Abstract
CC chemokine ligand 2 (CCL2, also known as monocyte chemoattractant protein-1) has been demonstrated to recruit monocytes to tumor sites. Monocytes are capable of being differentiated into tumor-associated macrophages (TAMs) and osteoclasts (OCs). TAMs have been shown to promote tumor growth in several cancer types. Osteoclasts have also been known to play an important role in cancer bone metastasis. To investigate the effects of CCL2 on tumorigenesis and its potential effects on bone metastasis of human prostate cancer, CCL2 was overexpressed into a luciferase-tagged human prostate cancer cell line PC-3. In vitro, the conditioned medium of CCL2 overexpressing PC-3luc cells (PC-3lucCCL2) was a potent chemoattractant for mouse monocytes in comparison to a conditioned medium from PC-3lucMock. In addition, CCL2 overexpression increased the growth of transplanted xenografts and increased the accumulation of macrophages in vivo. In a tumor dissemination model, PC-3lucCCL2 enhanced the growth of bone metastasis, which was associated with more functional OCs. Neutralizing antibodies targeting both human and mouse CCL2 inhibited the growth of PC-3luc, which was accompanied by a decrease in macrophage recruitment to the tumor. These findings suggest that CCL2 increases tumor growth and bone metastasis through recruitment of macrophages and OCs to the tumor site.
Introduction
Prostate cancer is the most commonly diagnosed lethal malignancy in men in the United States [1]. Although androgen-dependent metastatic prostate cancer is initially treatable by castration, advanced prostate cancer remains incurable owing to the inevitable emergence of androgen-independent cells [2]. New approaches to the treatment of metastatic prostate cancer are needed. One such approach is to identify novel targets based on cancer cell-tumor microenvironment interactions [3–7].
CC chemokine ligand 2 (CCL2, also known as monocyte chemotactic protein-1) is a member of the CC beta chemokine family that is produced by macrophages, fibroblasts, and endothelial cells to stimulate chemotaxis of monocyte/macrophages and other inflammatory cells through its receptor, CCR2 [8]. Monocytes recruited to the tumor site adopt a macrophage phenotype that is dictated by the presence of specific cytokines. Mature macrophages are divided into M1 (classically activated) and M2 (alternatively activated) phenotypes. Tumor-associated macrophages (TAMs) of the M2 phenotype secrete several growth factors (transforming growth factor β, vascular endothelial growth factor, etc.) that promote tumor growth [9–13]. In several cancers, CCL2 has previously been shown to be an important determinant of tumor growth [14–16]. CCL2 promotes prostate cancer cell proliferation, migration, and survival from autophagic cell death through Akt phosphorylation-dependent mechanisms in vitro and in vivo [16,17]. The PC-3 cell line was established from human prostatic adenocarcinoma bone metastasis [18]. Neither expression of androgen receptor nor response to androgens was reported; therefore, the PC-3 cell line has been used as an androgen-independent cell model [19]. To further investigate the effects of CCL2 on tumorigenesis, metastatic potential, and the tumor microenvironment of human prostate cancer cells, human CCL2 DNA was introduced into the human prostate cancer cell line with the luciferase gene, PC-3luc, by lentivirus stable transfection. In this study, CCL2 increased prostate cancer growth and bone metastasis in vivo and was accompanied by the recruitment of macrophages and osteoclasts (OCs).
Materials and Methods
Cell Culture and Transfection
Human prostate cancer PC-3luc cells were generated as previously described [16] and were cultured in RPMI-1640 (GIBCO, Grand Island, NY), 100 U/ml penicillin, and 100 µg/ml streptomycin supplemented with 10% fetal bovine serum (GIBCO) under a humidified atmosphere of 5% CO2 at 37°C. Human CCL2 DNA (Accession No. BC009716) was produced by polymerase chain reaction (Takara Bio, Inc, Otsu, Japan) and is subcloned into pLenti6/V5-DEST (Invitrogen, Carlsbad, CA) vector with Gateway System (Invitrogen). The plasmid was packaged into lentivirus by the University of Michigan Vector Core, using psPAX2 and pMD2.G mammalian expression lentiviral helper plasmids. Transfected cells were selected by treatment with 5 µg/ml Blasticidin (Invitrogen) for 14 days, and surviving cells were used for the following experiment. Empty pLenti6/V5-DEST vector was used as a mock vector.
