Abstract
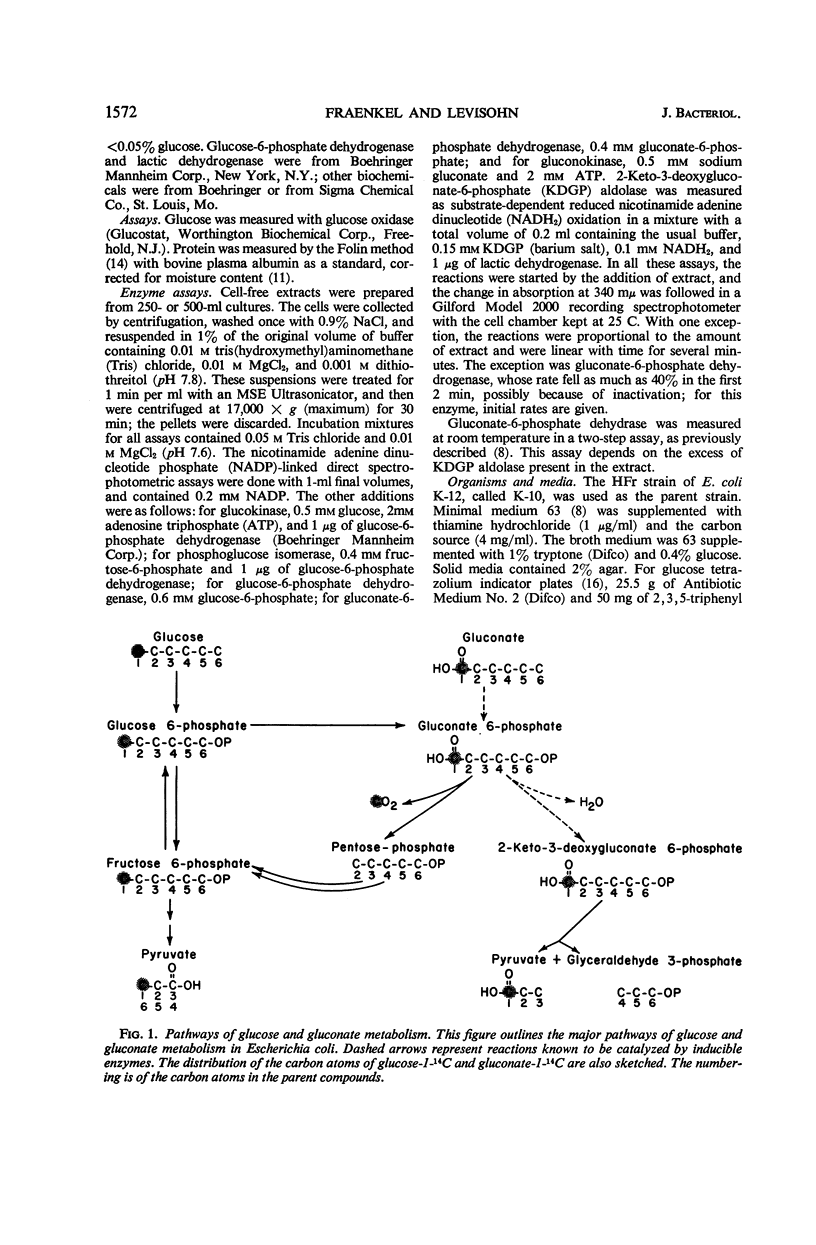
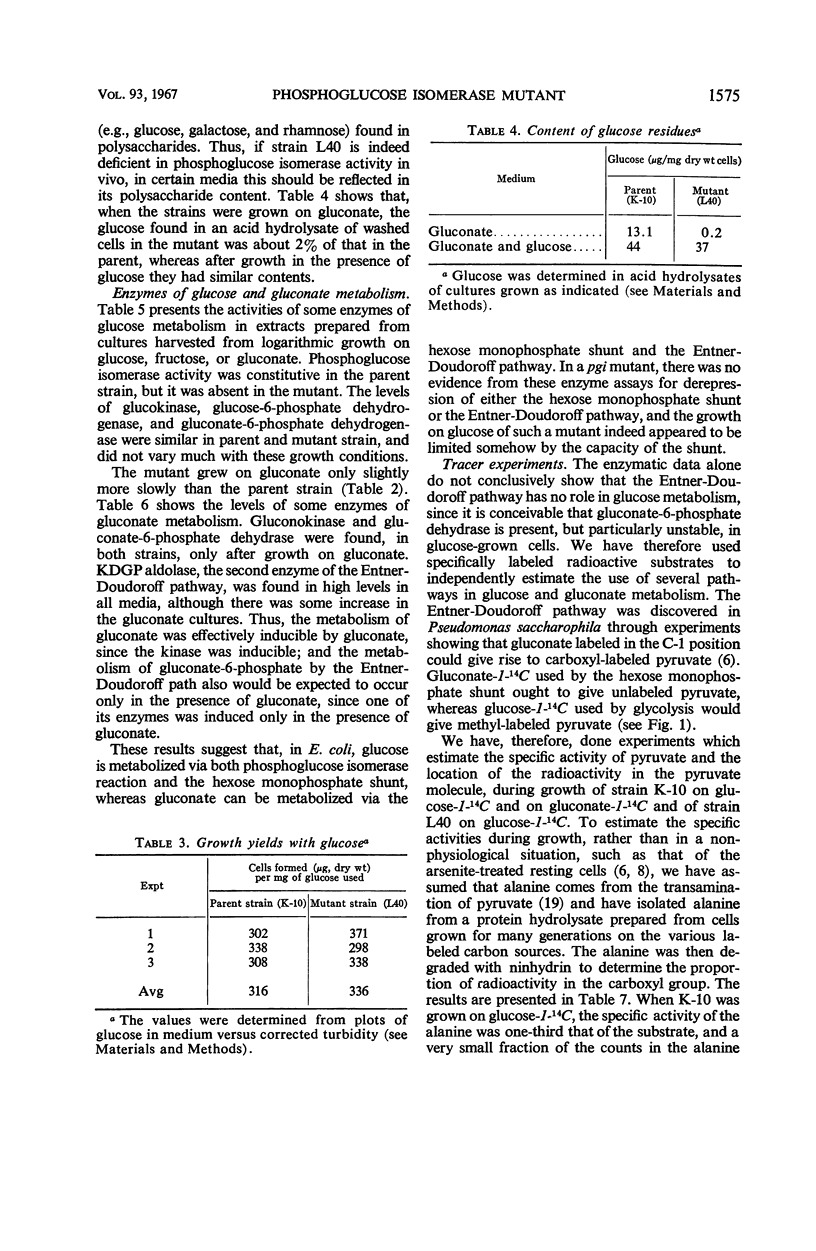
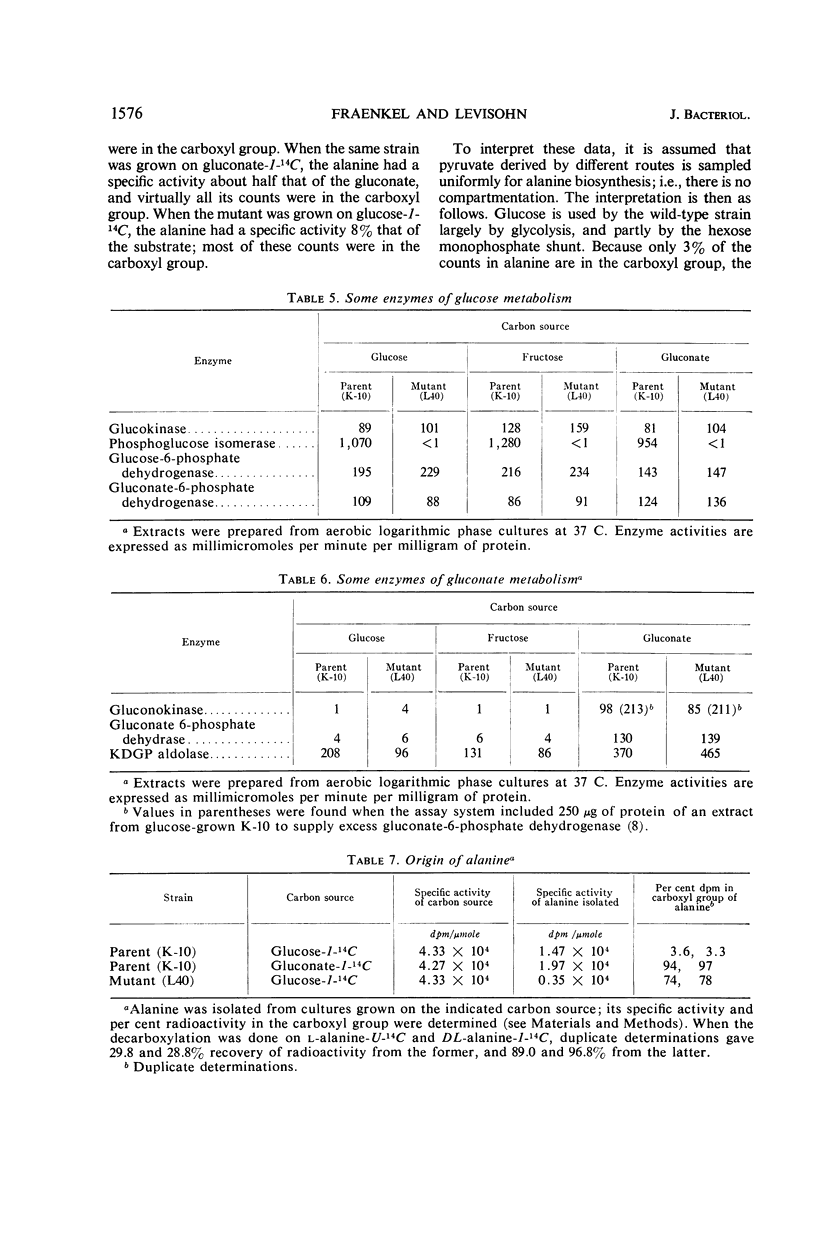
A single gene mutant lacking phosphoglucose isomerase (pgi) was selected after ethyl methane sulfonate mutagenesis of Escherichia coli strain K-10. Enzyme assays revealed no pgi activity in the mutant, whereas levels of glucokinase, glucose-6-phosphate dehydrogenase, and gluconate-6-phosphate dehydrogenase were similar in parent and mutant. The amount of glucose released by acid hydrolysis of the mutant cells after growth on gluconate was less than 2% that released from parent cells; when grown in the presence of glucose, mutant and parent cells contained the same amount of glucose residues. The mutant grew on glucose one-third as fast as the parent; it also grew much slower than the parent on galactose, maltose, and lactose. On fructose, gluconate, and other carbon sources, growth was almost normal. In both parent and mutant, gluconokinase and gluconate-6-phosphate dehydrase were present during growth on gluconate but not during growth on glucose. Assay and degradation of alanine from protein hydrolysates after growth on glucose-1-14C and gluconate-1-14C showed that in the parent strain glucose was metabolized by the glycolytic path and the hexose monophosphate shunt. Gluconate was metabolized by the Entner-Doudoroff path and the hexose monophosphate shunt. The mutant used glucose chiefly by the shunt, but may also have used the Entner-Doudoroff path to a limited extent.