Abstract
Human embryonic stem cells (hESCs) and induced pluripotent stem cells (iPSCs) provide remarkable cellular platforms to better understand human hematopoiesis and to develop clinically applicable hematopoietic cell–based therapies. Over the past decade, hESCs have been used to characterize molecular and cellular mechanisms underpinning the differentiation of hematopoietic progenitors and mature, functional hematopoietic cells. These advances are now poised to lead to clinical translation of hESC- and iPSC-derived hematopoietic cells for novel therapies in the next few years. On the basis of areas of recent success, initial clinical use of hematopoietic cells derived from human pluripotent stem cells will probably be in the areas of transfusion therapies (erythrocytes and platelets) and immune therapies (natural killer cells). In contrast, efficient development and isolation of hematopoietic stem cells capable of long-term, multilineage engraftment still remains a significant challenge. Technical, safety, and regulatory concerns related to clinical applications of human PSCs must be appropriately addressed. However, proper consideration of these issues should facilitate and not inhibit clinical translation of new therapies. This review outlines the current status of hematopoietic cell development and what obstacles must be surmounted to bring hematopoietic cell therapies from human PSCs from “bench to bedside.”
Introduction
A decade has now passed since the first report describing human embryonic stem cells (hESCs) provided an important landmark in studies of stem cell biology.1 Even that first report shows the potential to use hESCs to study hematopoiesis, because a figure of an hESC-derived teratoma showed bone elements surrounding immature hematopoietic cells. In the past decade, dozens of studies have now described the derivation of essentially all blood cell lineages from hESCs. The foundation for this work rests on decades of previous studies using mouse ESCs (mESCs) and other developmental models.2,3 These approaches have facilitated our ability to translate basic biologic mechanisms to novel cellular therapies now routinely used for transfusions, hematopoietic cell transplantation, and cell-based immunotherapy.
The more recent development of mouse and human induced pluripotent stem cells (iPSCs) also provide other key achievements in the stem cell field.4–10 Briefly, iPSCs are produced by reprogramming somatic cells (eg, fibroblasts) by transfer of defined genes using viral or other vectors.9,10 Initial studies by Yamanaka4,7 used Oct4, Sox2, Klf4, and c-Myc to derive first mouse and then human iPSCs. Thomson and colleagues8 found Oct4, Sox2, Nanog, and Lin28 could also produce human iPSCs. iPSCs can now be successfully produced with just 1 or 2 genes, and this gene expression can be done transiently rather than requiring stable genome integration.9,11–17 This premise is further advanced by derivation of iPSCs with the use of protein transduction of appropriate transcription factors.18,19 In addition, it is possible to convert many different mature cell lineages (including hematopoietic cells) into iPSCs.20–22 In an intriguing related study, transient expression of a limited number of genes can convert one mature cell population into another mature cell population without going through an iPSC intermediary, as shown for conversion of exocrine to endocrine pancreas.23
iPSCs have essentially the same phenotype, gene expression pattern, and developmental potential as ESCs. Mouse iPSCs can form viable chimeras and contribute to germline cells when injected into mouse blastocytes.5,24 This demonstration that an entire mouse can be derived from a single mouse iPSC is the most stringent test of pluripotency. Human iPSCs form teratomas with contributions of all 3 germ layers (endoderm, ectoderm, and mesoderm) and have been used to produce many differentiated cell lineages.6–8,25
Human iPSCs may provide an optimal source of patient-specific pluripotent stem cells for derivation of hematopoietic cells (or other cells of interest) suitable for transplantation without concern for immunologic barriers. Recent studies have shown derivation of hematopoietic cells from iPSCs with the same characteristics as those derived from hESCs.26 Although many questions about iPSCs remain, this technology has proven to be highly reproducible and rapidly evolving to become more efficient.
This review focuses on the potential clinically relevant use of hematopoietic cells from either hESCs or iPSCs (collectively considered human pluripotent stem cells, hPSCs). However, there are several key rationale or other important applications of hPSC biology (Table 1). These other rationale include using hPSCs as models of human development and human genetics, as well as using hPSCs and their derivatives as a platform for pharmaceutical testing. These considerations are especially relevant in hematology in which many therapies using “adult” (non–hPSC-derived) cell populations such as HSCs from bone marrow (BM), peripheral blood (PB), or cord blood already exist.
Table 1.
Key rationale to study hematopoiesis from hPSCs
| Rationale | Description | References |
|---|---|---|
| hPSCs provide an optimal model to study basic human developmental biology. | Distinct differences in hematopoietic development between humans and mice (and other species) are reflected in embryogenesis, globin gene expression, and other genes that regulate developmental pathways. | 10,27–29 |
| hPSCs provide a model for human genetics and platform for gene therapy. | Human iPSC lines have been derived from patients with diverse genetic diseases. These cell lines can be used to define how specific mutations affect development and function of cells of interest. These also provide potential for combined cell and gene therapy to cure these diseases. hESCs can also be derived from patients with genetic disease or engineered to model gene mutations. | 30–37 |
| Pharmaceutical testing with hPSC-derived cells can be used to evaluate efficacy of novel therapies. | hPSCs play a growing role in drug development and testing in the future. Current efforts by industry are mainly on testing of hPSC-derived cardiomyocytes but can also expand to testing of hematopoiesis and other cell lineages. | 38–40 |
| hPSCs can provide a source of cells for transfusion medicine. | hPSC-derived RBCs and platelets can provide an important adjunct population to that collected from donors, with reduced risk of transmissible infectious agents. | 41–45 |
| hPSCs can provide a novel source for immune therapies. | NK cells, T cells, and dendritic cells have been derived from hPSCs. Novel properties of hPSCs may allow improved targeting of hPSC-derived effector lymphocytes. | 46–52 |
| hPSCs may provide an alternative source of cells for HCT. | hPSC-derived cells may be engineered to allow beneficial properties such as drug resistance that may allow more effective administration of chemotherapy to kill tumors. Also hESC-derived HSCs may generate hematopoietic chimerism to promote engraftment of other hESC-derived lineages. | 53–56 |
hPSC indicates human pluripotent stem cell; iPSC, induced pluripotent stem cell; hESC, human embryonic stem cell; RBC, red blood cell; NK, natural killer; HCT, hematopoietic cell transplantation; and HSC, hematopoietic stem cell.
Approaches to hematopoietic differentiation from hESCs
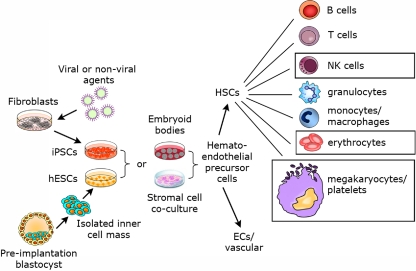
An overview of hematopoietic development from hPSCs is outlined (Figure 1). In studies that first showed hematopoietic development from hESCs, CD34+ hematopoietic precursor cells expressing hematopoietic transcription factors were derived using a coculture method with the murine BM cell line S17 or the yolk sac endothelial cell line C166 in the presence of fetal bovine serum (FBS), without any other added cytokines or growth factors.57 As an important validation for the normal characteristics of the hESC-derived cells, CD34+ cell selection lead to enrichment of the hematopoietic progenitor cells capable of forming characteristic myeloid, erythroid, and megakaryocytic colonies from clonogenic progenitors. Hematopoietic cells derived from these colonies also expressed typical hematopoietic cell morphology and phenotype. Multiple reports have subsequently used a variety of stromal cell lines, including OP9, M2-10B4, FH-B-hTERT, and primary human BM stroma, to support hematopoietic development from hESCs.54,58–61
Figure 1.
Derivation of hematopoietic cells from hESCs suitable for clinic therapies. Both hESCs derived from preimplantation blastocysts and iPSCs produced from somatic cells by gene transfer and/or chemical treatment can be used to investigate blood cell development. Two main methods have been used to produce hematopoietic cells from hESCs and iPSCs: embryoid body formation and stromal cell coculture. In vitro and in vivo studies have shown the development of essentially all mature hematopoietic cell populations from hESCs, with similar studies using human iPSCs being under way. The boxes around RBCs, platelets, and NK cells suggest these to be the most promising cell lineages to take into clinical trials, based on current status of the field.
More recent studies have evaluated what soluble or secreted “factors” are important for hematopoietic development in these cultures. Ledran et al54 demonstrated that coculture of hESCs on AM20.1B4 cell line lead to increased development of severe combined immunodeficient (SCID) mouse–repopulating cells (SRCs), a close surrogate for HSCs. Further analysis showed AM20 cells produced more TGFβ than other stromal cell lines tested, and the addition of exogenous TGFβ to hESCs cocultured with other stromal cell lines had a similar effect. The ability to modify stromal cells also provides a useful means to define specific “niche factors” that regulate human hematopoiesis. For example, hESCs express Frizzled receptors for Wnt protein, and expression of Wnt1 on S17 and M2-10B4 cells leads to increased hemato-endothelial cell development from hESCs.59 Another recent report by Bhatia and colleagues62 has shown noncanonical Wnt signaling also plays key role in this system.
