Abstract
Degradation of the extracellular matrix, which is an indispensable step in tissue remodelling processes such as embryonic development and wound healing of the skin, has been attributed to collagenolytic activity of members of the matrix metalloproteinase family (MMPs). Here, we employed mmp13 knockout mice to elucidate the function of MMP13 in embryonic skin development, skin homeostasis, and cutaneous wound healing. Overall epidermal architecture and dermal composition of non-injured skin were indistinguishable from wild-type mice. Despite robust expression of MMP13 in the early phase of wound healing, wild-type and mmp13 knockout animals did not differ in their efficiency of re-epithelialization, inflammatory response, granulation tissue formation, angiogenesis, and restoration of basement membrane. Yet, among other MMPs also expressed during wound healing, MMP8 was found to be enhanced in wounds of MMP13-deficient mice. In summary, skin homeostasis and also tissue remodelling processes like embryonic skin development and cutaneous wound healing are independent of MMP13 either owing to MMP13 dispensability or owing to functional substitution by other collagenolytic proteinases such as MMP8.
INTRODUCTION
Matrix metalloproteinases (MMPs), a family of structurally related neutral endopeptidases, play a central role in the breakdown of extracellular matrix (ECM). ECM degradation is essential for normal connective tissue remodelling in embryonic growth and development, morphogenesis, bone growth and resorption, wound healing, and uterine involution (Matrisian, 1992; Birkedal-Hansen et al., 1993; Nagase and Woessner, 1999; Vu and Werb, 2000; Stamenkovic, 2003). Additionally, incorrect regulation of MMPs is proposed to contribute to a variety of pathological processes characterized by increased matrix breakdown such as rheumatoid arthritis, atherosclerosis, and tumor invasion and metastasis (Mauch et al., 1993; Basset et al., 1997; Balbin et al., 2001, 2003; Neuhold et al., 2001). All MMPs display a conserved catalytic Zn2+ binding site and can be inhibited by specific physiologic inhibitors, the tissue inhibitor of metalloproteinase (for review, see Vu and Werb, 2000; Sternlicht and Werb, 2001; Stamenkovic, 2003). At present, 25 different human MMPs have been characterized at the amino-acid sequence level (for review, see Vu and Werb, 2000; McCawley and Matrisian, 2001; Lynch and Matrisian, 2002). Closely related members of the MMPs are often classified into different subfamilies of collagenases, gelatinases, stromelysins, matrilysins, membrane-type MMPs, and other MMPs (for review, see Nagase and Woessner, 1999; Vu and Werb, 2000; Sternlicht and Werb, 2001). The collagenase subfamily of human MMPs comprises three different members: the fibroblast interstitial collagenase (MMP1), the neutrophil collagenase (MMP8), and collagenase-3 (MMP13). To date, homologues in mice have been identified for MMP8 and MMP13 but not for MMP1. In mouse and rat, MMP1 appears to be functionally substituted by other collagenolytic enzymes such as MMP13, MMP14 (MT1-MMP), and McolA (Mariani et al., 1998; Balbin et al., 2001; Wu et al., 2003). Collagenases are the only members of the MMP family that degrade native fibrillar collagens in the triple helical domain in vivo (Krane et al., 1996; for review, see Massova et al., 1998; Vu and Werb, 2000; Balbin et al., 2001; Sternlicht and Werb, 2001; Egeblad and Werb, 2002; Stamenkovic, 2003). Therefore, collagenases are thought to execute a rate-limiting function in ECM re-organization that is essential not only for morphogenesis, but also for tissue remodelling processes in the adult organism, such as cutaneous wound healing.
This repair process, which provides an entirely restored barrier to protect the body from external environmental exposition, involves multiple distinct processes including (1) migration, proliferation, and re-stratification of keratinocytes into a multilayered structure, (2) infiltration of inflammatory cells and formation of the granulation tissue, (3) neovascularization, and (4) restoration of stable adhesive interaction with the underlying dermal stroma (for review, see Fuchs, 1990; Singer and Clark, 1999). During wound healing, the ECM undergoes a dramatic re-organization at the wound site, mainly accomplished by the action of MMPs (Clark, 1996; Ravanti and Kahari, 2000; Vu and Werb, 2000). In addition, MMP activity causes the release of cryptic information from the ECM. By cleaving large ECM components and ECM-associated molecules, MMPs liberate bioactive fragments and molecules (Schenk and Quaranta, 2003; Mott and Werb, 2004) and thereby regulate angiogenesis (Xu et al., 2001; Hangai et al., 2002) and release growth factors and cytokines (Yu and Stamenkovic, 2000; Dangelo et al., 2001; Mu et al., 2002; Belotti et al., 2003). Thus, ECM proteolysis executed by MMPs may be a prerequisite for proper wound repair and accurate tissue regeneration. Degradation of the ECM that enables the movement of migrating keratinocytes between the collagenous dermis and the fibrin matrix deposited within hours after injury (Singer and Clark, 1999) presumably depends on the production of collagenolytic proteases produced by the keratinocytes (Pilcher et al., 1997; for review, see Seiki, 2002; Beare et al., 2003; Stamenkovic, 2003). Moreover, secretion of different MMPs by inflammatory cells such as neutrophils and macrophages facilitates infiltration into the granulation tissue (Clark, 1996; Beare et al., 2003). MMPs produced by the endothelial cells of newly formed blood vessels (Hiraoka et al., 1998; Werb et al., 1999; Langlois et al., 2004; Warner et al., 2004) may participate in angiogenesis (Singer and Clark, 1999; Seandel et al., 2001). Although the list of MMPs found to be expressed in the wound is constantly growing (Okada et al., 1997; Madlener et al., 1998; Saarialho-Kere, 1998; Lund et al., 1999; Planus et al., 1999; Hieta et al., 2003), the exact spectrum of MMPs critically involved in cutaneous wound healing has not been completely unravelled so far. Clearly, collagen degradation is required for keratinocyte migration in culture (Pilcher et al., 1997) and in vivo (Beare et al., 2003). The strong expression of MMP13 by migrating keratinocytes at the leading wound edge in the early phase of the healing process (Madlener et al., 1998) and its secretion by the dermal fibroblasts in the granulation tissue (Wu et al., 2003) suggests that this enzyme plays an important role in wound healing.
In this study, MMP13-deficient mice were generated to analyze the physiological function of MMP13 during skin development, homeostasis, and healing of excisional skin wounds.
RESULTS
Generation and phenotype of MMP13-deficient mice
To determine the role of MMP13 in embryonic skin development, skin homeostasis, and cutaneous wound healing, we crossed mice with a floxed mmp13 (mmp13 >/>) locus (Stickens et al., 2004) to CMV-cre deleter (Schwenk et al., 1995) to obtain ubiquitous disruption of the mmp13 gene. By inter-crossing offspring, which were heterozygous for the mutated mmp13 and the cre transgene, we obtained mmp13 null (mmp13 −/− mmp13), heterozygous (+/−), and wild-type (mmp13 +/+) mice, transgenic and non-transgenic for cre, in the expected Mendelian ratio. Floxed mmp13 mice did not differ from wild-type mice in MMP13 expression (Stickens et al., 2004, and data not shown) and were used as controls in this study. Complete cre recombination and loss of MMP13 transcripts in all tissues of mmp13 null mice including skin were verified by Southern blotting (data not shown) and by in situ hybridization (Figure 2), Northern blot (Figure 5), and reverse transcription-PCR (data not shown).
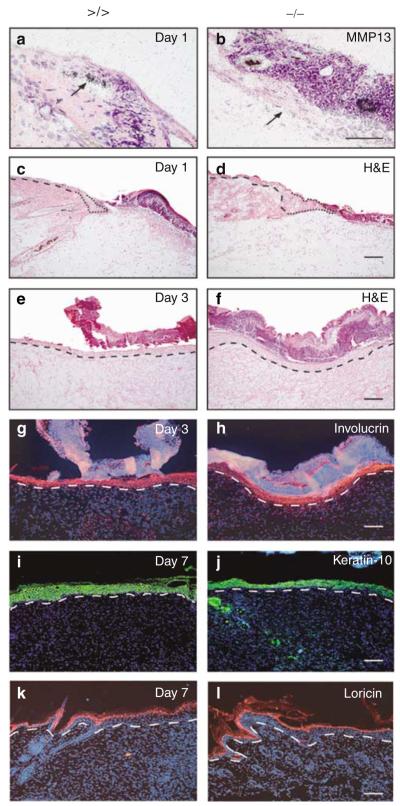
Figure 2. MMP13 expression analysis and microscopic assessment of wounds in MMP13-deficient mice.
