Abstract
Gβ subunits from heterotrimeric G-proteins directly bind diverse proteins, including effectors and regulators, to modulate a wide array of signaling cascades. These numerous interactions constrained the evolution of the molecular surface of Gβ. Though mammals contain five Gβ genes comprising two classes (Gβ1-like and Gβ5-like), plants and fungi have a single ortholog and organisms such as Caenorhabditis elegans and Drosophila melanogaster contain one copy from each class. A limited number of crystal structures of complexes containing Gβ subunits and complementary biochemical data highlight specific sites within Gβs needed for protein interactions. It is difficult to determine from these interaction sites what, if any, additional regions of the Gβ molecular surface comprise interaction interfaces essential to Gβ's role as a nexus in numerous signaling cascades. We used a comparative evolutionary approach to identify five known and eight previously-unknown putative interfaces on the surface of Gβ. We show that one such novel interface occurs between Gβ and phospholipase C β2 (PLC-β2), a mammalian Gβ interacting protein. Substitutions of residues within this Gβ-PLC-β2 interface reduce the activation of PLC-β2 by Gβ1, confirming that our de novo comparative evolutionary approach predicts previously unknown Gβ-protein interfaces. Similarly, we hypothesize the seven remaining untested novel regions contribute to putative interfaces for other Gβ interacting proteins. Finally, this comparative evolutionary approach is suitable for application to any protein involved in a significant number of protein-protein interactions.
Keywords: Heterotrimeric G proteins, Phospholipase C-β2, Protein surface evolution, Interface prediction, PLC-β2 – Gβγ interaction interface
Introduction
Gene duplication is a fundamental source of genetic and phenotypic novelty 1. After duplication, one of the new paralogs is freed from functional constraint, enabling it to evolve new functions. For genes encoding proteins that interact with other proteins, this process often liberates one copy to develop a new set of interactions. This is particularly true for signaling molecules that are used by organisms to communicate between cells and to perceive their environment. For example, evolution of increasingly complex organisms correlates with the enormous diversification of heterotrimeric guanine nucleotide-binding protein (G-protein) signaling complexes and cell surface G-protein coupled receptors. The G-protein complex consists of a heterotrimer comprised of Gα, Gβ, and Gγ subunits. Upon activation by cell surface receptors, the complex dissociates into a free Gα subunit and a Gβγ dimer, both of which bind to and signal through other proteins. Signaling typically terminates when the heterotrimer reforms 2; 3. Saccharomyces cerevisiae have two Gα but a single Gβ and a single Gγ gene; mammalian genomes encode sixteen Gα, five Gβ, and twelve Gγ genes 2. This complex array of subunit combinations allows for diverse signaling possibilities. It was previously thought that Gα was the primary signaling molecule in mammals while the sole function of Gβ was to inhibit Gα signaling and to provide for its membrane localization 4 It is now clear that Gβ also modulates downstream targets; a subset of signaling pathways is uniquely regulated by the Gβ subunit 5.
Several proteins that bind Gβ have been identified in mammals. In addition to Gα and Gγ, interacting proteins such as the localization chaperone phosducin, G-protein-coupled receptor kinases (GRKs), phospholipase C β2 (PLC-β2), regulator of G-protein signaling 9 (RGS9), calcium channels, potassium channels, and adenylyl cyclase 2 (AC2) bind Gβ proteins. Mammalian Gβ interacting proteins arose differentially over evolutionary time as not all eukaryotes contain homologs to all known interactors 2. For example, Gα and phosducin are present in all eukaryotes beginning with plants. Canonical RGS9, PLC-β2, and GRK2 originated and were maintained in metazoans at least by the time of the formation of annelids, since Caenorhabditis elegans contains bona fide RGS9, PLC-β2, and GRK2 orthologs 6; 7; 8 As the Gβ subunit acquired additional binding partners throughout evolution, new Gβ-protein interfaces likely evolved to accommodate these interactions. These new interfaces may have partially overlapped with existing interfaces since, for example, the interface from many Gβ interactors overlaps with the Gα-Gβ interface 9. However, these interfaces may have also utilized regions on the Gβ molecular surface that previously had no associated function.
Two major experimental approaches, structural and biochemical studies, have characterized some binding interfaces between Gβ and interacting proteins. Currently, there are four crystal structures of mammalian Gβ subunits in complex with signaling proteins: Gβ1γ1-Gα 10, Gβ1γ2-GRK2 11, Gβ1γ1-phosducin 12, and Gβ5-RGS9 13. These structures provide a three-dimensional view of where proteins interact, but provide limited information regarding the importance of individual contacts. Additionally, structures of Gβ in complex with peptides 14; 15 provide partial information on physiologically relevant interfaces within Gβ. Biochemical studies include both targeted mutational studies 9; 16; 17 and targeted domain-swapping or peptide binding experiments 18; 19; 20. Mutational studies directed by the structural studies are limited by the number of available structures. Interpretation of these studies is complicated by overlap between the implicated binding regions. When crystal structures of a particular Gβ complex are not available, mutations are frequently targeted to an interface identified in a solved Gβ complex structure. For example, Ford et al. 9 created several mutations in the Gα binding interface to show that this interface was utilized in part by five other interacting proteins. This type of approach does not, however, elucidate binding sites, or even those regions of the binding interface, that are not shared with the Gα interface. To locate these alternative sites, groups such as Panchenko et al. 17 performed mutational analyses outside of known binding areas. Although this study identified mutant regions of Gβ with reduced ability to activate PLC-β2, much refinement remains necessary to identify individual critical interaction sites. Moreover, this study left many regions of the Gβ surface unexplored.
Bioinformatic analyses have been developed to identify functional residues including those composing conserved patches on the surface of a protein that may function as a binding interface. Three such analyses include Evolutionary Trace (ET) 21, DIVERGE 22; 23, and ConSurf 24. All three rely on multiple sequence alignments (MSAs) and phylogenetic trees to identify structurally-clustered functional residues. ET has been applied to Gβ and the two predicted interfaces correlated with those of Gα and Gγ25. To date, none of these three methods have predicted previously uncharacterized Gβ interfaces.
