Summary
Porphyromonas gingivalis is an oral pathogen that is also associated with serious systemic conditions such as preterm delivery. Here we investigated the interaction between P. gingivalis and a cell line of extravillous trophoblasts (HTR-8) derived from the human placenta. P. gingivalis internalized within HTR-8 cells and inhibited proliferation through induction of arrest in the G1 phase of the cell cycle. G1 arrest was associated with decreased expression of cyclin D and of CDKs 2, 4 and 6. In addition, levels of CDK inhibitors p15, p16, p18 and p21 were increased following P. gingivalis infection. The amount of Rb was diminished by P. gingivalis, and transient overexpression of Rb, with concomitant upregulation of phospho-Rb, relieved P. gingivalis-induced G1 arrest. HTR-8 cells halted in the G1 phase became apoptotic, and apoptosis was accompanied by an increase in the ratio of Bax/Bcl-2 and increased activity of caspases 3, 7 and 9. HTR-8 cells infected with P. gingivalis also exhibited a sustained activation of ERK1/2, and knockdown of ERK1/2 activity with siRNA abrogated both G1 arrest and apoptosis. Thus, P. gingivalis can invade placental trophoblasts and induce G1 arrest and apoptosis through pathways involving ERK1/2 and its downstream effectors, properties that provide a mechanistic basis for pathogenicity in complications of pregnancy.
Introduction
Preterm delivery of low-birth-weight (PTLBW) infants occurs in about 12% of births and frequently contributes to infant mortality and morbidity (Hoyert et al., 2006). Bacterial infection is a major cause of PTLBW, either ascending from the urogenital tract such as occurs with Escherichia coli, or transplacental as is the case with Listeria monocytogenes and Salmonella (Goldenberg et al., 2003; Schloesser et al., 2004; Bakardjiev et al., 2005). Recently, an epidemiological association has emerged between periodontal bacteria and PTLBW delivery (Xiong et al., 2006; Michalowicz and Durand, 2007). Meta-analyses of studies examining the association between periodontal disease and PTLBW have reported odds ratios ranging from 1.1 to 20, although not all studies of this complex phenomenon find an association (Wimmer and Pihlstrom, 2008). The mechanistic basis for a causal association between periodontal bacteria and PTLBW delivery has yet to be defined; however, within the confines of the periodontal tissues bacteria and their antigens could initiate an inflammatory response with systemic consequences that could trigger preterm labour (Offenbacher et al., 1998; Garcia et al., 2001). Alternatively, direct oralhaematogenous spread of bacteria to the gestational tissues may occur. Bacteremias are common in patients with periodontal disease and are often associated with tooth brushing and dental treatment (Kinane et al., 2005; Lockhart et al., 2008; Perez-Chaparro et al., 2008). Moreover, Porphyromonas gingivalis, a major periodontal pathogen, has been detected in the amniotic fluid in preterm labour (Leon et al., 2007), and in the placenta in pre-eclampsia (Barak et al., 2007). A role for P. gingivalis in pregnancy complications is supported by animal models. Infection of P. gingivalis in subcutaneous chambers in mice leads to systemic spread and localization in the placental tissues, resulting in an increased rate of both low-birth-weight fetuses and fetal resorption (Lin et al., 2003). In pregnant rabbits, chronic exposure to P. gingivalis results in systemic dissemination, transplacental passage and fetal exposure (Boggess et al., 2005). Intravenous inoculation of rats with P. gingivalis results in invasion of both maternal and fetal tissues, along with chorioamnionitis and placentitis (Belanger et al., 2008).
In the oral cavity P. gingivalis and epithelial cells engage each other in a complex bidirectional molecular dialogue that results in the internalization of the organism (Lamont et al., 1995). Invasion is effectuated through reprogramming of host cell signal transduction pathways (Watanabe et al., 2001). Engagement of integrin receptors by the P. gingivalis long fimbriae results in activation of the signalling adaptor proteins paxillin and FAK and recruitment into focal adhesion complexes (Yilmaz et al., 2003). P. gingivalis also expresses a HAD family serine phosphatase (SerB) that induces cytoskeletal remodelling through impinging upon phospho-dependent signalling circuits (Tribble et al., 2006). In primary cultures of gingival epithelial cells, disruption signal transduction converges on negative regulation of NF-κB (Watanabe et al., 2001) and there is perturbation of the host cell transcriptome (Handfield et al., 2005). Both host cells and intracellular bacteria remain viable for extended periods and the phenotypic outcomes for gingival epithelial cells include suppression of apoptotic pathways along with resistance to chemically induced apoptosis, and acceleration through the cell cycle (Nakhjiri et al., 2001; Yilmaz et al., 2004; Mao et al., 2007; Kuboniwa et al., 2008). The physiological impact of P. gingivalis on the host, however, is cell and context dependent as P. gingivalis whole cells and components such as proteases, LPS, fimbriae and the metabolic end-product butyric acid can induce apoptosis in various cell types (Isogai et al., 1996; Hiroi et al., 1998; Ozaki and Hanazawa, 2001; Murray and Wilton, 2003; Sheets et al., 2006; Desta and Graves, 2007). In addition, in stromal/osteblastic cells P. gingivalis reduces cyclin expression and causes G1 arrest, leading to the inhibition of cellular proliferation (Kato et al., 2008).
The placental–fetal barrier in the monohemochorial placenta of humans consists of a single layer of fetally derived trophoblasts. This trophoblast barrier is formed from a highly proliferative, migratory and invasive sub-population of trophoblasts, known as extravillous trophoblasts, which invade the uterus and its vasculature. The resulting trophoblast layer is in direct contact with maternal blood and is essential for the adequate exchange of key molecules between the maternal and fetal circulation. Organisms that commonly cause fetoplacental infections are often intracellular, and include viruses such as CMV (Stagno et al., 1986), protozoa such as Toxoplasma gondii (Jones et al., 2001) and bacteria such as L. monocytogenes (Mylonakis et al., 2002). Listeria, for example, invades trophoblasts both in vitro and in vivo and can induce apoptosis in placental cells (Disson et al., 2008). The outcome of the interaction between P. gingivalis and placental trophoblasts has yet to be characterized. Here we report that P. gingivalis can invade a trophoblast cell line and induce G1 arrest and apoptosis. G1 arrest is associated with activation of ERK1/2 and signalling through cyclins and Rb.
Results
P. gingivalis invades and survives within HTR-8 trophoblasts
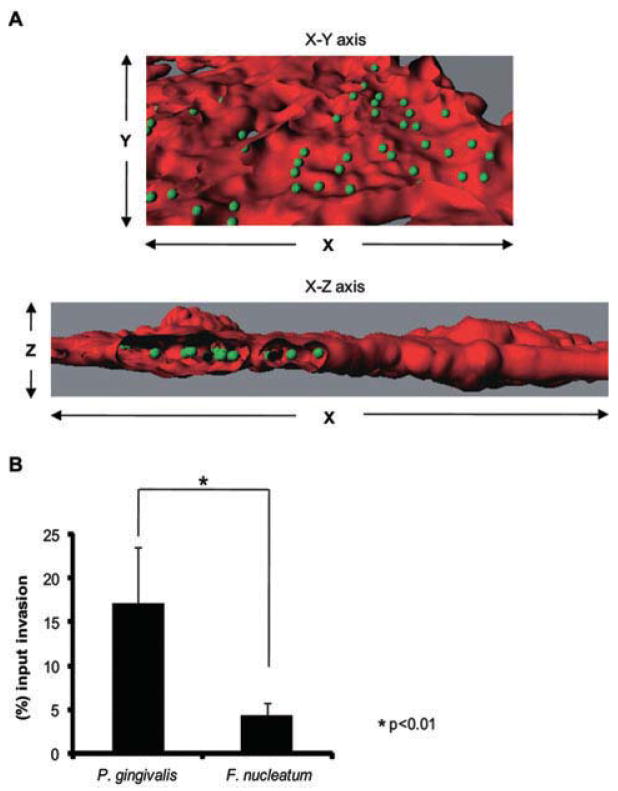
The ability to traverse the placental barrier is an essential attribute of organisms capable of causing fetoplacental infections. The physical association between P. gingivalis and HTR-8/SVneo trophoblast (henceforth called HTR-8) cells was examined by fluorescent confocal microscopy. HTR-8 cells are a non-tumorigenic cell line established from explant cultures of human first trimester placenta (8–10 weeks of gestation) by immortalization with the SV40 large T antigen (Graham et al., 1993). HTR-8 cells exhibit the phenotypic behaviour of freshly isolated primary trophoblasts with regard to Matrigel invasion (Kilburn et al., 2000), and response to the migration/invasion signals TGF-β, IGFII and IGFBP-1 (Graham et al., 1993; Chakraborty et al., 2003; Zygmunt et al., 2005). Furthermore, HTR-8 cells express all the cytokeratin markers of extravillous trophoblasts and secrete hCG. HTR-8 cells express TLRs and respond to challenge with bacterial LPS and peptidoglycan (Svinarich et al., 1996; Abrahams et al., 2004). These cells have been used widely as a model for study of early placental trophoblast invasion and responses to uterine growth regulatory and angiogenic factors at the maternal–fetal interface (Svinarich et al., 1996; Svinarich et al., 1998; Renaud et al., 2005). Confocal microscopy showed that P. gingivalis internalized within HTR-8 cells in high numbers (Fig. 1A). Internalization and viability was then quantified by an antibiotic protection assay (Fig. 1B). At a multiplicity of infection (moi) of 100, 17.2% of the initial inoculum of P. gingivalis could be recovered intracellularly, a level of invasion that is similar to that of gingival epithelial cells (Lamont et al., 1995). For comparison, we also examined the invasive properties of Fusobacterium nucleatum, another periodontal organism that is also capable of inducing preterm delivery in animal models (Liu et al., 2007). In the antibiotic protection assay, the invasion of F. nucleatum in HTR-8 cells was significantly lower than P. gingivalis (Fig. 1B), indicating that F. nucleatum and P. gingivalis exhibit differing potential virulence characteristics in placental tissues. P. gingivalis is more aggressively invasive for placental cells, and thus possibly more destructive to tissue and able to evade immune surveillance.
