Abstract
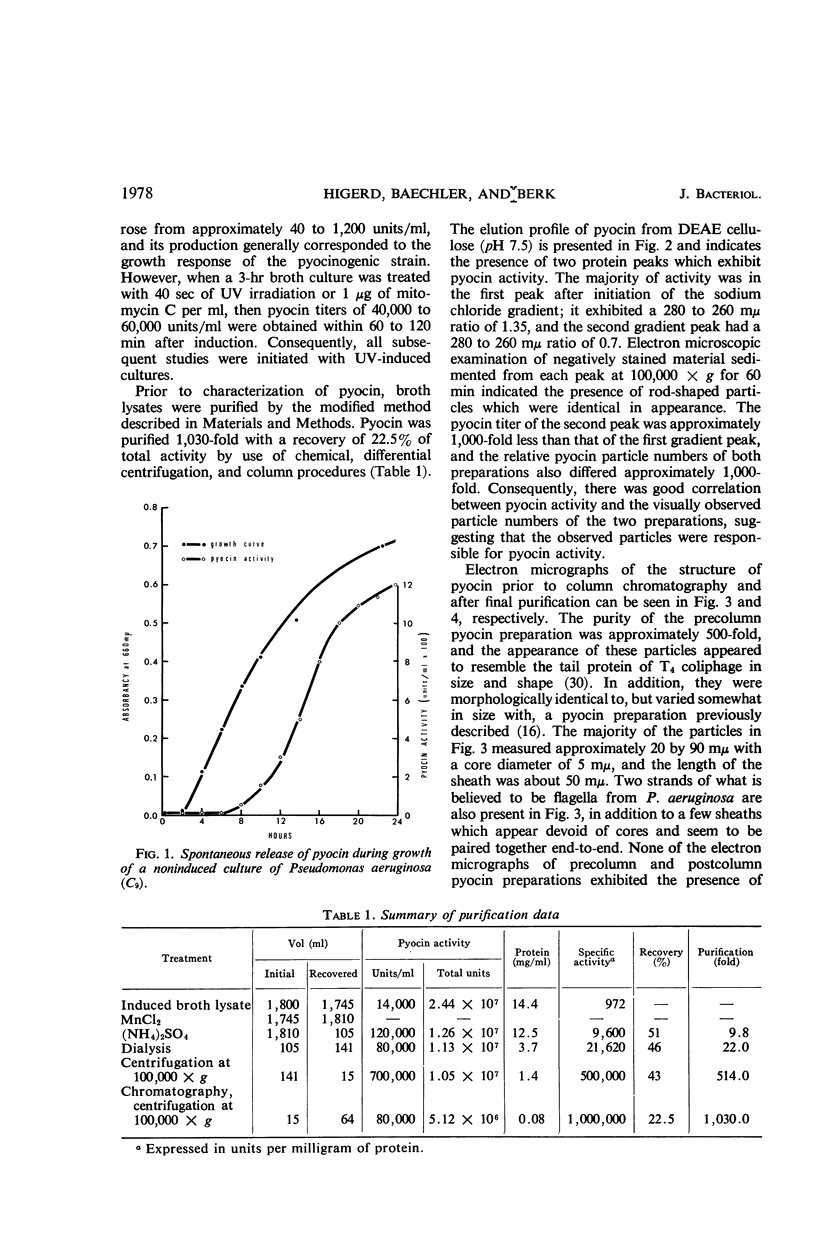
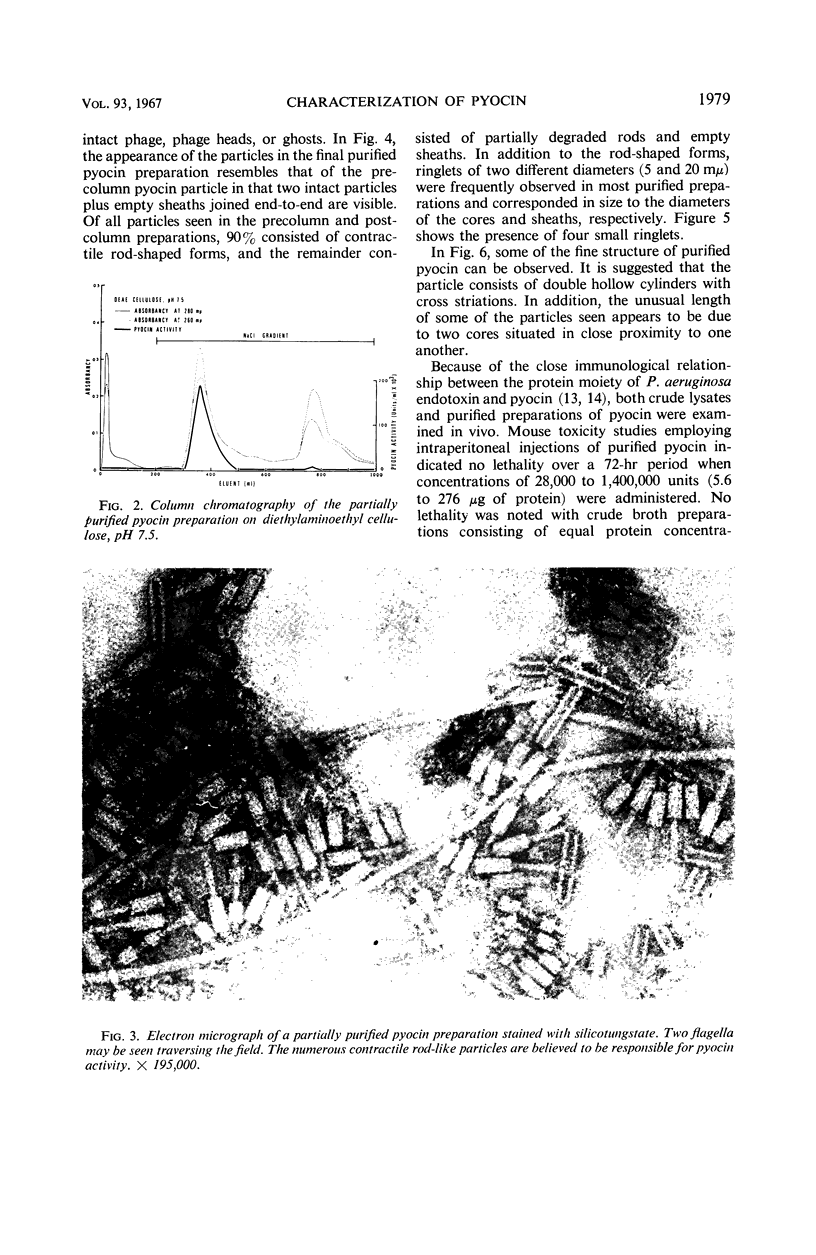
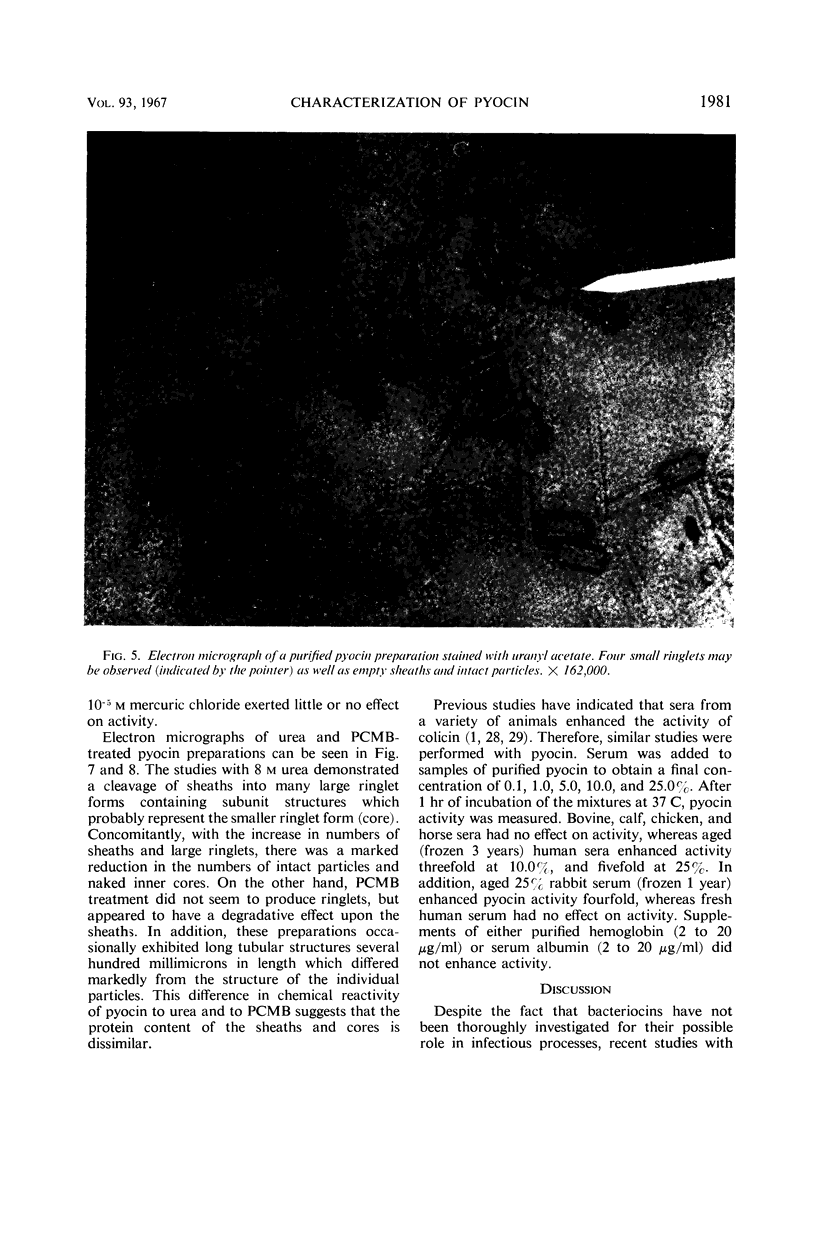
Pyocin, a bacteriocin obtained from lysates of ultraviolet-induced cultures of Pseudomonas aeruginosa was characterized in vitro and in vivo after 1,000-fold purification by chemical, column, and differential centrifugation procedures. Electron micrographs of negatively stained pyocin preparations contained rod-shaped particles which resembled the contractile tail protein of the T-even phages of Escherichia coli. Although two separate and distinct pyocin fractions were eluted from diethylaminoethyl cellulose (pH 7.5) during the purification procedure, the particles appeared identical. In addition, the two fractions exhibited a close correlation between their titers and the particle numbers as observed in the electron microscope. The particles were approximately 20 by 90 mμ with a core diameter of 5 mμ and a sheath length of 50 mμ. Neither intact phage nor ghosts were seen in any of the preparations, although ringlets of two different diameters, which appeared to correspond to the diameters of the sheath and inner core, were observed. Other studies indicated that, although crude preparations were stable to freezing and thawing, purified preparations lost all of their activity under similar treatment. However, the addition of 50% glycerol to purified preparations completely protected activity. Conversely, aged normal human or rabbit sera enhanced the antibacterial activity