We took advantage of both the rich source of divergent Gβ subunit sequences and the differential evolutionary emergences of known Gβ interactors to understand not only how to predict novel interaction interfaces on the Gβ surface in the absence of crystallized complexes, but also how these new interactions gave rise to new binding surfaces. These results can be utilized for more targeted and informed biochemical studies. Based on the hypothesis that the acquisition of mammalian-like sequence identity on the surface of Gβ reflects the utilization of new binding interfaces, we applied a suite of bioinformatic and phylogenetic techniques to follow the shift in patterns of amino acid conservation in Gβs, concentrating on changes between distinct points in the evolutionary history of the Gβ subunit represented by five reference species. By placing this conservation in a structural context, we predicted regions of interest (ROI) that are comprised of adjacent surface residues that simultaneously evolved to residues conserved with mammals. We identified a novel PLC-β2 interface by demonstrating that at least one ROI which became conserved in C. elegans is involved in PLC-β2 activation by Gβ. Similarly, we propose that the remaining ROIs also compose at least portions of binding interfaces.
Results and Discussion
Gβ Proteins Fall into Two Major Classes: Gβ1-like and Gβ5-like
Since most extant plant and fungal species have a single Gβ, while nematodes and later metazoans have at least two Gβ subunits, the first Gβ gene duplication occurred between the splitting of fungi and C. elegans from the mammalian lineage. To compare pre-duplication plant and fungal Gβ sequences to extant post-duplication Gβ sequences from metazoans, ancestral sequences to plants and to fungi were reconstructed from MSAs (Figs. S1 and S2, respectively) of extant plant and fungal Gβ sequences using the Bayesian ancestral reconstruction implemented in MrBayes (see Methods). These inferred pre-duplication ancestors were important as they, and not individual plant or fungal species, reflect sequence constraints common to all plants or fungi and, therefore, more closely reflect the predecessor of all extant post-duplication Gβ genes.
An MSA was created containing Gβ sequences from the plant ancestor, fungal ancestor, and extant C. elegans, Drosophila melanogaster, and human Gβ sequences (Fig. S3) for comparison of pre- and post-duplication Gβ genes. This MSA was used to generate a Bayesian phylogenetic tree (Fig. S4) that elucidates the evolutionary relationship between the different Gβ proteins. Human Gβ1, Gβ2, Gβ3, and Gβ4 sequences formed a monophyletic Gβ1-like clade also containing D. melanogaster and C. elegans Gβ1 sequences. Human Gβ5 formed a Gβ5-like monophyletic clade containing D. melanogaster Gβ5 and C. elegans sequences Gβ2. Both plant and fungal ancestors were outside of these two clades.
Based on the phylogenetic data (Fig. S4) and corroborating previous observations that Gβ5 often behaves differently than the other four mammalian Gβ proteins 5, metazoan Gβ proteins can be grouped into two major classes, Gβ1-like and Gβ5-like. The initial Gβ gene duplication between fungi and C. elegans gave rise to the Gβ5-like family since C. elegans is the earliest species examined to contain a Gβ5-like protein. The ancestral plant and fungal Gβ proteins each contain characteristics of both major classes, but neither strictly belongs to either class. Finally, the phylogeny revealed that after duplication, the Gβ5-like genes diverged from the ancestor more than the Gβ1-like genes while the Gβ1-like genes maintained more ancestral characteristics.
Interaction Interfaces Identified from Gβ Complex Structures Co-evolved with Interactors
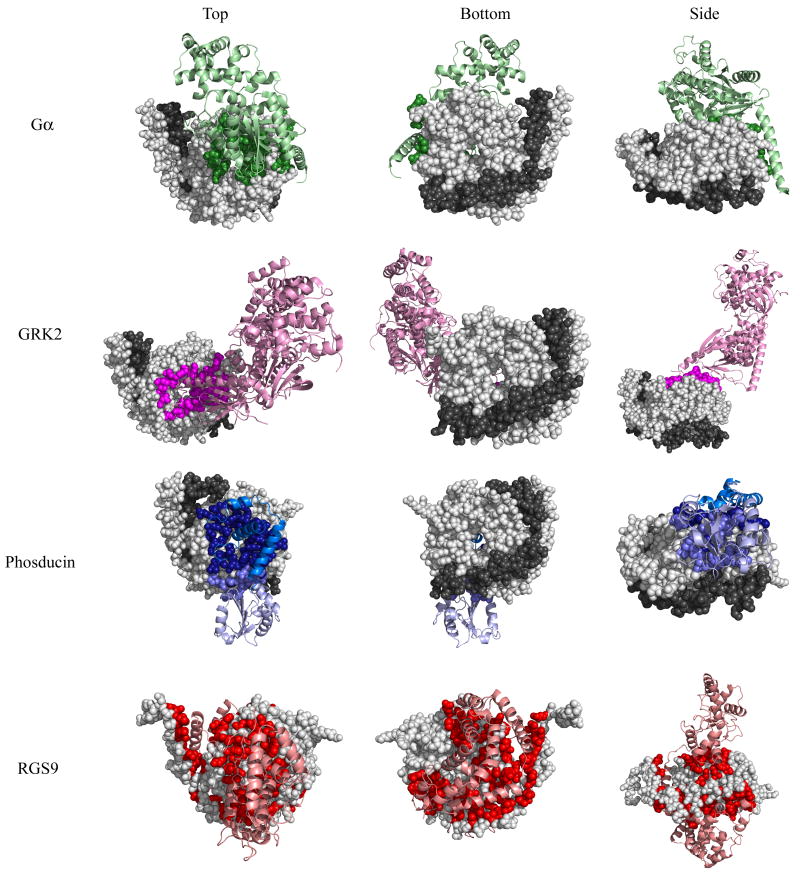
Complexes containing mammalian Gβ1-Gα, Gβ1-GRK2, Gβ1-phosducin, and Gβ5-RGS9 are shown in Fig. 1 (Gβ in spheres, interacting protein in ribbons). While these structures provide a three-dimensional view of Gβ-protein complexes, the interfaces between the proteins must still be defined 26. To define sites of interaction, we calculated the solvent accessibility of each residue in the four crystal structures using Naccess 27. Those Gβ residues whose side chains have lower relative solvent accessibility by more than five percent between the monomer and the protein complex, including those that hydrogen bond with the binding partner, were defined to comprise the interface and were noted on the structure (see Methods) (Fig. 1). These four Gβ-interacting proteins: 1) cover three major areas on the Gβ surface, 2) all partially overlap with each other, and 3) appeared at different times over Gβ evolution.
Fig 1. Binding interfaces of four Gβ-interacting proteins as determined by crystal structures.