Embryoid body (EB)–mediated differentiation can also be used to support hematopoietic development from hPSCs. EBs have been commonly used for studies of hematopoiesis from mESCs, although the mechanics and timing of EB-mediated differentiation from hESCs is somewhat different. Initial studies using EB-mediated hematopoietic differentiation from hESCs used a combination of FBS and defined cytokines.63 Numerous other studies have also used EB-mediated differentiation for development of both hematopoietic progenitor cells and more mature cell populations from hESCs.64–66 Notably, studies by several investigators, including Elefanty's group and Zandstra's group, who use EB-mediated differentiation of hESCs, highlight methods to make EB-mediated hematopoietic differentiation of hESCs more consistent and efficient.67–69
Development of hemato-endothelial cells from hESCs
As emphasized, hESCs provide an unparalleled means to analyze early stages of human development that are otherwise difficult to study in a systematic manner. Several groups have characterized progenitor cells with both hematopoietic and endothelial cell potential (termed hemogenic-endothelium or hemangioblasts) from hESCs.59,64,66,70,71 Although this characterization of hemato-endothelial cells from hESCs has used different hESC lines and differing methods to induce or support differentiation, the timing of development and phenotype of this cell population is reasonably similar. Bhatia and colleagues70 first characterized a population of CD31+Flk1(KDR, CD309)+VE-Cadherin(CD144)+CD45− cells with the use of EB-mediated differentiation. Similarly, we used stromal cell–mediated differentiation to generate CD34bright CD31+Flk1(KDR)+CD45− cells with hemato-endothelial potential.59 Key studies by Zambidis et al66 identified angiotensin-converting enzyme/CD143 (as recognized by the BB9 antibody) on hESC-derived hemangioblasts as not just a lineage “marker,” but also as a functionally important molecule to regulate hemato-endothelial cell development by the renin-angiotensin system. Inhibiting angiotensin-converting enzyme activity (by captopril) or blocking AGTR1 led to decreased hematopoietic development and increased endothelial cell development from hEB-derived progenitors. In contrast, blocking AGTR2 leads to a 5-fold increase in hematopoietic progenitor cells (colony-forming cells; CFCs) and increased hematopoietic development from hESC-derived BL-CFCs (clonal hemangioblast colonies). Therefore, these analyses not only provide insight into regulation of early human hematopoiesis, but they may also be applicable to clinical hematopoietic cell transplantation when prompt hematopoietic engraftment is desired and may be affected by patient medications. Keller and colleagues,72 who pioneered studies to characterize development of hemangioblasts from mESCs, have also isolated 2 separate populations of cells from hESCs that meet the criteria of hemangioblast (ie, blast colonies having both hematopoietic and vascular potential).64
The OP9 stromal cell line (genetically deficient in M-CSF production) commonly used for studies of hematopoiesis from mESCs has also been used for hESC-based studies. Vodyanik et al71 demonstrated that early progenitors committed to hematopoietic development could be identified by surface expression of leukosialin (CD43). The appearance of CD43 was found to precede that of CD45 on all types of emerging clonogenic progenitors, and CD43 can reliably separate the hematopoietic CD34+ population from CD34+CD43− endothelial and mesenchymal cells. Interestingly, a population of CD34+CD43−KDR+ cell population with dual hemato-endothelial potential was isolated, similar to the CD34brightCD31+Flk1(KDR)+ cell population isolated by our group.59
With the use of stromal cell lines engineered to express mediators of canonical and non–canonical Wnt signaling, we further tested the role of Wnt proteins to provide insight into mechanisms that mediate hESC-derived hemato-endothelial cell development. Wnts are known to play a key role in many developmental pathways.73 As mentioned, stromal cell expression of Wnt1 (activating canonical Wnt signaling pathway), but not Wnt5 (non–canonical Wnt signaling) lead to increased CD34brightCD31+Flk1+ hemato-endothelial cells and CD34dimCD45+ hematopoietic progenitor cells.59 A corresponding decrease in these cell populations was shown by the inhibition of canonical Wnt signaling. Here again, the hESC system may provide insight to translate this basic biology to clinical applications.
hESC-derived hematopoietic cells evaluated for long-term engraftment potential
This review highlights the development of essentially all mature hematopoietic cell lineages from hESCs. Therefore, isolation of putative HSCs from hESCs with long-term multilineage engraftment potential could be considered a straightforward goal. However, studies to date continue to find that SRCs, regarded as a close surrogate for HSCs, remain challenging to derive and characterize from hESCs. Notably, this same hurdle exists for studies of mESCs in which xenogeneic barriers are not a concern.2 Several groups have exploited strategies such as overexpression of HoxB4 or other genes in mESC-derived cells to enable development of cells capable of long-term, multilineage hematopoietic cell engraftment.74–76 Similar strategies have been less successful for improving development of hematopoietic cells capable of long-term multilineage engraftment from hESCs.55,77,78 These finding emphasize that understanding the role of HoxB4 and related genes in hematopoietic cell development remains incomplete.
Several studies have now evaluated the engraftment potential of hESC-derived hematopoietic cells.53–56 The first used an intra-BM transplantation (IBMT) technique to successfully engraft hESC-derived cells in 11 of 19 mice.55 However, evidence of human reconstitution was limited compared with umbilical cord blood (UCB)–derived cells. Interestingly, this same study was unable to show successful engraftment after intravenous (tail vein) injection of the mice with hESC-derived hematopoietic cells. Indeed, there was actually a decrease in survival of the mice after intravenous injection, probably because of aggregation of the cells after injection, resulting in pulmonary emboli.
We used both IBMT and intravenous injection of hESC-derived hematopoietic cells to demonstrate successful engraftment in the mice without any decreased survival or pulmonary emboli after intravenous injection.53 These results probably reflect differences in the cell populations derived by alternative methods (by coculture with stromal cells versus by EB formation). In this study, BM analyzed 3 or more months after intravenous injection showed an average 0.69% human CD45+ cells, still considerably reduced compared with mice injected with cells derived from UCB. In mice in which IBMT was used, the level of engraftment was seen to be approximately 2% in the femur directly injected with the cells and essentially the same in the contralateral femur. The engrafted cells were primarily CD45+CD33+ myeloid cells; however, some CD34+ cells were also seen, suggesting HSC survival. Secondary transplantation studies were done to more rigorously show successful engraftment, although at a level only detectable by polymerase chain reaction (PCR) analysis in the secondary recipients. NOD/SCID mice are reported as NK-cell deficient; however, several analyses have shown that these mice retain some NK cell activity.55 In this regard, mice treated with anti-asialo GM1 antiserum (which depletes NK cells) led to a modestly enhanced level of engraftment, probably related to lower HLA class I molecule expression on the hESC-derived progenitors compared with UCB-derived HSCs, a difference that would predispose them to NK cell–mediated lysis.
In light of the residual immunity in NOD/SCID mice, a more recent study used the more immunodeficient NOD/SCID/IL2Rγc−/− mice.54 Here, Ledran et al54 also used a stromal cell coculture system and transplanted a heterogenous population of unsorted hESC-derived cells into adult recipients. Coculture of hESCs with one stromal cell line (AM20.1B4) isolated from the aorta/mesenchyme region of day 10 mouse embryos lead to higher levels of engraftment than the other stromal cell lines isolated from other developmental niches. Up to 16% of human CD45+ cell engraftment could be seen in the PB of some of these mice that received a transplant, although engraftment in the BM remained at only 1% to 2%. There were at least two other important findings in these studies that compared efficiency of different mouse stromal cell lines on hematopoietic development from hESCs.54 First, the stromal cell lines that lead to the highest level of hematopoietic development in vitro did not necessarily lead to the best SRC development. For example, hESCs cocultured with the cell line UG26.1B6 produced more CD34+ cells and more CFCs in vitro, compared with hESCs cocultured with AM20.1B4 cells. However, the AM20-derived cells were markedly better at in vivo engraftment. Second, stromal cell expression of TGFβ superfamily members correlated with improved hematopoietic (CD45+ cell) development from hESCs. Addition of TGFβ1 and TGFβ3 lead to improved CD45+ cell development, although studies to evaluate the SRC potential of the TGFβ-treated cells were not reported.54
Narayan et al56 used a fetal sheep transplantation system; hESC-derived hematopoietic cells were injected in utero into the peritoneal cavity of fetal sheep at less then 65 days of gestation. Five to 17 months after birth, approximately 0.1% human CD34+ or CD45+ cells were seen in the BM and or PB. Again, this level of engraftment is deceased compared with the use of UCB-derived cells.56 However, the low level engraftment was confirmed in the hESC studies by PCR for human DNA; BM samples were chimeric in 6 of 8 animals analyzed at 33 to 39 months after transplantation. Furthermore, human hematopoiesis in sheep that received a secondary transplant was followed for up to 22 months.
In another interesting study, hESC-derived CD34+ cells were transplanted into chick embryos.79 Here, human CD45+ cells could be seen to develop, with highest levels in the bursa of Fabricius, including CD19+IgM+ cells consistent with B cells. Other erythroid, myeloid, and endothelial cell populations could also be identified by phenotype, although no T cells were found. Although these studies are limited to a short time course, this does provide an intriguing in vivo model for future analyses to better identify mediators of hematopoietic development and engraftment.