Reverse Transcription-Polymerase Chain Reaction
RNA was extracted by RNAeasy Micro Kit (QIAGEN GmbH, Hilden, Germany) according to the manufacturer's instructions. One microgram of total RNA of each sample was reverse transcribed by a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). To detect transfected vectors, Blasticidin sequence was used. The primers for Blasticidin were as follows: Blasticidin-sense, 5′-ATCAACAGCATCCCCATCTC-3′; Blasticidin-antisense, 5′-ATGCAGATCGAGAAGCACCT-3′. The housekeeping transcript, β-actin, was used as a control for semistandardization. The polymerase chain reaction (PCR) products were analyzed by electrophoresis on 1% agarose gels.
Proliferation Assays
Proliferation assays were previously described [16]. Briefly, cells were seeded at a density of 1 x 104 cells in 96-well plates in RPMI-1640 complete medium. Cell growth was determined every 24 hours using the WST-1 assay (Roche Diagnostic, Indianapolis, IN).
ELISA Assays
For preparing the conditioned medium, 3.0 x 105 PC-3lucMock and PC-3lucCCL2 cells were seeded on six-well culture plates and cultured for 72 hours with complete medium. The conditioned medium was centrifuged, and the supernatant was collected and stored in -80°C until use. ELISA analysis for human CCL2 (R&D Systems, Minneapolis, MN) was performed following the manufacturer's instructions. CCL2 concentrations were normalized for cell number (1.0 x 106 cells/ml).
Migration Assay
For preparing the conditioned medium for the transwell assay, PC-3lucMock and PC-3lucCCL2 cells were cultured in 75-mm2 tissue culture flasks to be 60% to 70% confluent. Next, cells were washed with PBS and cultured with RPMI-1640 supplemented with 1% serum for 72 hours. The conditioned medium was normalized for cell number. Mouse peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Paque PLUS (GE healthCare Bio-Sciences AB, Uppsala, Sweden) density gradient centrifugation from male severe combined immunodeficient mice (CB-17 SCID) peripheral blood (Charles River, Chicago, IL). Cells at a density of 2.5 x 104 cells in RPMI-1640 supplemented with 1% FBS were plated in the inner chamber of 24-well culture plates (8-µm pore size; Becton Dickinson, Franklin Lakes, NJ). The outer chamber was filled with RPMI (1% FBS) as a negative control, 100 ng/ml recombinant human CCL2 (PeproTech, Inc, Rocky Hill, NJ) as a positive control, and conditioned medium of each cell line. After incubation for 24 hours, cells were fixed and stained with 2% crystal violet, and cells inside the inserts were removed. The number of migrated cells in the whole membrane was counted under a microscope.
Prostate Cancer Xenograft Model In Vivo
Bioluminescent imaging of PC-3luc was done as previously described [16]. Briefly, PC-3lucMock and PC-3lucCCL2 cells (1 x 105 and 2 x 105 cells for subcutaneous and intracardiac injections, respectively) were introduced into CB-17 SCID mice (5–6 weeks of age). Mice were imaged weekly for 5 (subcutaneous) or 7 (intracardiac) weeks using a CCD IVIS system with a 50-mm lens (Xenogen, Corp). The results were analyzed using LivingImage software (Xenogen Corp, Alameda, CA). In antibody treatment studies, CNTO888, a human IgG1k antibody that neutralizes human CCL2, and CNTO1142, a rat/mouse chimeric antibody (provided by Dr. Linda Snyder, Centocor, Inc, Horsham, PA), were injected intraperitoneally twice weekly.
Flow Cytometry
A total of 1 x 106 cells of each cell line (PC-3lucMock and PC-3lucCCL2) were injected to generate tumors for flow cytometric analysis. At week 4, 8- to 10-mm-sized tumors were used. In the antibody treatment study, xenograft tumors were harvested after 6 weeks. Tumors were digested with collagenase (Sigma-Aldrich, St Louis, MO) and then made into single cell suspension. Mononuclear cells were collected by layering in Ficoll-Paque centrifuge. Macrophages were stained with fluorescein isothiocyanate-conjugated anti-mouse Mac-3 (M3/86) antibody (BD Bioscience Pharmingen, San Jose, CA) and their matching isotype controls according to the manufacturer's protocols. The cells were incubated with antibodies for 30 minutes at 4°C and washed with PBS. The samples were analyzed by using a FACSCalibur flow cytometer and CellQuest software (Becton Dickinson).
Histology
Xenograft tumors were harvested after 6 weeks, fixed in 10% buffered formalin, and then embedded in paraffin wax. Tumor metastases of bones and jaws were harvested after 7 weeks. They were fixed in a 10% buffered formalin and decalcified before paraffin embedding. Five-micrometer sections were cut and placed on glass slides and stained with anti-mouse Mac-3 (M3/86) antibody at a 1:200 dilution for identification of mouse macrophages. Bone samples were stained with a tartrate-resistant acid phosphatase (TRAP) staining kit (Sigma). TRAP-positive and multinuclear cells on the bone surface were counted as OCs in a blinded manner.