Fig. 1. P. gingivalis internalizes and survives within HTR-8 cells.
A. Fluorescent confocal microscopy of HTR-8 cells infected with P. gingivalis at an moi of 100 for 2 h. Bacterial cells were detected with P. gingivalis antibodies and FITC-secondary antibodies (green). The volume of the HTR-8 cells was visualized with TRIC-phalloidin staining for actin (red). Infected cells were viewed by confocal microscopy, and fluorescent optical x–y and x-z sections were reconstructed with Imaris software. Z stacks of the x–y sections of confocal microscopy were converted to composite images with ‘Iso Surface’ and ‘Spot Detection’ functions of the ‘Surpass’ option on Imaris. Section views were created using the ‘Clipping’ function.
B. Antibiotic protection invasion assay with P. gingivalis and F. nucleatum. HTR-8 cells were infected with P. gingivalis or F. nucleatum at an moi of 100 for 2 h. Extracellular bacteria were killed with gentamicin and metronidazole and intracellular bacteria released by lysis of the HTR-8 cells. Numbers of intracellular bacteria were determined by viable counting of the cell lysates and are expressed as percentage of input bacterial cell number. Data are means ± SD of three independent experiments and were analysed with a t-test.
P. gingivalis slows proliferation and induces morphological changes in HTR-8 cells
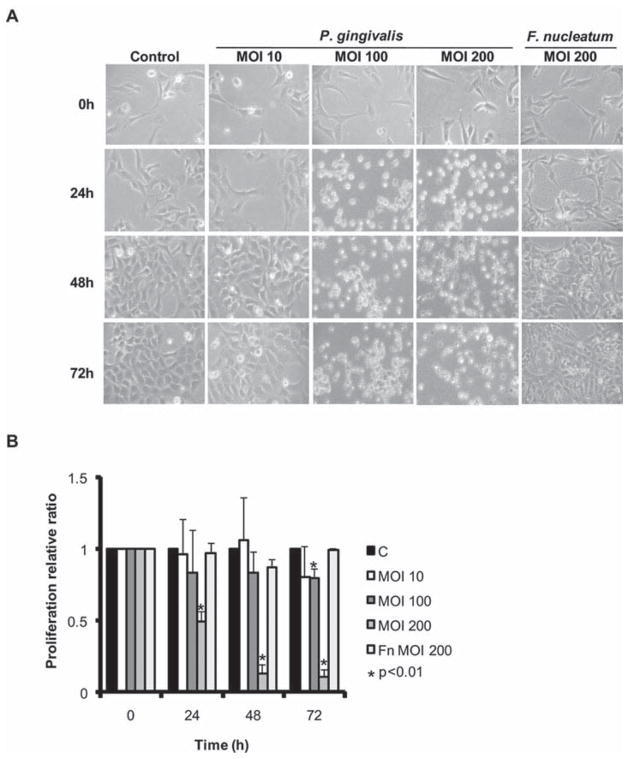
To begin the process of characterizing the response of HTR-8 cells to infection with P. gingivalis, the morphology of infected cells was examined by microscopy. With increasing moi and time, P. gingivalis caused HTR-8 cells to contract and round-up (Fig. 2A), while remaining attached to the substratum and capable of excluding trypan blue (not shown). F. nucleatum did not induce these changes, which, in combination with the invasion data, establishes a basis for proposing a specific cellular dialog between P. gingivalis and HTR-8 cells. The nature of morphological changes prompted us to investigate the proliferation rate of infected HTR-8 cells. In a dehydrogenase activity assay P. gingivalis but not F. nucleatum, inhibited cell proliferation in an moi and time dependent manner (Fig. 2B).
Fig. 2. P. gingivalis induces morphological changes and inhibits cell proliferation in HTR-8 cells.
A. Light microscopy showing morphology of HTR-8 cells infected with P. gingivalis at an moi of 10, 100 and 200, or F. nucleatum at an moi of 200, for 24, 48 and 72 h. Control cells were uninfected.
B. Proliferation of HTR-8 cells measured by tetrazolium following infection with P. gingivalis or F. nucleatum at the times and moi indicated. Data are expressed as relative ratio infected/uninfected (c) and are means ± SD from three independent experiments analysed with a t-test.
P. gingivalis causes G1 phase cell cycle arrest
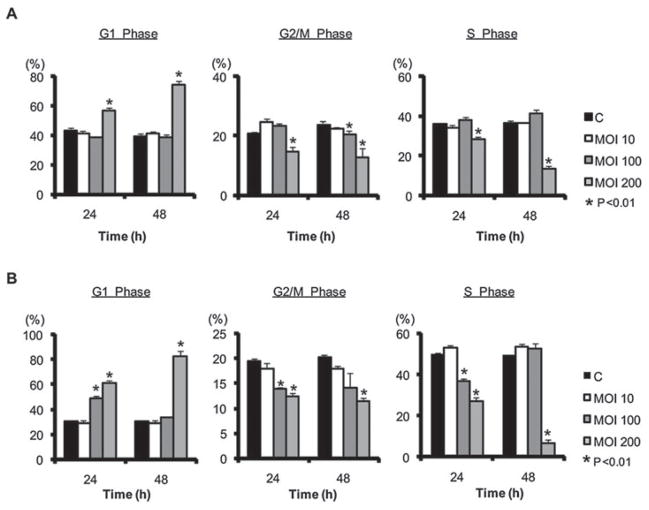
Inhibition of HTR-8 proliferation by P. gingivalis led us to predict that P. gingivalis would impinge upon HTR-8 cell cycle progression. To test this hypothesis, the proportion of infected HTR-8 cells in the various stages of cell cycle was determined by propidium iodide staining and flow cytometry. Infection with P. gingivalis caused a progressive increase in G1 phase cells and a corresponding decrease in S and G2/M phase cells, and this effect was evident in cells synchronized either by serum starvation or by double thymidine block (Fig. 3). P. gingivalis-induced G1 arrest was dose dependent and was maximal after 48 h. At 72 h the integrity of the cells after trypsinization was insufficient for flow cytometry analysis (Fig. S1) although the cells remained viable and attached to the culture dish prior to trypsinization. These results suggested that P. gingivalis-induced inhibition of cell growth was correlated with an arrest in G1 of the cell cycle. Subsequently, serum starvation synchronized cells were used in all experiments.
Fig. 3. P. gingivalis induces G1 arrest in HTR-8 cells. HTR-8 cells were infected with P. gingivalis, or uninfected (c), for the times and moi indicated.
A. Starvation synchronized HTR-8 cells.
B. Double thymidine block synchronized HTR-8 cells. DNA content was analysed by flow cytometry and cell cycle profiles obtained with ModFit software. Results are means ± SD of cell cycle distribution from three different experiments with flow cytometry parameters set to exclude cell debris and aggregates. *P < 0.01 compared with control uninfected cells (t-test).