(a, b) In situ hybridization for MMP13 on wounds of (a) mmp13 >/> controls and (b) mmp13 −/− mice followed by H&E staining to detect transcripts on sections of the left-hand site of wounds 1 day post injury. The arrow points to keratinocytes of the leading wound edge. Bar: 50μm. (c–f) H&E-stained sections of the left-hand site of wounds (c, d) 1 day and (e, f) 3 days after injury. The dashed line indicates basement membrane. The migrating tip is marked by the dotted line. Bar: 100μm. (g, h) Full re-epithelialization of mmp13 −/− and control wounds, as demonstrated by wound sections taken 3 days post injury and immunostained for involucrin (red signal). Nuclei were counterstained with Hoechst dye (blue signal), which also stained the scab. The basement membrane is indicated by a dashed line. Bar: 100μm. (i–l) Sections of wounds taken 7 days post excision were immunostained for the differentiation marker (i, j, green signal) keratin-10 and (k, l, red signal) loricrin to demonstrate complete keratinocyte differentiation. Nuclei were counterstained with Hoechst dye (blue signal). The dashed line indicates basement membrane. Bars: 100μm.
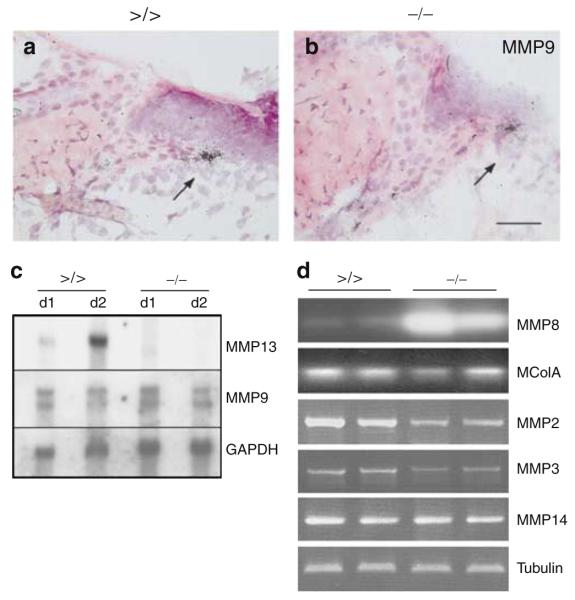
Figure 5. MMP expression analyses in the early phase of wound healing.
(a, b) In situ hybridization followed by H&E staining to detect MMP9 transcripts on sections of the left-hand site of wounds from (a) mmp13 >/> and (b) mmp13 −/− mice. The arrow points to keratinocytes of the leading wound edge. Bar: 50μm. (c) Northern blot analyses were performed with total RNA prepared from day 1 and 2 wounds of mmp13 >/> and mmp13 −/− mice and hybridized to MMP13 and MMP9 cDNA. Quality and quantity of the RNA was confirmed by visualization of GAPDH. (d) Semiquantitative PCR of different MMPs performed on cDNA prepared on total RNA isolated from day 1 wounds of mmp13 >/> and mmp13 −/− mice. After gel electrophoresis, PCR products of MMP8 and McolA were blotted and hybridized to the corresponding PCR fragments. The autoradiograph was scanned and reverted. β-Tubulin was used as an internal standard.
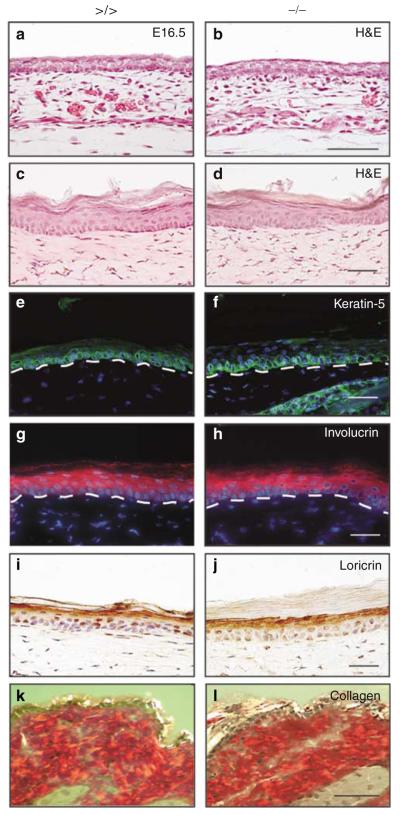
Despite MMP13 deficiency, mice developed normally, were fertile and showed no gross phenotypic abnormalities, and long-term survival rates were indistinguishable from their wild-type littermates and the floxed controls, being in agreement with published data (Stickens et al., 2004). Histological examination of back skin of embryonic day 16.5 embryos (Figures 1a and b) and of tail (Figure 1c and d) and back skin (data not shown) of 7- to 8-week-old mice revealed by hematoxylin and eosin (H&E) staining showed no obvious difference between mmp13 >/> and mmp13 −/− in overall morphology. As the epidermis of back skin comprises only 2–3 keratinocyte layers, we used tail skin sections to analyze keratinocyte proliferation and epidermal differentiation pattern in the mutant animals (Figure 1c–j, and data not shown). No significant difference was observed in terms of keratinocyte proliferation rate, as demonstrated by Ki67 staining of tail skin sections (data not shown). Likewise, expression analysis of (i) keratin-5 (Figure 1e and f), a cytokeratin protein expressed in the basal keratinocyte layer of the skin, (ii) involucrin (Figure 1g and h), detectable in all suprabasal layers, and (iii) loricrin (Figure 1i and j), a protein representative of the stratum granulosum, indicated normal keratinocyte differentiation in mmp13 knockout mice. Epidermal organization and dermal composition in skin of 6- and 12-month-old knockout mice were also unchanged when compared to controls (data not shown). In addition, we did not detect obvious increases in collagen deposition, fibrosis, or any other obvious abnormalities in the dermis of tail skin (data not shown) and back skin (Figure 1k and l). These data indicate that loss of MMP13 expression compromises neither proliferation and differentiation of epidermal keratinocytes nor the function of dermal fibroblasts and other cell types in mouse skin during embryonic development and homeostasis in adult mice.
Figure 1. Histopathological analyses of epidermal tissue morphology.
(a, b) H&E of sections from back skin of embryonic day (E)16.5 embryos of (a) controls and (b) mmp13 −/− mice. Bar: 100μm. (c–l) Tail skin sections (c–j) and back skin sections (k, l) of adult mice (left panel: mmp13 >/> and right panel: mmp13 −/−) were either stained with H&E (c, d) or analyzed by immunofluorescence for (e, f, green signal) keratin-5 and (g, h, red signal) involucrin expression. Nuclei were counterstained with Hoechst dye (blue signal). The basement membrane is indicated by a dashed line. (i, j, brown signal) Loricrin was visualized immunohistochemically and, for this analysis, nuclei were counterstained with hematoxylin (blue signal). (k, l) Collagen fibers in the dermis of back skin were stained with Picrosirius red and visualized by crossed polarization. Bars: 50μm.
Cutaneous wound healing in mmp13 −/− mice
To study the physiological role of MMP13 during the healing process, we generated full-thickness excisional wounds in the back skin of 8- to 12-week-old mmp13 −/− and mmp13 >/> mice. In agreement with published data (Madlener et al., 1998), during the early phase of wound healing, we observed an upregulation of MMP13 in the keratinocytes of the leading wound edge of wild-type and floxed controls, by in situ hybridization (Figure 2a, and data not shown). In contrast, in wounds of mmp13 −/− mice, MMP13 expression was extinguished (Figure 2b), confirming MMP13 deficiency in skin keratinocytes.
Macroscopic examination of wounds from mmp13 −/− and control animals revealed a comparable healing process in all individuals tested (data not shown). To assess the re-epithelialization potential of mmp13 −/− keratinocytes from day 1 to 10 post wounding, we applied detailed histological analysis of knockout and control wounds and studied keratinocyte differentiation pattern and wound closure kinetics (Figures 2c–l and Figure S1, and data not shown). In all cases, 24 hours post injury wound beds of both strains were just covered by a fibrin clot, and keratinocytes had not yet formed a continuous layer (Figure 2c and d). In some instances, wounds of mmp13 −/− as well as mmp13 >/> mice were already re-epithelialized at day 3 after wounding. This was evidenced by a complete keratinocyte layer (Figure 2e and f) and continuous involucrin staining throughout the former wound area (Figure 2g and h). In both strains, epidermal re-organization was completed 7 days post wounding, as proven by staining with antibodies against the early differentiation marker keratin-10 (Figure 2i and j) and the late differentiation marker loricrin (Figure 2k and l). Both proteins were expressed at normal levels and within the adequate layers. Measuring the distance between the leading wound edges on sections of mmp13 −/− and control mice at different time points after wounding revealed no significant difference in wound closure at days 2, 3, and 5 (Figure S1).