Statistics
Data were analyzed with GraphPad Prism4 software (GraphPad Software Inc, La Jolla, CA). Either the Student's t test (in vitro data analysis) or the Mann-Whitney test (in vivo data analysis) was used for the comparison between two groups as required. Significance was defined as P < .05.
Results
Characterization of PC-3lucMock and PC-3lucCCL2 Cells
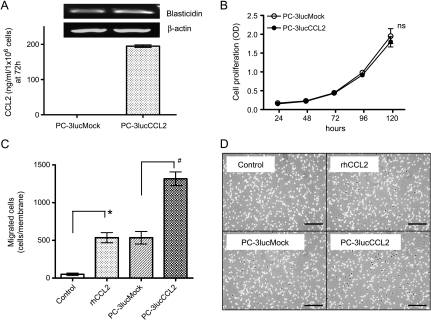
To confirm that both cell lines included vectors, mRNA of Blasticidin, a selection maker, was identified by reverse transcription-PCR (Figure 1A, upper panels). Blasticidin expression levels were similar. CCL2 secretion by each of the cell lines cultured with complete medium was measured by ELISA. PC-3lucCCL2 cells secreted 195.0 ng/ml per 106 cells in the conditioned medium (Figure 1A, lower panel) at 72 hours. The concentration of CCL2 secreted from PC-3lucMock cells was below the detection range of the ELISA kit (∼2 pg/ml). In vivo cell proliferation of these cell lines in complete medium (10% serum) did not show a significant difference (Figure 1B). It was previously demonstrated that human CCL2 partially binds to and activates the mouse CCR2 receptor [20]. Next, the chemoattractant activity of CCL2 from PC-3lucCCL2 in a transwell assay was examined. As expected, recombinant human CCL2 affected the migration activity of mouse PBMCs. The numbers of attracted PBMCs were similar in rhCCL2 (535.7 cells per membrane) and PC-3lucMock (535.3 cells per membrane). In addition, PC-3lucMock attracted more PBMCs compared with the control (50.3 cells per membrane). The conditioned medium from PC-3lucCCL2 cells attracted more mouse PBMCs compared with the conditioned medium from PC-3lucMock cells (mock = 535.3 cells per membrane, CCL2 = 1316.3 cells per membrane; Figure 1, C and D). This result demonstrates that human CCL2 from PC-3lucCCL2 cells attracts mouse PBMCs in vitro.
Figure 1.
Characterization of CCL2 overexpression PC-3luc cells in vitro. (A) Expression of CCL2 in PC-3luc cell. Vectors' transfection was detected by reverse transcription-PCR (upper panels). The quantification of CCL2 secretion in supernatant was detected by ELISA (lower panel). Values represent mean ± SD. (B) In vitro growth of PC-3lucMock and PC-3lucCCL2 with complete medium. Cell proliferation was analyzed by WST-1 assay. Values represent mean ± SD (ns, not significant). (C) Mouse PBMC migration was analyzed in response to rhCCL2 (100 ng/ml), PC-3lucMock, and PC-3lucCCL2 conditioned medium. The number of cells per whole membrane was manually counted by microscopy. Columns: *P < .05 compared with control, #P < .05 compared with PC-3lucMock. Bars, SD. (D) Representative pictures of migrated cells. Clockwise; control, rhCCL2 (100 ng/ml), conditioned medium from PC-3lucCCL2 and conditioned medium from PC-3lucMock. Bars, 200 µm.
Overexpression of CCL2 Correlates with Local Prostate Cancer Growth In Vivo
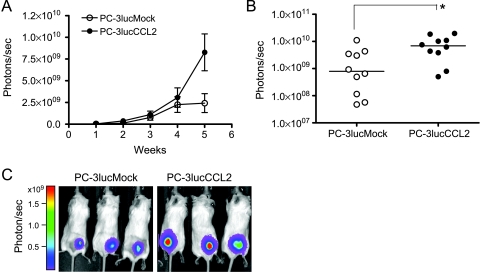
To evaluate the localized tumor growth of PC-3lucCCL2, an in vivo model of prostate cancer growth was used. A total of 1 x 105 cells were transplanted by subcutaneous injection (n = 10 per group) to male SCID mice, and tumor growth was monitored weekly for up to 5 weeks. As shown in Figure 2A, the average tumor growth of PC-3lucCCL2 was faster than that of the control. PC-3lucCCL2 continued growing after 4 weeks, whereas PC-3lucMock cells exhibited a trend of growth inhibition after 4 weeks. At 5 weeks, the tumor burden of PC-3lucCCL2 was significantly higher when compared with the control (Figure 2, B and C). The average tumor burden of PC-3lucCCL2 increased 3.4-fold. Given that PC-3lucCCL2 cells did not grow faster in vitro than control cells, it is likely that this increase was a result of CCL2 affects on cells of the tumor microenvironment (Figure 1B).