G1 arrest is correlated with differential expression of cell cycle effectors
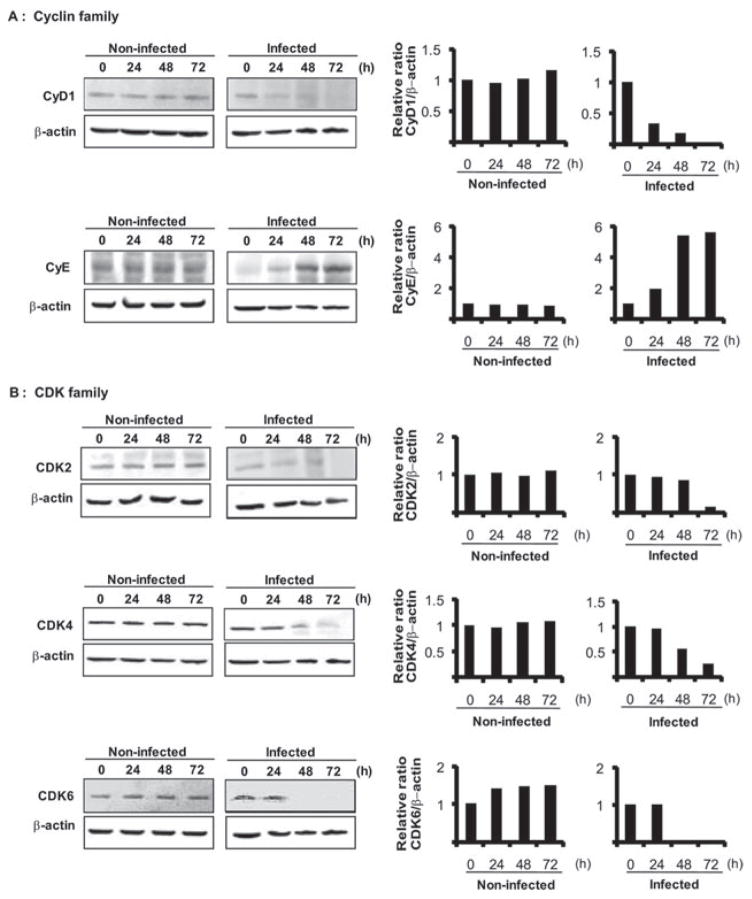
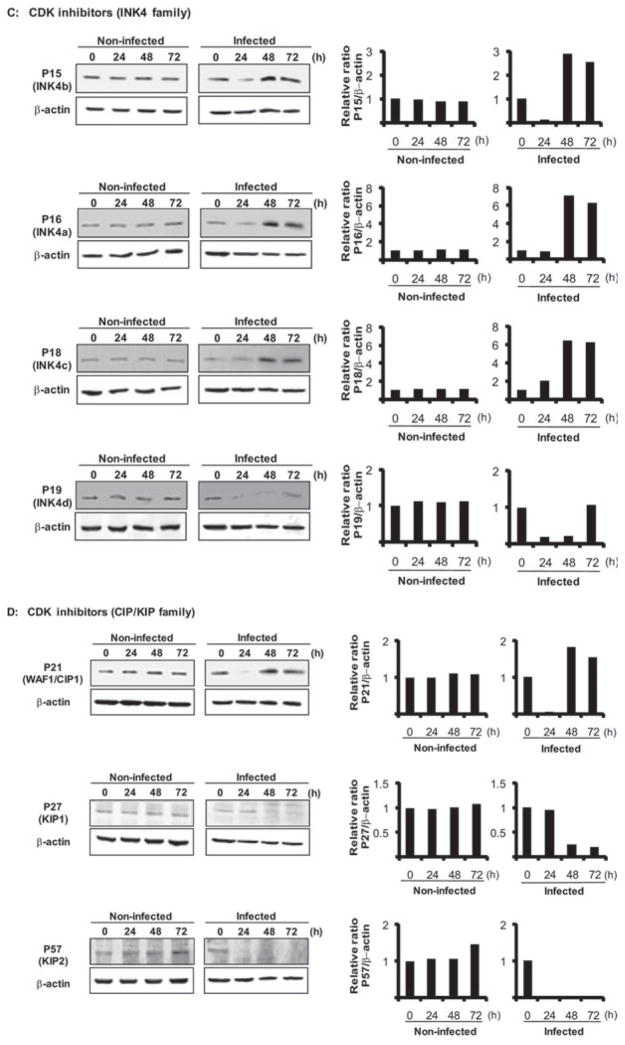
Progression from G1 to S depends on the coordinated activation/inactivation of a series of cyclins, CDKs and cyclin inhibitors (Malumbres and Barbacid, 2005; Bloom and Cross, 2007; Besson et al., 2008). Cyclins and CDKs form catalytically active complexes that phosphorylate effectors of directional cell cycle progression. Thus, we examined the effect of P. gingivalis on expression of cyclins, CDKs and CDK inhibitors. Densiometric analysis of immunoblots showed that after 24 h challenge with P. gingivalis, the levels of cyclin D were reduced and remained lower than in uninfected control cells for up to 72 h (Fig. 4A). Cyclin D binds to and activates CDK4 or CDK6, the activity of which is required for the G1 to S transition. The levels of CDK4 and CDK6 (Fig. 4B) were also reduced by P. gingivalis over 48–72 h, consistent with a failure of HTR-8 cells to progress beyond G1. Cyclin E, which accumulates at the G1-S phase boundary and is degraded as cells progress through S phase (Malumbres and Barbacid, 2005), accumulated in the presence of P. gingivalis (Fig. 4A), consistent with stalling of the HTR-8 cells in G1. Cyclin E also activates CDK2, which, along with CDK 4 and 6, is required for G1-S transition. However, CDK 2 levels were reduced after 72 h (Fig. 4B) and hence the higher levels of cyclin E alone are unlikely to push the cells through G1. The activity of CDK4 and CDK6 is opposed by members of the INK4 family (Jeffrey et al., 2000), while the CIP/KIP family bind to and inhibit cyclin/CDK complexes, also regulating G1-S transition (Besson et al., 2008). P. gingivalis elevated levels of INK4 family members P15, P16 and P18, over 48–72 h, although P19 did not increase in amount (Fig. 4C). The expression of P21 (CIP1) a cyclin D complex inhibitor increased slightly in response to P. gingivalis infection over 48–72 h; whereas levels of P27 (KIP1) and P57 (KIP2), which preferentially inhibit complexes of cyclin E and A (Besson et al., 2008), were reduced (Fig. 4D). Levels of P15, P19 and P21 transiently decreased prior to an increase (P15, P21) or a return to steady state levels (P19) at 72 h. This may be a reflection of the ability of P. gingivalis to interface with multiple pathways during the process of internalization and adaptation to an intracellular environment, and the impact of P. gingivalis thus reverberates through host cell systems. However, the effects of a transient decrease of expression of CDK inhibitors were not observed phenotypically in terms of accelerated cell cycle progression. Collectively, these results show that P. gingivalis selectively reduces cyclin D levels in HTR-8 cells after 24 h and this is followed by inhibition of CDK2, CDK 4 and CDK6 activity. These events will impede progression of HTR-8 cells through the G1-S phase boundary.
Fig. 4. Regulators of cell cycle progression are differentially expressed in HTR-8 cells infected with P. gingivalis. HTR-8 cells were infected with P. gingivalis at an moi of 200 for 24, 48 and 72 h. Lysates of infected and uninfected cells were analysed by immunoblotting.
A. Cyclin family (Cyclin D1 and E).
B. CDK family (CDK2, CDK4, CDK6).
C. CDK inhibitors (Ink4 family; P15, P16, P18, P19).
D. CDK inhibitors (CIP/KIP family; P21, P27, P57). Blots were analysed by scanning densitometry and ratios of protein/β-actin relative to the zero time point determined. Data are representative of three independent experiments.
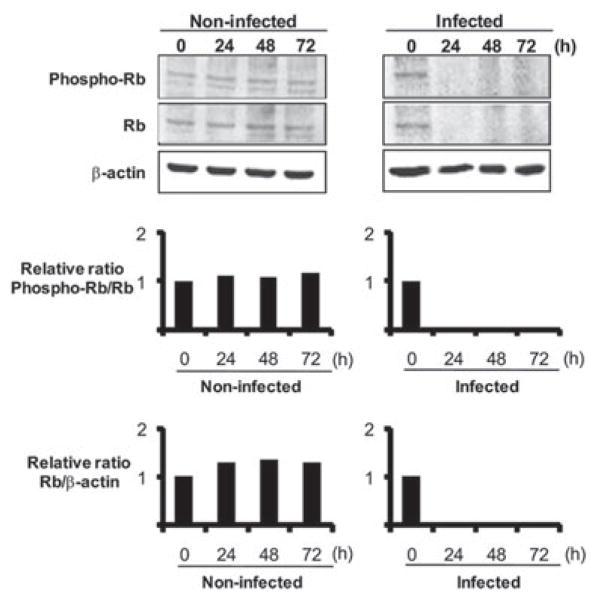
Rb is modulated by P. gingivalis and participates in cell cycle arrest
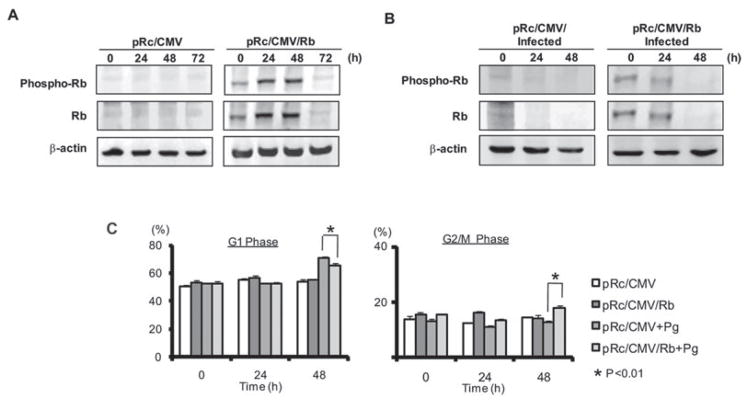
Retinoblastoma protein (Rb) is a tumor suppressor that exerts complex multilevel control over the cell cycle (Chau and Wang, 2003; Sun et al., 2007). Rb can be inactivated by CDK-dependent phosphorylation and by proteolytic degradation, and downregulation of CDK4/6 has also been associated with lower levels of Rb (Deshpande et al., 2005; Sarfaraz et al., 2006). As P. gingivalis reduced the amount and potential activity of CDK4 and CDK6 in HTR-8 cells, we examined the levels and phosphorylation status of Rb in infected cells. A decrease in expression of total Rb protein was observed following P. gingivalis infection (Fig. 5), an effect associated with G1 arrest in other cell types (Sarfaraz et al., 2006). Moreover, there was no observable increase in phospho-Rb following P. gingivalis infection (Fig. 5), indicating that any residual Rb remains active and capable of contributing to inhibition of cell cycle progression. To clarify the role of Rb in G1 arrest we transiently expressed Rb protein by transfection with plasmid pRc/CMV/Rb (Kim et al., 1994). Transfected HTR-8 cells overexpressed phospho- and total Rb for 48 h (Fig. 6A). At 24 h after transfection, cells were challenged with P. gingivalis and assayed immediately (time 0) and at 24 and 48 h after bacterial challenge. Immunoblotting showed that the decrease in phospho-and total Rb levels following P. gingivalis infection was delayed in comparison to infected, vector-transfected cells (Fig. 6B). Moreover, cell cycle analysis demonstrated that, compared with vector control, cells transfected with Rb and challenged with P. gingivalis were no longer delayed in the G1 phase and indeed had progressed through S phase and into G2/M (Fig. 6C and Fig. S2).
