a time course for the interaction of a T cell with a B cell. Time points of the interaction are given with the time before or after activation indicated below each panel. The second T cell activating on the central B cell is slightly above the plane of focus of the objective; thus the recorded GFP fluorescence is reduced. The sixth panel at the right shows the activation of T cells by an I-Ek-transfected CHO cell 2 min after the activation of the middle T cell. The movies from which these photos are derived can be viewed at http://cmgm.stanford.edu/hhmi/mdavis.
Next, we varied the peptide concentration on the ICAM-1/GFP-transfected CH27 cells by diluting the agonist peptide into a neutral peptide as described (25, 26). At a 1.000-fold dilution (10 nM agonist peptide), CH27 cells are no longer able to stimulate T cells. At a 100-fold dilution of the agonist peptide (0.1 μM) the rearrangement of ICAM-1 is indistinguishable from the behavior induced by maximal peptide concentrations. ICAM-1 redistribution is thus independent of the concentration of activating peptide as long as this peptide concentration is sufficient for T cell activation.
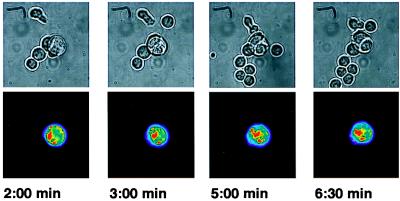
To be sure that the ICAM-1/GFP fusion was not behaving aberrantly, we stained T/B cell couples with an ICAM-1-specific antibody and performed confocal microscopy (data not shown). We find that the ICAM-1/GFP colocalizes with unmodified ICAM-1 on the B cell (both are expressed at equal levels). As most of the interfaces observed are oriented perpendicular to the observation plane, some or all of the observed accumulation could result from the flattening of the membranes at the cell–cell interface. However, we were also able to observe several T/B cell couples where the orientation of the interface relative to the focal plane changes during the experiment (Fig. 2). These couples show that the accumulation is not an artifact of the observation method. Furthermore, they allow us to determine the geometry of the accumulation: ICAM-1 is not homogeneously distributed through the interface but concentrated into patches, whose size and position can change somewhat over time (Fig. 2).
Figure 2.
Capturing the T/B cell interface in a different orientation. Two T cells, activated by one ICAM-1-transfected CH27 B cell, are shown. The T cells have moved under the larger CH27 cell after activation, with the result that the focal plane of the microscope is now at the B/T cell interface (versus the side view of Fig. 1). One cell lies under the top left part of the CH27 cell, the other under the bottom right part. The other T cells in the images are not activated. In the upper row are bright-field images, in the bottom row the ICAM-1/GFP fluorescence images. The fluorescence intensity is encoded in a rainbow color scheme, which represent linear variations in intensity, because the images have been collected with a cooled CCD camera. The time after the activation of the bottom right cell is indicated below each panel. The top left cell has activated 7 min before this. T cell activation was determined by monitoring the intracellular calcium concentration (data not shown).
Accumulation of ICAM-1 at the Interface Is T Cell Driven.
To establish whether the cytoskeleton of the B cell or the T cell is important for the ICAM-1 clustering phenomenon, we pretreated either cell type with 2 μM cytochalasin D before mixing the cells. In the case of the B cells, this treatment had no noticeable effect on T cell activation, including the formation of a tight interface and ICAM-1 redistribution, indicating that these are independent of the B cell cytoskeleton. Similarly, when the transmembrane and cytoplasmic domains of ICAM-1 are substituted with a signal sequence for GPI linkage, the same ICAM-1 redistribution is observed, arguing that the ICAM-1 cytoplasmic domain is not required for ICAM-1 rearrangement (data not shown). In contrast, pretreatment of the T cells with cytochalasin D caused a 10-fold drop in the activation frequency as determined by the elevation of the intracellular calcium concentration, reduced the degree of ICAM-1 redistribution, and resulted in the formation of a markedly less tight interface (data not shown). This is consistent with previous data showing that an intact actin cytoskeleton is important for T cell activation (33).
Elevation of Intracellular Calcium Is Not Essential to ICAM-1 Redistribution.