These data show that re-epithelialization after cutaneous injury is not significantly affected even though MMP13 expression is lost in the keratinocytes of the leading wound edge, suggesting either that MMP13 is not required during this process or can be functionally replaced by other MMPs.
Granulation tissue formation and angiogenesis at the wound sites
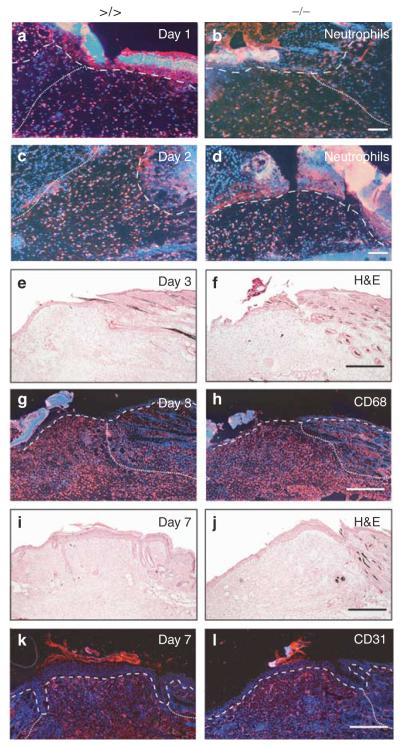
Dermal fibroblasts of the granulation tissue express reasonable amounts of MMP13 (Madlener et al., 1998; Wu et al., 2003), suggesting a contribution of this protease in granulation tissue formation and angiogenesis. Therefore, we explored the size and composition of the granulation tissue in wounds of mmp13 −/− and control animals. Concerning the size of the granulation tissue, which was visible in both strains 2–3 days post wounding (Figure 3c–h), no alterations were found in wounds of mmp13 null mice compared to the floxed controls (Figure 3, and data not shown). To determine the inflammatory response, tissue sections were analyzed for the infiltration of macrophages and neutrophils. As shown in Figure 3a–d, the number of infiltrated neutrophils was comparable in wounds of mmp13 −/− and mmp13 >/> mice. Similar data were observed for the number of infiltrated macrophages (Figure 3g and h).
Figure 3. Histological analyses of the granulation tissue at wound sites of mmp13 −/− mice.
(a–d) Neutrophils located in the granulation tissue of (a, b) day 1 and (c, d) day 2 wounds of mmp13 >/> (left panel) and mmp13 −/− (right panel) mice were stained by immunofluorescence (red signal) using the antibody neutrophils. Nuclei were counterstained with Hoechst dye (blue signal). The basement membrane is depicted by a dashed line and the granulation tissue is highlighted by a dotted line. (e–h) Bar: 100μm. (e, f) H&E staining and staining of macrophages by (g, h, red signal) CD68 immunofluorescence on wound sections 3 days post wounding. Bar: 300μm. (i–l) H&E staining (i, j) and staining of endothelial cells by (k, l, red signal) CD31 immunofluorescence on wound sections 7 days after wounding. Bars: 300μm.
Angiogenesis, one of the important events during wound healing, was explored on wound sections of MMP13-deficient and mmp13 floxed mice by CD31 immunostaining. No significant differences in vessel density and size were observed in uninjured skin (data not shown) or 7 days after injury (Figure 3i–l). These data imply that MMP13 deficiency interferes neither with the formation of granulation tissue nor with angiogenesis at cutaneous wound sites.
Basement membrane and dermal re-organization at the wound sites in MMP13-deficient mice
To re-establish proper dermal–epidermal junctions after re-epithelialization, an intact basement membrane has to be reconstituted by keratinocytes and fibroblasts. Recently, components of the basement membrane, such as perlecan and laminin-5, and cell surface receptors, such as integrins and dystroglycans, which interact with the basement membrane network, have been demonstrated to be substrates of a number of MMPs including MMP13 (Mott and Werb, 2004).
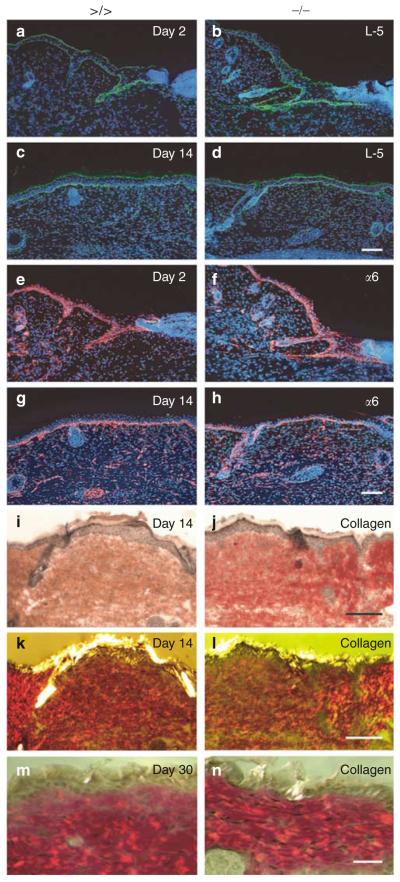
To demonstrate a possible function of MMP13 in the regeneration of basal lamina, we followed the deposition of various basement membrane components during the wound healing process. Immunostaining of histological sections for laminin-5 (Figure 4a–d), α6-integrin (Figure 4e–h), perlecan, and nidogen (data not shown) revealed no differences in protein expression pattern and intensity between wounds of mmp13 knockout and control mice. These data indicate that basement membrane re-organization after wound repair appears to be unaffected by the loss of MMP13 activity.
Figure 4. Immunofluorescence analyses of basement membrane re-organization and visualization of dermal collagen fibers in wounds of mmp13 −/− mice.
(a–h) Laminin-5 (a–d, green signal) and (e–h, red signal) α6-integrin were visualized by immunofluorescence on sections of mmp13 >/> (left panel) and mmp13 −/− (right panel) wounds analyzed 2 and 14 days after injury. Nuclei were counterstained with Hoechst dye (blue signal). Bars: 100μm. (i–n) To visualize collagen fibers of wounds taken at (i–l, bar: 50μm) day 14 and (m, n, bar: 30μm) day 30, sections were stained by Picrosirius red and documented by both (i, j) bright field and (k–n) crossed polarization.
Large amounts of ECM proteins, most prominently collagens, are synthesized, deposited, and remodelled during wound healing until the final reconstitution of the dermal matrix is achieved. To follow the expression level and quality of the collagen network, fibrillar collagen was visualized by Picrosirius staining throughout the course of wound healing on sections of control and mmp13 knockout mice from day 1 to 30 post wounding (Figure 4i–n, and data not shown). We could not detect any obvious abnormalities in the distribution, mass, or arrangement of the collagen fibers in the dermal compartment of the MMP13-deficient wounds. These data demonstrate that loss of MMP13 enzyme activity does not affect the formation and remodelling of fibrillar collagen during the entire wound healing process and indicate that a high degree of functional redundancy between MMP13 and other members of the MMP family occurs during initial and late phase of remodelling and tissue repair.
Expression pattern of MMPs in wounds of MMP13-deficient mice
In wild-type and mmp13 floxed mice, highest MMP13 expression levels were observed 1 and 2 days post wounding (Figure 5c, and data not shown; Madlener et al., 1998). To narrow down the potential candidates compensating for MMP13 deficiency in mutant mice during this early phase of wound healing, we determined the expression of other MMPs found in wounds shortly after injury (Figure 5).
The first candidate we analyzed was MMP9, which is frequently expressed at sites of active tissue remodelling such as the moving epidermal layer in cutaneous wounds (Salo et al., 1994; Mohan et al., 1998; Lund et al., 1999) and functionally cooperates with MMP13 in long bone development and homeostasis (Inada et al., 2004; Stickens et al., 2004). In agreement with published data (Lund et al., 1999), MMP9 expression originated predominantly from keratinocytes, which are located at the leading edge of the wound (Figure 5a and b), and completely overlapped with the pattern of MMP13-expressing cells in wild-type mice (Figure 2a). Upon quantification however, the amount of MMP9 transcripts was not significantly enhanced compared to wounds of floxed controls, as shown by Northern blot analysis of RNA prepared from day 1 and 2 wounds (Figure 5c).
Two other MMPs that are capable of cleaving native, undenatured fibrillar collagens in vivo are MMP8, which is predominantly expressed by neutrophils invading the wound area (Devarajan et al., 1991; Krane et al., 1996; Balbin et al., 1998), and McolA, the putative murine counterpart of the human interstitial collagenase MMP1 (Balbin et al., 2001). Although McolA expression has only been described for embryonic tissues so far (Balbin et al., 2001), McolA transcripts could be detected in wild-type mice from day 1 to 5 after wounding (data not shown), and in wounds of mmp13 −/− mice its expression level was marginally reduced compared to control (Figure 5d). By contrast, expression of neutrophil collagenase MMP8 was significantly enhanced in wounds of mmp13 −/− mice, as measured by Northern blot analysis and semiquantitative PCR (Figure 5d, and data not shown). Immunohistochemical analysis of wounds from control and mutant mice 3 days post injury revealed no sign of additional cell types expressing MMP8 at the wound sites of mmp13 −/− mice (Figure S2).