Top, bottom, and side views of Gβ or the Gβγ dimer (Gβ in light gray spheres, Gγ in dark gray spheres) bound to four different interacting proteins (ribbon): Gβ1-Gα in green (1got.pdb), Gβ1-GRK2 in magenta (1omw.pdb), Gβ1-phosducin in blue (N-terminal domain is dark, C-terminal domain is light, 1a0r.pdb), and Gβ5-RGS9 in red (2pbi.pdb). Gβ residues that contact each interacting protein are colored accordingly. Binding contacts were determined by evaluating the solvent accessibility difference between single molecules and those in complex.
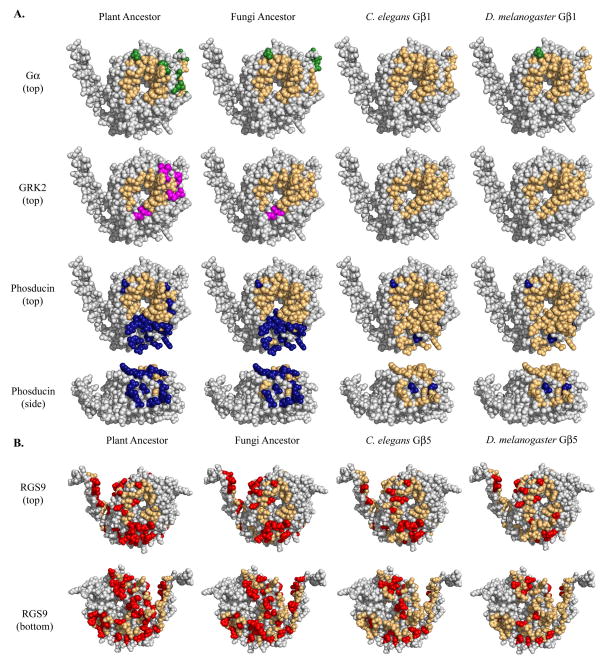
In order to examine the evolution of these known Gβ-protein interfaces, we compared the patterns of amino acid conservation within these regions. For each interface residue in the MSA, comparisons were made between each of the four reference sequences and the corresponding mammalian residue. For Gβ1 complexes (Gα, GRK2, and phosducin), the plant ancestor, fungal ancestor, C. elegans Gβ1, and D. melanogaster Gβ1 were compared to bovine Gβ1. Identical interface residues were mapped onto the bovine Gβ1 structure (1got.pdb). For the RGS9 complex, the plant ancestor, fungal ancestor, C. elegans Gβ5 and D. melanogaster Gβ5 were compared to mouse Gβ5 and identities were noted on the mouse Gβ5 structure (2pbi.pdb). As expected, conservation within known binding areas correlated with the known utilization of these regions (Fig. 2). The following was observed in the four crystal structure complexes:
Fig. 2. Conservation within known binding interfaces based on bovine Gβ1 crystal structures.
Conservation of the (A) Gα, GRK2, and phosducin binding interfaces on Gβ1 (1got.pdb) and the (B) RGS9 binding interface on Gβ5 (2pbi.pdb) in four reference organisms. While the interface is comprised of all colored residues, conserved residues are colored light orange while non-conserved residues are colored green (Gα), blue (phosducin), magenta (GRK2), or red (RGS9). Only residues in the binding area were analyzed.
Gβ1-Gα
The Gβ-Gα interaction is the most ancient, thus it would be expected that the Gα-Gβ interface is well-conserved in all organisms studied. As expected, the Gα binding interface was highly conserved (77% -100% identity) in all sequences analyzed (Fig. 2a, top row) as illustrated by the predominantly orange coloration of the interface.
Gβ1-GRK2
The GRK2 interface (Fig. 2a, second row) was completely conserved in C. elegans and D. melanogaster, correlating with the emergence of GRK2 between fungi and C. elegans. Though a large portion of the interface was also conserved in the plant (62%) and fungal (92%) ancestors, this can be attributed to the fact that the interface largely overlaps that of Gα.
Gβ1-phosducin
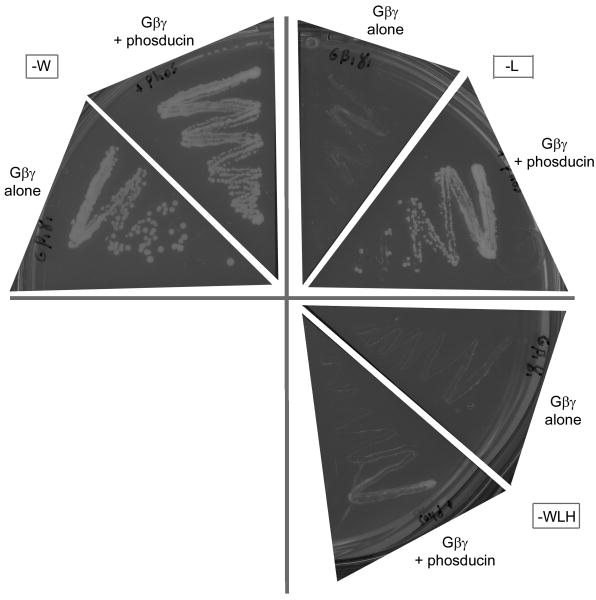
Plants contain several phosducin-like sequences 28 and our yeast-three-hybrid data suggest that at least one phosducin in Arabidopsis thaliana interacts with the A. thaliana Gβ (Fig. 3). The phosducin interface is highly conserved in C. elegans and D. melanogaster (94% identity); however, it is only partly conserved in plant and fungal ancestors (55% and 64%, respectively). Mammalian phosducin has two domains: a helical N-terminal domain and a C-terminal domain (see Fig. 1 for locations of N- and C-terminal binding interfaces), both of which bind mammalian Gβ 29. The plant and fungal ancestors show high conservation in the N-terminal binding area (68% and 76%, respectively) but not in the C-terminal binding area (25% and 38%, respectively) (Fig. 2a, third and fourth rows). Two possible explanations for these results are that plants and fungi bind only one phosducin domain but not the second or that the second domain binds in a species-specific manner.
Fig. 3. An Arabidopsis phosducin and Gβ interact physically.
Growth of yeast strain AH109 containing the genes indicated (AtGβ1γ1 alone or AtGβ1γ1 and phosducin) on yeast dropout media. Media missing tryptophan (-W) selects for the AtGβ1γ1 vector, resulting in positive growth for both genotypes. Media missing leucine (-L) selects for the phosducin vector, resulting in no growth for the strain lacking phosducin and positive growth for the strain containing phosducin. Media missing leucine, tryptophan, and histidine (-LWH) selects for a positive interaction between the two genes, resulting in no growth for the strain containing AtGβ1γ1 alone and positive growth for the strain containing both AtGβ1γ1 and phosducin. The latter growth indicates that the two genes physically interact.