Most T and B lymphocytes require both calcium mobilization and protein kinase C (PKC) translocation to be activated. To determine the relation between the calcium signal and ICAM-1 redistribution, we treated T cells with 5 μM thapsigargin. This treatment elevates the intracellular calcium concentration without triggering a signal through the TCR. We allowed the thapsigargin-treated T cells to interact with nonpeptide-loaded CH27 cells transfected with ICAM-1/GFP. No tight T/B cell couples form, even after prolonged cell–cell contact. We also do not observe any ICAM-1 redistribution (data not shown). In addition, we inhibited the rise in intracellular calcium by chelating cytoplasmic calcium with BAPTA and inhibiting calcium influx with 5 mM Ni2+. We found that tight B/T cell interfaces still form normally and, except for a delay in the first occurrence of the interface from 20 ± 4 sec to 61 ± 36 sec, the accumulation of ICAM-1 is not disturbed. Thus the calcium signal is neither necessary nor sufficient for the formation of a B/T cell couple and the redistribution of ICAM-1. In contrast, treatment of both T and B cells with the PKC activator phorboldibutyrate (at 50 ng/ml) produces tight cell–cell interfaces with very strong ICAM-1 accumulation in the absence of peptide. These occur both in T/B cell couples, as well as between B cells (data not shown). This markedly different behavior by the B cells under these conditions shows that they can also mediate ICAM-1 clustering but require PKC activation to do so.
The Presence of ICAM-1 Sustains the Calcium Signal.
To study the role of ICAM-1 in T cell activation we used two substitutes for the CH27 cells as APCs, either supported lipid bilayers or transfected CHO cells. On supported lipid bilayers reconstituted with I-Ek and ICAM-1, we find that the presence of ICAM-1 increases both the activation frequency and the percentage of “sustained” calcium responses, especially at the lower peptide concentrations (Table 1, data set 1). We define a sustained calcium response as one in which calcium rises and stays elevated throughout the 20-min observation period (Fig. 3). A T cell whose intracellular calcium returns to baseline in this time is counted as partial. To study the specificity of the effect of ICAM-1 on the form of the calcium signal, we increased the strength of the T cell stimulus by an I-Ek-transfected CHO cell at limiting peptide concentrations in two different ways. We either cotransfected with the ICAM-1/GFP fusion protein or increased the expression levels of I-Ek 3-fold. Even though the stronger stimulus is reflected in an increase in the activation frequency in both cases, only the cotransfection of ICAM-1 assures that most of the calcium signals are sustained, thus showing the specificity of the ICAM-1-induced signal (Table 1, data set 2).
Table 1.
Activation frequencies and signal type in 5C.C7 T cells activated in the presence or absence of ICAM
| Data set | Peptide concentration | I-Ek
|
I-Ek + ICAM
|
I-Ek-high
|
I-Ek + ICAM-low
|
I-Ek + ICAM-high
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| % act. | % sust. Ca | % act. | % sust. Ca | % act. | % sust. Ca | % act. | % sust. Ca | % act. | % sust. Ca | ||
| 1 | 25 nM | 8 ± 3 (2) | 40 ± 14 (2) | 25 ± 11 (7) | 81 ± 10 (7) | ||||||
| 250 nM | 23 ± 10 (4) | 49 ± 23 (4) | 45 ± 13 (5) | 81 ± 10 (5) | |||||||
| 2.5 μM | 26 ± 5 (3) | 85 ± 18 (3) | 60 ± 14 (2) | 92 ± 3 (2) | |||||||
| 2 | 0.25 nM | 11 ± 2 | 54 ± 30 | 29 ± 1 | 65 ± 6 | 17 ± 2 | 91 ± 11 | ||||
| 3 | 0.15 nM | 27 ± 23 | 45 ± 26 | 50 ± 7 | 72 ± 9 | 40 ± 18 | 96 ± 4 | ||||
| 2.5 nM | 49 ± 30 | 82 ± 8 | 46 ± 12 | 77 ± 15 | 36 ± 12 | 95 ± 5 | |||||
Peptide concentration refers to the amount of agonist peptide (MCC 88-103). The percentage of T cells which are activated is given in the “% act.” column with standard deviations. The percentages of those among the activated ones that have a sustained phase above the baseline are given in the “% sust. Ca” with standard deviations. Data set 1: T cell are activated by supported lipid bilayers. The number of experiments is indicated in parentheses. Supported lipid bilayers containing I-Ek alone or in combination with ICAM are indicated. Data set 2: T cell are activated by transfected CHO cells. The 0.25 nM peptide is the minimal peptide concentration at which T cell activation is observed. CHO lines expressing two different levels of I-Ek (“high” corresponds to three times the I-Ek cells) and ICAM-1/GFP (Data set 3) are indicated on the top of the table. The differences in % activation and % sustained calcium between CHO cells containing ICAM or not are statistically significant with P<0.05 (independent t test). Each data point is based on the analysis of 60–120 cells. Data set 3: T cell are activated by transfected CHO cells. The 0.25 nM peptide is the minimal peptide concentration at which T cell activation is observed. CHO lines expressing I-Ek (see data set 2) and different levels of ICAM-1/GFP (“high” corresponds to four times the ICAM-1 “low” cells) are indicated on the top of the table. The differences in % sustained calcium between ICAM-high and ICAM-low/CHO cells without ICAM are statistically significant to P<0.005 (0.25 nM peptide) and P<0.06 (2.5 nM peptide) (independent t test). Differences in activation percentage are not significant. Each data point is based on the analysis of >120 cells. Therefore, for the determination of the type of calcium signal, >50 activated cells have been analyzed for each data point.