of pyocin approximately fourfold, although serum albumin and hemoglobin had no effect. In vivo studies indicated that purified pyocin was not lethal for mice when injected intraperitoneally in concentrations of 28,000 to 1,400,000 units (5.6 to 276 μg of protein), nor was 7,200 to 36,000 units dermonecrotic for rabbits.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARBER W., KELLENBERGER G. Study of the properties of seven defective-lysogenic strains derived from Escherichia coli K12 (lambda). Virology. 1958 Jun;5(3):458–475. doi: 10.1016/0042-6822(58)90039-4. [DOI] [PubMed] [Google Scholar]
- HALL C. E. Electron densitometry of stained virus particles. J Biophys Biochem Cytol. 1955 Jan;1(1):1–12. doi: 10.1083/jcb.1.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOMMA J. Y., SUZUKI N. "CELL-WALL PROTEIN A" OF PSEUDOMONAS AERUGINOSA AND ITS RELATIONSHIP TO "ORIGINAL ENDOTOXIN PROTEIN". J Bacteriol. 1964 Mar;87:630–640. doi: 10.1128/jb.87.3.630-640.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IKEDA K., KAGEYAMA M., EGAMI F. STUDIES OF A PYOCIN. II. MODE OF PRODUCTION OF THE PYOCIN. J Biochem. 1964 Jan;55:54–58. doi: 10.1093/oxfordjournals.jbchem.a127840. [DOI] [PubMed] [Google Scholar]
- Ishii S. I., Nishi Y., Egami F. The fine structure of a pyocin. J Mol Biol. 1965 Sep;13(2):428–431. doi: 10.1016/s0022-2836(65)80107-3. [DOI] [PubMed] [Google Scholar]
- Mennigmann H. D. Electron microscopy of the anti-bacterial agent produced by Escherichia coli 15. J Gen Microbiol. 1965 Nov;41(2):151–154. doi: 10.1099/00221287-41-2-151. [DOI] [PubMed] [Google Scholar]