Finally, we analyzed the expression levels of MMP2, MMP3, and MMP14 (MT1-MMP), which exhibit collagenolytic activity, at least in vitro (Overall and Sodek, 1992; Sodek and Overall, 1992; Ohuchi et al., 1997; Seandel et al., 2001; Tam et al., 2002, 2004). The amount of these transcripts was even slightly reduced in MMP13-deficient wounds except for MMP14, which remained unchanged (Figure 5d).
These data demonstrate that a series of MMPs are expressed during re-epithelialization. Some MMPs showed enhanced or reduced expression in MMP13-deficient wounds. Thus, most likely, the activity of one or multiple members of the MMP family appears to be responsible for the functional compensation of MMP13 deficiency.
DISCUSSION
MMPs are important players in tissue remodelling and repair processes, in which they mediate proteolysis of the ECM predominantly composed of fibrillar collagens. In the mouse, in vivo collagenolytic activity has been widely attributed to MMP13 activity, the major interstitial collagenase in this species. In this study, we functionally determined the role of this protease during tissue remodelling and repair processes, such as skin development and cutaneous wound repair, employing the powerful knockout technology. To achieve MMP13 deficiency in mice, a targeted mmp13 locus with floxed exons (Stickens et al., 2004) was inter-crossed with the CMV-cre strain (Schwenk et al., 1995). Despite the fact that MMP13 was suggested to be involved in postpartum involution, at least in rats (Wolf et al., 1996), as well as in very early embryo development (Yamamoto et al., 1998) and in microvessel formation (Burbridge et al., 2002), we found no impact of MMP13 deficiency on reproduction or embryonic survival. Yet, in line with the massive expression of MMP13 during long bone development (Gack et al., 1995) and homeostasis (Tuckermann et al., 2000), MMP13 inactivation caused abnormal skeletal growth plate development (Inada et al., 2004; Stickens et al., 2004; unpublished data). To date, MMP13 expression has only been detected during endochondral ossification but not in embryonic or newborn skin (Gack et al., 1995). In line with these data, the unaltered skin architecture of the mmp13 −/− mice shows that this member of the family of interstitial collagenases obviously does not play a role during skin development and skin homeostasis.
Mice that express a collagenase-resistant version of collagen suffer from impaired tissue remodelling and spontaneously develop skin fibrosis in aged unchallenged animals (Liu et al., 1995), underscoring the need for collagenolytic enzymes. However, our data strongly imply that MMP13 is not the critical candidate, as no sign of fibrotic depositions was seen in the skin of the mmp13 −/− mice, even after 12 months of age. Thus, the assigned function of MMP13, as an interstitial collagenase cleaving fibrillar collagen in skin homeostasis, may be compensated by other members of the MMP family such as MMP2 and MMP14 (MT1-MMP) in mmp13 mutant mice. It was shown previously that both MMP2 and MP14 are expressed in unchallenged skin (Madlener et al., 1998) and are capable of cleaving fibrillar collagen, at least in vitro (Overall and Sodek, 1992; Sodek and Overall, 1992; Ohuchi et al., 1997; Seandel et al., 2001; Tam et al., 2002, 2004).
Similar to MMP3, MMP9, MMP8, MMP10, MMP11, and Mcol-A (Madlener et al., 1998; Nwomeh et al., 1998; our unpublished data), MMP13 expression is strongly upregulated shortly after injury and declines when re-epithelialization is completed (Madlener et al., 1998; Nwomeh et al., 1998; Lund et al., 1999). In the early phase of wound healing, MMP13 transcripts are found in the migrating keratinocytes located at the wound edges, and at later phases of wound repair when re-epithelialization is completed, insular fibroblasts express the enzyme in the granulation tissue (Madlener et al., 1998; Wu et al., 2003; our unpublished data). Mice expressing a collagenase-resistant version of collagen displayed a severely delayed wound healing (Beare et al., 2003), demonstrating the need for collagenases in healing of the injured skin, even though the identity of the specific MMP in charge is not clear. Upon performing excisional wounding experiments on the back skin of mmp13 −/− and control mice, we observed no significant effects on the wound healing process. Angiogenesis and collagen network assembly in the area of the former wound bed are unimpaired. Although MMP13 is strongly upregulated during the early phase of wound healing, we found no changes in re-epithelialization, epidermal re-organization, granulation tissue formation, inflammatory response, re-assembly of the basement membrane, and tissue remodelling in mmp13 −/− mice. These results are in agreement with data from wound healing studies performed in mice deficient for other MMPs. Except for mice lacking MMP14, which develop fibrosis of the dermis in unchallenged skin (Holmbeck et al., 1999, 2004), none of the MMP knockout mice generated so far displayed a severe phenotype in wound healing (Bullard et al., 1999; Mohan et al., 2002; Mauch, 2003).
Based on these results, it is reasonable to postulate that skin development, homeostasis, and wound healing do not rely on a single proteinase, but rather benefit from the availability of an array of MMPs that are simultaneously expressed. Facing the fact that most MMPs exhibit broad substrate specificity and that many MMPs share the same substrates (McCawley and Matrisian, 2001; Sternlicht and Werb, 2001), redundancy among the MMPs can be postulated. Collagen type I, being the preferential substrate of MMP13, together with collagen type II and III (Knäuper et al., 1996; McCawley and Matrisian, 2001; Sternlicht and Werb, 2001) can also be cleaved by non-MMP proteolytic enzymes such as serine proteases (Hibbs et al., 1983; Dano et al., 1985; Kofford et al., 1997; Ashcroft et al., 2000; Behrendt, 2004; Iba et al., 2004; Provenzano et al., 2005). A role in ECM degradation and tissue remodelling during cutaneous wound healing has already been demonstrated for the serine protease plasmin, which is the effector protease of the urokinase plasminogen activator system (Romer et al., 1991, 1994, 1996). Hence, we suggest that collagenolytic proteases other than MMP13 may also substitute for the loss of collagenolytic activity in mmp13 −/− mice during these processes.
One obvious candidate for the compensatory assignment by MMP family members is MMP9, which, like MMP13, is expressed by keratinocytes of the leading wound edge. Moreover, MMP9 was found in mmp13 −/− mice to be upregulated during embryonic bone development and was shown to synergize with MMP13 during endochondral ossification (Inada et al., 2004; Stickens et al., 2004). However, monitoring MMP9 expression in wounds of MMP13-deficient mice 1 and 2 days post injury revealed no significant increases in the level of MMP9 transcripts. Furthermore, the mild epidermal wound closure phenotype observed in mmp9 knockout mice was not further impaired by an additional loss of MMP13 in mmp9/mmp13 double-knockout animals (B. Hartenstein, B. Dittrich, B. Arnold, and P. Angel, unpublished data). These data suggest that in contrast to the process of endochondral ossification, MMP9 does not compensate for the loss of collagenase activity during cutaneous wound healing in MMP13-deficient mice.
Similarly, another candidate for substitution of MMP13, MMP14, a proteinase with the capacity to cleave native collagen (Tam et al., 2004) and which is expressed during wound healing (Madlener et al., 1998) and upregulated in bones of mmp13 −/− embryos (Inada et al., 2004), also remained unchanged at the mRNA transcript level in MMP13-deficient wounds.
Interestingly, we detected enhanced expression of MMP8 in the early phase of cutaneous wound healing in MMP13-deficient mice, corresponding with the induced expression level of this neutrophil-specific collagenase in bone marrow cavities of newborn mmp13−/− mice (Inada et al., 2004). Although expression of MMP8 has recently been detected in a number of other cell types (Wang et al., 2004), neutrophils that infiltrate the granulation tissue are postulated to be the main source of MMP8 in wounds (Balbin et al., 1998; Madlener et al., 1998; Armstrong and Jude, 2002). As quantification of the number of neutrophils in the granulation tissue of mmp13−/− wounds was unchanged and no additional cell types, such as keratinocytes, exhibited MMP8 protein in mutant wounds, augmented MMP8 transcript levels in skin very likely originated from enhanced expression of this gene in neutrophils by an as yet unknown mechanism. Thus, increased MMP8 activity may be one of the events during the process of cutaneous wound healing in mmp13 null mice that counteracts the harmful consequences of reduced collagenolytic activity caused by MMP13 deficiency.