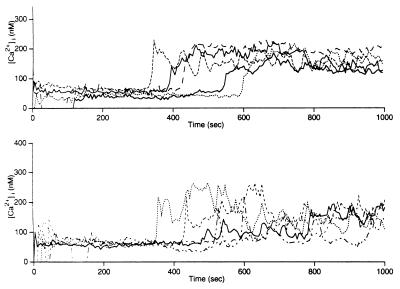
Figure 3.
Types of calcium signals. Five traces were taken from one experiment each, where 5C.C7 T cells have been stimulated with CHO cells transfected with I-Ek and ICAM-GFP. (Upper) A stable sustained phase induced by high ICAM-1 expression levels. (Lower) A reduced sustained phase found with low ICAM-1 expression levels (see Table 1).
An Increased Density of ICAM-1 Sustains the Calcium Signal.
On CHO cells transfected with I-Ek and the ICAM-1/GFP fusion protein, 5C.C7 T cells activate and adhere in a peptide-dependent manner, but, surprisingly, no significant redistribution of ICAM-1 is seen (Fig. 1), whether the transfected CHO cells used were adherent or in a suspension. To ask what effect the increase in ICAM-1 density brought about by clustering might have on T cell activation, we established two different CHO cell lines, one of which expresses a density of ICAM-1 GFP similar to that of the CH27 transfectants prior to mobilization and one which expresses 4-fold more, similar to the density after mobilization. When the calcium signal induced by these two cell lines at limiting activation conditions is compared with the one induced by CHO cells bearing only I-Ek, we find that the ICAM-1high line is significantly better at maintaining a sustained calcium response (Table 1, data set 3, and Fig. 3). Thus we conclude that the mobilization of ICAM-1 serves to keep calcium concentrations high within the cell once activation is initiated.
T Cell Flattening and Strong Adhesion Do Not Require ICAM-1.
We also asked whether the presence of ICAM-1 is necessary for either the formation or the maintenance of the tight T-cell/APC interface, the major morphological feature of T cell activation. By using a laminar flow chamber with an attached syringe pump, we determined the adhesion strength of activated 5C.C7 T cells to CHO cells transfected with I-Ek and loaded with peptide to be more than 90 dynes/cm2, the maximum force we are able to generate. This finding compares with less than 2 dynes/cm2 prior to activation and is an order of magnitude above maximal physiological shear stresses (34, 35). Cotransfection of ICAM-1 does not change this behavior. In all experiments the adhesion is not affected by antibodies against ICAM-1 or LFA-1, even with a density of agonist peptide loaded I-Ek of at most 1 per μm2. Thus, under these conditions, T cells behave similarly with respect to their adhesion characteristics regardless of whether ICAM-1 is present.