Gβ5-RGS9
The RGS9 binding interface (Fig. 2b) is only 42% identical in the plant ancestor and 50% in the fungal ancestor. Correlating with the genesis of the RGS9 protein, conservation in the binding area rises to 67% and 76% in C. elegans and D. melanogaster, respectively. Though this is markedly less than the 90% to 100% identity seen in corresponding Gβ1 binding areas, it is important to note the size of the RGS9 interface. Encompassing 103 residues, the RGS9 interface is over three times larger than the Gα (31 residues) and GRK2 (26 residues) interfaces and twice the size of the phosducin interface (47 residues). Thus, we expect the RGS9 interface could tolerate more substitutions than its smaller counterparts and that only those residues making energetically critical contacts in the interaction interface are conserved.
Newly Conserved Regions of Adjacent Surface Residues Arose Over Time and Likely Contribute to Gβ-protein Interfaces
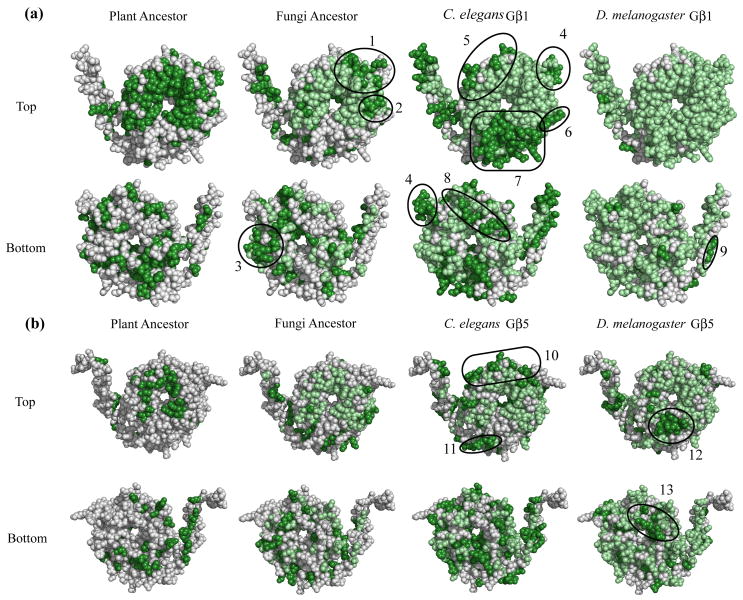
As conservation within known Gβ-protein interfaces correlated with the emergence of proteins utilizing these interfaces, we next used a similar method of analysis to predict novel binding interfaces. This unbiased comparative evolutionary analysis was not restricted to predetermined binding interfaces and was performed on the entire molecule, but was otherwise similar to the methods used in the validation stage described in the previous section. Bovine Gβ1 was compared at each residue in the MSA to the plant ancestor, fungal ancestor, C. elegans Gβ1, and D. melanogaster Gβ1 (Fig. 4a). Identical residues between the plant ancestor and bovine Gβ1 were located on the surface of Gβ1 and colored dark green. These 162 residues form region zero (ROI0) and represent the primordial function of the Gβ molecule, since the plant molecule is the most ancestral-like of the Gβ family. For all other reference species, conserved residues that continued to be conserved from the previous species were colored light green (indicating the persistence of an existing function) while newly conserved residues were colored dark green (indicating the emergence of potential novel function). Similarly, mouse Gβ5 was compared to the plant ancestor, fungal ancestor, C. elegans Gβ5, and D. melanogaster Gβ5 and conserved residues were mapped onto the structure (Fig. 4b).
Fig. 4. Gβ regions of interest as determined by a comparative evolutionary analysis.
Three views (top, bottom, side) of the evolution of conserved regions in the plant ancestor, fungal ancestor, C. elegans Gβ, and D. melanogaster Gβ (a, Gβ1 [1got.pdb] and b, Gβ5 [2pbi.pdb]). The dark green regions of the plant ancestor highlight primordial function and form region of interest (ROI) 0. In all other organisms, newly conserved residues (those that matched the mammalian value) were colored dark green, while conserved residues present in a previous organism were colored light green. Thus, dark green patches represent acquisition of new function. ROIs are indicated and corresponding residues are listed in Table 1.
Due to its primordial nature, ROI0 contains residues conserved both for structural maintenance as well as for interaction surfaces. ROI0 contains the residues – e.g. W99, M101 K57, Y59, L117, D186, D228, and W332 – previously indicated as interacting with multiple effectors and which also made energetically critical contacts in various interaction interfaces9; 14. Subsequent non-ancestral ROIs in each organism (for both Gβ1 and Gβ5) were defined as clusters of three or more structurally adjacent (within 5 Å30) newly conserved surface residues and are encircled on the structure (Fig. 4, for residue designations see Table 1). It is important to note that though all surface-exposed residues could form a part of an interface, we chose a larger cluster size (containing three or more newly-fixed residues) in order to predict clusters that likely make significant energetic contributions to binding. We also ignored several large clusters near the Gγ binding area due to their likely involvement in the Gβ-Gγ interface.
Table 1. ROI residue numbers.
Mammalian residue positions for each Gβ residue within the regions of interest highlighted in Fig. 4.
| ROI# | Residues | First conserved in |
|---|---|---|
| 1 | R96, S97, S98, I120, R134, E138, E172 | Fungi Gβ1 |
| 2 | L55, A56, S334 | Fungi Gβ1 |
| 3 | D66, R68, Y85, N88, V90, Y105 | Fungi Gβ1 |
| 4 | K127, R129, E130, N132 | C. elegans Gβ1 |
| 5 | T143, T173, Q175, Q176, T181, T184, M217 | C. elegans Gβ1 |
| 6 | R46, T47, R52, D312, F335 | C. elegans Gβ1 |
| 7 | R42, Q44, D267, N268, I269, I270, C271, G272, I273, D290, D291, N293, N295, V307, A309 | C. elegans Gβ1 |
| 8 | L152, D153, N155, D195, R197, L210 | C. elegans Gβ1 |
| 9 | D20, A24 | D. melanogaster Gβ1 |
| 10 | T102, P104, T106, N141, K146, N154 | C. elegans Gβ5 |
| 11 | E43, K279, E280, S281 | C. elegans Gβ5 |
| 12 | F284, N303, Y305 | D. melanogaster Gβ5 |
| 13 | N163, L203, P205, E207 | D. melanogaster Gβ5 |
Five of the thirteen ROIs (7, 10, 11, 12, and 13) could be explained by existing structural data. ROI7 lies in the phosducin binding region on Gβ1 (Fig 1). Portions of ROIs 10-13 localize to the RGS9 binding interface on Gβ5 (Fig 1). Additionally, a portion of ROI2 was shown to interact with Gα, AC2, calcium channels, potassium channels, and PLC-β2 in mutational studies 9. These data support the hypothesis that regions of adjacent surface residues that become conserved to the mammalian state at the same time are likely sites of Gβ-protein interactions. Similarly, we hypothesize that the remaining ROIs are binding interfaces with yet-to-be identified interacting proteins or additional, undescribed binding interfaces with previously identified interacting proteins.