Figure 2.
In vivo growth of PC-3lucMock and PC-3lucCCL2. (A) Local tumor xenografts were established by subcutaneous injection of PC-3lucMock and PC-3lucCCL2 to SCID mice (n = 10 mice per group), and tumor growth was monitored as described in Materials and Methods. Values represent mean ± SEM. (B) Scatter plots of photons per second from PC-3lucMock and PC-3lucCCL2 on the fifth week. Values represent median (*P < .05). (C) Representative pictures illustrate week 5 images from each group (left panel, PC-3lucMock; right panel, PC-3lucCCL2).
CCL2 Recruits Macrophages to Tumor Sites
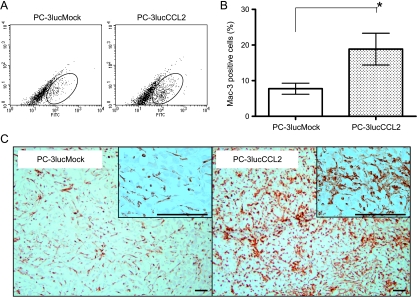
The effects of tumor derived CCL2 on macrophage recruitment in prostate cancer was assessed in vivo using flow cytometry and immunohistochemistry (Figure 3, B and C). Flow cytometric analysis of tumor infiltrated macrophages revealed that the PC-3lucCCL2 tumor contained 16.62% macrophages (18.29% in the representative experiment shown in Figure 3A), whereas PC-3lucMock contained 7.76% macrophages (8.09% in the representative experiment shown in Figure 3A). This difference was also observed in immunostaining analysis for Mac-3 (Figure 3C).
Figure 3.
Macrophage recruitment to prostate cancer xenografts. (A) Representative results show macrophage infiltration into tumors. Local tumor xenografts for flow cytometric analysis were generated as described in Materials and Methods. At week 4, tumors were harvested, and mouse macrophages stained with fluorescein isothiocyanate-conjugated anti-mouse Mac-3 antibody were analyzed by flow cytometry. (B) Graphical representation of the percentage recruited macrophages percentage in tumor. Values represent mean ± SEM (*P < .05). (C) Representative pictures of immunostaining analysis of Mac-3-positive cells in each group tumors. Bars, 50 µm.
Neutralizing Antibodies Targeting Both Mouse and Human CCL2 Reduce Tumor Volume and Macrophage Recruitment
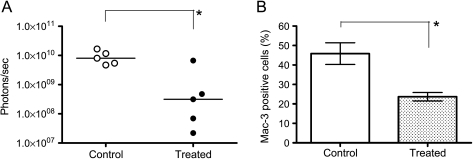
To inhibit CCL2 from both tumor and host cells, SCID mice were treated with CNTO888, a human IgG1k antibody that neutralizes human CCL2, and CNTO1142, a rat/mouse chimeric antibody that neutralizes mouse CCL2/JE, 1 week before PC-3luc cell transplantation (control group, n = 5; treatment group, n = 5). The mice were treated twice per week with anti-CCL2 antibodies at 10mg/kg until the end of the study, and serial bioluminescent images were taken. At 5 weeks of transplantation, CCL2 neutralizing antibody reduced tumor growth by 83.7% on average compared with the control group (Figure 4A). To further determine the efficacy of CCL2 inhibition in macrophage recruitment, macrophages in the tumor were counted by flow cytometry at 6 weeks. Of the control tumor cells, 45.8% were mouse macrophage, whereas the percentage in the treatment tumor was reduced to 23.7% on average (48.3% decrease compared with control; Figure 4B).
Figure 4.
Effects of target treatment of CCL2 on local PC-3luc tumor growth and macrophage recruitment. (A) Mice received PBS (control) or a combination of anti-human CCL2 CNTO888 (10 mg/kg) and anti-mouse CCL2/JE CNTO1142 (10 mg/kg) from 1 week before PC-3luc subcutaneous injection (n = 5 per group) to the end of the experiment twice weekly. Tumor growth was recorded as photons per second, and data from the fifth week were plotted. Values represent median (*P < .05). (B) Macrophage recruitment to tumor. Tumors were harvested on the sixth week, and the percentage of Mac-3-positive cells were analyzed as described in Materials and Methods. Values represent mean ± SEM (*P < .05, Student's t test).