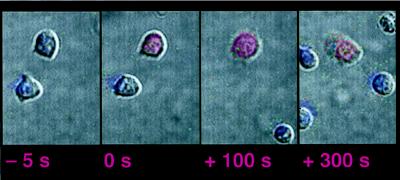
To ask whether ICAM-1 is required for the morphological changes that are characteristic of T cell activation, we also performed video microscopy on supported lipid bilayers that contain only I-Ek/peptide at different densities (Fig. 4). Because the experiments are performed in the presence of calf serum to prevent nonspecific adsorption of the T cells to the bilayers, it is likely that extracellular matrix proteins are present on the bilayer too. Here T cells flux calcium, flatten out against the bilayers, and adhere as in the above experiments (Fig. 1); thus it seems that the morphological changes associated with activation are dependent only on the presence of MHC/peptide and possibly the extracellular matrix, even at an average of less than 20 peptide/I-Ek complexes per μm2 (assuming all I-Ek molecules contain the peptide, a likely over-estimate). Inclusion of ICAM-1 into the supported lipid bilayers at this density of peptide loaded MHC does not change the morphological order of events, but has a strong influence on the activation frequency of the T cells and on the form of the calcium signal (Table 1, data set 1).
Figure 4.
Activation of T cells on supported lipid bilayers containing I-Ek. The figure setup is as in the upper panels in Fig. 1. The original movie is available at http://cmgm.stanford.edu/hhmi/mdavis.
DISCUSSION
Mechanism of ICAM-1 Clustering.
At this point our understanding of the mechanism behind the ICAM-1 clustering described here is only fragmentary. It appears T cell driven, as cytochalasin treatment of T cells, but not B cell APCs interferes with it. On the APC side it has some cell-type specificity, as CHO cells bearing the same ICAM-GFP construct do not cluster these molecules at the T cell interface. This is not a function of cell geometry, because CHO cells made nonadherent also do not show this clustering. This finding raises the possibility that some receptor(s) on the B cell may be necessary to trigger this effect. Interestingly, the formation of a tight interface that is linked to the calcium signal as well and always precedes the ICAM-1 accumulation can be induced by the transfected CHO cells. This flattening of the B/T cell interface after T cell activation brings a larger number of complementary receptors into close vicinity, potentially allowing increased binding. Nonetheless, as transfected CHO cells cannot induce ICAM-1 accumulation, the flattening of the interface alone could not drive redistribution.
The other salient feature of this phenomenon is the close connection we observe between the rise in the intracellular calcium concentration in the T cell and the onset of ICAM-1 clustering. This occurs despite the fact that inhibition of the calcium elevation only slows down the process. Thus some other TCR-triggered signaling pathway must drive ICAM-1 clustering. One possible candidate would be the activation or translocation of one or several of the PKC isoforms. In particular Monks et al. (36) have recently shown that PKC θ is translocated to the T/B cell interface shortly after activation. Consistent with this is the finding that phorbol ester treatment can promote the formation of tight interfaces and ICAM-1/GFP accumulation at the interfaces of either T/B or B/B cell couples. Because phorbol ester treatment activates PKCs in both B and T cells and has been shown to increase the avidity of LFA-1 (13), it seems likely that this is a major signaling pathway underlying ICAM-1 accumulation.
The Role of ICAM-1 in T Cell Activation.
Because the avidity of LFA-1 is up-regulated as a result of T cell activation, most attention has been focused on the contribution of LFA-1/ICAM-1 binding to adhesion (8, 9, 37). Nevertheless, previous work has suggested a signaling role for this interaction (38, 39), especially in the stimulation of naive CD4 T cells (as reviewed in ref. 40). Particularly relevant to the work presented here is the finding by Van Seventer et al. (38) of a partial stabilization of the calcium flux in activated T cells by ICAM-1-coated beads. Here we examine the contribution of ICAM-1 to both adhesion and signaling in the same experimental system and find a clear effect on the stabilization of the calcium signal but no obvious effect on adhesion. Why might this be? The most dramatic effects seen with LFA-1/ICAM-1 mediated adhesion are with T cells activated by cross-linking antibodies or in the presence of phorbol 12-myristate 13-acetate, whereas here we use limiting amounts of the normal ligand, namely peptide/MHC complexes on B cells. In addition Sung et al. (41) directly measured the effect of different levels of ICAM-1 on transfected APCs binding to activated T cells by using micropipette suction. They found that the adhesive force ranges from 1,400 dyn/cm2 in the absence of ICAM-1 to 6,250 dyn/cm2 in the presence of ICAM-1. This is well beyond the adhesion forces tested here (≤90 dyn/cm2) and also beyond what is thought to be necessary for T cell activation in vivo (34, 35). Thus the adhesive properties of the LFA-1/ICAM-1 interaction may not be strictly necessary in T/B cell recognition over the peptide range studied, although we have clearly not surveyed all possible APCs or all possible types of T cells. In particular we note the work of Dustin et al. (42) who found that very high densities of peptide/MHC (>1,000/μm2) were required to cause a T cell hybridoma to adhere to a bilayer. We would also like to point out that the formation of a tight, flat interface might facilitate adhesion already by itself. Such an interface would allow a large number of receptors of whatever kind to bind to their ligands and would, by virtue of its geometry, confer increased resistance to shear stress.