To produce HSCs with better long-term multilineage engraftment potential from hESCs, it is probably necessary to develop culture techniques that more closely resemble the in vivo microenvironment needed to stimulate a genetic program needed for not only the hematopoietic specification of the hESCs but also for the transition from primitive to definitive hematopoiesis. In this regard we need better understanding of the pathways involved in this complex process. Several signaling pathways, including Wnt, Notch, Hedgehog, and TGFβ/Smad, are likely to play a prominent role in this developmental process.3,80,81 Furthermore, the in vivo environment may skew development of the hESC-derived cells, as shown by recent studies that used luciferase (luc)–expressing hESCs. Here, transplantation of luc+hESC-derived CD34+ cells that are known to have both hematopoietic and endothelial potential in vitro lead to long-term engraftment of the luc+ cells when transplanted into neonatal NOD/SCID/IL2Rγc−/− recipient mice, as visualized by bioluminescent imaging. However, although the engrafted luc+hESC-derived cells could be seen to expand and migrate systemically to diverse anatomic regions for several months after transplantation, analysis of the surviving and expanding cells found them to be mainly endothelial cells, again with little long-term hematopoietic cell engraftment.82 In another report, Lu et al83 also transplanted hESC-derived cells with hemato-endothelial cell potential into xenogeneic models of vascular injury and demonstrated endothelial cell engraftment and repair, without evidence of hematopoietic engraftment.
One important and often overlooked outcome of these multiple studies concerns the safety of hESC-based therapies because of the ability of the undifferentiated hESCs to form teratomas on injection into animals. However, no teratoma formation has been seen in any engraftment studies that used hematopoietic progenitors derived from hESCs. This is despite the use of irradiated immunocompromised mice that are very susceptible to teratoma development. Although a more rigorous study would be needed to prove that transplantation of hESC-derived hematopoietic cells pose no risk of teratoma development, these studies suggest that this is the case.
In vitro production of specific hematopoietic lineages from hESCs
Derivation of several mature cell lineages from hESCs has been a very fruitful endeavor, with potential to translate use of these cells to clinical testing sooner than HSCs derived from hESCs.
Cells for transfusion medicine
Erythroid development
Derivation of erythroid cells from hESCs may translate into a new and safer source of RBCs for transfusions. Although millions of units of RBCs are donated and used each year, new sources of RBCs that do not require volunteer donations remain of considerable interest. These alternatives include either cell-free systems such as polymerized hemoglobin84 or production of RBCs in bioreactor systems. Because each unit of transfused RBCs contains approximately 1012 mature, enucleated erythrocytes, efficient development of a cellular RBC population to be used routinely on a clinical scale remains a daunting (although not impossible) obstacle. Derivation of RBCs from UCB has been done,85 although it is unclear if a yield such as shown in those studies (∼ 3 units of RBCs from 1 donated UCB unit) is clinically feasible on a wide scale. Because hESCs can be used as a starting cell population and expanded essentially indefinitely, several studies that aim at large-scale production of RBCs from hESCs or iPSCs have been recently published.41–43
Analysis of erythroid development from hESCs provides key biologic insight about the developmental stage of hESC-derived cells. Globin gene regulation has distinct switches during normal human erythropoiesis.86 The α-globin locus switches from ζ-globin to α-globin expression, and the β-globin locus switches from ϵ-globin to γ-globin at approximately 6 to 7 weeks of development. Subsequently, γ-globin switches to β-globin expression around the time of birth. Of course, many human hemoglobinopathies and thalassemic syndromes result in defective or deficient globin expression and abnormal erythroid development. Models exploiting hESCs and iPSCs provide a promising approach to define molecular mechanisms that may be altered to repair these defects.30
Initial studies on hematopoietic differentiation from hESCs have shown derivation of erythroid progenitor cells, as shown by their ability to form BFU-E and CFU-E colonies in a methylcellulose-based colony-forming cell assay.57 Subsequent studies used EB-mediated differentiation with media supplemented by defined cytokines whereby VEGF and EPO improved development of erythroid cells and expression of embryonic ζ and ϵ globins, although no change in fetal/adult hemoglobin was noticed.87 More specific analysis of globin gene expression in erythroid cells derived from hESCs used hESCs cocultured on FH-B or S17 cells.61 Analysis of mRNA expression from the β-globin locus showed that hESC-derived erythroid cells produced ϵ- and γ-globin mRNAs but only minimal β-globin expression. Over time in culture, the mean ratio of γ/ϵ increased by more than 10-fold, recapitulating the ϵ-globin to γ-globin switch but not the later γ-globin to β-globin switch. Another analysis that used both EB and stromal cell (OP9)–mediated differentiation, as well as different cytokine combinations, also showed almost exclusive expression of ϵ- and γ-globin genes, with little β-globin expression.87
Production of RBCs from hESCs on a scale large enough for clinical use is intriguing from a therapeutic standpoint for several reasons. Blood from an hESC (or iPSC) line that was blood group O and RhD− would provide a source of “universal donor” RBCs. Such hESC-derived erythroid cells would originate from a defined source selected so that it was free of infectious pathogens. Furthermore, it may be possible to engineer hESC-derived cells to have a longer “shelf-life” and be more stable in storage. Recently, 3 groups have used different methods to scale up production of erythroid cells from hESCs.41–43 Together, these results have been impressive, not only in terms of the number of cells produced but also in the functional maturity of the cells. One study used a multistage protocol involving EB formation, defined cytokines plus a recombinant tPTD-HOXB4 protein to produce up to 1010 to 1011 RBCs from one 6-well plate of undifferentiated hESCs (probably ∼ 107 starting cells).42 In addition, the hESC-derived erythrocytes enucleate over time in culture, show near-normal O2–disassociation curves, and respond to 2,3-DPG and pH, similar to other RBCs.42,43 Although considerable effort is still needed to bring hESC-derived RBCs to a scale needed for clinical trials, these efforts certainly provide a promising direction.
Megakaryocytes and platelets
Platelets derived from hESCs also have the potential for transfusion medicine purposes, for much of the same safety and supply reasons as hESC-derived RBCs. Although our initial studies on hematopoietic development from hESCs showed CFU-megakaryocytes, identified by CD41 (αIIb) staining,57 these cells were not further characterized. Subsequently, the OP9 stromal cell coculture system was also used to generate megakaryocytes from hESCs with characteristic polyploid nucleus, cytoskeletal proteins, and ability to signal through integrin αIIbβ3.44 Although that report only showed developmental maturation to the megakaryocyte stage (without platelet production), subsequent work used hESC coculture with either OP9 or C3H10T1/2 cells in the presence of thrombopoietin for longer periods of time (2-4 weeks) to generate platelets with morphology and function similar to those isolated from fresh plasma.45
As discussed later in this review, safety of hPSC-derived cells is a key concern for clinical translation. Importantly, because RBCs and platelets do not have a nucleus and only minimal genetic material, malignant transformation of these particular cell types should not be an issue.
Cell for immune therapies
NK cells
Although some earlier reports suggested development of lymphocytes from hESCs based on limited reverse transcription–PCR analysis or surface staining for markers such as CD3 (T cells) or CD19 (B cells),46,60 studies to derive NK cells were the first to show development of functional lymphocytes from hESCs.47 A 2-step culture method was used to support development of CD56+CD45+ hESC-derived lymphocytes that function like mature NK cells. Specifically, these hESC-derived NK cells express killer cell Ig-like receptors, natural cytotoxicity receptors, and CD16, and they were able to lyse human tumor cells by both direct cell-mediated cytotoxicity and antibody-dependent cellular cytotoxicity.47
More recently, we have extended these studies to show killing not only of leukemia cells but also prostate cancer, lymphoma, glioma, germ cell tumor, and breast cancer cell lines by hESC-derived NK (hESC-NK) cells.88 To advance these studies to a more relevant preclinical system, the in vivo activity of hESC-NK cells was investigated in a xenogeneic mouse model. All mice treated with hESC-NK cells showed complete clearance of the primary tumor 2 weeks after tumor inoculation. In contrast, mice treated with UCB-derived NK (UCB-NK) cells had significantly less antitumor activity in vivo. Some mice treated with hESC-NK cells were monitored up to 8 weeks with no evidence of tumor development, showing complete tumor eradication after just one injection of hESC-NK cells. Furthermore, tissues were analyzed for the presence of micrometastasis, finding a significant reduction of metastases in the hESC-NK–treated animals. These results suggest that hESC-NK cells are capable of clearing human tumor cells in vivo more effectively than UCB-NK cells.
We postulated several potential mechanisms to account for the increased killing of tumors by the hESC-NK cells compared with UCB-NK cells. Analysis of homing receptors on the 2 cell populations did not show any significant differences, and studies to determine whether there was increased in vivo survival of the hESC-NK cells were inconclusive. Instead, we found a significant difference in the phenotype of the hESC-NK and UCB-NK cells based on expression of the receptor tyrosine kinase CD117 and the c-type lectin receptor CD94. Interesting studies find CD117+CD94−CD56+ NK cells represent an immature stage whereby the cells have committed to the NK-cell lineage but lack expression of NK-cell receptors and have no cytotoxic capacity. In contrast, a second population of more mature NK cells can be identified that are CD117−/lowCD94+ cells and acquire NK receptors, cytolytic activity, and IFNγ production.89,90 Notably, UCB-NK cells are a heterogeneous population with both immature CD117+CD94− and mature CD117−/lowCD94+ NK cells.88,89 In contrast, hESC-NK cells are a homogeneous population of mature CD117−/lowCD94+ NK cells.88 This suggests that, although cytolytic cells are generated from UCB progenitors, the proportion of mature and functional cytolytic NK cells is higher from the hESC-derived progenitor cells. This probably accounts for increased antitumor activity by hESC-NK cells.