Several ROIs Contain Gβ Residues That Activate PLC-β2
To test our hypothesis that ROIs represent Gβ-protein interaction interfaces, we mutated residues likely to interact with PLC-β2. Despite the absence of a crystal structure of the Gβγ-PLC-β2 complex, several studies implicated multiple potential interfaces. Peptide-binding assays implicated a region at the base of Gβ's N-terminal helix 18; 20. A patch of residues near this region was implicated as an interface when it was revealed that the C-terminal tail of Gγ was responsible for the activation of PLC-β2 19. Therefore, ROIs 4, 6, and 7 were chosen for mutational studies due to their proximity to the general region implicated in these peptide-binding and domain-swapping experiments. Additionally, all three regions became conserved between the fungi and C. elegans split, corresponding with the emergence of PLC-β proteins.
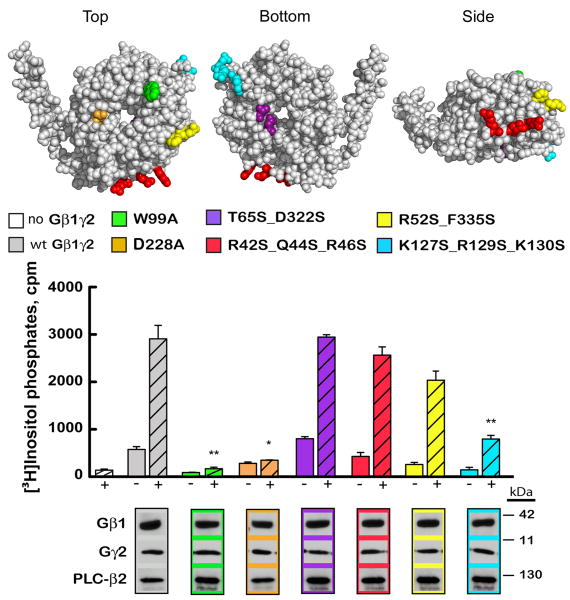
In order to evaluate the extent of PLC-β2 activation, COS-7 cells were transfected with wild-type and mutant forms of Gβ1 and wild-type Gγ2 in the presence or absence of PLC-β2. Equal expression of all Gβ1, Gγ2, and PLC-β2 constructs was confirmed by immunoblot analysis. Additionally, equivalent expression of Gγ2 was observed in the presence of all forms of Gβ1. The location of each mutation on the surface of Gβ1 is depicted in Fig. 5. The construct containing the double mutation T65A_D322A, which is located outside of the predicted binding region and lacks sequence conservation with bovine Gβ1, activates PLC-β2 to the same extent as wild-type Gβγ. Conversely, two single mutations W99A and D228A abolish PLC-β2 activation as previously shown 9. Within the newly predicted binding areas, the mutations that most significantly reduced PLC-β2 activation were found in ROIs 4 and 6 (Figs. 4a and 5). R52S_F335S slightly reduced PLC-β2 activation while K127S_R129S_E130S nearly abolished PLC-β2 activation. In summary, when residues within the new binding areas predicted by our de novo analysis are mutated there is a concordant drop in activity; conversely, when residues we identified as not part of binding surfaces are mutated there is no change in activation. These results indicate that our evolutionary structural analysis predicts the non-overlapping portions of interaction interfaces and can be used to further dissect the PLC-β2 interface on Gβ1.
Fig. 5. Regions of Gβ involved in PLC-β2 activation.
Groups of residues mutated on the Gβ surface [1got.pdb] are colored and coordinate with colors in the bar graph and associated blots. Wild-type and mutant Gβ1 subunits were tested in the absence (-) or presence (+) of PLC-β2 for their ability to activate PLC-β2 (*p<0.01 **p<0.005, error bars represent standard error). Immunoblot analysis confirmed equal expression of all proteins utilized.
Molecular Evolution Can Be Used as a Tool to Predict Novel Binding Interfaces
Conservation is a hallmark of a residue's functional importance 23 and as the plant ancestor is most similar to the most ancient Gβ molecule, primordial Gβ function can be inferred to correlate with those residues which were conserved in the plant ancestor (ROI0) (Fig. 4). These primordial functional regions comprise both conserved protein-binding interfaces as well as regions of the protein important for structural integrity. With each comparative step (e.g. between plants and fungi, fungi and C. elegans, etc.), new functional regions became conserved. These regions may represent sites of novel Gβ-protein interaction or new interaction sites with existing proteins (co-evolution). Lineage-specific protein interactions, however, will not be revealed by this type of analysis. Lineage-specific interactions would be indicated by conservation of a residue within a particular organismal group (e.g. within all plants or within all fungi) that is not conserved outside of that group (e.g. between plants and fungi). All interfaces identified in our analysis are ones that are maintained in the mammalian lineage. Though species-specific interactions are also of great interest, a different approach would be necessary to reveal those regions.
The Gβ gene family was produced by a series of gene duplications. After these events, the new Gβ paralogs likely evolved novel functions as a result of relaxed functional constraint. Eventually, as these new Gβ functions become critical to the organism, these new functional regions become highly conserved regions of the Gβ structure and include the ROIs we identified. We hypothesize that these ROIs represent regions of newly-acquired function in the organism in which they first appeared. For instance, portions of six ROIs presented in Fig. 4 – ROI2, ROI7, and ROIs10-13 – contain residues that likely bind interactors as previously identified in mutational or structural studies. The remaining ROIs in Fig. 4 are in regions that lie outside of known regions presented thus far. Although interactions with many proteins have been determined by mutational studies, many studies focus on known interfaces and therefore indirectly introduce bias. For example, since the Gα binding area is utilized in many Gβ-protein interactions, studies historically focused on these residues. Our de novo analysis eliminates this bias, generating a set of potential binding interfaces (ROIs) that are yet to be explored by mutational studies. As with the residues implicated in the PLC-β2 binding interface, these ROIs would make ideal targets for site-directed mutagenesis. In this manner, we can identify new Gβ-interacting proteins as well as to further characterize interactions with existing proteins. Ultimately, this will allow us to characterize new Gβ-mediated signaling pathways.