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Böck A., Neidhardt F. C. Properties of a Mutant of Escherichia coli with a Temperature-sensitive Fructose-1,6-Diphosphate Aldolase. J Bacteriol. 1966 Aug;92(2):470–476. doi: 10.1128/jb.92.2.470-476.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consden R., Gordon A. H., Martin A. J. Qualitative analysis of proteins: a partition chromatographic method using paper. Biochem J. 1944;38(3):224–232. doi: 10.1042/bj0380224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzarelli N. R., Koch J. P., Hayashi S., Lin E. C. Growth stasis by accumulated L-alpha-glycerophosphate in Escherichia coli. J Bacteriol. 1965 Nov;90(5):1325–1329. doi: 10.1128/jb.90.5.1325-1329.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENTNER N., DOUDOROFF M. Glucose and gluconic acid oxidation of Pseudomonas saccharophila. J Biol Chem. 1952 May;196(2):853–862. [PubMed] [Google Scholar]
- FRAENKEL D. G., HORECKER B. L. PATHWAYS OF D-GLUCOSE METABOLISM IN SALMONELLA TYPHINMURIUM. A STUDY OF A MUTANT LACKING PHOSPHOGLUCOSE ISOMERASE. J Biol Chem. 1964 Sep;239:2765–2771. [PubMed] [Google Scholar]
- FRAENKEL D., OSBORN M. J., HORECKER B. L., SMITH S. M. Metabolism and cell wall structure of a mutant of Salmonella typhimurium deficient in phosphoglucose isomerase. Biochem Biophys Res Commun. 1963 Jun 20;11:423–428. doi: 10.1016/0006-291x(63)90086-x. [DOI] [PubMed] [Google Scholar]