T and B cells
Studies of T-cell development from hESCs have also lead to interesting and surprising results. Work by Galic et al48 initially used OP9 stromal cells to differentiate GFP-expressing hESCs into CD34+ and CD133+ cells that were injected into human thymic tissues engrafted into immunodeficient mice (SCID-hu mouse model). These hESC-derived T cells expressed surface antigens such as CD4, CD8, CD1a, and CD7 typical of T cells. More recently, this group has used EB-mediated differentiation to also derive T-cell progenitor cells, as assayed in the SCID-hu model.49 These studies also showed function of the hESC-derived T cells based on increased expression of CD25 after CD3/CD28-mediated activation. Although these studies clearly show development of hESC-derived T cells, the engraftment of these cells derived in the SCID-hu model is typically 1% or less, as measured by GFP+ cells. In contrast, previous studies that used SCID-hu mice to derive T cells from human BM, UCB, or fetal liver would be expected to have significantly higher levels of engraftment.91 These results suggest some inhibition or unexplained inefficiency in the development of hESC-derived T cells in this model.
To study hESC-based T-cell development in an in vitro model that might allow more access to developing cells and manipulation of conditions that support or inhibit development of these cells, we used the Notch ligand–expressing OP9-DL1 stromal cells that have been used to derive T cells from multiple progenitor cell populations such as human BM, UCB, and mouse ES cells.92–95 Surprisingly, the same hESC-derived CD34+CD45+ cells that effectively produce NK cells from hESCs were unable to produce T cells in this in vitro system. Use of fetal thymic organ culture also failed to produce T cells, although both the OP9-DL1 stromal cells and fetal thymic organ culture system effectively produced T cells from UCB.92 In addition, we also explored derivation of B cells from hESC-derived CD34+CD45+ cells with the use of different stromal cells lines and cytokine combinations, but again we failed to demonstrate effective B-cell development in the in vitro cultures that worked well from UCB progenitor cells.92
To identify potential mechanisms that may account for effective development of NK cells from hESC-derived progenitor cells, but inhibit development of mediators or adaptive immunity (T and B cells), we evaluated the expression of ID family genes in the hESC and UCB populations. ID genes encode for helix-loop-helix proteins that regulate many developmental pathways by the interaction with E proteins and other bHLH transcription factors.96 We found that undifferentiated hESCs expressed all 4 ID family members (ID1-4), and differentiation of the hESCs even further stimulated ID2 and ID3 expression.92 In contrast, UCB progenitor cells had only low levels of ID2 expressed and no ID3. In addition, E2A-responsive gene expression is inhibited in the hESC-derived cells, as would be expected by the function of more highly expressed ID proteins. Notably, several studies have shown ID2 and ID3 expression in mouse and human lymphoid progenitor cells promotes NK cell development and inhibits B- and T-cell development.97–100 Therefore, the in vitro conditions used to culture undifferentiated hESCs, as well as to promote hematopoietic differentiation (eg, with serum or BMP4), may induce ID expression and skewed lymphoid development of the hESC-derived cells toward NK-cell and away from T- and B-cell development. In addition, SLUG, a gene known to be activated by SCF-mediated signaling, is also more highly expressed in hESC-derived CD34+CD45+ cells than in similar cells from UCB.92 Because this SCF/c-kit–SLUG pathway stimulates NK-cell expansion, the presence of SCF and/or activation of this signaling pathway by other agonists probably also promote NK-cell growth from hESC-derived progenitor cells. These findings provide an important lesson for clinical translation of hESCs, because the hESC culture conditions may mediate unrecognized effects on the quantity or quality of mature cells of defined lineages that may be of interest for novel therapies.
Recently, Timmermans et al101 demonstrated in vitro development of T cells from hESCs with the use of a different hESC line (H1) and a different stromal cell line (OP9) to mediate differentiation. In addition, they demonstrated that a specific population of CD34+CD43low cells that were present in hematopoietic zones morphologically similar to blood islands were uniquely suited to produce T cells when cultured on OP9-DL1 cells with a cocktail of cytokines. This promising study suggests a process to use hESCs to generate T cells for novel immunotherapy. However, additional work is needed to better understand the differences between hESC lines and/or culture conditions that may effect the development of T cells in this system.
Other mediators of immunity: monocytes/macrophages, granulocytes, and dendritic cells
CFC assays to quantify hematopoietic progenitor cells have been perhaps the most useful means to show the development of bona fide hESC-derived hematopoietic cells,57 because other tests such as surface antigens tested by flow cytometry and gene expression evaluated by reverse transcription PCR are not always completely specific for hematopoietic cell properties. Subsequently, several studies have evaluated more specific means to produce and characterize hESC-derived monocyte/macrophages and granulocytes. In an initial report about producing more purified macrophages from hESCs, CD34+ cells derived by S17 stromal cell–mediated culture were isolated. These hESC-derived CD34+ cells were sorted and cultured with defined cytokines to generate macrophages. The hESC-derived macrophages could be cultured in bulk and displayed functions including phagocytosis, up-regulation of the costimulatory molecule B7.1, cytokine secretion in response to lipopolysaccharide stimulation, and maintenance of productive HIV infection.102 Another study used EB-mediated differentiation of 3 different hESC lines to first produce monocytes then mature macrophages with defined cytokines in a 3-step culture process.65 Although demonstration of granulocytes from hESCs is common with CFC assay conditions,57 2 studies produced a highly enriched population of neutrophils by a 2-step culture system with partially defined conditions (feeder-free, but with serum).103,104 These neutrophils were characterized based on morphology, surface antigen and gene expression, as well as phagocytosis and in vivo chemotactic activity.
Dendritic cells (DCs) are principle antigen-presenting cells that trigger and support immune responses. One study by Cheng's group used EB-mediated differentiation to derive both functional DCs and macrophages.46 These antigen-presenting cells expressed high levels of HLA class II molecules and were able to stimulate mixed leukocyte reactions as an in vitro measure of immune activity. A second study by Slukvin et al50 used the OP9 coculture to promote differentiation of hESCs into myeloid DCs. Function was shown by antigen (ovalbumin) uptake and processing, as well as well by the ability to stimulate allogeneic and antigen-specific T-cell responses. Other studies have also shown similar development of hESC-derived DCs also using OP9 cell coculture, but with different cytokines, or in a stromal-fee system that might be more amenable to clinical translation.51,52
Induced pluripotent SCs for hematopoietic cell development and therapies
The derivation of iPSCs from mouse and then human somatic cells is yet another key landmark in stem cell biology.4–8 Although this review does not detail technical aspects of iPSC production, the ability to now to produce iPSCs with transient gene expression and by combined use of drugs or small molecules with just 1 or 2 transgenes and without need for stable integration of the transgenes is a key development.11–17 These advances foreshadow an expected means to produce iPSCs without any gene transfer, instead relying on defined exogenous agents to reprogram differentiated cells into a pluripotent cell population, an advance very recently described to generate mouse and human iPSCs solely using protein transduction.18,19 Although the efficiency of iPSC production may be reduced with the use of fewer transgenes, it is quite easy to treat 106 or more cells with agents that lead to iPSC development. Therefore, even low-frequency events should readily lead to iPSC outgrowth.
Although iPSCs offer many potential advantages (detailed in part below), they may not be optimal for some purposes. Specifically, hESCs will probably remain the preferred starting point for studies of normal human development, because it is certainly not “normal” for a somatic cell to be reprogrammed to a pluripotent state. Consequently, using iPSCs as a starting point to determine genes, proteins, and other stimuli that mediate differentiation into defined cell lineages may be less accurate or helpful than starting from hESCs. In addition, safety concerns for iPSCs are still the same as for hESCs, because by definition iPSCs are able to form teratomas. However, iPSCs offer an unparalleled means to study human genetic disease and to evaluate how defined mutations may affect developmental pathways. Therefore, although hESCs provide a “gold standard” for human pluripotent stem cells, hESCs and iPSCs offer complementary opportunities.
Cure of a mouse model of human sickle cell anemia shows how human iPSCs may lead to new combined cell and gene therapies. Here, fibroblasts from a mouse carrying homozygous expression of the human β-globin sickle cell gene were made into iPSCs. The βs mutation was then corrected in these iPSCs, and those βs-expressing cells corrected to be normal βA-expressing cells were then made into hematopoietic cells by EB formation, HoxB4 was expressed in these cells to allow successful engraftment and an effective cure for the affected βs mouse.30 Similar cures have now been achieved for the mouse model of hemophilia,31 as well as nonhematologic diseases.32 Translation of these strategies to treat human genetic disease is inevitable,33,105 although the same barriers that exist for bringing hESC-based therapies to clinical reality also certainly apply to iPSC-based therapies. In addition, for autoimmune diseases such as type 1 diabetes, it is unclear whether iPSCs are an optimal starting cell population, because they would still be subject to the underlying autoimmune process, although immune suppressive or immune-evasive strategies can be proposed for iPSCs, much like for hESCs.106,107 Remarkable work showing the potential to directly convert 1 mature cell population to another without the need to go through an iPSC intermediate, as was shown for conversion of exocrine to endocrine pancreas,23 provides another therapeutic pathway.
Hematopoietic cell development from human iPSCs was shown in one of the initial studies.6 In addition, Slukvin's group26 has more closely analyzed hematopoietic development from several iPSC lines. Although there is variation between the lines, the overall developmental pattern based on phenotype, gene expression, and production of CFCs is essentially the same as for hESCs.26 Because these studies have found significant differences in developmental potential of iPSCs derived from the same person, an unknown question is how many iPSC lines are needed from a person if they are to be used for therapeutic purposes.