Fig. 5.
Rb and phospho-Rb expression is reduced in HTR-8 cells infected with P. gingivalis. HTR-8 cells were infected with P. gingivalis at the times and moi indicated. Lysates of infected and non-infected cells were analysed by immunoblotting with antibodies to phosphorylated (phospho-) Rb or total Rb. Blots were analysed by scanning densitometry and ratios of phospho-Rb/Rb and of Rb/β-actin relative to the zero time point determined. Data are representative of three independent experiments.
Fig. 6. Overexpression of Rb prevents P. gingivalis induced G1 arrest. HTR-8 cells were transfected with plasmid pRc/CMV/Rb expressing Rb or control pRc/CMV empty vector.
A. Immunoblots of HTR-8 lysates at 0, 24, 48 and 72 h after transfection.
B. Rb or control transfected HTR-8 cells at 24 h after transfection were infected with P. gingivalis at an moi of 200 for the times indicated, and analysed by immunoblotting with antibodies to Rb or to phospho-Rb.
C. Quantitative analysis of cell cycle distribution of cells transfected (24 h) cells infected with P. gingivalis at an moi of 200 for the times indicated. Data are means ± SD from three independent experiments. *P < 0.01 (t-test).
P. gingivalis induces apoptosis in HTR-8 cells
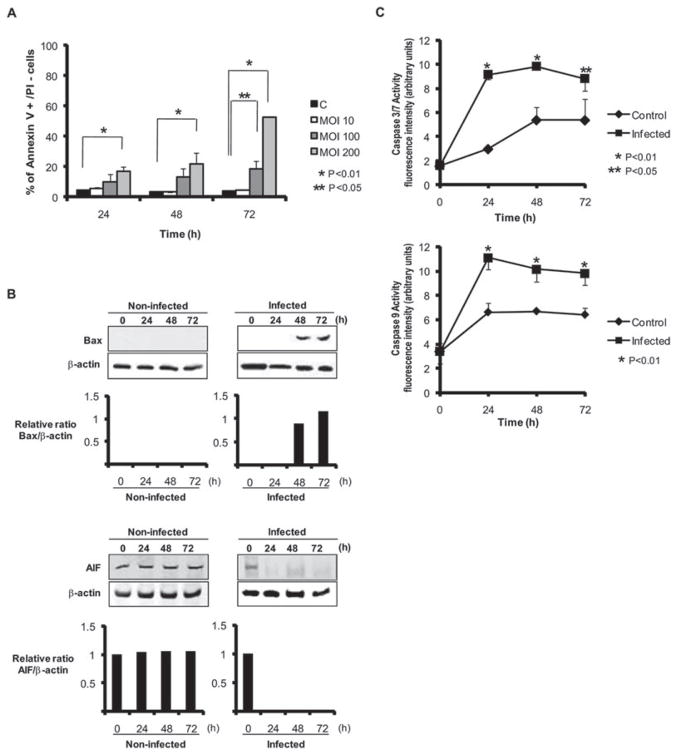
Arrest in the G1 phase is known to lead to apoptotic cell death (Massague, 2004; Sarfaraz et al., 2006), and loss of Rb sensitizes cells to apoptosis (Chau and Wang, 2003). In addition, examination of the flow cytometry results shows a sub-G1 peak, indicative of apoptotic cells, after 48 h challenge with P. gingivalis (Fig. S1). Hence, we assessed the extent of apoptosis in HTR-8 cells infected with P. gingivalis using Annexin V/propidium iodide staining and flow cytometry. As shown in Fig. 7A, the percentage of cells positive for Annexin V was increased by P. gingivalis in a time and moi dependent manner. In addition, expression of the Bcl-2 family apoptotic activator Bax was upregulated by P. gingivalis (Fig. 7B) whereas the anti-apoptotic Bcl-2 itself was unaffected by P. gingivalis (not shown). Alteration of the Bax/Bcl-2 ratio can initiate the caspase cascade (Chowdhury et al., 2006; Hail et al., 2006); therefore, we next quantified the activity of caspase 3/7 and of caspase 9. In a fluorescent substrate assay, P. gingivalis challenge was found to significantly increase activity of caspase 3/7 and of caspase 9 (Fig. 7C). In contrast, AIF expression was reduced by P. gingivalis (Fig. 7B), and caspase 8 was elevated (not shown), supporting the concept that P. gingivalis-induced apoptosis is caspase dependent. Furthermore, as P. gingivalis-induced apoptosis in fibroblasts is AIF-dependent (Desta and Graves, 2007), these results provide additional evidence for cell type-specific responses to P. gingivalis.
Fig. 7. HTR-8 cells infected with P. gingivalis become apoptotic. HTR-8 cells were infected with P. gingivalis at the moi indicated for 24, 48 and 72 h.
A. Cells were stained with Annexin V/propidium iodide (PI) and analysed by flow cytometry. Mean percentage ± SD of apoptotic (Annexin V+/PI−) cells from three independent experiments and analysed with a t-test.
B. Immunoblots of P. gingivalis-infected or uninfected HTR-8 cell lysates probed with Bax or AIF antibodies. Blots were analysed by scanning densitometry and ratios of target protein/β-actin determined relative to the zero time point. Data are representative of three independent experiments.
C. Caspase 3/7 and 9 activity in P. gingivalis-infected and uninfected cells measured by fluorescent substrate assays. Data are expressed as arbitrary fluorescent units and are means ± SD from three independent experiments analysed with a t-test.
ERK1/2 activity is increased by P. gingivalis
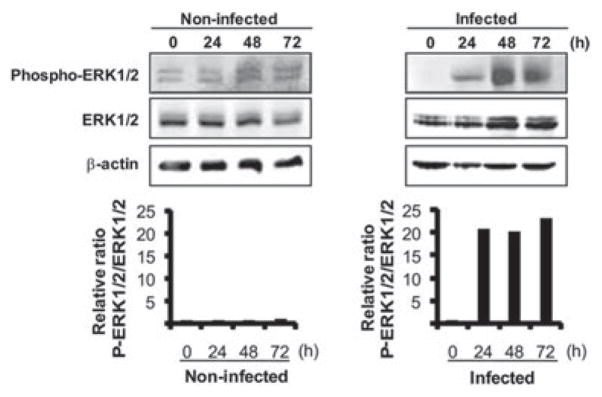
ERK has a dual behaviour in cell proliferation or death. On the one hand, activation of ERK1/2 can contribute to cell proliferation and cell survival, while on the other hand, more sustained activation of ERK1/2 can lead to G1 arrest and apoptosis (Sarfaraz et al., 2006; Meloche and Pouyssegur, 2007). Therefore, we next examined the effect of P. gingivalis on ERK1/2 levels and phosphorylation status. Treatment of HTR-8 cells with P. gingivalis resulted in a significant and sustained activation of ERK1/2 as compared with uninfected cells. After 72 h of P. gingivalis challenge, ERK1/2 phosphorylation was over 20-fold higher than resting levels (Fig. 8).
Fig. 8.
Phospho-ERK1/2 and ERK1/2 expression is upregulated in HTR-8 cells infected with P. gingivalis. HTR-8 cells were infected with P. gingivalis at an moi of 200 for 24, 48 and 72 h. Cell lysates were immunoblotted with antibodies to phosphorylated (Phospho-) ERK1/2 or ERK1/2. Blots were analysed by scanning densitometry and ratios of phospho-ERK1/2 to ERK1/2 were determined relative to the zero time point. Data are representative of three independent experiments.
Activation of ERK1/2 by P. gingivalis controls G1 arrest and apoptosis
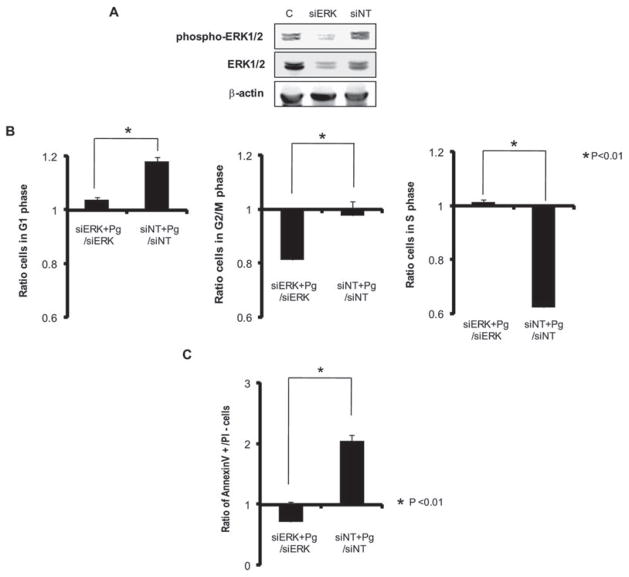
To evaluate the role of ERK1/2 activation in P. gingivalis-induced cell cycle arrest and apoptosis, we transcriptionally silenced ERK1/2 expression with siRNA. Expression of ERK1/2 protein was significantly reduced in knockdown cells (Fig. 9A). Following infection with P. gingivalis, cells in which ERK1/2 was silenced demonstrated significantly less (21%) arrest in G1 phase compared with cells transfected with no-target siRNA (Fig. 9B). Moreover, knock-down of ERK1/2 abrogated that ability of P. gingivalis to induce apoptosis (Fig. 9C). Collectively, these results indicate that P. gingivalis diverts ERK1/2 signalling events resulting in both cell cycle disturbance and ultimately cell death.