Materials and Methods
Ancestral Reconstruction
To reconstruct plant and fungal ancestors, sequence databases were queried with known plant and mammalian Gβ sequences for full-length plant and fungal Gβ homologs. Additionally, Gβ sequences were also retrieved for two outgroups: entamoeba (Entamoeba dispar) and diatom (Thalassiosira pseudonana). MSAs were generated in ClustalX 31. Expasy and Genbank IDs for each gene used in the alignment are denoted in Figs. S1 and S2. For each MSA generated, all gaps except those found exclusively in the two outgroups were removed. From the resulting NEXUS file, ancestors were generated using MrBayes 32; 33 using the fixed equalin model, using the inverse gamma rate, and sampling 100,000 generations at a frequency of 100. In order to eliminate ancestral residue values chosen with low confidence, only those residues predicted >90% of the time with a maximum value >0.8 were accepted and included in the ancestral sequence. All other residues were assigned a value of “X”, as they were too variable to be called with confidence.
Sequence Collection, Alignment, and Phylogeny Generation
All Gβ sequences from Drosophila melanogaster, Caenorhabditis elegans, and human (Expasy and Genbank sequence IDs are denoted in Fig. S3) were combined with the plant and fungal ancestral sequences and the diatom and entamoeba outgroups to create a MSA (see previous section for alignment and NEXUS file generation). The alignments were made robust by structural comparisons between bovine Gβ1 and mouse Gβ5. MrBayes 32; 33 was run using a fixed equalin model, using the inverse gamma rate, and sampling 1,000,000 generations at a frequency of 100 for 3 independent runs with a burn in of 250,000 generations to generate a consensus phylogenetic tree.
Interface Determination
In order to identify binding interfaces from structures of Gβ in complex with interacting proteins, Naccess 27 was used to calculate relative solvent accessibility (RSA) of all Gβ residue side chains both as a monomer and in complex with other proteins. All residue side chains with an RSA decrease > 5% (RSA of Gβ monomer – RSA of complex > 5) between monomer and complex were denoted as being present in the interface. This group of residues also included all Gβ residues that formed hydrogen bonds with the interacting protein as determined in the PyMol Molecular Modeling System 34. The 5% ΔRSA value was chosen because it is more stringent than the commonly utilized 1Å2 change in absolute solvent accessibility 26; 35 but still included all residues which form hydrogen bonds. Additionally, a residue with >=5% RSA is often designated as a surface residue while a residue with <5% RSA is designated as an interior residue 26; 35.
Yeast-Three Hybrid Protein Interaction
Arabidopsis thaliana Gβ (AGB1) and Gγ1 (AGG1) were cloned into the pBridge vector (Clontech, Palo Alto, CA). A. thaliana phosducin (At5g14240) was cloned into the p-ENTR/D-TOPO vector (Invitrogen, Carlsbad, CA) and then recombined into the pACTGW-attR Gateway vector 36 which contains an activation domain and is compatible with the pBridge vector. The prey (phosducin) was transformed into yeast strain AH109 which had previously been transformed with the bait (AGB1/AGG1). Both strains (that containing the bait alone and that containing both bait and prey) were grown on nutritionally selective media. Presence of the bait and prey were confirmed by the expression of nutritional markers (positive growth on media lacking tryptophan and leucine, respectively). Interaction was confirmed by the expression of an additional nutritional marker (positive growth on media lacking histidine).
Transfection of COS-7 Cells with Gβ1, Gγ2, and PLC-β2
The accumulation of [3H]inositol phosphates was measured in transiently transfected COS-7 cells as previously described 37. Briefly, COS-7 cells were plated in 12-well dishes at a cell density of 60,000 cells per well in Dulbecco's Modified Eagles Medium (DMEM) supplemented with 10% fetal bovine serum, 10 units / ml penicillin, and 10 units / ml streptomycin. Following incubation for 24 hrs at 37°C in an atmosphere of 95% air / 5% CO2, cells were transfected with 200 or 300 ng each of the indicated human Gβ1 and Gγ2 DNA in the presence and absence of 30 ng of PLC-β2 and empty vector for a total of 700 ng of DNA per well. DNA was complexed with FuGENE 6 (Roche Applied Sciences, Indianapolis, IN) per manufacturer's protocol prior to transfection. Twenty-four hours post-transfection, the medium was aspirated and cells were metabolically labeled with 1 μCi / well of myo-[2-3H(N)]inositol (American Radiolabeled Chemicals, St. Louis, MO) in inositol-free DMEM for 12 -16 hours. Subsequently, 10 mM LiCl was added. One hour after incubation with LiCl, reactions were stopped by aspiration of the medium and addition of 50 mM formic acid. Samples were neutralized by the addition of 150 mM NH4OH, and [3H]inositol phosphates were quantified by Dowex chromatography. Statistical significance for comparisons between the activation of PLC-β2 by wt Gβ1γ2 and each mutant Gβγ was determined by performing a Student's t-test assuming equal variance between the log-transformed number of [3H]inositol phosphates for each construct. Western blotting was performed to confirm equal expression of each construct in COS-7 cells using an antibody directed toward the c-Myc epitope (Invitrogen) on hGβ1, the HA epitope (Roche Applied Sciences) on Gγ2, and a monoclonal antibody (Santa Cruz, Santa Cruz, CA) against PLC-β2.
Supplementary Material
Plant sequences were aligned using Clustal X. Diatom and entamoeba Gβ sequences served as outgroups during ancestral reconstruction. Expasy and Genbank accession numbers are indicated within the sequence name in the alignment. Residues are colored according to their identity and symbols above each residue indicate residues that are strongly conserved (“*” full conservation of a single residue, “:” full conservation within a “strong” group, “.” full conservation within a “weak” group). The bar graph beneath the alignment represents the conservation score at each position in the alignment.
Fungal sequences were aligned using Clustal X. Diatom and entamoeba Gβ sequences served as outgroups during ancestral reconstruction. Expasy and Genbank accession numbers are indicated within the sequence name in the alignment. Residues are colored according to their identity and symbols above each residue indicate residues that are strongly conserved (“*” full conservation of a single residue, “:” full conservation within a “strong” group, “.” full conservation within a “weak” group). The bar graph beneath the alignment represents the conservation score at each position in the alignment.
