Abstract
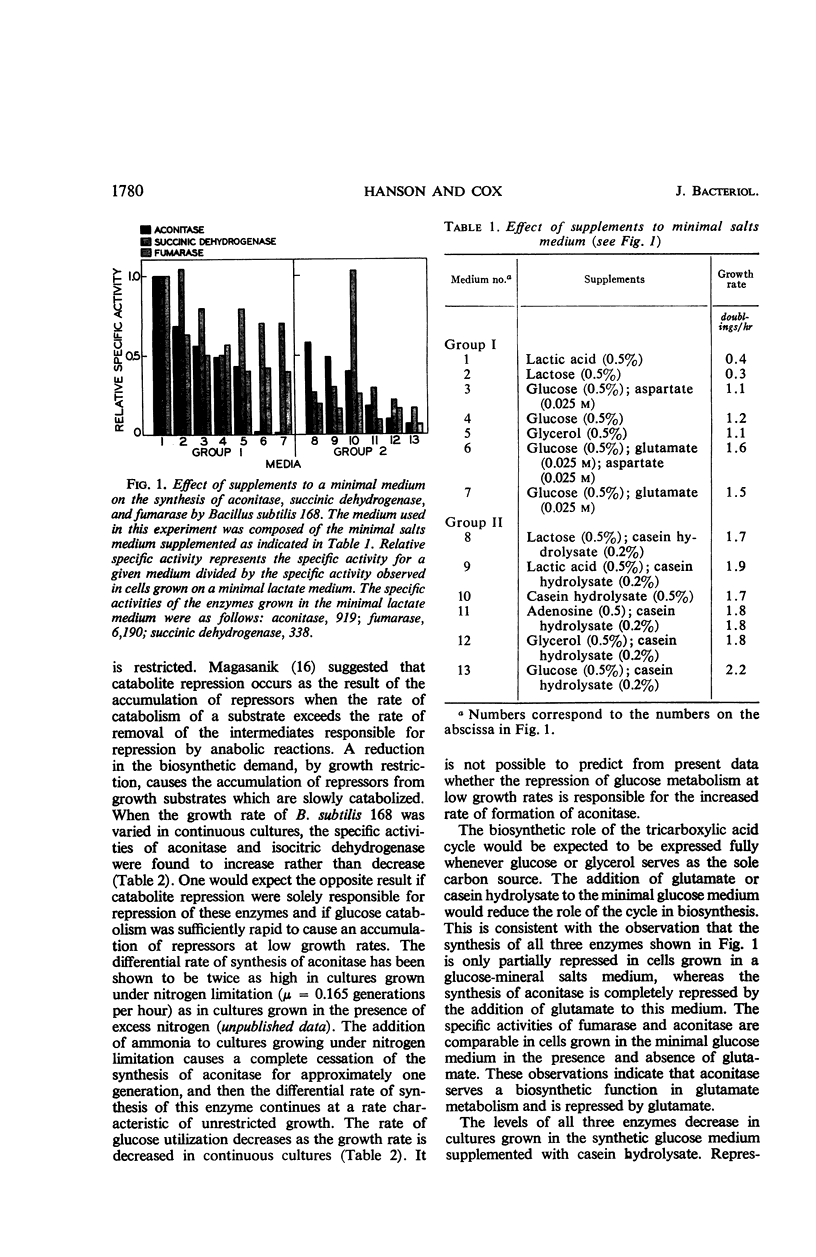
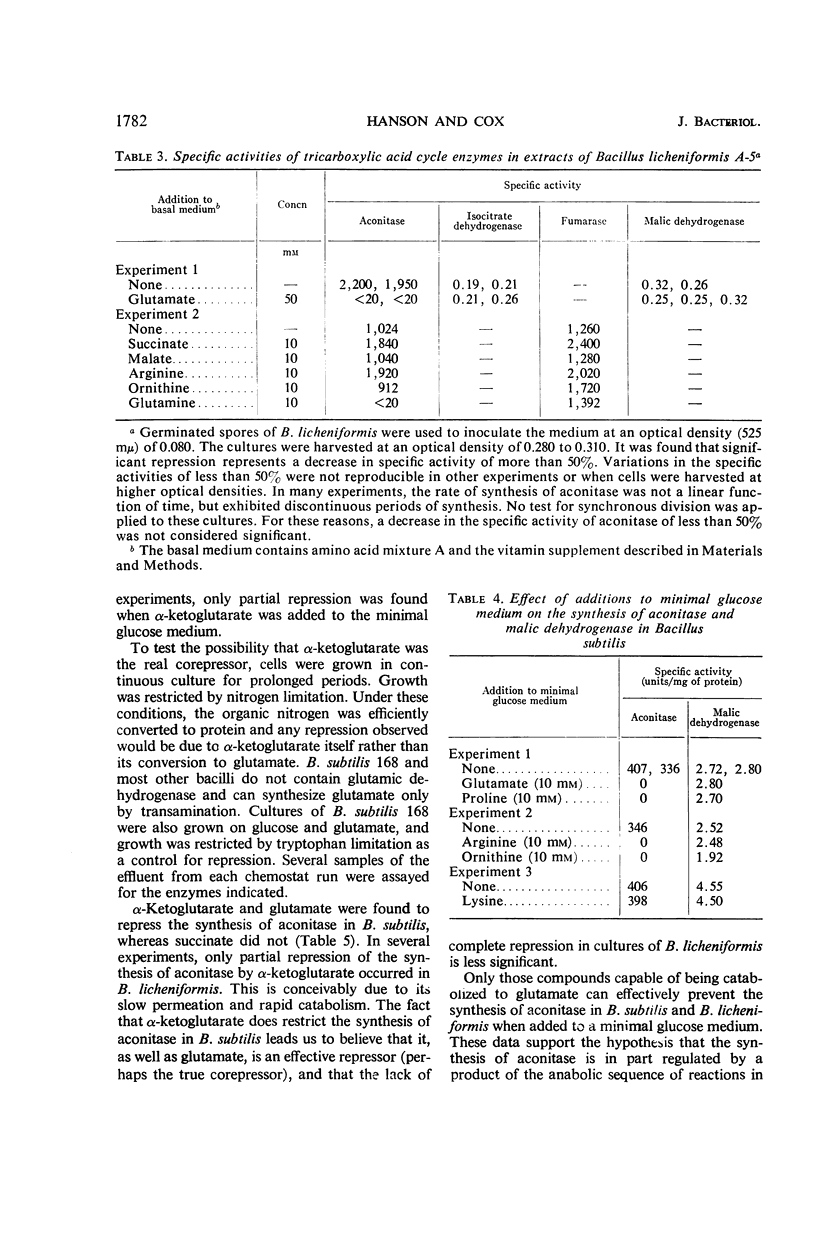
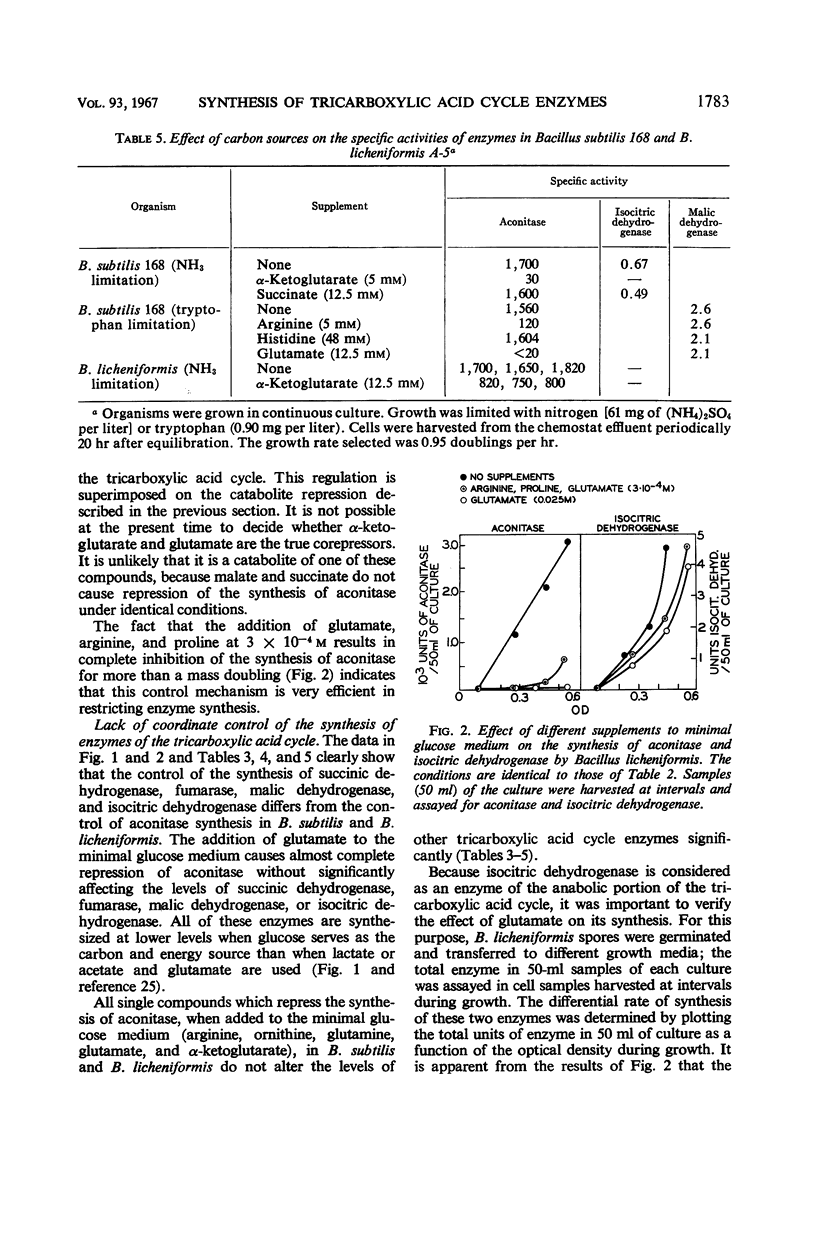
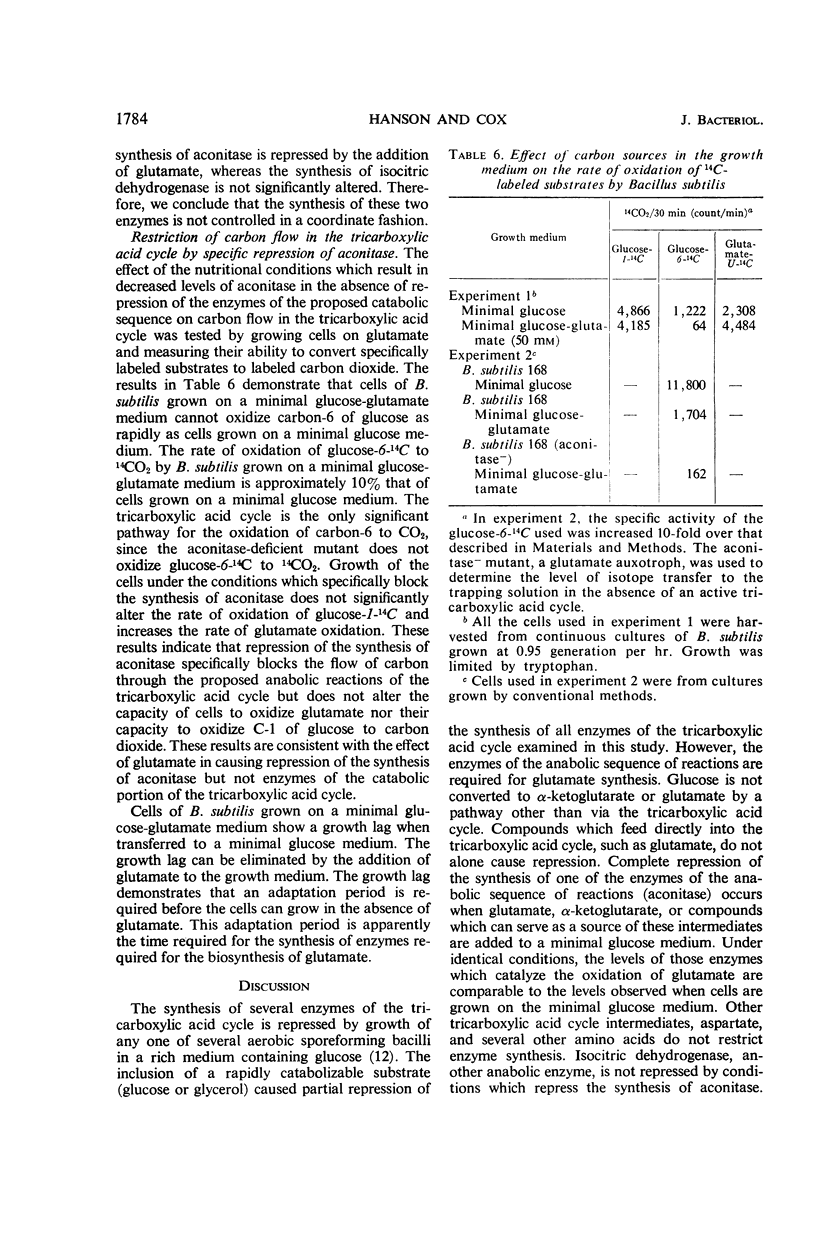
The effect of various nutritional conditions on the levels of Krebs cycle enzymes in Bacillus subtilis, B. licheniformis, and Escherichia coli was determined. The addition of glutamate, α-ketoglutarate, or compounds capable of being catabolized to glutamate, to a minimal glucose medium resulted in complete repression of aconitase in B. subtilis and B. licheniformis. The synthesis of fumarase, succinic dehydrogenase, malic dehydrogenase, and isocitric dehydrogenase was not repressed by these compounds. It is postulated that glutamate or α-ketoglutarate is the true corepressor for the repression of aconitase. A rapidly catabolizable carbon source and α-ketoglutarate or glutamate must be simultaneously present for complete repression of the formation of aconitase. Conditions which repress the synthesis of aconitase in B. subtilis restrict the flow of carbon in the sequence of reactions leading to α-ketoglutarate but do not prevent glutamate oxidation