Fig. 9. Knock-down of ERK1/2 by siRNA represses P. gingivalis-mediated G1 arrest and apoptosis. HTR-8 cells were transfected with siRNA to ERK1/2 or with a no-target control (siNT).
A. Transfected (72 h) or control (c) cells probed with antibodies to ERK1/2 or to phospho-ERK1/2.
B. Transfected (72 h) HTR-8 cells were infected with P. gingivalis at an moi of 200 for 48 h, and cell cycle distribution was determined by flow cytometry. The fold change in numbers of cells in each phase of the cell cycle was calculated in cells transfected with siRNA to ERK1/2 or siNT and infected with P. gingivalis, and compared to transfected, uninfected cells. Data are means ± SD of three independent experiments and analysed with a t-test.
C. Transfected (72 h) HTR-8 cells were infected with P. gingivalis at an moi of 200 for 48 h, stained with Annexin V/PI and analysed by flow cytometry. The fold change in the number of Annexin V+/PI− cells was calculated in cells transfected with siRNA to ERK1/2 or siNT and infected with P. gingivalis, and compared to transfected, non-infected cells. Data are means ± SD of three independent experiments and analysed with a t-test.
Discussion
Epidemiological, culture and animal data provide evidence of a role for P. gingivalis in PTLBW deliveries; however, the molecular and cellular interactions between P. gingivalis and placental cells and tissues have yet to be investigated. This study demonstrates that P. gingivalis has the capacity to invade and remain viable within extravillous trophoblasts. The placental barrier normally shields the fetus and gestational tissues from microbial intrusion, however, organisms with the ability to cause haematogenously derived fetal infections possess the ability to traverse the placental barrier. L. monocytogenes, for example, reaches the villous tissue of the placenta by invading trophoblast cells (Disson et al., 2008). Of additional relevance to the potential of P. gingivalis as a systemic pathogen is the capability of the organism to survive in the bloodstream (Perez-Chaparro et al., 2008). Indeed, P. gingivalis can resist complement mediated killing (Slaney et al., 2006), and the production of superoxide dismutase (SOD) and rubrerythrin by P. gingivalis can protect the organism from oxygen-dependent killing within professional phagocytic cells (Amano et al., 1992; Mydel et al., 2006). P. gingivalis thus possesses virulence attributes consistent with those of a successful haematogenously delivered fetoplacental pathogen.
In addition to invasion of trophoblasts, P. gingivalis also disrupted normal host cell function, specifically cell cycle and programmed cell death. The cell cycle of mammalian cells is a fundamental aspect of their biological status and uncontrolled proliferation leads to tumorgenesis (Deshpande et al., 2005). Furthermore, immune responses, barrier function and cellular differentiation can all vary with cell cycle progression. A complex network of interconnected regulatory pathways controls the cell cycle and ensures that each step is performed in the proper sequence. These regulatory pathways involve highly choreographed protein kinase/phosphatase and proteolysis events (Malumbres and Barbacid, 2005; Nakayama and Nakayama, 2006; Meloche and Pouyssegur, 2007). P. gingivalis infection of placental cells resulted in a reprogramming of signal transduction pathways to induce an arrest in the G1 phase and activate apoptotic cell death pathways. G1 arrest correlated with an accumulation of proteins that inhibit G1/S transition, along with loss or inactivation of proteins that facilitate progression of G1 to S. Most notably, a significant reduction in Cyclin D levels was observed in P. gingivalis-infected cells. Cyclin D is the first cyclin produced in the cell cycle in response to mitotic signals, and binds to existing CDK 4 and 6 forming active cyclin D-CDK complexes that effectuate transition of cells from G1 to S phase (Malumbres and Barbacid, 2005). Reinforcing the impact on cyclin D activity, P. gingivalis also reduced levels of CDK 4 and CDK 6. In addition, expression of CDK2, which acts subsequent to CDK4/6 action at the G1-S phase transition (Malumbres and Barbacid, 2005) was diminished by P. gingivalis, after reductions in CDK4 and 6 were observed. Lower levels of CDK2 will render increases cyclin E expression redundant, also contributing to failure of the cells to exit G1 phase. The pro-mitotic activity of cyclin D-CDK complexes is counteracted by two families of cell cycle inhibitors, the INK4 and the CIP/KIP families. These multilevel control mechanisms provide additional opportunities for interference by infecting organisms, and indeed P. gingivalis induced expression of the INK4 family members p15, p16 and p18, and of the CIP/KIP constituent p21, all of which can halt the cell cycle in the G1 phase (Besson et al., 2008). P. gingivalis thus can selectively target several components of the cell cycle control machinery to stall trophoblasts in the G1 phase. The broadly based impact of P. gingivalis suggests that the trophoblasts are irreversibly locked in G1 and that normal mitotic processes are unlikely to occur.
Cyclin D-CDK complexes funnel through Rb, a tumor suppressor protein and an important node in the network of cell cycle control (Deshpande et al., 2005; Sun et al., 2007). Consistent with the reduction in potential for CDK4/6 activity that characterized P. gingivalis challenge, infected trophoblasts did not show an increase in levels of phosphorylated Rb protein. Rb will thus remain active in P. gingivalis-infected cells and act to block G1 to S progression. In addition, P. gingivalis induced G1 arrest was associated with degradation of Rb protein, a phenomenon recently characterized in prostate cancer cells (Sarfaraz et al., 2006). The importance of Rb in integrating the response of trophoblasts to P. gingivalis infection was validated by knock in experiments. A transient increase in the level and phosphorylation of Rb relieved G1 arrest and reduced the accumulation of cells in the G1 phase following P. gingivalis infection.
Apoptosis is a highly regulated process of cell deletion that is involved in development, differentiation, homoeostasis and regulation of immune function. Dysfunction or dysregulation of the apoptotic program is involved in a variety of pathological conditions including autoimmune disease and cancer (Fadeel and Orrenius, 2005; Hail et al., 2006). Cell cycle progression and apoptosis are interconnected on several levels, and arrest in G1 often leads to apoptotic cell death (Massague, 2004; Sarfaraz et al., 2006). Accordingly, HTR-8 cells infected with P. gingivalis showed an increase in cell surface phosphatidylserine, a marker of apoptosis. Multiple signalling circuits are involved in controlling apoptosis in eukaryotic cells in response to both extrinsic or intrinsic stimuli. The Bcl-2 family of proteins are one of the critical regulators of apoptosis. Bcl-2 itself is anti-apoptotic in part through preventing cytochrome c release from the mitochondria (Kluck et al., 1997), while Bax facilitates release of cytochrome c, and is pro-apoptotic (Hail et al., 2006). Bcl-2 and Bax form heterodimers and the Bcl-2/Bax ratio is considered a decisive factor in determining the apoptotic fate of cells. P. gingivalis elevated expression of Bax without affecting Bcl-2 levels, indicating that imbalance of the Bcl-2/Bax ratio is a mechanism by which the organism induces apoptosis. Alteration of the Bcl-2/Bax ratio also activates the caspase signalling. Caspases are a family of cysteine proteases that are cleaved and activated during apoptosis through a self-amplification cascade (Chowdhury et al., 2006). Pro-apoptotic signals stimulate the activation of the initiator caspases 8, 9 and 10, which in turn proteolytically activate the executioner caspases 3, 6 and 7. The effector caspases cleave a set of physiologically active proteins such as PARP, which initiates the mechanism of apoptotic cell death. Consistent with pro-apoptotic signalling through alteration of the Bcl-2/Bax ratio, both caspase 3/7 and caspase 9 were activated by P. gingivalis.
ERK1/2 is a member of the mitogen activated protein kinase signalling cascade that plays a central role in transmitting information from extracellular stimuli to nuclear transcription factors. ERK1/2 is well established as a positive regulator of cell cycle progression; however, accumulating evidence suggests that prolonged and excessive activation of ERK1/2 can instead lead to cell cycle arrest and apoptosis (Sarfaraz et al., 2006; Meloche and Pouyssegur, 2007). Indeed, sustained ERK1/2 can induce the expression p15, p16 and p21 and inhibit CDK2 (Meloche and Pouyssegur, 2007), as we observed following P. gingivalis infection. The finding that P. gingivalis increased the amount of phosphorylated ERK1/2 in trophoblasts over several days suggests that ERK1/2 is an upstream target of P. gingivalis and activation of ERK1/2 then contributes both to G1 arrest and apoptosis. Support for this concept was provided by knock-down of ERK1/2 with siRNA, which relieved the G1 block and abrogated apoptotic cell death. Given the central role of the ERK1/2 pathway in effectuating the response to P. gingivalis, ERK inhibitors such as AZD6244, may have potential therapeutic value for prevention of P. gingivalis associated placental disruption.