It is also unclear how similar or how different hESCs are in their ability to efficiently produce specific cell populations. An important study by the International Stem Cell Initiative compared phenotype, gene expression, and imprinting of 59 hESCs lines produced by 17 different groups.108 This analysis showed that overall the undifferentiated hESCs were quite similar to one another, although some differences, especially in imprinted genes, were noted. However, Melton's group109 evaluated 17 different hESC lines induced to differentiate by EB formation. They analyzed representative gene expression for the 3 germ layers and 8 derivative organs and found significant differences between the hESC lines.109 Similar variation between hESC lines was described in a more detailed study of cardiac differentiation.110 Additional rigorous analyses are clearly needed that use functional studies and not just phenotype or limited gene expression to compare the developmental potential of different hESC lines, as well as how iPSCs compare with hESCs.26
Current barriers to clinical translation
Within a decade, we have moved from the initial derivation of hESCs to approval by the Food and Drug Administration (FDA) of the first clinical trials with hESC-derived cells. These initial trials will evaluate oligodendrocyte cells or progenitors for patients with spinal cord injury.111 This is indeed a remarkable achievement to initiate trials of these novel therapies for diseases or disorders such as spinal cord injury in which there are currently no effective therapies. However, there are still several barriers that will be important to overcome for clinical trials of hPSC-derived cells to be effective. Although these barriers hold true whether treating disorders such as spinal cord injury or other conditions, there are some barriers that may be more relevant for clinical trials of hematopoietic cell therapies from hPSCs (Figure 2).
Figure 2.
Hurdles to overcome for clinical translation of hPSC-derived therapies. Although novel therapies starting from hPSCs are an enticing prospect, multiple technical, safety, and regulatory hurdles outlined in this figure and the text will need to be surmounted for successful clinical translation. It is essential that, although regulatory and other nonscientific interests should be considered and appropriately accommodated, these considerations should facilitate and not inhibit clinical translation of hPSCs as these therapies move to the clinic.
Function and integration of hPSC-derived cells
It is not enough to produce cells from hPSCs of appropriate phenotype and gene expression patterns to be used for clinical purposes. Cells used for clinical therapies must have proper function and will have to integrate or interact appropriately with an injured or diseased environment. In some respects, hematopoietic cells that primarily exist in the blood and BM do not integrate with their environment. However, HSCs still have to mature in a defined hematopoietic cell niche, and potentially therapeutic cells such as lymphocytes have to recognize appropriate targets through receptor repertoire as well as properly home to areas of disease or injury. hPSC-derived cells must also reach an appropriate degree of functional maturity. Several lineages, including cardiomyocytes, pancreatic endoderm cells, and hematopoietic cells derived from hESCs, have embryonic/fetal developmental characteristics rather than fully adult characteristics.61,112,113 For example, as described, erythroid cells derived from hESCs primarily express ϵ- and γ-globin and relatively little β-globin. Of course, RBCs that express fetal globins can still be functional (and, in fact, may be particularly desirable in conditions such as sickle cell anemia), but they are not the same as typical adult erythroid cells.
Administration of novel cell products will obviously be an area of key consideration for clinical use of hPSC-derived hematopoietic cells, whether they be HSCs, RBCs, platelets, or lymphocytes. It is fortunate that these cells can probably be administered systemically by intravenous method as is standard for current transfusion therapies and hematopoietic cell transplantation. Even antitumor lymphocytes are routinely infused systemically, with no apparent need or benefit to directly inject cells at sites of persistent malignancies. Other related cell populations such as endothelial cells may need to be injected at local sites of ischemia (cardiac or peripheral vascular disease), as has been done for clinical trials of other cell-based therapies for ischemia.
Clinical scale
The number of cells necessary for specific therapies will clearly depend on the underlying disease or condition being treated. The number of glial cells needed for spinal cord injury or the number of retinal-pigmented epithelial cells required to treat blindness caused by macular degeneration is approximately 105 to 106. This is a scale that is readily achieved in a single culture dish or flask. However, for some hematopoietic cell therapies, especially development of RBCs for transfusion medicine, the number of cells needed is enormous. Each unit of RBCs contains approximately 1012 enucleated, mature RBCs. Although considerable efforts have been made to derive erythroid cells suitable for transfusions from hESCs, the scale required will probably require novel bioengineering methods. How long it will take to routinely and efficiently achieve the numbers of hESC- or iPSC-derived cells for immunotherapies or transfusion therapies remains a matter of speculation. However, this field is moving ahead rapidly. If, as expected, the initial trials of hESC-derived oligodendrocyte progenitor cells for spinal cord injury show safety and, potentially, some clinical success, then the impetus for other therapies will grow. With the progress reviewed here, and in recognition of the outline obstacles, it is possible for hES- or iPSC-derived hematopoietic cells to begin clinical trials in the next 3 to 5 years.
Safety
Investigators have expressed concern about the possible development of teratomas or other tumors from undifferentiated hESCs that might be transplanted along with the more mature functional tissue. Obviously, no trial would use undifferentiated hPSCs known to lead to teratoma development. Indeed, almost no preclinical test in which hESC-derived cells were transplanted into immunodeficient animals has shown teratoma development in a system in which these tumors would probably be evident.53,54 However, one study in which differentiated neuronal cells were transplanted into a rat Parkinson disease model did show that some animals developed neuroectoderm tumors.114 Of course, other “adult” cell-based therapies also confer the risk of tumors such as sarcomas that can develop from mesenchymal stem cells,115 and even in clinical hematopoietic cell transplantation, donor-derived leukemia can develop on occasion.116 Therefore, although safety of the hPSC-derived cells is of utmost concern, this risk will have to be weighed against the potential benefits of the therapy being administered.
Immune response
Because most hESC-derived cells have been tested in preclinical models using immunodeficient mice and rats, the actual immune response against the hESC-derived cells is difficult to determine. Cells derived from hESCs would almost certainly be an allogeneic tissue source and be subject to typical immune rejection.117 Recent studies have shown that hESCs and hESC-derived cells can be rejected by a T cell–mediated process,117 and other studies have shown that immune effectors (NK cells) can also mediate rejection of hESC-derived hematopoietic cells that may have low levels of HLA class I expression.53 Multiple methods have been proposed to inhibit the host immune response against these transplanted tissues.106,107 These options range from standard pharmaceutical immunosuppressive drugs to using the novel properties of hPSCs to allow potentially more creative and effective means to prevent immunorejection such as decreasing HLA expression or promoting expression of immunosuppressant molecules. For clinical use of iPSCs, these can be derived from an autologous cell source, and host immune rejection would not be an expected issue. Of course, for some therapies such as pancreatic endocrine cells given to patients with type 1 diabetes, the ability of these patients to still have an underlying immune response against the islet cells needs to be considered. In addition, allogeneic lymphocytes from hESCs may be preferable to autologous lymphocytes from iPSCs to better mediate antitumor or other beneficial immune responses.
Regulatory issues
The FDA must approve clinical trials of hPSC-derived cells performed in the United States. Similar regulatory bodies will need to approve trials in other parts of the world. These regulatory bodies will not only be concerned with the safety of the cells but also with standard, clinical grade cell production (GMP methods).118 In many cases this has been inferred to mean avoidance of xenogeneic tissue such as murine cells that are used for maintenance of undifferentiated hPSCs, or stromal cell lines that are commonly utilized to induce differentiation. Notably, use of stromal cells and serum does not preclude the use of the cells for clinical trials. Indeed, the initial trials of oligodendrocytes from hESCs for spinal cord will use hESCs that were derived on mouse embryonic fibroblasts and cultured for at least a time with FBS. Other cell-based clinical therapies such as keratinocytes grown for the treatment of burns use mouse stromal cells and FBS. This treatment for severe burns (used in over 1000 patients) is now FDA approved and available as Epicel. Therefore, although there is strong interest to develop cell-free and serum-free methods for hESC growth and differentiation, the use of xenogeneic reagents does not, in and of themselves, preclude translation to clinical therapies. Another regulatory issue to consider will be intellectual property rights. This is a complex area within the stem cell and hPSC literature and beyond the scope of this review. Although business and other interests should be considered and appropriately accommodated, these considerations should facilitate and not inhibit clinical translation of hPSCs as these therapies move to the clinic. Importantly, intellectual property issues may differ in areas of the world, such that the European patent office has not approved a patent on hESCs.
Conclusion
Hematologists have been using hematopoietic stem cells to cure malignant and nonmalignant hematologic diseases for more than 40 years. Therefore, the study and clinical use of stem cells are not a new concept. However, the derivation of hESCs and the remarkable ability to induce pluripotency from somatic cells has captured both the imagination of the public and scientific communities. These PSCs present both new opportunities and challenges for novel cell-based therapies. A decade of studies with hESCs has produced remarkable progress, despite some political inhibition. The recent change in the US federal policy to permit federal (National Institutes of Health) funding for a greater number of hESC lines will accelerate the momentum of this work. In addition, work by organizations such as the US National Academies of Science and the International Society for Stem Cell Research to provide policy recommendation for oversight of hESCs and other new stem cell research and clinical translation are extremely beneficial in setting uniform standards.119 As clinical trials of hPSC-based therapies are now being initiated to better treat and cure diseases, it is now paramount to define the efficacy and safety of the novel therapies. The clinical need for new and better therapies remains greater than any barriers and unanswered questions. The next few years will undoubtedly continue to produce great strides to move hematopoietic cells derived from hPSCs to new clinical treatments and cures.