As to the implications of the data presented here indicating a role for LFA-1/ICAM-1 in sustaining the rise in intracellular calcium that accompanies T cell activation: it seems clear that the stabilization of the calcium signal is very important for T cell activation (4). In particular Timmerman et al. (43) demonstrated that sustained elevated calcium levels are necessary to keep the transcription factor NFATc in the nucleus. Similarly, Negulescu et al. (44) have shown that an elevated calcium concentration must be maintained for 30–60 min to express a lacZ gene driver by an interleukin 2 promoter. In our own work, we have shown that only a sustained calcium signal correlates with subsequent T cell proliferation (25, 26). Of particular relevance here is our finding that an incidence of more than 20% partial signals in a population correlates with a drastic reduction in T cell proliferation. This result makes the differences observed here (Table 1, data set 3) between T cells stimulated with the low levels of ICAM-1 on the APCs (72%) and those stimulated with the high ICAM-1-expressing cells (96%) very significant. Thus in the context of T cell recognition it may be that the primary role of the LFA-1/ICAM-1 interaction is to stabilize the calcium signal. This does not preclude an adhesive function for LFA-1 in TCR-independent processes such as during the extravasation of T cells.
LFA-1/ICAM-1 Clustering Could Amplify a Weak MHC/Peptide Signal.
Because a very small number of MHC II/peptide complexes (20–200 or less) is sufficient to fully activate a T cell (1, 16–19), creating a sustained signal might be difficult because of the weakness of the stimulus. One way in which a weak signal might be amplified has been suggested by Valitutti et al. (20) who have shown evidence that each MHC/peptide ‘serially engages’ many TCRs. Another suggestion comes from the work of Reich et al. (45), who have found that TCR-peptide/MHC complexes have an intrinsic ability to cluster, thus possibly focusing interacting molecules. The phenomenon of rapid ICAM-1 clustering that we describe here may represent a completely different “amplifier” mechanism. In this scenario, an initial TCR engagement, even one that involves very few ligands, triggers the clustering of ICAM-1/LFA-1 pairs at the interface. Consistent with this is our finding that the ICAM-1 accumulates at the interface even at very low concentrations of stimulating peptide, and that the rate of accumulation does not change with higher concentrations of antigen. The engagement of many thousands of high affinity LFA-1 molecules (13) with ICAM-1 could then produce additional second messenger signals that act to sustain the elevated intracellular calcium levels and promote full T cell activation in synergy with the TCR-induced signals. This mechanism would be most noticeable at limiting concentrations of peptide, consistent with the data in Table 1. It is also consistent with previous work showing that LFA-1 engagement can trigger the activation of phospholipase C-γ and can synergize with the TCR as judged by proliferation of a T cell clone stimulated with an APC as well as by early signaling intermediates of T cells stimulated with an anti-CD3 antibody and ICAM-1 on beads (38, 46).
Acknowledgments
We thank M. Anderson and L. A. Herzenberg for GFP-Bex1, Jen-Tsan Chi for cDNA, and R. Lewis for help with instrumentation. C.W. was supported by an European Molecular Biology Organization fellowship. This work was supported by the Howard Hughes Medical Institute.