in vivo. The data indicate that separate and independent mechanisms regulate the activity of the anabolic and catabolic reactions of the Krebs cycle in B. subtilis and B. licheniformis. The addition of glutamate to the minimal glucose medium results in the repression of aconitase, isocitric dehydrogenase, and fumarase, but not malic dehydrogenase in E. coli K-38.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amarasingham C. R., Davis B. D. Regulation of alpha-ketoglutarate dehydrogenase formation in Escherichia coli. J Biol Chem. 1965 Sep;240(9):3664–3668. [PubMed] [Google Scholar]
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERNLOHR R. W., NOVELLI G. D. BACITRACIN BIOSYNTHESIS AND SPORE FORMATION: THE PHYSIOLOGICAL ROLE OF AN ANTIBIOTIC. Arch Biochem Biophys. 1963 Oct;103:94–104. doi: 10.1016/0003-9861(63)90014-6. [DOI] [PubMed] [Google Scholar]
- COLLINS F. M., LASCELLES J. The effect of growth conditions on oxidative and dehydrogenase activity in Staphylococcus aureus. J Gen Microbiol. 1962 Nov;29:531–535. doi: 10.1099/00221287-29-3-531. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D. The teleonomic significance of biosynthetic control mechanisms. Cold Spring Harb Symp Quant Biol. 1961;26:1–10. doi: 10.1101/sqb.1961.026.01.005. [DOI] [PubMed] [Google Scholar]
- ENGLESBERG E., GIBOR A., LEVY J. B. Adaptive control of terminal respiration in Pasteurella pestis. J Bacteriol. 1954 Aug;68(2):146–151. doi: 10.1128/jb.68.2.146-151.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREUNDLICH M., BURNS R. O., UMBARGER H. E. Control of isoleucine, valine, and leucine biosynthesis. I. Multivalent repression. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1804–1808. doi: 10.1073/pnas.48.10.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALPERN Y. S., UMBARGER H. E. Conversion of ammonia to amino groups in Escherichia coli. J Bacteriol. 1960 Sep;80:285–288. doi: 10.1128/jb.80.3.285-288.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANSON R. S., SRINIVASAN V. R., HALVORSON H. O. BIOCHEMISTRY OF SPORULATION. II. ENZYMATIC CHANGES DURING SPORULATION OF BACILLUS CEREUS. J Bacteriol. 1963 Jul;86:45–50. doi: 10.1128/jb.86.1.45-50.