As the placenta develops it undergoes constant tissue remodelling, which is characterized by the functional loss of trophoblasts by apoptosis. While apoptosis is important for normal placental development, it can also be involved in the pathophysiology of pregnancy-related diseases. In pregnancies that are complicated by pre-eclampsia or intrauterine growth retardation there can be higher levels of apoptosis (Straszewski-Chavez et al., 2005), and moreover one cause of increased trophoblast apoptosis during pregnancy is infection (Abbasi et al., 2003; Straszewski-Chavez et al., 2005; Arechavaleta-Velasco et al., 2006). Hence the ability of P. gingivalis to invade trophoblasts and induce G1 arrest and apoptosis is likely to contribute to complications of pregnancy such as PTLBW deliveries. Interestingly, the outcome of P. gingivalis infection differs markedly between trophoblasts and gingival epithelial cells. While P. gingivalis can invade both cell types, gingival epithelial cells acquire a phenotype that renders then resistant to chemically induced apoptosis (Mao et al., 2007). In addition, cell cycle progression is accelerated in gingival epithelial cells infected with P. gingivalis (Kuboniwa et al., 2008). Responses of the host to P. gingivalis therefore appear to be tissue specific and to contribute to differing disease outcomes at different sites. The effect of P. gingivalis on gingival tissue is less harmful than on placental tissue, likely the result of co-evolution of the organism for oral colonization of the host. The response of placental cells to P. gingivalis also differs from that to F. nucleatum, another periodontal pathogen implicated in PTLBW. Apart from the point of origin therefore, the pathogenic processes of periodontal bacteria may be organism-specific. Collectively, our results support the status of P. gingivalis as a systemic pathogen, and provide a mechanistic basis for pathogenicity of P. gingivalis in PTLBW deliveries.
Experimental procedures
Bacterial and cell culture
Porphyromonas gingivalis ATCC 33277 and Fusobacterium nucleatum ATCC 25586 were grown anaerobically at 37°C in Trypticase Soy Broth supplemented with yeast extract (1 mg ml−1), hemin (5 μg ml−1) and menadione (1 μg ml−1). The HTR-8/SVneo trophoblast cell line was provided by Dr Charles Graham (Kingston, Ontario). Cells were cultured in RPMI 1640 medium (Sigma-Aldrich, St Louis, MO) supplemented with 5% fetal bovine serum at 37°C in 5% CO2. For synchronization by serum starvation (G0-G1), HTR-8 cells at approximately 50% confluence were incubated in media without serum for 48 h. Serum conditions were then restored and the cells reacted with bacteria at the moi and times indicated in individual experiments. For synchronization by double thymidine block (S1), cells were incubated in 2 mM thymidine for 20 h, washed extensively with PBS and released for 14 h, followed by a second thymidine block for 16 h and release for 1 h.
Bacterial invasion
Antibiotic protection assay: HTR-8 cells were reacted with bacteria at an moi of 100 for 2 h and then incubated with metronidazole (200 μg ml−1) and gentamicin (300 μg ml−1) for 1 h to kill extracellular bacteria (Lamont et al., 1995). Cells were lysed with sterile, distilled water for 15 min and the intracellular bacteria released were enumerated by viable counting on blood agar plates. Confocal microscopy: HTR-8 cells were cultured in a Laboratory Tek II 4 cell cover glass culture system (Thermo Fisher Scientific, Rochester, NY). After reacting with bacteria, cells were fixed with 4% paraformaldehyde, blocked with 10% Goat serum/Dulbecco’s PBS (DPBS) at room temperature for 10 min, then permeabilized with blocking buffer containing 0.2% saponin for 10 min. Internalized bacteria were detected with polyclonal rabbit anti-P. gingivalis antibody and FITC-conjugated anti-rabbit secondary antibody. Phalloidin-TRITC (1:200; Sigma) was used to visualize cytoskeletal F-actin. Images were acquired with a Bio-Rad MRC1024 confocal laser scanning microscope (Kr/Ar) system with an Olympus IMT-2 inverted light microscope and MS plan 40 × 0.85 NA objective using reflected laser light of combined 488 and 546 wavelengths. A series of fluorescent optical x–y sections were collected to create digitally reconstructed images with Image J V1.33 u (National Institutes of Health) and Imaris 5.0.1 (Bitplane AG; Zurich, Switzerland) software packages. z-stacks of the x–y sections were converted to composite images with ‘Iso Surface’ and ‘Spot Detection’ functions of the ‘Surpass’ option on Imaris software. ‘Iso Surface’ represented the outline of HTR-8 cells. ‘Spot’ was defined as a particle of 2 μm diameter, indicating a bacterial cell.
Tetrazolium proliferation assay
Infected or control HTR-8 cells were incubated with CellTiter 96 Aqueous One Solution reagent (Promega, Madison, MI) for 1 h at 37°C and absorbance at 490 nm recorded.
Flow cytometry
Cell cycle analysis: Infected or control HTR-8 cells were trypsinized, washed with cold PBS and then fixed in 70% ethanol at −20°C overnight. Ethanol-fixed cells were washed with PBS and incubated in 1 ml 0.1 mg ml−1 RNase A solution at 37°C for 30 min. The cells were stained with 50 μg ml−1 Propidium iodide (Invitrogen, Frederick, MD). Cell cycle analysis of 30 000 cells per sample was with excitation at 488 nm in a flow cytometer (FACScan, Becton-Dickinson, San Jose, CA). Data were analysed with Cell-Quest software (BD biosciences) and ModFit LT V 3.1 (Verity software). Apopotosis: For Annexin V staining, cells were harvested and stained with the Annexin V-FITC Apoptosis Detection Kit I (BD Pharmingen, San Diego, CA) according to the manufacturer’s protocol, and flow cytometric analysis performed.
Western immunoblotting
HTR-8 cells were solubilized in CelLytic MT Lysis/Extraction regent (Sigma-Aldrich) containing a protease and phosphatase inhibitor cocktail (Thermo Scientific, Rockford, IL). Lysates were separated by SDS-PAGE and proteins were transferred to nitrocellulose membranes by blotting and blocked with 5% BSA in TBS (PBS containing 0.1% Tween 20) for 1 h at room temperature. Blots were probed with antibodies obtained from Cell Signaling Technology (Beverly, MA) or Santa Cruz Biotechnology (Santa Cruz, CA), and reacted at 4°C overnight. Proteins or phosphorylated proteins were detected using the Pierce ECL Substrate (Thermo Scientific). Blots were stripped and probed with anti-β-actin antibody (Cell Signaling Technology) as a loading control. Densiometric analysis was performed on bands using ImageJ software.
Caspase activity
Caspase 3/7 or 9 activity was measured using the Caspase-Glo Assay (Promega). A 1:1 mixture of caspase reagent to supernatant was incubated at room temperature for 3 h and end-point luminescence measured on a Victor microplate fluorescence reader (Perkin Elmer, Wellesley, MA) at excitation and emission wavelengths of 355 and 460 nm respectively.
Transient expression
For Rb overexpression, HTR-8 cells were transfected with pRc/CMV/Rb plasmid (Addgene, Cambridge, MA). The pRc/CMV vector prepared by Hind III digestion of pRc/CMV/Rb and self-ligation, was used as a vector control. Transfection was carried out for 18 h with 4 μg of DNA using Lipofectamine 2000 (Invitrogen).
RNA interference
For suppressing ERK1/2, small interfering RNAs (siRNAs) against ERK1 and ERK2, or signalsilence control siRNA (Cell Signaling Technology) were transfected at 300 nM well−1 into HTR-8 cells using Oligefectamine (Invitrogen). After 24 h of transfection, the medium was replaced and cells were incubated for a further 24 h prior to challenge with P. gingivalis.
Supplementary Material
Acknowledgments
This study was supported by NIDCR grants DE11111, DE16593 and DE07200.