Acknowledgments
I thank Drs Petter Woll, Eric Bouhassira, Phil McGlave, Jeff Miller, Dan Wesidorf, and Greg Vercellotti for helpful review of the manuscript, and Carol Taubert for assistance with figures. I apologize for any relevant studies that could not be included in this review because of space limitations.
This work was supported by the National Heart, Lung, and Blood Institute; the Bill and Melinda Gates Foundation; and the Leukemia Research Fund of the University of Minnesota Masonic Cancer Center.
Authorship
Contribution: D.S.K. wrote the manuscript.
Conflict-of-interest disclosure: The author declares no competing financial interests.
Correspondence: Dan S. Kaufman, University of Minnesota, Department of Medicine and Stem Cell Institute, 420 Delaware St SE, MMC 480, Minneapolis, MN 55455; e-mail: kaufm020@umn.edu.
References
- 1.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2.Kyba M, Daley GQ. Hematopoiesis from embryonic stem cells: lessons from and for ontogeny. Exp Hematol. 2003;31(11):994–1006. doi: 10.1016/j.exphem.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132(4):631–644. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 5.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448(7151):313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 6.Park IH, Zhao R, West JA, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2007;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 8.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 9.Hochedlinger K, Plath K. Epigenetic reprogramming and induced pluripotency. Development. 2009;136(4):509–523. doi: 10.1242/dev.020867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishikawa S, Goldstein RA, Nierras CR. The promise of human induced pluripotent stem cells for research and therapy. Nat Rev Mol Cell Biol. 2008;9(9):725–729. doi: 10.1038/nrm2466. [DOI] [PubMed] [Google Scholar]
- 11.Stadtfeld M, Nagaya M, Utikal J, Weir G, Hochedlinger K. Induced pluripotent stem cells generated without viral integration. Science. 2008;322(5903):945–949. doi: 10.1126/science.1162494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carey BW, Markoulaki S, Hanna J, et al. Reprogramming of murine and human somatic cells using a single polycistronic vector. Proc Natl Acad Sci U S A. 2009;106(1):157–162. doi: 10.1073/pnas.0811426106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huangfu D, Osafune K, Maehr R, et al. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotech. 2008;26(11):1269–1275. doi: 10.1038/nbt.1502. [DOI] [PubMed] [Google Scholar]
- 14.Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322(5903):949–953. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
- 15.Woltjen K, Michael IP, Mohseni P, et al. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature. 2009;458(7239):766–770. doi: 10.1038/nature07863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaji K, Norrby K, Paca A, Mileikovsky M, Mohseni P, Woltjen K. Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature. 2009;458(7239):771–775. doi: 10.1038/nature07864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu J, Hu K, Smuga-Otto K, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324(5928):797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou H, Wu S, Joo JY, et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009;4(5):381–384. doi: 10.1016/j.stem.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim D, Kim C-H, Moon J-I, et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009;4(6):472–476. doi: 10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aasen T, Raya A, Barrero MJ, et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotech. 2008;26(11):1276–1284. doi: 10.1038/nbt.1503. [DOI] [PubMed] [Google Scholar]
- 21.Loh YH, Agarwal S, Park IH, et al. Generation of induced pluripotent stem cells from human blood. Blood. 2009;113(22):5476–5479. doi: 10.1182/blood-2009-02-204800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aoi T, Yae K, Nakagawa M, et al. Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science. 2008;321(5889):699–702. doi: 10.1126/science.1154884. [DOI] [PubMed] [Google Scholar]
- 23.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455(7213):627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wernig M, Meissner A, Foreman R, et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448(7151):318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- 25.Lowry WE, Richter L, Yachechko R, et al. Generation of human induced pluripotent stem cells from dermal fibroblasts. Proc Natl Acad Sci U S A. 2008;105(8):2883–2888. doi: 10.1073/pnas.0711983105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi KD, Yu J, Smuga-Otto K, et al. Hematopoietic and endothelial differentiation of human induced pluripotent stem cells. Stem Cells. 2009;27(3):559–567. doi: 10.1634/stemcells.2008-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Odorico JS, Kaufman DS, Thomson JA. Multilineage differentiation from human embryonic stem cell lines. Stem Cells. 2001;19(3):193–204. doi: 10.1634/stemcells.19-3-193. [DOI] [PubMed] [Google Scholar]
- 28.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172(5):2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 29.Palis J, Yoder MC. Yolk-sac hematopoiesis: the first blood cells of mouse and man. Exp Hematol. 2001;29(8):927–936. doi: 10.1016/s0301-472x(01)00669-5. [DOI] [PubMed] [Google Scholar]
- 30.Hanna J, Wernig M, Markoulaki S, et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007;318(5858):1920–1923. doi: 10.1126/science.1152092. [DOI] [PubMed] [Google Scholar]
- 31.Xu D, Alipio Z, Fink LM, et al. Phenotypic correction of murine hemophilia A using an iPS cell-based therapy. Proc Natl Acad Sci U S A. 2009;106(3):808–813. doi: 10.1073/pnas.0812090106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wernig M, Zhao J-P, Pruszak J, et al. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson's disease. Proc Natl Acad Sci U S A. 2008;105(15):5856–5861. doi: 10.1073/pnas.0801677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soldner F, Hockemeyer D, Beard C, et al. Parkinson's disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell. 2009;136(5):964–977. doi: 10.1016/j.cell.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen G, Ye Z, Yu X, et al. Trophoblast differentiation defect in human embryonic stem cells lacking PIG-A and GPI-anchored cell-surface proteins. Cell Stem Cell. 2008;2(4):345–355. doi: 10.1016/j.stem.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park IH, Arora N, Huo H, et al. Disease-specific induced pluripotent stem cells. Cell. 2008;134(5):877–886. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ebert AD, Yu J, Rose FF, Jr, et al. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature. 2009;457(7227):277–280. doi: 10.1038/nature07677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dimos JT, Rodolfa KT, Niakan KK, et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321(5893):1218–1221. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- 38.North TE, Goessling W, Walkley CR, et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447(7147):1007–1011. doi: 10.1038/nature05883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stummann TC, Bremer S. The possible impact of human embryonic stem cells on safety pharmacological and toxicological assessments in drug discovery and drug development. Curr Stem Cell Res Ther. 2008;3(2):118–131. doi: 10.2174/157488808784223104. [DOI] [PubMed] [Google Scholar]
- 40.Goh G, Self T, Barbadillo Munoz MD, Hall IP, Young L, Denning C. Molecular and phenotypic analyses of human embryonic stem cell-derived cardiomyocytes: opportunities and challenges for clinical translation. Thromb Haemost. 2005;94(4):728–737. doi: 10.1160/TH05-04-0268. [DOI] [PubMed] [Google Scholar]
- 41.Olivier EN, Qiu C, Velho M, Hirsch RE, Bouhassira EE. Large-scale production of embryonic red blood cells from human embryonic stem cells. Exp Hematol. 2006;34(12):1635–1642. doi: 10.1016/j.exphem.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 42.Lu SJ, Feng Q, Park JS, et al. Biologic properties and enucleation of red blood cells from human embryonic stem cells. Blood. 2008;112(12):4475–4484. doi: 10.1182/blood-2008-05-157198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma F, Ebihara Y, Umeda K, et al. Generation of functional erythrocytes from human embryonic stem cell-derived definitive hematopoiesis. Proc Natl Acad Sci U S A. 2008;105(35):13087–13092. doi: 10.1073/pnas.0802220105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gaur M, Kamata T, Wang S, Moran B, Shattil SJ, Leavitt AD. Megakaryocytes derived from human embryonic stem cells: a genetically tractable system to study megakaryocytopoiesis and integrin function. J Thromb Haemost. 2006;4(2):436–442. doi: 10.1111/j.1538-7836.2006.01744.x. [DOI] [PubMed] [Google Scholar]
- 45.Takayama N, Nishikii H, Usui J, et al. Generation of functional platelets from human embryonic stem cells in vitro via ES-sacs, VEGF-promoted structures that concentrate hematopoietic progenitors. Blood. 2008;111(11):5298–5306. doi: 10.1182/blood-2007-10-117622. [DOI] [PubMed] [Google Scholar]