CCL2 Increases Tumor Burden and Bone Metastasis of Prostate In Vivo
To further determine the effects of CCL2 overexpression on whole body tumor burden and bone metastases, PC-3lucMock and PC-3lucCCL2 (2 x 105 cells per mouse) were introduced into male SCID mice (mock, n = 14; CCL2, n = 15) by intracardiac injection, and tumor burden was monitored weekly for up to 7 weeks. By 4 weeks, the average tumor burden of PC-3lucCCL2 was slightly lower than the control, and at week 7, the average tumor burden of PC-3lucCCL2 increased 6.3-fold compared with PC-3lucMock (P < .05; Figure 5, A and B). To determine the effect of CCL2 in tumor sites within bone, growth was assessed by counting photons within regions of interest on the seventh week (jaw and bilateral lower limbs). The number of apparent metastases in the jaw and bilateral lower limbs was counted (PC-3lucMock = 15 metastases/14 mice, PC-3lucCCL2 = 24 metastases/15 mice); however, a statistically significant difference was not achieved. Comparison of region of interest values of apparent bone metastatic sites between control and PC-3lucCCL2 revealed a significant increase in tumor growth of PC-3lucCCL2 in bone (P < .05; Figure 5, C and D) at week 7.
Figure 5.
In vivo analysis of PC-3lucMock and PC-3lucCCL2 metastases. (A) Mice received PC-3lucMock and PC-3lucCCL2 cells by intracardiac injection (PC-3lucMock, n = 14; PC-3lucCCL2, n = 15), and overall tumor burden was monitored as photons per second. Values represent mean ± SEM. (B) Scatter plots of photons per second from whole body at week 7. Values represent median (*P < .05). (C) On the seventh week, jaw and bilateral lower limb-specific tumor burden was recorded as bone metastases and plotted (PC-3lucMock = 15 metastases/14 mice, PC-3lucCCL2 = 24 metastases/15 mice). Values represent median (*P < .05). Representative pictures illustrate week 7 images from each group (left panel, PC-3lucMock; right panel, PC-3lucCCL2).
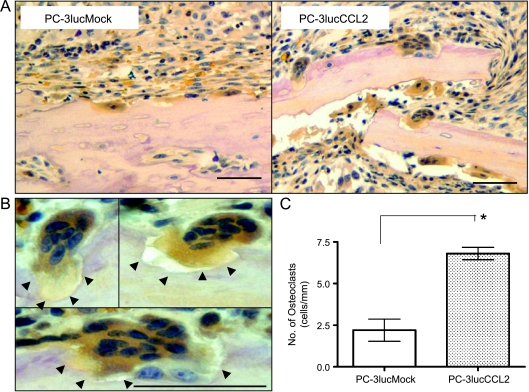
CCL2 Increases OCs in Bone Metastasis
Prostate cancer bone metastasis is generally considered to be osteoblastic owing to the unstructured bone formation [21–23]; however, OC formation and recruitment in bone metastasis are essential to osteoblast activation [24–27]. To address the mechanism of increased growth of PC-3lucCCL2 in the bone, OCs, recognized as TRAP staining positive and multinuclei cells in bone metastases, were analyzed (n = 5 per group). As shown in Figure 6, the number of OCs in PC-3lucCCL2 bone metastases is significantly higher than that in PC-3lucMock (P < .05; Figure 6C). Many defined Howship lacunae as pits or concavities in bone activity undergoing bone resorption [28], suggesting that OCs found in PC-3lucCCL2 bone metastases (Figure 6D, arrowheads) were activated.
Figure 6.
Osteoclasts in bone metastases. (A) Tartrate-resistant acid phosphatase staining of PC-3lucMock and PC-3lucCCL2 bone metastasis. (B) High-magnification picture of TRAP-positive multinuclear cells in PC-3lucCCL2. Howship lacunae (bone resorption lacunae) by OC were observed (arrowheads). (C) The number of TRAP-positive multinuclear cells on bone surface per 1 mm was manually counted by microscopy. Values represent mean ± SEM (*P < .05). Bars, 100 µm.
Discussion
Prostate cancer is an incurable disease once it metastasizes to other organs. It is well established that the tumor microenvironment plays a critical role in the “vicious cycle” of tumorigenesis by the enrichment of growth factors from component cells [4–7,9–13]. CCL2 is produced by macrophages, fibroblasts, endothelial cells, and cancer cells. CCL2 is one of the tumor microenvironment-derived signals that recruit TAMs, originating from circulating monocytes in tumor-related vasculature [5,29]. It has been previously demonstrated that CCL2-neutralizing antibodies effectively inhibited prostate cancer growth in vivo [16]. Here, the in vivo data further demonstrate the importance of CCL2 in prostate cancer bone metastasis by mediating TAM and OC recruitment and function.