Footnotes
Additional Supporting Information may be found in the online version of this article:
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Abbasi M, Kowalewska-Grochowska K, Bahar MA, Kilani RT, Winkler-Lowen B, Guilbert LJ. Infection of placental trophoblasts by Toxoplasma gondii. J Infect Dis. 2003;188:608–616. doi: 10.1086/377132. [DOI] [PubMed] [Google Scholar]
- Abrahams VM, Bole-Aldo P, Kim YM, Straszewski-Chavez SL, Chaiworapongsa T, Romero R, Mor G. Divergent trophoblast responses to bacterial products mediated by TLRs. J Immunol. 2004;173:4286–4296. doi: 10.4049/jimmunol.173.7.4286. [DOI] [PubMed] [Google Scholar]
- Amano A, Ishimoto T, Tamagawa H, Shizukuishi S. Role of superoxide dismutase in resistance of Porphyromonas gingivalis to killing by polymorphonuclear leukocytes. Infect Immun. 1992;60:712–714. doi: 10.1128/iai.60.2.712-714.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arechavaleta-Velasco F, Ma Y, Zhang J, McGrath CM, Parry S. Adeno-associated virus-2 (AAV-2) causes trophoblast dysfunction, and placental AAV-2 infection is associated with preeclampsia. Am J Pathol. 2006;168:1951–1959. doi: 10.2353/ajpath.2006.050781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakardjiev AI, Stacy BA, Portnoy DA. Growth of Listeria monocytogenes in the guinea pig placenta and role of cell-to-cell spread in fetal infection. J Infect Dis. 2005;191:1889–1897. doi: 10.1086/430090. [DOI] [PubMed] [Google Scholar]
- Barak S, Oettinger-Barak O, Machtei EE, Sprecher H, Ohel G. Evidence of periopathogenic microorganisms in placentas of women with preeclampsia. J Periodontol. 2007;78:670–676. doi: 10.1902/jop.2007.060362. [DOI] [PubMed] [Google Scholar]
- Belanger M, Reyes L, von Deneen K, Reinhard MK, Progulske-Fox A, Brown MB. Colonization of maternal and fetal tissues by Porphyromonas gingivalis is strain-dependent in a rodent animal model. Am J Obstet Gynecol. 2008;199:e1–7. doi: 10.1016/j.ajog.2007.11.067. [DOI] [PubMed] [Google Scholar]
- Besson A, Dowdy SF, Roberts JM. CDK inhibitors: cell cycle regulators and beyond. Dev Cell. 2008;14:159–169. doi: 10.1016/j.devcel.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Bloom J, Cross FR. Multiple levels of cyclin specificity in cell-cycle control. Nat Rev Mol Cell Biol. 2007;8:149–160. doi: 10.1038/nrm2105. [DOI] [PubMed] [Google Scholar]
- Boggess KA, Madianos PN, Preisser JS, Moise KJ, Jr, Offenbacher S. Chronic maternal and fetal Porphyromonas gingivalis exposure during pregnancy in rabbits. Am J Obstet Gynecol. 2005;192:554–557. doi: 10.1016/j.ajog.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Chakraborty C, Barbin YP, Chakrabarti S, Chidiac P, Dixon SJ, Lala PK. Endothelin-1 promotes migration and induces elevation of [Ca2+]i and phosphorylation of MAP kinase of a human extravillous trophoblast cell line. Mol Cell Endocrinol. 2003;201:63–73. doi: 10.1016/s0303-7207(02)00431-8. [DOI] [PubMed] [Google Scholar]
- Chau BN, Wang JY. Coordinated regulation of life and death by RB. Nat Rev Cancer. 2003;3:130–138. doi: 10.1038/nrc993. [DOI] [PubMed] [Google Scholar]
- Chowdhury I, Tharakan B, Bhat GK. Current concepts in apoptosis: the physiological suicide program revisited. Cell Mol Biol Lett. 2006;11:506–525. doi: 10.2478/s11658-006-0041-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande A, Sicinski P, Hinds PW. Cyclins and cdks in development and cancer: a perspective. Oncogene. 2005;24:2909–2915. doi: 10.1038/sj.onc.1208618. [DOI] [PubMed] [Google Scholar]
- Desta T, Graves DT. Fibroblast apoptosis induced by Porphyromonas gingivalis is stimulated by a gingipain and caspase-independent pathway that involves apoptosis-inducing factor. Cell Microbiol. 2007;9:2667–2675. doi: 10.1111/j.1462-5822.2007.00987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disson O, Grayo S, Huillet E, Nikitas G, Langa-Vives F, Dussurget O, et al. Conjugated action of two species-specific invasion proteins for fetoplacental listeriosis. Nature. 2008;455:1114–1118. doi: 10.1038/nature07303. [DOI] [PubMed] [Google Scholar]
- Fadeel B, Orrenius S. Apoptosis: a basic biological phenomenon with wide-ranging implications in human disease. J Intern Med. 2005;258:479–517. doi: 10.1111/j.1365-2796.2005.01570.x. [DOI] [PubMed] [Google Scholar]
- Garcia RI, Henshaw MM, Krall EA. Relationship between periodontal disease and systemic health. Periodontol 2000. 2001;25:21–36. doi: 10.1034/j.1600-0757.2001.22250103.x. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Thompson C. The infectious origins of stillbirth. Am J Obstet Gynecol. 2003;189:861–873. doi: 10.1067/s0002-9378(03)00470-8. [DOI] [PubMed] [Google Scholar]
- Graham CH, Hawley TS, Hawley RG, MacDougall JR, Kerbel RS, Khoo N, Lala PK. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp Cell Res. 1993;206:204–211. doi: 10.1006/excr.1993.1139. [DOI] [PubMed] [Google Scholar]
- Hail N, Jr, Carter BZ, Konopleva M, Andreeff M. Apoptosis effector mechanisms: a requiem performed in different keys. Apoptosis. 2006;11:889–904. doi: 10.1007/s10495-006-6712-8. [DOI] [PubMed] [Google Scholar]
- Handfield M, Mans JJ, Zheng G, Lopez MC, Mao S, Progulske-Fox A, et al. Distinct transcriptional profiles characterize oral epithelium–microbiota interactions. Cell Microbiol. 2005;7:811–823. doi: 10.1111/j.1462-5822.2005.00513.x. [DOI] [PubMed] [Google Scholar]
- Hiroi M, Shimojima T, Kashimata M, Miyata T, Takano H, Takahama M, Sakagami H. Inhibition by Porphyromonas gingivalis LPS of apoptosis induction in human peripheral blood polymorphonuclear leukocytes. Anticancer Res. 1998;18:3475–3479. [PubMed] [Google Scholar]
- Hoyert DL, Mathews TJ, Menacker F, Strobino DM, Guyer B. Annual summary of vital statistics: 2004. Pediatrics. 2006;117:168–183. doi: 10.1542/peds.2005-2587. [DOI] [PubMed] [Google Scholar]
- Isogai E, Isogal H, Kimura K, Fujii N, Takagi S, Hirose K, Hayashi M. In vivo induction of apoptosis and immune responses in mice by administration of lipopolysaccharide from Porphyromonas gingivalis. Infect Immun. 1996;64:1461–1466. doi: 10.1128/iai.64.4.1461-1466.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffrey PD, Tong L, Pavletich NP. Structural basis of inhibition of CDK-cyclin complexes by INK4 inhibitors. Genes Dev. 2000;14:3115–3125. doi: 10.1101/gad.851100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JL, Lopez A, Wilson M, Schulkin J, Gibbs R. Congenital toxoplasmosis: a review. Obstet Gynecol Surv. 2001;56:296–305. doi: 10.1097/00006254-200105000-00025. [DOI] [PubMed] [Google Scholar]
- Kato T, Tsuda T, Inaba H, Kawai S, Okahashi N, Shibata Y, et al. Porphyromonas gingivalis gingipains cause G(1) arrest in osteoblastic/stromal cells. Oral Microbiol Immunol. 2008;23:158–164. doi: 10.1111/j.1399-302X.2007.00405.x. [DOI] [PubMed] [Google Scholar]
- Kilburn BA, Wang J, Duniec-Dmuchowski ZM, Leach RE, Romero R, Armant DR. Extracellular matrix composition and hypoxia regulate the expression of HLA-G and integrins in a human trophoblast cell line. Biol Reprod. 2000;62:739–747. doi: 10.1095/biolreprod62.3.739. [DOI] [PubMed] [Google Scholar]
- Kim YW, Otterson GA, Kratzke RA, Coxon AB, Kaye FJ. Differential specificity for binding of retinoblastoma binding protein 2 to RB, p107, and TATA-binding protein. Mol Cell Biol. 1994;14:7256–7264. doi: 10.1128/mcb.14.11.7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinane DF, Riggio MP, Walker KF, MacKenzie D, Shearer B. Bacteraemia following periodontal procedures. J Clin Periodontol. 2005;32:708–713. doi: 10.1111/j.1600-051X.2005.00741.x. [DOI] [PubMed] [Google Scholar]
- Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- Kuboniwa M, Hasegawa Y, Mao S, Shizukuishi S, Amano A, Lamont RJ, Yilmaz O. P. gingivalis accelerates gingival epithelial cell progression through the cell cycle. Microbes Infect. 2008;10:122–128. doi: 10.1016/j.micinf.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont RJ, Chan A, Belton CM, Izutsu KT, Vasel D, Weinberg A. Porphyromonas gingivalis invasion of gingival epithelial cells. Infect Immun. 1995;63:3878–3885. doi: 10.1128/iai.63.10.3878-3885.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon R, Silva N, Ovalle A, Chaparro A, Ahumada A, Gajardo M, et al. Detection of Porphyromonas gingivalis in the amniotic fluid in pregnant women with a diagnosis of threatened premature labor. J Periodontol. 2007;78:1249–1255. doi: 10.1902/jop.2007.060368. [DOI] [PubMed] [Google Scholar]