- 46.Zhan X, Dravid G, Ye Z, et al. Functional antigen-presenting leucocytes derived from human embryonic stem cells in vitro. Lancet. 2004;364(9429):163–171. doi: 10.1016/S0140-6736(04)16629-4. [DOI] [PubMed] [Google Scholar]
- 47.Woll PS, Martin CH, Miller JS, Kaufman DS. Human embryonic stem cell-derived NK cells acquire functional receptors and cytolytic activity. J Immunol. 2005;175(8):5095–5103. doi: 10.4049/jimmunol.175.8.5095. [DOI] [PubMed] [Google Scholar]
- 48.Galic Z, Kitchen SG, Kacena A, et al. T lineage differentiation from human embryonic stem cells. Proc Natl Acad Sci U S A. 2006;103(31):11742–11747. doi: 10.1073/pnas.0604244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Galić Z, Kitchen SG, Subramanian A, et al. Generation of T lineage cells from human embryonic stem cells in a feeder free system. Stem Cells. 2009;27(1):100–107. doi: 10.1634/stemcells.2008-0813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Slukvin II, Vodyanik MA, Thomson JA, Gumenyuk ME, Choi KD. Directed differentiation of human embryonic stem cells into functional dendritic cells through the myeloid pathway. J Immunol. 2006;176(5):2924–2932. doi: 10.4049/jimmunol.176.5.2924. [DOI] [PubMed] [Google Scholar]
- 51.Senju S, Suemori H, Zembutsu H, et al. Genetically manipulated human embryonic stem cell-derived dendritic cells with immune regulatory function. Stem Cells. 2007;25(11):2720–2729. doi: 10.1634/stemcells.2007-0321. [DOI] [PubMed] [Google Scholar]
- 52.Su Z, Frye C, Bae K-M, Kelley V, Vieweg J. Differentiation of human embryonic stem cells into immunostimulatory dendritic cells under feeder-free culture conditions. Clin Cancer Res. 2008;14(19):6207–6217. doi: 10.1158/1078-0432.CCR-08-0309. [DOI] [PubMed] [Google Scholar]
- 53.Tian X, Woll PS, Morris JK, Linehan JL, Kaufman DS. Hematopoietic engraftment of human embryonic stem cell-derived cells is regulated by recipient innate immunity. Stem Cells. 2006;24(5):1370–1380. doi: 10.1634/stemcells.2005-0340. [DOI] [PubMed] [Google Scholar]
- 54.Ledran MH, Krassowska A, Armstrong L, et al. Efficient hematopoietic differentiation of human embryonic stem cells on stromal cells derived from hematopoietic niches. Cell Stem Cell. 2008;3(1):85–98. doi: 10.1016/j.stem.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 55.Wang L, Menendez P, Shojaei F, et al. Generation of hematopoietic repopulating cells from human embryonic stem cells independent of ectopic HOXB4 expression. J Exp Med. 2005;201(10):1603–1614. doi: 10.1084/jem.20041888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Narayan AD, Chase JL, Lewis RL, et al. Human embryonic stem cell-derived hematopoietic cells are capable of engrafting primary as well as secondary fetal sheep recipients. Blood. 2006;107(5):2180–2183. doi: 10.1182/blood-2005-05-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaufman DS, Hanson ET, Lewis RL, Auerbach R, Thomson JA. Hematopoietic colony-forming cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A. 2001;98(18):10716–10721. doi: 10.1073/pnas.191362598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tian X, Morris JK, Linehan JL, Kaufman DS. Cytokine requirements differ for stroma and embryoid body-mediated hematopoiesis from human embryonic stem cells. Exp Hematol. 2004;32(10):1000–1009. doi: 10.1016/j.exphem.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 59.Woll PS, Morris JK, Painschab MS, et al. Wnt signaling promotes hematoendothelial cell development from human embryonic stem cells. Blood. 2008;111(1):122–131. doi: 10.1182/blood-2007-04-084186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vodyanik MA, Bork JA, Thomson JA, Slukvin II. Human embryonic stem cell-derived CD34+ cells: efficient production in the coculture with OP9 stromal cells and analysis of lymphohematopoietic potential. Blood. 2005;105(2):617–626. doi: 10.1182/blood-2004-04-1649. [DOI] [PubMed] [Google Scholar]
- 61.Qiu C, Hanson E, Olivier E, et al. Differentiation of human embryonic stem cells into hematopoietic cells by coculture with human fetal liver cells recapitulates the globin switch that occurs early in development. Exp Hematol. 2005;33(12):1450–1458. doi: 10.1016/j.exphem.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 62.Vijayaragavan K, Szabo E, Bosse M, Ramos-Mejia V, Moon RT, Bhatia M. Noncanonical Wnt signaling orchestrates early developmental events toward hematopoietic cell fate from human embryonic stem cells. Cell Stem Cell. 2009;4(3):248–262. doi: 10.1016/j.stem.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chadwick K, Wang L, Li L, et al. Cytokines and BMP-4 promote hematopoietic differentiation of human embryonic stem cells. Blood. 2003;102(3):906–915. doi: 10.1182/blood-2003-03-0832. [DOI] [PubMed] [Google Scholar]
- 64.Kennedy M, D'Souza SL, Lynch-Kattman M, Schwantz S, Keller G. Development of the hemangioblast defines the onset of hematopoiesis in human ES cell differentiation cultures. Blood. 2007;109(7):2679–2687. doi: 10.1182/blood-2006-09-047704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Karlsson KR, Cowley S, Martinez FO, Shaw M, Minger SL, James W. Homogeneous monocytes and macrophages from human embryonic stem cells following coculture-free differentiation in M-CSF and IL-3. Exp Hematol. 2008;36(9):1167–1175. doi: 10.1016/j.exphem.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zambidis ET, Park TS, Yu W, et al. Expression of ACE (CD143) identifies and regulates primitive hemangioblasts derived from human pluripotent stem cells. Blood. 2008;112(9):3601–3614. doi: 10.1182/blood-2008-03-144766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ng ES, Davis RP, Azzola L, Stanley EG, Elefanty AG. Forced aggregation of defined numbers of human embryonic stem cells into embryoid bodies fosters robust, reproducible hematopoietic differentiation. Blood. 2005;106(5):1601–1603. doi: 10.1182/blood-2005-03-0987. [DOI] [PubMed] [Google Scholar]
- 68.Bauwens CL, Peerani R, Niebruegge S, et al. Control of human embryonic stem cell colony and aggregate size heterogeneity influences differentiation trajectories. Stem Cells. 2008;26(9):2300–2310. doi: 10.1634/stemcells.2008-0183. [DOI] [PubMed] [Google Scholar]
- 69.Cameron CM, Hu WS, Kaufman DS. Improved development of human embryonic stem cell-derived embryoid bodies by stirred vessel cultivation. Biotechnol Bioeng. 2006;94(5):938–948. doi: 10.1002/bit.20919. [DOI] [PubMed] [Google Scholar]
- 70.Wang L, Li L, Shojaei F, et al. Endothelial and hematopoietic cell fate of human embryonic stem cells originates from primitive endothelium with hemangioblastic properties. Immunity. 2004;21(1):31–41. doi: 10.1016/j.immuni.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 71.Vodyanik MA, Thomson JA, Slukvin II. Leukosialin (CD43) defines hematopoietic progenitors in human embryonic stem cell differentiation cultures. Blood. 2006;108(6):2095–2105. doi: 10.1182/blood-2006-02-003327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development. 1998;125(4):725–732. doi: 10.1242/dev.125.4.725. [DOI] [PubMed] [Google Scholar]
- 73.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 74.Kyba M, Perlingeiro RC, Daley GQ. HoxB4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell. 2002;109(1):29–37. doi: 10.1016/s0092-8674(02)00680-3. [DOI] [PubMed] [Google Scholar]
- 75.Kyba M, Perlingeiro RC, Hoover RR, Lu CW, Pierce J, Daley GQ. Enhanced hematopoietic differentiation of embryonic stem cells conditionally expressing Stat5. Proc Natl Acad Sci U S A. 2003;100(suppl 1):11904–11910. doi: 10.1073/pnas.1734140100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chan KM, Bonde S, Klump H, Zavazava N. Hematopoiesis and immunity of HOXB4-transduced embryonic stem cell-derived hematopoietic progenitor cells. Blood. 2008;111(6):2953–2961. doi: 10.1182/blood-2007-10-117366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Unger C, Karner E, Treschow A, et al. Lentiviral-mediated HoxB4 expression in human embryonic stem cells initiates early hematopoiesis in a dose-dependent manner but does not promote myeloid differentiation. Stem Cells. 2008;26(10):2455–2466. doi: 10.1634/stemcells.2007-0876. [DOI] [PubMed] [Google Scholar]
- 78.Bowles KM, Vallier L, Smith JR, Alexander MR, Pedersen RA. HOXB4 overexpression promotes hematopoietic development by human embryonic stem cells. Stem Cells. 2006;24(5):1359–1369. doi: 10.1634/stemcells.2005-0210. [DOI] [PubMed] [Google Scholar]
- 79.Park TS, Zambidis ET, Lucitti JL, Logar A, Keller BB, Pèault B. Human embryonic stem cell-derived hematoendothelial progenitors engraft chicken embryos. Exp Hematol. 2009;37(1):31–41. doi: 10.1016/j.exphem.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 80.Cerdan C, Rouleau A, Bhatia M. VEGF-A165 augments erythropoietic development from human embryonic stem cells. Blood. 2004;103(7):2504–2512. doi: 10.1182/blood-2003-07-2563. [DOI] [PubMed] [Google Scholar]
- 81.Blank U, Karlsson G, Karlsson S. Signaling pathways governing stem-cell fate. Blood. 2008;111(2):492–503. doi: 10.1182/blood-2007-07-075168. [DOI] [PubMed] [Google Scholar]