To confirm the role of CCL2 in the tumor microenvironment, a human CCL2 high-expression cell line, PC-3lucCCL2 was generated. Proliferation of PC-3lucMock and PC-3lucCCL2 in vitro was not significantly different. These data suggest that the main effect of CCL2 on cancer cell growth is not through direct stimulation of growth but rather through indirect effects on host cells of the microenvironment.
Migration assay data confirmed the cross-reactivity of human CCL2 from PC-3lucCCL2 cells on SCID mouse macrophages. In accordance with these findings, other researchers have shown that human CCL2 has cross-reactivity on mouse macrophages [20]. Thus, it is reasonable to believe that human CCL2 in these experiments can act on mouse macrophages in vivo. The numbers of migrated cells were similar in PC-3lucMock conditioned medium and rhCCL2-induced migrated cells and were higher than those in the control. Numerous factors that recruit PBMCs to the tumor microenvironment were reported [11]. Our data regarding several chemotactic factors (e.g., transforming growth factor β and macrophage inflammatory protein-1) from PC-3 cells (data not shown) may explain this result.
These studies demonstrated that overexpression of CCL2 in human prostate cancer cells significantly increased local tumor burden in vivo. Tumor cell growth rate in vivo showed a significant increase compared with the in vitro data. One possible explanation for this phenomenon includes the attraction of tumor promoting TAMs [9–12]. In the present study, the number of macrophages identified with anti-mouse Mac-3 antibody in PC-3lucCCL2 is higher compared with that in PC-3lucMock. Targeted treatment of human and mouse CCL2 reduced recruitment of macrophages to the tumor with a subsequent decrease in tumor growth. Further experiments to determine the precise characterization of macrophages and the factors that contribute to tumor progression from TAMs are ongoing.
Bone is the most preferred metastasis site of advanced prostate cancer. More than 80% of patients who died with prostate cancer had evidence of gross bone metastasis. [4,5]. Although prostate cancer is a prototypic osteoblastic tumor, bone resorption by OCs is known to be the key step of bone metastasis [24–27]. A large number of researchers have published their findings on tumor-induced OC formation in recent years [30–32]. CCL2 has been reported to be a key mediator of prostate cancer-induced osteoclastogenesis in vivo and in vitro [28,33]. We have reported that CCL2 did not affect human CD11b+ PBMCs OC formation and bone resorption in vitro [34]. This discrepancy may come from the population difference in human bone marrow mononuclear cells and purified PBMCs. To explore this discrepancy, tumor burden by intracardiac injection was studied to investigate the role of CCL2 in metastasis, especially in bone. The present data indicate that overexpression of CCL2 promotes prostate cancer bone metastasis. Although there was no statistically significant difference in bone metastasis numbers, PC-3lucCCL2 grew at a significantly higher rate in bone. This is expected because CCL2 is known to be important in attracting monocytes to the tumor microenvironment. Hematopoietic stem cells give rise to several types of blood cells including macrophages and OCs [35]. CCL2 was identified as the promoting migration factor of monocytes and macrophages to sites of inflammation and as an activator of OCs. Osteoclasts play a critical role in the “vicious cycle” of the tumor bone microenvironment [4,5]. Bone is a growth factor-rich soil, and the destruction of bone by OCs releases many growth factors, resulting in tumor growth [21,23,24,30,36]. Previous studies with a neutralizing antibody and short hairpin RNA for CCL2 have reported that CCL2 is a critical mediator for prostate cancer-induced osteoclastogenesis and bone resorption [30,33]. The combination of previous and current data suggests that CCL2 recruits OC precursor cells to bone metastasis sites and promotes OC formation and the consequent release of numerous factors that promote tumor growth. Precise analysis of dominant growth factors in a CCL2-rich bone microenvironment is needed.
In summary, these results indicate that CCL2 plays a positive regulatory role on human prostate cancer growth and bone metastasis in vivo. CCL2 promotes tumor growth through recruitment of macrophages and OCs. Clinical investigation is necessary to determine the efficacy of an anti-CCL2 antibody in prostate cancer patients who has metastatic disease, especially those with bone metastasis.
Acknowledgments
The authors thank Rhonda Hotchkin for manuscript preparation.
Footnotes
K.J. Pienta is supported by a National Institutes of Health grant PO1 CA093900, an American Cancer Society Clinical Research Professorship, National Institutes of Health Specialized Program of Research Excellence in prostate cancer grant P50 CA69568, Cancer Center support grant P30 CA46592, SouthWest Oncology Group CA32102, and the Prostate Cancer Foundation.