In humans, cutaneous wound healing is associated with the induction of another interstitial collagenase, MMP1, which shares a broad overlap in substrate specificity with MMP13, at least in vitro (McCawley and Matrisian, 2001; Sternlicht and Werb, 2001). Facing the fact that a true homologue for MMP1 has not been identified in mice, the issue of functional redundancy in humans might be even more complicated and has to additionally take into account MMP1.
In the face of functional redundancy in skin homeostasis and remodelling, future analyses of isolated cell types, such as keratinocytes, in appropriate tissue culture systems mimicking the in vivo situation, as well as wound healing studies on skin of a variety of knockout mice that are deficient for multiple MMPs, will have to be performed in order to identify the nature of cooperating MMPs and to unravel the underlying molecular mechanism of this functional collagenolytic network, which is obviously absolutely required for survival of a complex higher organism.
MATERIALS AND METHODS
Mice
To generate MMP13-deficient mice, floxed mmp13 mice (Stickens et al., 2004) were crossed to CMV-cre mice (Schwenk et al., 1995), ensuring ubiquitous disruption of the mmp13 gene. The mice used were in a mixed genetic background (C57BL/6;129/SV). The recombination of the floxed mmp13 alleles was analyzed by Southern and Northern blotting. All mice were housed in specific pathogen-free environment and under light-, temperature-, and humidity-controlled conditions. Food and water were available ad libitum. The procedures for performing animal experiments were in accordance with the principles and guidelines of the ATBW (officials for animal welfare) and were approved by the Regierungspräsidium Karlsruhe, Germany.
Wounding study
Control (floxed mmp13) and MMP13-deficient female mice aged 7–8 weeks were anesthetized with Ketanest and four paired 4mm diameter, full-thickness, excisional wounds were made using a sterile biopsy punch through the shaved and fumigated skin on the back of each mouse. At 1, 2, 3, 5, 7, and 14 days post wounding, mice were killed by cervical dislocation and wound tissue and surrounding wound margin skin were harvested and embedded in O.C.T.™ Compound (Tissue Tek, Vogel, Giessen, Germany). For each time point, 6–8 wounds originating from different mice were analyzed. Cryosections (5μm) of the wounds were stained with H&E and documented using a Leica (DMLB) microscope.
Immunofluorescence analyses and immunohistochemistry
Cryosections (5μm) of wounds and tail skin were stained with the following antibody solutions: rabbit anti-mouse loricrin (1:100; BAbCo, Richmond, CA), rabbit anti-mouse keratin-10 (1:250; BAbCo, Richmond, CA), rat anti-mouse CD68 (1:100; Serotec, Düsseldorf, Germany), rat anti-mouse neutrophils (1:400; Serotec, Düsseldorf, Germany), rat anti-mouse CD31 (1:100; Pharmingen, Heidelberg, Germany), rabbit anti-human MMP8 (1:500; Chemicon Europe, Hampshire, UK), rabbit anti-human laminin-5 γ2 (1:2,000; kindly provided by Dr G. Meneguzzi), rat anti-mouse α6-integrin (1:400; kindly provided by Professor A. Sonnenberg), rabbit anti-mouse involucrin (1:800; kindly provided by Professor F. Watt), and guinea-pig anti-human keratin-5 (1:500; generous gift from Dr L. Langbein).
For immunofluorescence analyses, cryosections were fixed in 100% methanol (4°C) for 1minute and acetone (−20°C) for 2 minutes (α6-integrin, laminin-5), in 80% methanol (4°C) for 5 minutes and acetone (−20°C) for 2 minutes (neutrophils, CD31, CD68), in acetone (−20°C) for 2 minutes (keratin-5, keratin-10, loricrin), in 80% methanol (room temperature) for 5 minutes (MMP8), or left unfixed (involucrin). For MMP8, cryosections were incubated for 20 minutes in citrate buffer (2mM citric acid, 10mM tri-sodium citrate, pH 6.0) at 85°C with subsequent rinsing in phosphate-buffered saline (PBS). Cryosections were then blocked with 1% BSA (Sigma, Deisenhofen, Germany) in PBS and incubated with the primary antibodies diluted in the blocking solution for 2 hours at room temperature (α6-integrin, keratin-10, loricrin, laminin-5) or overnight at 4°C (CD31, CD68, neutrophils, MMP8, involucrin, keratin-5). Subsequently, sections were washed with PBS and incubated with Cy3-conjugated goat anti-rat secondary antibody (for α6-integrin, CD31, neutrophils, CD68; 1:400; Dianova, Hamburg, Germany), Cy3-conjugated goat anti-rabbit secondary antibody (for involucrin, loricrin; 1:400; Dianova, Hamburg, Germany), Cy2-conjugated goat anti-rabbit secondary antibody (for keratin-10; 1:400; Dianova, Hamburg, Germany), Cy2-conjugated donkey anti-guinea-pig secondary antibody (for keratin-5; 1:400; Dianova, Hamburg, Germany), or Alexa-488-conjugated goat anti-rabbit secondary antibody (for laminin-5, MMP8; 1:400; Molecular Probes, Göttingen, Germany) for 1hour at room temperature. The nuclei were counterstained with Hoechst dye H33342 (1:1,000; Calbiochem-Novabiochem, Schwalbach, Germany). Finally, the cryosections were rinsed and mounted.
For immunohistochemistry, cryosections were fixed in 100% methanol (4°C) for 5 minutes and acetone (4°C) for 10 minutes. After rinsing with PBS, endogenous peroxidase was quenched with 3% H2O2 in methanol for 10 minutes at room temperature. The cryosections were then blocked with 1% BSA (Sigma, Deisenhofen, Germany) in PBS and incubated with the primary antibodies (keratin-10, loricrin) diluted in blocking solution for 1 hour at room temperature and followed by incubation with a peroxidase-coupled secondary antibody plus streptavidin–peroxidase complex and DAB staining (Vectastain ABC kit, Vector Laboratories, Burlingame, UK). Sections were counterstained with hematoxylin.
Collagen staining
Cryosections (5μm) of wounds and back skin were stained with Picrosirius red as described elsewhere (Junqueira et al., 1979) and imaged on a Zeiss Axioplan microscope. Images were captured using a Hamamatsu 3 CCD cooled camera (Hamamatsu, Hamamatsu City, Japan) and Simple PCI imaging software (Compix Imaging Systems, Pittsburgh, PA).
RNA extraction, Northern blotting, and reverse transcription-PCR
Total RNA was extracted from wounds 1 day after injury using the peqGold RNAPure™ kit (PeqLab, Erlangen, Germany) following the manufacturer’s protocol. For Northern blot analysis, 10μg of total RNA was denatured in glyoxal/DMSO buffer (1M Glyoxal, 10mM Na2HPO4 (pH 6.9), and 50% DMSO) and electrophoresed on a 1.4% agarose gel in 10mM Na2HPO4 (pH 6.9), blotted onto Hybond N+ membrane (Amersham Pharmacia Biotechnology, Freiburg, Germany), and hybridized at 65°C overnight with Church-Gilbert hybridization buffer (1mM EDTA, 0.25M NaPi, 7% SDS). As probes, the complete cDNA for MMP13 (Gack et al., 1995) and a BamHI/XhoI fragment of the MMP9 cDNA spanning exon 3 to exon 8 (Reponen et al., 1995) were used. A cDNA probe for rat GAPDH was taken as an internal control for RNA quality and quantity. Probe labelling with [32P]dCTP was performed using the RediPrime™ Labelling kit (Amersham Pharmacia Biotechnology, Freiburg, Germany).
Single-strand cDNAs were synthesized from 1μg of total RNA using AMV reverse transcriptase and oligo(dT) as primer (Promega, Mannheim Germany); reverse transcriptase products were amplified by semiquantitative PCR for different MMPs to compare transcript amounts. All PCR primer pairs used are intron-spanning on separate exons: MMP2 (495bp) sense 5′-ccgggagcgcaagatgga-3′ and antisense 5′-gagaaaagcgcagcggagtgacg-3′, MMP3 (673bp) sense 5′-attgcatgacagtgcaaggg-3′ and antisense 5′-tggaggacttgtagactggg-3′, MMP8 (484bp) sense 5′-ggcagaggaaggcaggagagg-3′ and antisense 5′-aaggtcaggggcgatgctacact-3′, MMP14 (516bp) sense 5′-ggcctgcctgcatccatcaata-3′ and antisense 5′-actgccctcctcatccacctcaat-3′, Mcol-A (374bp) sense 5′-gaccttccccaaatcccatcc-3′ and antisense 5′-ctcttcctcacaaacagcagcatc-3′. β-Tubulin (401bp) was used as an internal standard using the primers sense 5′-gcgacctgcagctggaccgaatct-3′ and antisense 5′-gggcgagggcaccacactgaagg-3′. For Mcol-A and MMP8, after electrophoresis the PCR products were blotted onto Hybond N+ membrane and hybridized at 65°C overnight with Church-Gilbert hybridization buffer. Hybridizations were performed with the corresponding PCR fragments as probes.