- 82.Tian X, Hexum MK, Penchev VR, Taylor RJ, Shultz LD, Kaufman DS. Bioluminescent imaging demonstrates transplanted human embryonic stem cell-derived CD34+ cells preferentially develop into endothelial cells. Stem Cells. 2009 Aug 26; doi: 10.1002/stem.204. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lu SJ, Feng Q, Caballero S, et al. Generation of functional hemangioblasts from human embryonic stem cells. Nat Methods. 2007;4(6):501–509. doi: 10.1038/nmeth1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Natanson C, Kern SJ, Lurie P, Banks SM, Wolfe SM. Cell-free hemoglobin-based blood substitutes and risk of myocardial infarction and death: a meta-analysis. JAMA. 2008;299(19):2304–2312. doi: 10.1001/jama.299.19.jrv80007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Giarratana MC, Kobari L, Lapillonne H, et al. Ex vivo generation of fully mature human red blood cells from hematopoietic stem cells. Nat Biotechnol. 2005;23(1):69–74. doi: 10.1038/nbt1047. [DOI] [PubMed] [Google Scholar]
- 86.Stamatoyannopoulos G. Control of globin gene expression during development and erythroid differentiation. Exp Hematol. 2005;33(3):259–271. doi: 10.1016/j.exphem.2004.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chang K-H, Nelson AM, Cao H, et al. Definitive-like erythroid cells derived from human embryonic stem cells coexpress high levels of embryonic and fetal globins with little or no adult globin. Blood. 2006;108(5):1515–1523. doi: 10.1182/blood-2005-11-011874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Woll PS, Grzywacz B, Tian X, et al. Human embryonic stem cells differentiate into a homogeneous population of natural killer cells with highly potent in vivo anti-tumor activity. Blood. 2009;113(24):6094–6101. doi: 10.1182/blood-2008-06-165225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Grzywacz B, Kataria N, Sikora M, et al. Coordinated acquisition of inhibitory and activating receptors and functional properties by developing human natural killer cells. Blood. 2006;108(12):3824–3833. doi: 10.1182/blood-2006-04-020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Freud AG, Yokohama A, Becknell B, et al. Evidence for discrete stages of human natural killer cell differentiation in vivo. J Exp Med. 2006;203(4):1033–1043. doi: 10.1084/jem.20052507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fraser CC, Kaneshima H, Hansteen G, Kilpatrick M, Hoffman R, Chen BP. Human allogeneic stem cell maintenance and differentiation in a long-term multilineage SCID-hu graft. Blood. 1995;86(5):1680–1693. [PubMed] [Google Scholar]
- 92.Martin CH, Woll PS, Ni Z, Zuniga-Pflucker JC, Kaufman DS. Differences in lymphocyte developmental potential between human embryonic stem cell and umbilical cord blood-derived hematopoietic progenitor cells. Blood. 2008;112(7):2730–2737. doi: 10.1182/blood-2008-01-133801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.de Pooter RF, Cho SK, Carlyle JR, Zuniga-Pflucker JC. In vitro generation of T lymphocytes from embryonic stem cell-derived prehematopoietic progenitors. Blood. 2003;102(5):1649–1653. doi: 10.1182/blood-2003-01-0224. [DOI] [PubMed] [Google Scholar]
- 94.Schmitt TM, de Pooter RF, Gronski MA, Cho SK, Ohashi PS, Zuniga-Pflucker JC. Induction of T cell development and establishment of T cell competence from embryonic stem cells differentiated in vitro. Nat Immunol. 2004;5(4):410–417. doi: 10.1038/ni1055. [DOI] [PubMed] [Google Scholar]
- 95.La Motte-Mohs RN, Herer E, Zuniga-Pflucker JC. Induction of T-cell development from human cord blood hematopoietic stem cells by Delta-like 1 in vitro. Blood. 2005;105(4):1431–1439. doi: 10.1182/blood-2004-04-1293. [DOI] [PubMed] [Google Scholar]
- 96.Yokota Y. Id and development. Oncogene. 2001;20(58):8290–8298. doi: 10.1038/sj.onc.1205090. [DOI] [PubMed] [Google Scholar]
- 97.Ikawa T, Fujimoto S, Kawamoto H, Katsura Y, Yokota Y. Commitment to natural killer cells requires the helix-loop-helix inhibitor Id2. Proc Natl Acad Sci U S A. 2001;98(9):5164–5169. doi: 10.1073/pnas.091537598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yokota Y, Mansouri A, Mori S, et al. Development of peripheral lymphoid organs and natural killer cells depends on the helix-loop-helix inhibitor Id2. Nature. 1999;397(6721):702–706. doi: 10.1038/17812. [DOI] [PubMed] [Google Scholar]
- 99.Heemskerk MH, Blom B, Nolan G, et al. Inhibition of T cell and promotion of natural killer cell development by the dominant negative helix loop helix factor Id3. J Exp Med. 1997;186(9):1597–1602. doi: 10.1084/jem.186.9.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Murre C. Helix-loop-helix proteins and lymphocyte development. Nat Immunol. 2005;6(11):1079–1086. doi: 10.1038/ni1260. [DOI] [PubMed] [Google Scholar]
- 101.Timmermans F, Velghe I, Vanwalleghem L, et al. Generation of T cells from human embryonic stem cell-derived hematopoietic zones. J Immunol. 2009;182(11):6879–6888. doi: 10.4049/jimmunol.0803670. [DOI] [PubMed] [Google Scholar]
- 102.Anderson JS, Bandi S, Kaufman DS, Akkina R. Derivation of normal macrophages from human embryonic stem (hES) cells for applications in HIV gene therapy. Retrovirology. 2006;3:24. doi: 10.1186/1742-4690-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Saeki K, Saeki K, Nakahara M, et al. A feeder-free and efficient production of functional neutrophils from human embryonic stem cells. Stem Cells. 2009;27(1):59–67. doi: 10.1634/stemcells.2007-0980. [DOI] [PubMed] [Google Scholar]
- 104.Yokoyama Y, Suzuki T, Sakata-Yanagimoto M, et al. Derivation of functional mature neutrophils from human embryonic stem cells. Blood. 2009;113(26):6584–6592. doi: 10.1182/blood-2008-06-160838. [DOI] [PubMed] [Google Scholar]
- 105.Raya A, Rodriguez-Piza I, Guenechea G, et al. Disease-corrected haematopoietic progenitors from Fanconi anaemia induced pluripotent stem cells. Nature. 2009;460(7251):53–59. doi: 10.1038/nature08129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kaufman DS, Thomson JA. Human ES cells–haematopoiesis and transplantation strategies. J Anat. 2002;200(Pt 3):243–248. doi: 10.1046/j.1469-7580.2002.00028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bradley JA, Bolton EM, Pedersen RA. Stem cell medicine encounters the immune system. Nat Rev Immunol. 2002;2(11):859–871. doi: 10.1038/nri934. [DOI] [PubMed] [Google Scholar]
- 108.Adewumi O, Aflatoonian B, Ahrlund-Richter L, et al. Characterization of human embryonic stem cell lines by the International Stem Cell Initiative. Nat Biotechnol. 2007;25(7):803–816. doi: 10.1038/nbt1318. [DOI] [PubMed] [Google Scholar]
- 109.Osafune K, Caron L, Borowiak M, et al. Marked differences in differentiation propensity among human embryonic stem cell lines. Nat Biotechnol. 2008;26(3):313–315. doi: 10.1038/nbt1383. [DOI] [PubMed] [Google Scholar]
- 110.Pekkanen-Mattila M, Kerkela E, Tanskanen JM, et al. Substantial variation in the cardiac differentiation of human embryonic stem cell lines derived and propagated under the same conditions–a comparison of multiple cell lines. Ann Med. 2009;41(5):360–370. doi: 10.1080/07853890802609542. [DOI] [PubMed] [Google Scholar]
- 111.Keirstead HS, Nistor G, Bernal G, et al. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci. 2005;25(19):4694–4705. doi: 10.1523/JNEUROSCI.0311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kroon E, Martinson LA, Kadoya K, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26(4):443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- 113.He JQ, Ma Y, Lee Y, Thomson JA, Kamp TJ. Human embryonic stem cells develop into multiple types of cardiac myocytes: action potential characterization. Circ Res. 2003;93(1):32–39. doi: 10.1161/01.RES.0000080317.92718.99. [DOI] [PubMed] [Google Scholar]
- 114.Roy NS, Cleren C, Singh SK, Yang L, Beal MF, Goldman SA. Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nat Med. 2006;12(11):1259–1268. doi: 10.1038/nm1495. [DOI] [PubMed] [Google Scholar]
- 115.Tolar J, Nauta AJ, Osborn MJ, et al. Sarcoma derived from cultured mesenchymal stem cells. Stem Cells. 2007;25(2):371–379. doi: 10.1634/stemcells.2005-0620. [DOI] [PubMed] [Google Scholar]
- 116.Flynn CM, Kaufman DS. Donor cell leukemia: insight into cancer stem cells and the stem cell niche. Blood. 2007;109(7):2688–2692. doi: 10.1182/blood-2006-07-021980. [DOI] [PubMed] [Google Scholar]
- 117.Swijnenburg R-J, Schrepfer S, Govaert JA, et al. Immunosuppressive therapy mitigates immunological rejection of human embryonic stem cell xenografts. Proc Natl Acad Sci U S A. 2008;105(35):12991–12996. doi: 10.1073/pnas.0805802105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Unger C, Skottman H, Blomberg P, Dilber MS, Hovatta O. Good manufacturing practice and clinical-grade human embryonic stem cell lines. Hum Mol Genet. 2008;17(R1):R48–R53. doi: 10.1093/hmg/ddn079. [DOI] [PubMed] [Google Scholar]
- 119.Hyun I, Lindvall O, Ahrlund-Richter L, et al. New ISSCR guidelines underscore major principles for responsible translational stem cell research. Cell Stem Cell. 2008;3(6):607–609. doi: 10.1016/j.stem.2008.11.009. [DOI] [PubMed] [Google Scholar]