References
- 1.American Cancer Society, author. Surveillance Research. 2008. [September 19, 2009]. Available at: http://www.cancer.org/downloads/stt/CFF2008Age-AdjustedCDR_Male.pdf.
- 2.Pienta KJ, Bradley D. Mechanisms underlying the development of androgen-independent prostate cancer. Clin Cancer Res. 2006;12:1665–1671. doi: 10.1158/1078-0432.CCR-06-0067. [DOI] [PubMed] [Google Scholar]
- 3.Rubin MA. Targeted therapy of cancer: new roles for pathologists—prostate cancer. Mod Pathol. 2008;21:S44–S55. doi: 10.1038/modpathol.2008.11. [DOI] [PubMed] [Google Scholar]
- 4.Loberg RD, Logothetis CJ, Keller ET, Pienta KJ. Pathogenesis and treatment of prostate cancer bone metastases: targeting the lethal phenotype. J Clin Oncol. 2005;23:8232–8241. doi: 10.1200/JCO.2005.03.0841. [DOI] [PubMed] [Google Scholar]
- 5.Taichman RS, Loberg RD, Mehra R, Pienta KJ. The evolving biology and treatment of prostate cancer. J Clin Invest. 2007;117:2351–2361. doi: 10.1172/JCI31791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lorusso G, Rüegg C. The tumor microenvironment and its contribution to tumor evolution toward metastasis. Histochem Cell Biol. 2008;130:1091–1103. doi: 10.1007/s00418-008-0530-8. [DOI] [PubMed] [Google Scholar]
- 7.Pienta KJ, McGregor N, Axelrod R, Axelrod DE. Ecological therapy for cancer: defining tumors using an ecosystem paradigm suggests new opportunities for novel cancer treatments. Transl Oncol. 2008;1:158–164. doi: 10.1593/tlo.08178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.New DC, Wong YH. CC chemokine receptor-coupled signalling pathways. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao (Shanghai) 2003;35:779–788. [PubMed] [Google Scholar]
- 9.Sica A, Larghi P, Mancino A, Rubino L, Porta C, Totaro MG, Rimoldi M, Biswas SK, Allavena P, Mantovani A. Macrophage polarization in tumour progression. Semin Cancer Biol. 2008;18:349–355. doi: 10.1016/j.semcancer.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Sica A, Allavena P, Mantovani A. Cancer related inflammation: the macrophage connection. Cancer Lett. 2008;267:204–215. doi: 10.1016/j.canlet.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 11.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allavena P, Sica A, Solinas G, Porta C, Mantovani A. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol. 2008;66:1–9. doi: 10.1016/j.critrevonc.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Halin S, Rudolfsson SH, Van Rooijen N, Bergh A. Extratumoral macrophages promote tumor and vascular growth in an orthotopic rat prostate tumor model. Neoplasia. 2009;11:177–186. doi: 10.1593/neo.81338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conti I, Rollins BJ. CCL2 (monocyte chemoattractant protein-1) and cancer. Semin Cancer Biol. 2004;14:149–154. doi: 10.1016/j.semcancer.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Loberg RD, Day LL, Harwood J, Ying C, St John LN, Giles R, Neeley CK, Pienta KJ. CCL2 is a potent regulator of prostate cancer cell migration and proliferation. Neoplasia. 2006;8:578–586. doi: 10.1593/neo.06280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loberg RD, Ying C, Craig M, Day LL, Sargent E, Neeley C, Wojno K, Snyder LA, Yan L, Pienta KJ. Targeting CCL2 with systemic delivery of neutralizing antibodies induces prostate cancer tumor regression in vivo. Cancer Res. 2007;67:9417–9424. doi: 10.1158/0008-5472.CAN-07-1286. [DOI] [PubMed] [Google Scholar]
- 17.Roca H, Varsos Z, Pienta KJ. CCL2 protects prostate cancer PC3 cells from autophagic death via phosphatidylinositol 3-kinase/AKT-dependent survivin up-regulation. J Biol Chem. 2008;283:25057–25073. doi: 10.1074/jbc.M801073200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaighn ME, Narayan KS, Ohnuki Y, Lechner JF, Jones LW. Establishment and characterization of a human prostatic carcinoma cell line (PC-3) Invest Urol. 1979;17:16–23. [PubMed] [Google Scholar]