- Fraenkel D. G. Genetic mapping of mutations affecting phosphoglucose isomerase and fructose diphosphatase in Escherichia coli. J Bacteriol. 1967 May;93(5):1582–1587. doi: 10.1128/jb.93.5.1582-1587.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HATTMAN S. THE FUNCTIONING OF T-EVEN PHAGES WITH UNGLUCOSYLATED DNA IN RESTRICTING ESCHERICHIA COLI HOST CELLS. Virology. 1964 Nov;24:333–348. doi: 10.1016/0042-6822(64)90171-0. [DOI] [PubMed] [Google Scholar]
- LOVELESS A., HOWARTH S. Mutation of bacteria at high levels of survival by ethyl methane sulphonate. Nature. 1959 Dec 5;184:1780–1782. doi: 10.1038/1841780a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Loomis W. F., Jr, Magasanik B. Nature of the effector of catabolite repression of beta-galactosidase in Escherichia coli. J Bacteriol. 1966 Jul;92(1):170–177. doi: 10.1128/jb.92.1.170-177.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signer E. R., Beckwith J. R., Brenner S. Mapping of suppressor loci in Escherichia coli. J Mol Biol. 1965 Nov;14(1):153–166. doi: 10.1016/s0022-2836(65)80237-6. [DOI] [PubMed] [Google Scholar]
- TROLL W., CANNAN R. K. A modified photometric ninhydrin method for the analysis of amino and imino acids. J Biol Chem. 1953 Feb;200(2):803–811. [PubMed] [Google Scholar]
- Zablotny R., Fraenkel D. G. Glucose and gluconate metabolism in a mutant of Escherichia coli lacking gluconate-6-phosphate dehydrase. J Bacteriol. 1967 May;93(5):1579–1581. doi: 10.1128/jb.93.5.1579-1581.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]