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANSON R. S., SRINIVASAN V. R., HALVORSON H. O. Biochemistry of sporulation. I. Metabolism of acetate by vegetative and sporulating cells. J Bacteriol. 1963 Feb;85:451–460. doi: 10.1128/jb.85.2.451-460.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laishley E. J., Bernlohr R. W. Catabolite repression of "three sporulation enzymes" during growth of Bacillus licheniformis. Biochem Biophys Res Commun. 1966 Jul 6;24(1):85–90. doi: 10.1016/0006-291x(66)90414-1. [DOI] [PubMed] [Google Scholar]
- MAGASANIK B. Catabolite repression. Cold Spring Harb Symp Quant Biol. 1961;26:249–256. doi: 10.1101/sqb.1961.026.01.031. [DOI] [PubMed] [Google Scholar]
- MANDELSTAM J. Induction and repression of beta-galactosidase in non-growing Escherichia coli. Biochem J. 1961 Jun;79:489–496. doi: 10.1042/bj0790489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCFALL E. Effects of 32P decay on enzyme synthesis. J Mol Biol. 1961 Apr;3:219–224. doi: 10.1016/s0022-2836(61)80048-x. [DOI] [PubMed] [Google Scholar]
- NAKATA H. M., HALVORSON H. O. Biochemical changes occurring during growth and sporulation of Bacillus cereus. J Bacteriol. 1960 Dec;80:801–810. doi: 10.1128/jb.80.6.801-810.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEIDHARDT F. C., MAGASANIK B. Reversal of the glucose inhibition of histidase biosynthesis in Aerobacter aerogenes. J Bacteriol. 1957 Feb;73(2):253–259. doi: 10.1128/jb.73.2.253-259.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMOS F., WIAME J. M., WYNANTS J., BECHET J. Effect of mutant of Bacillus subtilis on the specific transport of aconitase and dicarboxylic acid. Nature. 1962 Jan 6;193:70–71. doi: 10.1038/193070a0. [DOI] [PubMed] [Google Scholar]
- STRASTERS K. C., WINKLER K. C. CARBOHYDRATE METABOLISM OF STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1963 Nov;33:213–229. doi: 10.1099/00221287-33-2-213. [DOI] [PubMed] [Google Scholar]
- Schaeffer P., Millet J., Aubert J. P. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci U S A. 1965 Sep;54(3):704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZINDER N. D., COOPER S. HOST-DEPENDENT MUTANTS OF THE BACTERIOPHAGE F2. I. ISOLATION AND PRELIMINARY CLASSIFICATION. Virology. 1964 Jun;23:152–158. doi: 10.1016/0042-6822(64)90277-6. [DOI] [PubMed] [Google Scholar]