In situ hybridization
For in situ hybridization, 5μm cryosections from wounds were treated and hybridized with 35S-UTP-labelled sense and antisense probes, as described previously (Tuckermann et al., 2000). MMP13 cRNA antisense probe spanning exon 5 to exon 3 (246bp including nucleotides 595 to 429 of the published sequence) was derived by sp6 in vitro transcription from a plasmid containing an MMP13 cDNA fragment spanning exon 1 to exon 5 linearized at an internal StuI site. MMP9 cRNA antisense probe spanning exon 8 to exon 2 (904bp including nucleotides 1,275 to 371 of the published sequence) was derived by T7 in vitro transcription from plasmid linearized at a NotI site.
Supplementary Material
Figure S1. Distance measurements between wound edges of mmp13 >/> and mmp13 −/− mice. Distances were measured by ruler tool of Adobe Photoshop 7.0® software on different H&E and involucrin stained sections of different wounds (n) of day 1, 2, 3, 5 and 7 from mmp13 >/> (black dots) and mmp13−/− mice (red dots).
Figure S2. Histological localization of MMP8 protein at wound sites of mmp13 >/> (upper panel) and mmp13 −/− (lower panel) 3 days post injury (a, b) Neutrophils were stained by immunofluorescence using the antibody neutrophils (red signal). Nuclei were counterstained with Hoechst dye (blue signal). (c–f) MMP8 (c–d, green signal) was visualized by immunofluorescence on parallel sections to (a) and (b), respectively. Nuclei were counterstained with Hoechst dye (e-f, blue signal). Basement membrane is depicted by a dashed line and the granulation tissue is highlighted by a dotted line. Bar: 100μm.
ACKNOWLEDGMENTS
We thank Cornelia Mauch and Gerhard Fürstenberger for generous advice in wound healing experimentations, Fiona Watt, Guerrino Meneguzzi, Dirk Breitkreuz and Lutz Langbein for antibodies, Bernd Arnold for providing CMV-cre mice, and Lore Florin and Jochen Hess for critically reading the manuscript. We gratefully acknowledge Marco Marcello for excellent help with the documentation of the collagen staining by crossed polarized exposures. This work was supported by grants from the National Institutes of Health (CA72006 and AR46238 to ZW), the Deutsche Forschungsge-meinschaft (An 182/6-2) and the Research Training Network (RTN) Program of the European Community.
Abbreviations
- ECM
extracellular matrix
- >
floxed allele
- MMP
matrix metalloproteinase
- PBS
phosphate-buffered saline
Footnotes
CONFLICT OF INTEREST The authors state no conflict of interest.
REFERENCES
- Armstrong DG, Jude EB. The role of matrix metalloproteinases in wound healing. J Am Podiatr Med Assoc. 2002;92:12–18. doi: 10.7547/87507315-92-1-12. [DOI] [PubMed] [Google Scholar]
- Ashcroft GS, Lei K, Jin W, Longenecker G, Kulkarni AB, Greenwell-Wild T, et al. Secretory leukocyte protease inhibitor mediates non-redundant functions necessary for normal wound healing. Nat Med. 2000;6:1147–53. doi: 10.1038/80489. [DOI] [PubMed] [Google Scholar]
- Balbin M, Fueyo A, Knauper V, Pendas AM, Lopez JM, Jimenez MG, et al. Collagenase 2 (MMP-8) expression in murine tissue-remodeling processes. Analysis of its potential role in postpartum involution of the uterus. J Biol Chem. 1998;273:23959–68. doi: 10.1074/jbc.273.37.23959. [DOI] [PubMed] [Google Scholar]
- Balbin M, Fueyo A, Knauper V, Lopez JM, Alvarez J, Sanchez LM, et al. Identification and enzymatic characterization of two diverging murine counterparts of human interstitial collagenase (MMP-1) expressed at sites of embryo implantation. J Biol Chem. 2001;276:10253–62. doi: 10.1074/jbc.M009586200. [DOI] [PubMed] [Google Scholar]
- Balbin M, Fueyo A, Tester AM, Pendas AM, Pitiot AS, Astudillo A, et al. Loss of collagenase-2 confers increased skin tumor susceptibility to male mice. Nat Genet. 2003;35:252–7. doi: 10.1038/ng1249. [DOI] [PubMed] [Google Scholar]
- Basset P, Okada A, Chenard MP, Kannan R, Stoll I, Anglard P, et al. Matrix metalloproteinases as stromal effectors of human carcinoma progression: therapeutic implications. Matrix Biol. 1997;15:535–41. doi: 10.1016/s0945-053x(97)90028-7. [DOI] [PubMed] [Google Scholar]
- Beare AH, O’Kane S, Krane SM, Ferguson MW. Severely impaired wound healing in the collagenase-resistant mouse. J Invest Dermatol. 2003;120:153–63. doi: 10.1046/j.1523-1747.2003.12019.x. [DOI] [PubMed] [Google Scholar]
- Behrendt N. The urokinase receptor (uPAR) and the uPAR-associated protein (uPARAP/Endo180): membrane proteins engaged in matrix turnover during tissue remodeling. Biol Chem. 2004;385:103–36. doi: 10.1515/BC.2004.031. [DOI] [PubMed] [Google Scholar]
- Belotti D, Paganoni P, Manenti L, Garofalo A, Marchini S, Taraboletti G, et al. Matrix metalloproteinases (MMP9 and MMP2) induce the release of vascular endothelial growth factor (VEGF) by ovarian carcinoma cells: implications for ascites formation. Cancer Res. 2003;63:5224–9. [PubMed] [Google Scholar]
- Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, et al. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- Bullard KM, Lund L, Mudgett JS, Mellin TN, Hunt TK, Murphy B, et al. Impaired wound contraction in stromelysin-1-deficient mice. Ann Surg. 1999;230:260–5. doi: 10.1097/00000658-199908000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbridge MF, Coge F, Galizzi JP, Boutin JA, West DC, Tucker GC. The role of the matrix metalloproteinases during in vitro vessel formation. Angiogenesis. 2002;5:215–26. doi: 10.1023/a:1023889805133. [DOI] [PubMed] [Google Scholar]
- Clark RA. The molecular and cellular biology of wound repair. Plenum Press; New York: 1996. Wound repair: overview and general consideration; pp. 3–50. [Google Scholar]
- Dangelo M, Sarment DP, Billings PC, Pacifici M. Activation of transforming growth factor beta in chondrocytes undergoing endochondral ossification. J Bone Miner Res. 2001;16:2339–47. doi: 10.1359/jbmr.2001.16.12.2339. [DOI] [PubMed] [Google Scholar]
- Dano K, Andreasen PA, Grondahl-Hansen J, Kistensen P, Nielson LS, Skriver L. Plasminogen activators, tissue degradation, and cancer. Adv Cancer Res. 1985;44:139–266. doi: 10.1016/s0065-230x(08)60028-7. [DOI] [PubMed] [Google Scholar]
- Devarajan P, Mookhtiar K, Van Wart H, Berliner N. Structure and expression of the cDNA encoding human neutrophil collagenase. Blood. 1991;77:2731–8. [PubMed] [Google Scholar]
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–74. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- Fuchs E. Epidermal differentiation: the bare essentials. J Cell Biol. 1990;111:2807–14. doi: 10.1083/jcb.111.6.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gack S, Vallon R, Schmidt J, Grigoriadis A, Tuckermann J, Schenkel J, et al. Expression of interstitial collagenase during skeletal development of the mouse is restricted to osteoblast-like cells and hypertrophic chondrocytes. Cell Growth Differ. 1995;6:759–67. [PubMed] [Google Scholar]
- Hangai M, Kitaya N, Xu J, Chan CK, Kim JJ, Werb Z, et al. Matrix metalloproteinase-9-dependent exposure of a cryptic migratory control site in collagen is required before retinal angiogenesis. Am J Pathol. 2002;161:1429–37. doi: 10.1016/S0002-9440(10)64418-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbs MS, Postlethwaite AE, Mainardi CL, Seyer JM, Kang AH. Alterations in collagen production in mixed mononuclear leukocyte-fibroblast cultures. J Exp Med. 1983;157:47–59. doi: 10.1084/jem.157.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hieta N, Impola U, Lopez-Otin C, Saarialho-Kere U, Kahari VM. Matrix metalloproteinase-19 expression in dermal wounds and by fibroblasts in culture. J Invest Dermatol. 2003;121:997–1004. doi: 10.1046/j.1523-1747.2003.12533.x. [DOI] [PubMed] [Google Scholar]