ABBREVIATIONS
- ICAM-1
intracellular adhesion molecule 1
- LFA-1
lymphocyte function-associated antigen 1
- MHC
major histocompatibility complex
- APC
antigen-presenting cell
- TCR
T cell antigen receptor
- GFP
green fluorescent protein
- GPI
glycoprotein I
- BAPTA
bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate
- CCD
charge-coupled device
- PKC
protein kinase C
References
- 1.Dustin M L, Bromley S K, Kan Z, Peterson D A, Unanue E R. Proc Natl Acad Sci USA. 1997;94:3909–3913. doi: 10.1073/pnas.94.8.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Negulescu P A, Krasieva T B, Khan A, Kerschbaum H H, Cahalan M D. Immunity. 1996;4:421–430. doi: 10.1016/s1074-7613(00)80409-4. [DOI] [PubMed] [Google Scholar]
- 3.Donnadieu E, Bismuth G, Trautmann A. Curr Biol. 1994;4:584–595. doi: 10.1016/s0960-9822(00)00130-5. [DOI] [PubMed] [Google Scholar]
- 4.Weiss A, Littman D R. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 5.Premack B A, Gardner P. Am J Physiol. 1992;263:C1119–C1140. doi: 10.1152/ajpcell.1992.263.6.C1119. [DOI] [PubMed] [Google Scholar]
- 6.Lewis R S, Cahalan M D. Annu Rev Immunol. 1995;13:623–653. doi: 10.1146/annurev.iy.13.040195.003203. [DOI] [PubMed] [Google Scholar]
- 7.Croft M, Dubey C. Crit Rev Immunol. 1997;17:89–118. doi: 10.1615/critrevimmunol.v17.i1.40. [DOI] [PubMed] [Google Scholar]
- 8.Dustin M L, Springer T A. Nature (London) 1989;341:619–624. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- 9.van Kooyk Y, van de Wiel-van Kemenade P, Weder P, Kuijpers T W, Figdor C G. Nature (London) 1989;342:811–813. doi: 10.1038/342811a0. [DOI] [PubMed] [Google Scholar]
- 10.Kupfer A, Singer S J. J Exp Med. 1989;170:1697–1713. doi: 10.1084/jem.170.5.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark E A, Brugge J S. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- 12.Lub M, van Kooyk Y, Figdor C G. Immunol Today. 1995;16:479–483. doi: 10.1016/0167-5699(95)80031-x. [DOI] [PubMed] [Google Scholar]
- 13.Lollo B A, Chan K W, Hanson E M, Moy V T, Brian A A. J Biol Chem. 1993;268:21693–21700. [PubMed] [Google Scholar]
- 14.Xu H, Gonzalo J A, St Pierre Y, Williams I R, Kupper T S, Cotran R S, Springer T A, Gutierrez-Ramos J C. J Exp Med. 1994;180:95–109. doi: 10.1084/jem.180.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sligh J E, Jr, Ballantyne C M, Rich S S, Hawkins H K, Smith C W, Bradley A, Beaudet A L. Proc Natl Acad Sci USA. 1993;90:8529–8533. doi: 10.1073/pnas.90.18.8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harding C V, Unanue E R. Nature (London) 1990;346:574–576. doi: 10.1038/346574a0. [DOI] [PubMed] [Google Scholar]
- 17.Demotz S, Grey H M, Sette A. Science. 1990;249:1028–30. doi: 10.1126/science.2118680. [DOI] [PubMed] [Google Scholar]
- 18.Sykulev Y, Joo M, Vturina I, Tsomides T J, Eisen H N. Immunity. 1996;4:565–571. doi: 10.1016/s1074-7613(00)80483-5. [DOI] [PubMed] [Google Scholar]
- 19.Brower R C, England R, Takeshita T, Kozlowski S, Margulies D H, Berzofsky J A, Delisi C. Mol Immunol. 1994;31:1285–1293. doi: 10.1016/0161-5890(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 20.Valitutti S, Müller S, Cella M, Padovan E, Lanzavecchia A. Nature (London) 1995;375:148–151. doi: 10.1038/375148a0. [DOI] [PubMed] [Google Scholar]
- 21.Siu G, Hedrick S M, Brian A A. J Immunol. 1989;143:3813–3820. [PubMed] [Google Scholar]
- 22.Anderson M T, Tjioe I M, Lorincz M C, Parks D R, Herzenberg L A, Nolan G P, Herzenberg L A. Proc Natl Acad Sci USA. 1996;93:8508–8511. doi: 10.1073/pnas.93.16.8508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin A Y, Devaux B, Green A, Sagerström C, Elliott J F, Davis M M. Science. 1990;249:677–679. doi: 10.1126/science.1696397. [DOI] [PubMed] [Google Scholar]