- Lin D, Smith MA, Elter J, Champagne C, Downey CL, Beck J, Offenbacher S. Porphyromonas gingivalis infection in pregnant mice is associated with placental dissemination, an increase in the placental Th1/Th2 cytokine ratio, and fetal growth restriction. Infect Immun. 2003;71:5163–5168. doi: 10.1128/IAI.71.9.5163-5168.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Redline RW, Han YW. Fusobacterium nucleatum induces fetal death in mice via stimulation of TLR4-mediated placental inflammatory response. J Immunol. 2007;179:2501–2508. doi: 10.4049/jimmunol.179.4.2501. [DOI] [PubMed] [Google Scholar]
- Lockhart PB, Brennan MT, Sasser HC, Fox PC, Paster BJ, Bahrani-Mougeot FK. Bacteremia associated with toothbrushing and dental extraction. Circulation. 2008;117:3118–3125. doi: 10.1161/CIRCULATIONAHA.107.758524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malumbres M, Barbacid M. Mammalian cyclin-dependent kinases. Trends Biochem Sci. 2005;30:630–641. doi: 10.1016/j.tibs.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Mao S, Park Y, Hasegawa Y, Tribble GD, James CE, Handfield M, et al. Intrinsic apoptotic pathways of gingival epithelial cells modulated by Porphyromonas gingivalis. Cell Microbiol. 2007;9:1997–2007. doi: 10.1111/j.1462-5822.2007.00931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J. G1 cell-cycle control and cancer. Nature. 2004;432:298–306. doi: 10.1038/nature03094. [DOI] [PubMed] [Google Scholar]
- Meloche S, Pouyssegur J. The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene. 2007;26:3227–3239. doi: 10.1038/sj.onc.1210414. [DOI] [PubMed] [Google Scholar]
- Michalowicz BS, Durand R. Maternal periodontal disease and spontaneous preterm birth. Periodontol 2000. 2007;44:103–112. doi: 10.1111/j.1600-0757.2006.00197.x. [DOI] [PubMed] [Google Scholar]
- Murray DA, Wilton JM. Lipopolysaccharide from the periodontal pathogen Porphyromonas gingivalis prevents apoptosis of HL60-derived neutrophils in vitro. Infect Immun. 2003;71:7232–7235. doi: 10.1128/IAI.71.12.7232-7235.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mydel P, Takahashi Y, Yumoto H, Sztukowska M, Kubica M, Gibson FC, 3rd, et al. Roles of the host oxidative immune response and bacterial antioxidant rubrerythrin during Porphyromonas gingivalis infection. PLoS Pathog. 2006;2:e76. doi: 10.1371/journal.ppat.0020076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylonakis E, Paliou M, Hohmann EL, Calderwood SB, Wing EJ. Listeriosis during pregnancy: a case series and review of 222 cases. Medicine (Baltimore) 2002;81:260–269. doi: 10.1097/00005792-200207000-00002. [DOI] [PubMed] [Google Scholar]
- Nakayama KI, Nakayama K. Ubiquitin ligases: cell-cycle control and cancer. Nat Rev Cancer. 2006;6:369–381. doi: 10.1038/nrc1881. [DOI] [PubMed] [Google Scholar]
- Nakhjiri SF, Park Y, Yilmaz O, Chung WO, Watanabe K, El-Sabaeny A, et al. Inhibition of epithelial cell apoptosis by Porphyromonas gingivalis. FEMS Microbiol Lett. 2001;200:145–149. doi: 10.1111/j.1574-6968.2001.tb10706.x. [DOI] [PubMed] [Google Scholar]
- Offenbacher S, Jared HL, O’Reilly PG, Wells SR, Salvi GE, Lawrence HP, et al. Potential pathogenic mechanisms of periodontitis associated pregnancy complications. Ann Periodontol. 1998;3:233–250. doi: 10.1902/annals.1998.3.1.233. [DOI] [PubMed] [Google Scholar]
- Ozaki K, Hanazawa S. Porphyromonas gingivalis fimbriae inhibit caspase-3-mediated apoptosis of monocytic THP-1 cells under growth factor deprivation via extracellular signal-regulated kinase-dependent expression of p21 Cip/WAF1. Infect Immun. 2001;69:4944–4950. doi: 10.1128/IAI.69.8.4944-4950.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Chaparro PJ, Gracieux P, Lafaurie GI, Donnio PY, Bonnaure-Mallet M. Genotypic characterization of Porphyromonas gingivalis isolated from sub-gingival plaque and blood sample in positive bacteremia subjects with periodontitis. J Clin Periodontol. 2008;35:748–753. doi: 10.1111/j.1600-051X.2008.01296.x. [DOI] [PubMed] [Google Scholar]
- Renaud SJ, Postovit LM, Macdonald-Goodfellow SK, McDonald GT, Caldwell JD, Graham CH. Activated macrophages inhibit human cytotrophoblast invasiveness in vitro. Biol Reprod. 2005;73:237–243. doi: 10.1095/biolreprod.104.038000. [DOI] [PubMed] [Google Scholar]
- Sarfaraz S, Afaq F, Adhami VM, Malik A, Mukhtar H. Cannabinoid receptor agonist-induced apoptosis of human prostate cancer cells LNCaP proceeds through sustained activation of ERK1/2 leading to G1 cell cycle arrest. J Biol Chem. 2006;281:39480–39491. doi: 10.1074/jbc.M603495200. [DOI] [PubMed] [Google Scholar]
- Schloesser RL, Schaefer V, Groll AH. Fatal transplacental infection with non-typhoidal Salmonella. Scand J Infect Dis. 2004;36:773–774. doi: 10.1080/00365540410020802. [DOI] [PubMed] [Google Scholar]
- Sheets SM, Potempa J, Travis J, Fletcher HM, Casiano CA. Gingipains from Porphyromonas gingivalis W83 synergistically disrupt endothelial cell adhesion and can induce caspase-independent apoptosis. Infect Immun. 2006;74:5667–5678. doi: 10.1128/IAI.01140-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaney JM, Gallagher A, Aduse-Opoku J, Pell K, Curtis MA. Mechanisms of resistance of Porphyromonas gingivalis to killing by serum complement. Infect Immun. 2006;74:5352–5361. doi: 10.1128/IAI.00304-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagno S, Pass RF, Cloud G, Britt WJ, Henderson RE, Walton PD, et al. Primary cytomegalovirus infection in pregnancy. Incidence, transmission to fetus, and clinical outcome. JAMA. 1986;256:1904–1908. [PubMed] [Google Scholar]
- Straszewski-Chavez SL, Abrahams VM, Mor G. The role of apoptosis in the regulation of trophoblast survival and differentiation during pregnancy. Endocr Rev. 2005;26:877–897. doi: 10.1210/er.2005-0003. [DOI] [PubMed] [Google Scholar]
- Sun A, Bagella L, Tutton S, Romano G, Giordano A. From G0 to S phase: a view of the roles played by the retinoblastoma (Rb) family members in the Rb-E2F pathway. J Cell Biochem. 2007;102:1400–1404. doi: 10.1002/jcb.21609. [DOI] [PubMed] [Google Scholar]
- Svinarich DM, Bitonti OM, Romero R, Gonik B. Induction and posttranslational expression of cytokines in a first-trimester trophoblast cell line by lipopolysaccharide. Am J Obstet Gynecol. 1996;175:970–973. doi: 10.1016/s0002-9378(96)80034-2. [DOI] [PubMed] [Google Scholar]
- Svinarich DM, DiCerbo JA, Zaher FM, Yelian FD, Gonik B. Ethanol-induced expression of cytokines in a first-trimester trophoblast cell line. Am J Obstet Gynecol. 1998;179:470–475. doi: 10.1016/s0002-9378(98)70381-3. [DOI] [PubMed] [Google Scholar]
- Tribble GD, Mao S, James CE, Lamont RJ. A Porphyromonas gingivalis haloacid dehalogenase family phosphatase interacts with human phosphoproteins and is important for invasion. Proc Natl Acad Sci USA. 2006;103:11027–11032. doi: 10.1073/pnas.0509813103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Yilmaz O, Nakhjiri SF, Belton CM, Lamont RJ. Association of mitogen-activated protein kinase pathways with gingival epithelial cell responses to Porphyromonas gingivalis infection. Infect Immun. 2001;69:6731–6737. doi: 10.1128/IAI.69.11.6731-6737.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer G, Pihlstrom BL. A critical assessment of adverse pregnancy outcome and periodontal disease. J Clin Periodontol. 2008;35:380–397. doi: 10.1111/j.1600-051X.2008.01284.x. [DOI] [PubMed] [Google Scholar]
- Xiong X, Buekens P, Fraser WD, Beck J, Offenbacher S. Periodontal disease and adverse pregnancy outcomes: a systematic review. BJOG. 2006;113:135–143. doi: 10.1111/j.1471-0528.2005.00827.x. [DOI] [PubMed] [Google Scholar]
- Yilmaz O, Young PA, Lamont RJ, Kenny GE. Gingival epithelial cell signalling and cytoskeletal responses to Porphyromonas gingivalis invasion. Microbiology. 2003;149:2417–2426. doi: 10.1099/mic.0.26483-0. [DOI] [PubMed] [Google Scholar]
- Yilmaz O, Jungas T, Verbeke P, Ojcius DM. Activation of the phosphatidylinositol 3-kinase/Akt pathway contributes to survival of primary epithelial cells infected with the periodontal pathogen Porphyromonas gingivalis. Infect Immun. 2004;72:3743–3751. doi: 10.1128/IAI.72.7.3743-3751.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt M, McKinnon T, Herr F, Lala PK, Han VK. HCG increases trophoblast migration in vitro via the insulin-like growth factor-II/mannose-6 phosphate receptor. Mol Hum Reprod. 2005;11:261–267. doi: 10.1093/molehr/gah160. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.