- Hiraoka N, Allen E, Apel IJ, Gyetko MR, Weiss SJ. Matrix metalloproteinases regulate neovascularization by acting as pericellular fibrinolysins. Cell. 1998;95:365–77. doi: 10.1016/s0092-8674(00)81768-7. [DOI] [PubMed] [Google Scholar]
- Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA, et al. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99:81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- Holmbeck K, Bianco P, Yamada S, Birkedal-Hansen H. MT1-MMP: a tethered collagenase-1. J Cell Physiol. 2004;200:11–9. doi: 10.1002/jcp.20065. [DOI] [PubMed] [Google Scholar]
- Iba Y, Shibata A, Kato M, Mosukawa T. Possible involvement of mast cells in collagen remodeling in the late phase of cutaneous wound healing in mice. Int Immunopharmacol. 2004;4:1873–80. doi: 10.1016/j.intimp.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Inada M, Wang Y, Byrne MH, Rahman MU, Miyaura C, Lopez-Otin C, et al. Critical roles for collagenase-3 (Mmp13) in development of growth plate cartilage and in endochondral ossification. Proc Natl Acad Sci USA. 2004;101:17192–7. doi: 10.1073/pnas.0407788101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979;11:447–55. doi: 10.1007/BF01002772. [DOI] [PubMed] [Google Scholar]
- Knäuper V, Lopez-Otin C, Smith B, Knight G, Murphy G. Biochemical characfterization of human collagenase-3. J Biol Chem. 1996;271:1544–50. doi: 10.1074/jbc.271.3.1544. [DOI] [PubMed] [Google Scholar]
- Kofford MW, Schwartz LB, Schechter NM, Yager DR, Diegelmann RF, Graham MF. Cleavage of type I procollagen by human mast cell chymase initiates collagen fibril formation and generates a unique carboxyl-terminal propeptide. J Biol Chem. 1997;272:7127–31. doi: 10.1074/jbc.272.11.7127. [DOI] [PubMed] [Google Scholar]
- Krane SM, Byrne MH, Lemaitre V, Henriet P, Jeffrey JJ, Witter JP, et al. Different collagenase gene products have different roles in degradation of type I collagen. J Biol Chem. 1996;271:28509–15. doi: 10.1074/jbc.271.45.28509. [DOI] [PubMed] [Google Scholar]
- Langlois S, Gingras D, Beliveau R. Membrane type 1-matrix metalloproteinase (MT1-MMP) cooperates with sphingosine 1-phosphate to induce endothelial cell migration and morphogenic differentiation. Blood. 2004;103:3020–8. doi: 10.1182/blood-2003-08-2968. [DOI] [PubMed] [Google Scholar]
- Liu X, Wu H, Byrne M, Jeffrey J, Krane S, Jaenisch R. A targeted mutation at the known collagenase cleavage site in mouse type I collagen impairs tissue remodeling. J Cell Biol. 1995;130:227–37. doi: 10.1083/jcb.130.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund LR, Romer J, Bugge TH, Nielsen BS, Frandsen TL, Degen JL, et al. Functional overlap between two classes of matrix-degrading proteases in wound healing. EMBO J. 1999;18:4645–56. doi: 10.1093/emboj/18.17.4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch CC, Matrisian LM. Matrix metalloproteinases in tumor–host cell communication. Differentiation. 2002;70:561–73. doi: 10.1046/j.1432-0436.2002.700909.x. [DOI] [PubMed] [Google Scholar]
- Madlener M, Parks WC, Werner S. Matrix metalloproteinases (MMPs) and their physiological inhibitors (TIMPs) are differentially expressed during excisional skin wound repair. Exp Cell Res. 1998;242:201–10. doi: 10.1006/excr.1998.4049. [DOI] [PubMed] [Google Scholar]
- Mariani TJ, Sandefur S, Roby JD, Pierce RA. Collagenase-3 induction in rat lung fibroblasts requires the combined effects of tumor necrosis factor-alpha and 12-lipoxygenase metabolites: a model of macrophage-induced, fibroblast-driven extracellular matrix remodeling during inflammatory lung injury. Mol Biol Cell. 1998;9:1411–24. doi: 10.1091/mbc.9.6.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massova I, Kotra LP, Fridman R, Mobashery S. Matrix metalloproteinases: structures, evolution, and diversification. FASEB J. 1998;12:1075–95. [PubMed] [Google Scholar]
- Matrisian LM. The matrix-degrading metalloproteinases. BioEssays. 1992;14:455–63. doi: 10.1002/bies.950140705. [DOI] [PubMed] [Google Scholar]
- Mauch C. Matrix metalloproteinase-19: what role does this enzyme play in wound healing? J Invest Dermatol. 2003;121:xix–xx. doi: 10.1046/j.1523-1747.2003.12582.x. [DOI] [PubMed] [Google Scholar]
- Mauch C, Eckes B, Hunzelmann N, Oono T, Kozlowska E, Krieg T. Control of fibrosis in systemic scleroderma. J Invest Dermatol. 1993;100:92S–6S. doi: 10.1111/1523-1747.ep12356293. [DOI] [PubMed] [Google Scholar]
- McCawley LJ, Matrisian LM. Matrix metalloproteinases: they’re not just for matrix anymore! Curr Opin Cell Biol. 2001;13:534–40. doi: 10.1016/s0955-0674(00)00248-9. [DOI] [PubMed] [Google Scholar]
- Mohan R, Chintala SK, Jung JC, Villar WV, McCabe F, Russo LA, et al. Matrix metalloproteinase gelatinase B (MMP-9) coordinates and effects epithelial regeneration. J Biol Chem. 2002;277:2065–72. doi: 10.1074/jbc.M107611200. [DOI] [PubMed] [Google Scholar]
- Mohan R, Rinehart WB, Bargagna-Mohan P, Fini ME. Gelatinase B/lacZ transgenic mice, a model for mapping gelatinase B expression during developmental and injury-related tissue remodeling. J Biol Chem. 1998;273:25903–14. doi: 10.1074/jbc.273.40.25903. [DOI] [PubMed] [Google Scholar]
- Mott JD, Werb Z. Regulation of matrix biology by matrix metalloproteinases. Curr Opin Cell Biol. 2004;16:558–64. doi: 10.1016/j.ceb.2004.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H, et al. The integrin alpha(v)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. J Cell Biol. 2002;157:493–507. doi: 10.1083/jcb.200109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagase H, Woessner JF. Matrix metalloproteinases. J Biol Chem. 1999;274:21491–4. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- Neuhold LA, Killar L, Zhao W, Sung ML, Warner L, Kulik J, et al. Postnatal expression in hyaline cartilage of constitutively active human collagenase-3 (MMP-13) induces osteoarthritis in mice. J Clin Invest. 2001;107:35–44. doi: 10.1172/JCI10564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwomeh BC, Liang HX, Diegelmann RF, Cohen IK, Yager DR. Dynamics of the matrix metalloproteinases MMP-1 and MMP-8 in acute open human dermal wounds. Wound Repair Regen. 1998;6:127–34. doi: 10.1046/j.1524-475x.1998.60206.x. [DOI] [PubMed] [Google Scholar]
- Ohuchi E, Imai K, Fujii Y, Sato H, Seiki M, Okada Y. Membrane type 1 matrix metalloproteinase digests interstitial collagens and other extracellular matrix macromolecules. J Biol Chem. 1997;272:2446–51. doi: 10.1074/jbc.272.4.2446. [DOI] [PubMed] [Google Scholar]
- Okada A, Tomasetto C, Lutz Y, Bellocq JP, Rio MC, Basset P. Expression of matrix metalloproteinases during rat skin wound healing: evidence that membrane type-1 matrix metalloproteinase is a stromal activator of pro-gelatinase A. J Cell Biol. 1997;137:67–77. doi: 10.1083/jcb.137.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall CM, Sodek J. Reciprocal regulation of collagenase, 72 kDa-gelatinase, and TIMP gene expression and protein synthesis in human fibroblasts induced by concanavalin A. Matrix. 1992;(Suppl 1):209–11. [PubMed] [Google Scholar]
- Pilcher BK, Dumin JA, Sudbeck BD, Krane SM, Welgus HG, Parks WC. The activity of collagenase-1 is required for keratinocyte migration on a type I collagen matrix. J Cell Biol. 1997;137:1445–57. doi: 10.1083/jcb.137.6.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planus E, Galiacy S, Matthay M, Laurent V, Gavrilovic J, Murphy G, et al. Role of collagenase in mediating in vitro alveolar epithelial wound repair. J Cell Sci. 1999;112(Part 2):243–52. doi: 10.1242/jcs.112.2.243. [DOI] [PubMed] [Google Scholar]