- 24.Whitehorn E A, Tate E, Yanofsky L, Kochersperger L, Davis A, Mortensen R B, Yonkovich S, Bell K, Dower W J, Barrett R W. BioTechnology. 1995;13:1215–1219. doi: 10.1038/nbt1195-1215. [DOI] [PubMed] [Google Scholar]
- 25.Wülfing C, Rabinowitz J D, Beeson C, Sjaastad M D, McConnell H M, Davis M M. J Exp Med. 1997;185:1815–1825. doi: 10.1084/jem.185.10.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rabinowitz J D, Beeson C, Wülfing C, Tate K, Allen P M, Davis M M, McConnell H M. Immunity. 1996;5:125–135. doi: 10.1016/s1074-7613(00)80489-6. [DOI] [PubMed] [Google Scholar]
- 27.Takei F. J Immunol. 1985;134:1403–1407. [PubMed] [Google Scholar]
- 28.Sanchez-Madrid F, Davignon D, Martz E, Springer T A. Cell Immunol. 1982;73:1–11. doi: 10.1016/0008-8749(82)90431-2. [DOI] [PubMed] [Google Scholar]
- 29.Groves J T, Wülfing C, Boxer S G. Biophys J. 1996;71:2716–2723. doi: 10.1016/S0006-3495(96)79462-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsien R Y. Annu Rev Neurosci. 1989;12:227–253. doi: 10.1146/annurev.ne.12.030189.001303. [DOI] [PubMed] [Google Scholar]
- 31.Fink P J, Matis L A, McElligott D L, Bookman M, Hedrick S M. Nature (London) 1986;321:219–226. doi: 10.1038/321219a0. [DOI] [PubMed] [Google Scholar]
- 32.Seder R A, Paul W E, Davis M M, Fazekas de St. Groth B. J Exp Med. 1992;176:1091–1098. doi: 10.1084/jem.176.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valitutti S, Dessing M, Aktories K, Gallati H, Lanzavecchia A. J Exp Med. 1995;181:577–584. doi: 10.1084/jem.181.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heisig N. Adv Microcirc. 1968;1:89–94. [Google Scholar]
- 35.Atherton A, Born G V R. J Physiol (London) 1972;222:447–474. doi: 10.1113/jphysiol.1972.sp009808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monks C R F, Kupfer H, Tamir I, Barlow A, Kupfer A. Nature (London) 1997;385:83–86. doi: 10.1038/385083a0. [DOI] [PubMed] [Google Scholar]
- 37.van de Stolpe A, van der Saag P T. J Mol Med. 1996;74:13–33. doi: 10.1007/BF00202069. [DOI] [PubMed] [Google Scholar]
- 38.Van Seventer G A, Bonvini E, Yamada H, Conti A, Stringfellow S, June C H, Shaw S. J Immunol. 1992;149:3872–3880. [PubMed] [Google Scholar]
- 39.Kuhlman P, Moy V T, Lollo B A, Brian A A. J Immunol. 1991;146:1773–1782. [PubMed] [Google Scholar]
- 40.Swain S L, Croft M, Dubey C, Haynes L, Rogers P, Zhang X, Bradley L M. Immunol Rev. 1996;150:143–167. doi: 10.1111/j.1600-065x.1996.tb00700.x. [DOI] [PubMed] [Google Scholar]
- 41.Sung K L, Kuhlman P, Maldonado F, Lollo B A, Chien S, Brian A A. J Cell Sci. 1992;103:259–266. doi: 10.1242/jcs.103.1.259. [DOI] [PubMed] [Google Scholar]
- 42.Dustin M L, Miller J M, Ranganath S, Vignali D A, Viner N J, Nelson C A, Unanue E R. J Immunol. 1996;157:2014–2021. [PubMed] [Google Scholar]
- 43.Timmerman L A, Clipstone N A, Ho S N, Northrop J P, Crabtree G R. Nature (London) 1996;383:837–840. doi: 10.1038/383837a0. [DOI] [PubMed] [Google Scholar]
- 44.Negulescu P A, Shastri N, Cahalan M D. Proc Natl Acad Sci USA. 1994;91:2873–2877. doi: 10.1073/pnas.91.7.2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reich Z, Boniface J J, Lyons D S, Borochov N, Wachtel E J, Davis M M. Nature (London) 1997;387:617–620. doi: 10.1038/42500. [DOI] [PubMed] [Google Scholar]
- 46.Kanner S B, Grosmaire L S, Ledbetter J A, Damle N K. Proc Natl Acad Sci USA. 1993;90:7099–7103. doi: 10.1073/pnas.90.15.7099. [DOI] [PMC free article] [PubMed] [Google Scholar]