Gβ sequences from the plant ancestor, fungal ancestor, C. elegans, D. melanogaster, and human were aligned in ClustalX. These alignments were corrected to match the structural alignment between mammalian Gβ1 and Gβ5. Expasy and Genbank accession numbers are indicated within the sequence name in the alignment. Residues are colored according to their identity and symbols above each residue indicate residues that are strongly conserved (“*” full conservation of a single residue, “:” full conservation within a “strong” group, “.” full conservation within a “weak” group). The bar graph beneath the alignment represents the conservation score at each position in the alignment.
The MSA (Fig. S3) was used to generate a consensus phylogenetic tree using MrBayes with Thalassioria (diatom) and Entamoeba serving as outgroups. Metazoan Gβ sequences form two main groups, Gβ1-like and Gβ5-like. Confidence intervals are noted on the phylogeny.
Acknowledgments
Work in A.M.J.'s lab on the Arabidopsis G proteins is supported by the NIGMS (GM065989-01), the DOE (DE-FG02-05ER15671), and the NSF (MCB-0718202 and MCB-0723515). Work in J.S.'s lab is supported by the NIH (5-50921-2311).
Abbreviations
- AC2
adenylyl cyclase 2
- ET
evolutionary trace
- GRK
G-protein coupled receptor kinase
- MSA
multiple sequence alignment
- PLC-β2
phospholipase C beta 2
- RGS9
Regulator of G-protein signaling 9
- ROI
region of interest
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ohno S. Evolution by gene duplication. Springer; Berlin: 1970. [Google Scholar]
- 2.Jones AM, Assmann SM. Plants: the latest model system for G-protein research. EMBO Rep. 2004;5:572–8. doi: 10.1038/sj.embor.7400174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCudden CR, Hains MD, Kimple RJ, Siderovski DP, Willard FS. G-protein signaling: back to the future. Cell Mol Life Sci. 2005;62:551–77. doi: 10.1007/s00018-004-4462-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Milligan G, Kostenis E. Heterotrimeric G-proteins: a short history. Br J Pharmacol. 2006;147 1:S46–55. doi: 10.1038/sj.bjp.0706405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cabrera-Vera TM, Vanhauwe J, Thomas TO, Medkova M, Preininger A, Mazzoni MR, Hamm HE. Insights into G protein structure, function, and regulation. Endocr Rev. 2003;24:765–81. doi: 10.1210/er.2000-0026. [DOI] [PubMed] [Google Scholar]
- 6.van der Linden AM, Simmer F, Cuppen E, Plasterk RH. The G-protein beta-subunit GPB-2 in Caenorhabditis elegans regulates the G(o)alpha-G(q)alpha signaling network through interactions with the regulator of G-protein signaling proteins EGL-10 and EAT-16. Genetics. 2001;158:221–35. doi: 10.1093/genetics/158.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller KG, Emerson MD, Rand JB. Goalpha and diacylglycerol kinase negatively regulate the Gqalpha pathway in C. elegans. Neuron. 1999;24:323–33. doi: 10.1016/s0896-6273(00)80847-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukuto HS, Ferkey DM, Apicella AJ, Lans H, Sharmeen T, Chen W, Lefkowitz RJ, Jansen G, Schafer WR, Hart AC. G protein-coupled receptor kinase function is essential for chemosensation in C. elegans. Neuron. 2004;42:581–93. doi: 10.1016/s0896-6273(04)00252-1. [DOI] [PubMed] [Google Scholar]
- 9.Ford CE, Skiba NP, Bae H, Daaka Y, Reuveny E, Shekter LR, Rosal R, Weng G, Yang CS, Iyengar R, Miller RJ, Jan LY, Lefkowitz RJ, Hamm HE. Molecular basis for interactions of G protein betagamma subunits with effectors. Science. 1998;280:1271–4. doi: 10.1126/science.280.5367.1271. [DOI] [PubMed] [Google Scholar]
- 10.Lambright DG, Sondek J, Bohm A, Skiba NP, Hamm HE, Sigler PB. The 2.0 A crystal structure of a heterotrimeric G protein. Nature. 1996;379:311–9. doi: 10.1038/379311a0. [DOI] [PubMed] [Google Scholar]
- 11.Lodowski DT, Pitcher JA, Capel WD, Lefkowitz RJ, Tesmer JJ. Keeping G proteins at bay: a complex between G protein-coupled receptor kinase 2 and Gbetagamma. Science. 2003;300:1256–62. doi: 10.1126/science.1082348. [DOI] [PubMed] [Google Scholar]
- 12.Loew A, Ho YK, Blundell T, Bax B. Phosducin induces a structural change in transducin beta gamma. Structure. 1998;6:1007–19. doi: 10.1016/s0969-2126(98)00102-6. [DOI] [PubMed] [Google Scholar]
- 13.Cheever ML, Snyder JT, Gershburg S, Siderovski DP, Harden TK, Sondek J. Crystal structure of the multifunctional Gbeta5-RGS9 complex. Nat Struct Mol Biol. 2008;15:155–62. doi: 10.1038/nsmb.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis TL, Bonacci TM, Sprang SR, Smrcka AV. Structural and molecular characterization of a preferred protein interaction surface on G protein beta gamma subunits. Biochemistry. 2005;44:10593–604. doi: 10.1021/bi050655i. [DOI] [PubMed] [Google Scholar]
- 15.Johnston CA, Kimple AJ, Giguere PM, Siderovski DP. Structure of the parathyroid hormone receptor C terminus bound to the G-protein dimer Gbeta1gamma2. Structure. 2008;16:1086–94. doi: 10.1016/j.str.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Li Y, Sternweis PM, Charnecki S, Smith TF, Gilman AG, Neer EJ, Kozasa T. Sites for Galpha binding on the G protein beta subunit overlap with sites for regulation of phospholipase Cbeta and adenylyl cyclase. J Biol Chem. 1998;273:16265–72. doi: 10.1074/jbc.273.26.16265. [DOI] [PubMed] [Google Scholar]
- 17.Panchenko MP, Saxena K, Li Y, Charnecki S, Sternweis PM, Smith TF, Gilman AG, Kozasa T, Neer EJ. Sites important for PLCbeta2 activation by the G protein betagamma subunit map to the sides of the beta propeller structure. J Biol Chem. 1998;273:28298–304. doi: 10.1074/jbc.273.43.28298. [DOI] [PubMed] [Google Scholar]
- 18.Bonacci TM, Ghosh M, Malik S, Smrcka AV. Regulatory interactions between the amino terminus of G-protein betagamma subunits and the catalytic domain of phospholipase Cbeta2. J Biol Chem. 2005;280:10174–81. doi: 10.1074/jbc.M412514200. [DOI] [PubMed] [Google Scholar]
- 19.Myung CS, Lim WK, DeFilippo JM, Yasuda H, Neubig RR, Garrison JC. Regions in the G protein gamma subunit important for interaction with receptors and effectors. Mol Pharmacol. 2006;69:877–87. doi: 10.1124/mol.105.018994. [DOI] [PubMed] [Google Scholar]