- Provenzano PP, Alejandro-Osorio AL, Valhmu WB, Jensen KT, Vanderby R., Jr Intrinsic fibroblast-mediated remodeling of damaged collagenous matrices in vivo. Matrix Biol. 2005;23:543–55. doi: 10.1016/j.matbio.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Ravanti L, Kahari VM. Matrix metalloproteinases in wound repair (review) Int J Mol Med. 2000;6:391–407. [PubMed] [Google Scholar]
- Reponen P, Leivo I, Sahlberg C, Apte SS, Olsen BR, Thesleff I, et al. 92-kDa type IV collagenase and TIMP-3, but not 72-kDa type IV collagenase or TIMP-1 or TIMP-2, are highly expressed during mouse embryo implantation. Dev Dyn. 1995;202:388–96. doi: 10.1002/aja.1002020408. [DOI] [PubMed] [Google Scholar]
- Romer J, Bugge TH, Pyke C, Lund LR, Flick MJ, Degen JL, et al. Impaired wound healing in mice with a disrupted plasminogen gene. Nat Med. 1996;2:287–92. doi: 10.1038/nm0396-287. [DOI] [PubMed] [Google Scholar]
- Romer J, Lund LR, Eriksen J, Pyke C, Kristensen P, Dano K. The receptor for urokinase-type plasminogen activator is expressed by keratinocytes at the leading edge during re-epithelialization of mouse skin wounds. J Invest Dermatol. 1994;102:519–22. doi: 10.1111/1523-1747.ep12373187. [DOI] [PubMed] [Google Scholar]
- Romer J, Lund LR, Eriksen J, Ralfkiaer E, Zeheb R, Gelehrter TD, et al. Differential expression of urokinase-type plasminogen activator and its type-1 inhibitor during healing of mouse skin wounds. J Invest Dermatol. 1991;97:803–11. doi: 10.1111/1523-1747.ep12486833. [DOI] [PubMed] [Google Scholar]
- Saarialho-Kere UK. Patterns of matrix metalloproteinase and TIMP expression in chronic ulcers. Arch Dermatol Res. 1998;290(Suppl):S47–54. doi: 10.1007/pl00007453. [DOI] [PubMed] [Google Scholar]
- Salo T, Makela M, Kylmaniemi M, Autio-Harmainen H, Larjava H. Expression of matrix metalloproteinase-2 and -9 during early human wound healing. Lab Invest. 1994;70:176–82. [PubMed] [Google Scholar]
- Schenk S, Quaranta V. Tales from the crypt[ic] sites of the extracellular matrix. Trends Cell Biol. 2003;13:366–75. doi: 10.1016/s0962-8924(03)00129-6. [DOI] [PubMed] [Google Scholar]
- Schwenk F, Baron U, Rajewsky K. A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res. 1995;23:5080–1. doi: 10.1093/nar/23.24.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seandel M, Noack-Kunnmann K, Zhu D, Aimes RT, Quigley JP. Growth factor-induced angiogenesis in vivo requires specific cleavage of fibrillar type I collagen. Blood. 2001;97:2323–32. doi: 10.1182/blood.v97.8.2323. [DOI] [PubMed] [Google Scholar]
- Seiki M. The cell surface: the stage for matrix metalloproteinase regulation of migration. Curr Opin Cell Biol. 2002;14:624–32. doi: 10.1016/s0955-0674(02)00363-0. [DOI] [PubMed] [Google Scholar]
- Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–46. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- Sodek J, Overall CM. Matrix metalloproteinases in periodontal tissue remodelling. Matrix. 1992;1(Suppl):352–62. [PubMed] [Google Scholar]
- Stamenkovic I. Extracellular matrix remodelling: the role of matrix metalloproteinases. J Pathol. 2003;200:448–64. doi: 10.1002/path.1400. [DOI] [PubMed] [Google Scholar]
- Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickens D, Behonick DJ, Ortega N, Heyer B, Hartenstein B, Yu Y, et al. Altered endochondral bone development in matrix metalloproteinase 13-deficient mice. Development. 2004;131:5883–95. doi: 10.1242/dev.01461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam EM, Moore TR, Butler GS, Overall CM. Characterization of the distinct collagen binding and cleavage mechanisms of matrix metalloproteinase 2 and 14 (gelatinase A and MT1-MMP): the differential roles of the MMP hemopexin C domains and the MMP-2 fibronectin type II modules in collagen triple helicase activities. J Biol Chem. 2004;279:43336–44. doi: 10.1074/jbc.M407186200. [DOI] [PubMed] [Google Scholar]
- Tam EM, Wu YI, Butler GS, Stack MS, Overall CM. Collagen binding properties of the membrane type-1 matrix metalloproteinase (MT1-MMP) hemopexin C domain. The ectodomain of the 44-kDa autocatalytic product of MT1-MMP inhibits cell invasion by disrupting native type I collagen cleavage. J Biol Chem. 2002;277:39005–14. doi: 10.1074/jbc.M206874200. [DOI] [PubMed] [Google Scholar]
- Tuckermann JP, Pittois K, Partridge NC, Merregaert J, Angel P. Collagenase-3 (MMP-13) and integral membrane protein 2a (Itm2a) are marker genes of chondrogenic/osteoblastic cells in bone formation: sequential temporal, and spatial expression of Itm2a, alkaline phosphatase, MMP-13, and osteocalcin in the mouse. J Bone Miner Res. 2000;15:1257–65. doi: 10.1359/jbmr.2000.15.7.1257. [DOI] [PubMed] [Google Scholar]
- Vu TH, Werb Z. Matrix metalloproteinases: effectors of development and normal physiology. Genes Dev. 2000;14:2123–33. doi: 10.1101/gad.815400. [DOI] [PubMed] [Google Scholar]
- Wang H, Parry S, Macones G, Sammel MD, Ferrand PE, Kuivaniemi H, et al. Functionally significant SNP MMP8 promoter haplotypes and preterm premature rupture of membranes (PPROM) Hum Mol Genet. 2004;13:2659–69. doi: 10.1093/hmg/ddh287. [DOI] [PubMed] [Google Scholar]
- Warner RL, Bhagavathula N, Nerusu KC, Lateef H, Younkin E, Johnson KJ, et al. Matrix metalloproteinases in acute inflammation: induction of MMP-3 and MMP-9 in fibroblasts and epithelial cells following exposure to pro-inflammatory mediators in vitro. Exp Mol Pathol. 2004;76:189–95. doi: 10.1016/j.yexmp.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Werb Z, Vu TH, Rinkenberger JL, Coussens LM. Matrix-degrading proteases and angiogenesis during development and tumor formation. APMIS. 1999;107:11–8. doi: 10.1111/j.1699-0463.1999.tb01521.x. [DOI] [PubMed] [Google Scholar]
- Wolf K, Sandner P, Kurtz A, Moll W. Messenger ribonucleic acid levels of collagenase (MMP-13) and matrilysin (MMP-7) in virgin, pregnant, and postpartum uterus and cervix of rat. Endocrinology. 1996;137:5429–34. doi: 10.1210/endo.137.12.8940367. [DOI] [PubMed] [Google Scholar]
- Wu N, Jansen ED, Davidson JM. Comparison of mouse matrix metalloproteinase 13 expression in free-electron laser and scalpel incisions during wound healing. J Invest Dermatol. 2003;121:926–32. doi: 10.1046/j.1523-1747.2003.12497.x. [DOI] [PubMed] [Google Scholar]
- Xu J, Rodriguez D, Petitclerc E, Kim JJ, Hangai M, Moon YS, et al. Proteolytic exposure of a cryptic site within collagen type IV is required for angiogenesis and tumor growth in vivo. J Cell Biol. 2001;154:1069–79. doi: 10.1083/jcb.200103111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Flannery ML, Kupriyanov S, Pearce J, McKercher SR, Henkel GW, et al. Defective trophoblast function in mice with a targeted mutation of Ets2. Genes Dev. 1998;12:1315–26. doi: 10.1101/gad.12.9.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163–76. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Distance measurements between wound edges of mmp13 >/> and mmp13 −/− mice. Distances were measured by ruler tool of Adobe Photoshop 7.0® software on different H&E and involucrin stained sections of different wounds (n) of day 1, 2, 3, 5 and 7 from mmp13 >/> (black dots) and mmp13−/− mice (red dots).
Figure S2. Histological localization of MMP8 protein at wound sites of mmp13 >/> (upper panel) and mmp13 −/− (lower panel) 3 days post injury (a, b) Neutrophils were stained by immunofluorescence using the antibody neutrophils (red signal). Nuclei were counterstained with Hoechst dye (blue signal). (c–f) MMP8 (c–d, green signal) was visualized by immunofluorescence on parallel sections to (a) and (b), respectively. Nuclei were counterstained with Hoechst dye (e-f, blue signal). Basement membrane is depicted by a dashed line and the granulation tissue is highlighted by a dotted line. Bar: 100μm.