- 19.van Bokhoven A, Varella-Garcia M, Korch C, Johannes WU, Smith EE, Miller HL, Nordeen SK, Miller GJ, Lucia MS. Molecular characterization of human prostate carcinoma cell lines. Prostate. 2003;57:205–225. doi: 10.1002/pros.10290. [DOI] [PubMed] [Google Scholar]
- 20.Luini W, Sozzani S, Van Damme J, Mantovani A. Species-specificity of monocyte chemotactic protein-1 and -3. Cytokine. 1994;6:28–31. doi: 10.1016/1043-4666(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 21.Yin JJ, Pollock CB, Kelly K. Mechanisms of cancer metastasis to the bone. Cell Res. 2005;15:57–62. doi: 10.1038/sj.cr.7290266. [DOI] [PubMed] [Google Scholar]
- 22.Morrissey C, Vessella RL. The role of tumor microenvironment in prostate cancer bone metastasis. J Cell Biochem. 2007;101:873–886. doi: 10.1002/jcb.21214. [DOI] [PubMed] [Google Scholar]
- 23.Kingsley LA, Fournier PG, Chirgwin JM, Guise TA. Molecular biology of bone metastasis. Mol Cancer Ther. 2007;6:2609–2617. doi: 10.1158/1535-7163.MCT-07-0234. [DOI] [PubMed] [Google Scholar]
- 24.Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350:1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 25.Datta HK, Ng WF, Walker JA, Tuck SP, Varanasi SS. The cell biology of bone metabolism. J Clin Pathol. 2008;61:577–587. doi: 10.1136/jcp.2007.048868. [DOI] [PubMed] [Google Scholar]
- 26.Keller ET, Brown J. Prostate cancer bone metastases promote both osteolytic and osteoblastic activity. J Cell Biochem. 2004;91:718–729. doi: 10.1002/jcb.10662. [DOI] [PubMed] [Google Scholar]
- 27.Dougall WC, Chaisson M. The RANK/RANKL/OPG triad in cancer-induced bone diseases. Cancer Metastasis Rev. 2006;25:541–549. doi: 10.1007/s10555-006-9021-3. [DOI] [PubMed] [Google Scholar]
- 28.Anderson MA. Dorland's Illustrated Medical Dictionary. Philadelphia, PA: WB Saunders; 2000. [Google Scholar]
- 29.Craig MJ, Loberg RD. CCL2 (monocyte chemoattractant protein-1) in cancer bone metastases. Cancer Metastasis Rev. 2006;25:611–619. doi: 10.1007/s10555-006-9027-x. [DOI] [PubMed] [Google Scholar]
- 30.Lu Y, Cai Z, Xiao G, Keller ET, Mizokami A, Yao Z, Roodman GD, Zhang J. Monocyte chemotactic protein-1 mediates prostate cancer-induced bone resorption. Cancer Res. 2007;67:3646–3653. doi: 10.1158/0008-5472.CAN-06-1210. [DOI] [PubMed] [Google Scholar]
- 31.Nicolin V, Bortul R, Bareggi R, Baldini G, Martinelli B, Narducci P. Breast adenocarcinomaMCF-7 cell line induces spontaneous osteoclastogenesis via a RANK-ligand-dependent pathway. Acta Histochem. 2008;110:388–396. doi: 10.1016/j.acthis.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 32.Lau YS, Sabokbar A, Giele H, Cerundolo V, Hofstetter W, Athanasou NA. Malignant melanoma and bone resorption. Br J Cancer. 2006;94:1496–1503. doi: 10.1038/sj.bjc.6603103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu Y, Chen Q, Corey E, Xie W, Fan J, Mizokami A, Zhang J. Activation of MCP-1/CCR2 axis promotes prostate cancer growth in bone. Clin Exp Metastasis. 2009;26:161–169. doi: 10.1007/s10585-008-9226-7. [DOI] [PubMed] [Google Scholar]
- 34.Mizutani K, Sud S, Pienta KJ. Prostate cancer promotes CD11b positive cells to differentiate into osteoclasts. J Cell Biochem. 2009;106:563–569. doi: 10.1002/jcb.22021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bar-Shavit Z. The osteoclast: a multinucleated, hematopoietic-origin, bone-resorbing osteoimmune cell. J Cell Biochem. 2007;102:1130–1139. doi: 10.1002/jcb.21553. [DOI] [PubMed] [Google Scholar]
- 36.Sato S, Futakuchi M, Ogawa K, Asamoto M, Nakao K, Asai K, Shirai T. Transforming growth factor beta derived from bone matrix promotes cell proliferation of prostate cancer and osteoclast activation-associated osteolysis in the bone microenvironment. Cancer Sci. 2008;99:316–323. doi: 10.1111/j.1349-7006.2007.00690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]