- 20.Yoshikawa DM, Bresciano K, Hatwar M, Smrcka AV. Characterization of a phospholipase C beta 2-binding site near the amino-terminal coiled-coil of G protein beta gamma subunits. J Biol Chem. 2001;276:11246–51. doi: 10.1074/jbc.M006073200. [DOI] [PubMed] [Google Scholar]
- 21.Lichtarge O, Bourne HR, Cohen FE. An evolutionary trace method defines binding surfaces common to protein families. J Mol Biol. 1996;257:342–58. doi: 10.1006/jmbi.1996.0167. [DOI] [PubMed] [Google Scholar]
- 22.Gu X, Vander Velden K. DIVERGE: phylogeny-based analysis for functional-structural divergence of a protein family. Bioinformatics. 2002;18:500–1. doi: 10.1093/bioinformatics/18.3.500. [DOI] [PubMed] [Google Scholar]
- 23.Zheng Y, Xu D, Gu X. Functional divergence after gene duplication and sequence-structure relationship: a case study of G-protein alpha subunits. J Exp Zoolog B Mol Dev Evol. 2007;308:85–96. doi: 10.1002/jez.b.21140. [DOI] [PubMed] [Google Scholar]
- 24.Armon A, Graur D, Ben-Tal N. ConSurf: an algorithmic tool for the identification of functional regions in proteins by surface mapping of phylogenetic information. J Mol Biol. 2001;307:447–63. doi: 10.1006/jmbi.2000.4474. [DOI] [PubMed] [Google Scholar]
- 25.Lichtarge O, Bourne HR, Cohen FE. Evolutionarily conserved Galphabetagamma binding surfaces support a model of the G protein-receptor complex. Proc Natl Acad Sci U S A. 1996;93:7507–11. doi: 10.1073/pnas.93.15.7507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones S, Thornton JM. Principles of protein-protein interactions. Proc Natl Acad Sci U S A. 1996;93:13–20. doi: 10.1073/pnas.93.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hubbard SJ, Thornton JM. Department of Biochemistry and Molecular Biology. University College London; 1993. ‘NACCESS,’ computer program. [Google Scholar]
- 28.Blaauw M, Knol JC, Kortholt A, Roelofs J, Ruchira, Postma M, Visser AJ, van Haastert PJ. Phosducin-like proteins in Dictyostelium discoideum: implications for the phosducin family of proteins. Embo J. 2003;22:5047–57. doi: 10.1093/emboj/cdg508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaudet R, Bohm A, Sigler PB. Crystal structure at 2.4 angstroms resolution of the complex of transducin betagamma and its regulator, phosducin. Cell. 1996;87:577–88. doi: 10.1016/s0092-8674(00)81376-8. [DOI] [PubMed] [Google Scholar]
- 30.Madabushi S, Yao H, Marsh M, Kristensen DM, Philippi A, Sowa ME, Lichtarge O. Structural clusters of evolutionary trace residues are statistically significant and common in proteins. J Mol Biol. 2002;316:139–54. doi: 10.1006/jmbi.2001.5327. [DOI] [PubMed] [Google Scholar]
- 31.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–82. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huelsenbeck JP, Ronquist F, Nielsen R, Bollback JP. Bayesian inference of phylogeny and its impact on evolutionary biology. Science. 2001;294:2310–4. doi: 10.1126/science.1065889. [DOI] [PubMed] [Google Scholar]
- 33.Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–4. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- 34.DeLano WL. The PyMOL Molecular Graphics System. DeLano Scientific; San Carlos, CA, USA: 2002. [Google Scholar]
- 35.Jones S, Marin A, Thornton JM. Protein domain interfaces: characterization and comparison with oligomeric protein interfaces. Protein Eng. 2000;13:77–82. doi: 10.1093/protein/13.2.77. [DOI] [PubMed] [Google Scholar]
- 36.Nakayama M, Kikuno R, Ohara O. Protein-protein interactions between large proteins: two-hybrid screening using a functionally classified library composed of long cDNAs. Genome Res. 2002;12:1773–84. doi: 10.1101/gr.406902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wing MR, Snyder JT, Sondek J, Harden TK. Direct activation of phospholipase C-epsilon by Rho. J Biol Chem. 2003;278:41253–8. doi: 10.1074/jbc.M306904200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Plant sequences were aligned using Clustal X. Diatom and entamoeba Gβ sequences served as outgroups during ancestral reconstruction. Expasy and Genbank accession numbers are indicated within the sequence name in the alignment. Residues are colored according to their identity and symbols above each residue indicate residues that are strongly conserved (“*” full conservation of a single residue, “:” full conservation within a “strong” group, “.” full conservation within a “weak” group). The bar graph beneath the alignment represents the conservation score at each position in the alignment.
Fungal sequences were aligned using Clustal X. Diatom and entamoeba Gβ sequences served as outgroups during ancestral reconstruction. Expasy and Genbank accession numbers are indicated within the sequence name in the alignment. Residues are colored according to their identity and symbols above each residue indicate residues that are strongly conserved (“*” full conservation of a single residue, “:” full conservation within a “strong” group, “.” full conservation within a “weak” group). The bar graph beneath the alignment represents the conservation score at each position in the alignment.
Gβ sequences from the plant ancestor, fungal ancestor, C. elegans, D. melanogaster, and human were aligned in ClustalX. These alignments were corrected to match the structural alignment between mammalian Gβ1 and Gβ5. Expasy and Genbank accession numbers are indicated within the sequence name in the alignment. Residues are colored according to their identity and symbols above each residue indicate residues that are strongly conserved (“*” full conservation of a single residue, “:” full conservation within a “strong” group, “.” full conservation within a “weak” group). The bar graph beneath the alignment represents the conservation score at each position in the alignment.
The MSA (Fig. S3) was used to generate a consensus phylogenetic tree using MrBayes with Thalassioria (diatom) and Entamoeba serving as outgroups. Metazoan Gβ sequences form two main groups, Gβ1-like and Gβ5-like. Confidence intervals are noted on the phylogeny.