- SEAMAN E., TARMY E., MARMUR J. INDUCIBLE PHAGES OF BACILLUS SUBTILIS. Biochemistry. 1964 May;3:607–613. doi: 10.1021/bi00893a001. [DOI] [PubMed] [Google Scholar]
- Takeya K., Amako K. A rod-shaped Pseudomonas phage. Virology. 1966 Jan;28(1):163–165. doi: 10.1016/0042-6822(66)90317-5. [DOI] [PubMed] [Google Scholar]
- To C. M., Eisenstark A., Töreci H. Structure of mutator phage Mu1 of Escherichia coli. J Ultrastruct Res. 1966 Mar;14(5):441–448. doi: 10.1016/s0022-5320(66)80074-6. [DOI] [PubMed] [Google Scholar]
- VANVUNAKIS H., RUFFILLI A., LEVINE L. ENHANCEMENTS OF ANTIBIOTIC ACTIVITY OF COLICINE K AND ALTERATION OF SEROLOGICAL PROPERTIES OF COLICINE K AND ENDOTOXINS. J Exp Med. 1965 Feb 1;121:261–278. doi: 10.1084/jem.121.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vunakis H., Ruffilli A., Levine L. Colicine K and endotoxins: effect of hemoglobin and its subunits on their antibiotic and serological properties. Biochem Biophys Res Commun. 1964 Jul 1;16(4):293–299. doi: 10.1016/0006-291x(64)90029-4. [DOI] [PubMed] [Google Scholar]
- WILLIAMS R. C., FRASER D. Structural and functional differentiation in T2 bacteriophage. Virology. 1956 Jun;2(3):289–307. doi: 10.1016/0042-6822(56)90024-1. [DOI] [PubMed] [Google Scholar]
- ZAK B., COHEN J. Automatic analysis of tissue culture proteins with stable Folin reagents. Clin Chim Acta. 1961 Sep;6:665–670. doi: 10.1016/0009-8981(61)90112-7. [DOI] [PubMed] [Google Scholar]