Abstract
West Nile virus, which is transmitted by Culex mosquitoes while feeding on birds and humans, has emerged as the dominant vector borne disease in North America. We have identified natural compounds from humans and birds, which are detected with extreme sensitivity by olfactory receptor neurons (ORNs) on the antennae of Culex pipiens quinquefasciatus (Cx. quinquefasciatus). One of these semiochemicals, nonanal, dominates the odorant spectrum of pigeons, chickens, and humans from various ethnic backgrounds. We determined the specificity and sensitivity of all ORN types housed in different sensilla types on Cx. quinquefasciatus antennae. Here, we present a comprehensive map of all antennal ORNs coding natural ligands and their dose-response functions. Nonanal is detected by a large array of sensilla and is by far the most potent stimulus; thus, supporting the assumption that Cx. quinquefasciatus can smell humans and birds. Nonanal and CO2 synergize, thus, leading to significantly higher catches of Culex mosquitoes in traps baited with binary than in those with individual lures.
Keywords: bird odorants, human odorants across different ethnicities, nonanal-baited traps, semiochemicals, single sensillum recordings
Olfaction has a major role in host seeking in mosquitoes (1–4). They perceive semiochemicals with antennae and maxillary palps. Sensilla in these appendages house olfactory receptor neurons (ORNs) that detect a plethora of chemicals originating from skin, breath, plant, nectar, and oviposition sites. There are ≈1,300 antennal sensilla in the Southern house mosquito, Culex pipiens quinquefasciatus (Cx. quinquefasciatus) Say (Diptera: Culicidae) that have olfactory function (5). Long-range host detection in mosquitoes starts with interactions between odorants (semiochemicals) and a distinct subpopulation of odorant receptors (ORs) embedded in the dendritic membrane of ORNs. Odorant-binding proteins (OBPs), present in large concentrations in the sensillar lymph surrounding the ORNs, are postulated to participate in the early perireceptor events in olfaction (6), including uptake of odorants, transport through the sensillar lymph, and delivery to ORs. Several OBPs have been isolated and cloned from Culex spp. (7–9), and CquiOBP1 has been mapped to a specific sensilla type on antenna, and functional studies showed binding of this OBP to semiochemicals involved in the chemical ecology of this mosquito species (10).
Mosquitoes occupy diverse habitats, feed on a range of animals, and transmit many life-threatening diseases. West Nile virus (WNV) emerged in North America in 1999, and has since spread across the United States, Canada, Mexico, and the Caribbean Basin, causing morbidity and mortality in humans, horses, and hundreds of bird species (11). Most of the early transmission of WNV was mediated by Cx. pipiens, but the introduction of WNV into the warmer southern United States, where Cx. quinquefasciatus feeds as adults throughout the year, exacerbated the situation. Also, it enabled frequent transmission opportunities to migrating birds close to their wintering quarters in Central and South America (12).
Captive and free-ranging birds have been used for decades as living sentinels in arbovirus (arthropod borne viruses) surveillance programs (13). Birds and bird derived odorants have been demonstrated as attractive to Culex spp. (14, 15). A high degree of anthropophily was also shown for Cx. quinquefasciatus in Africa (16). Also, feeding shifts from birds to humans in Culex are well documented in the literature (17).
Here, we describe how nonanal, a dominant constituent in odorant profiles from birds and humans, is detected with remarkable sensitivity by ORNs on Cx. quinquefasciatus antennae. We also provide a list of potent semiochemicals that are detected with high sensitivity by other ORNs. Also, we discuss how nonanal could be involved in the unique shifts in Culex feeding between birds and humans (17).
Results
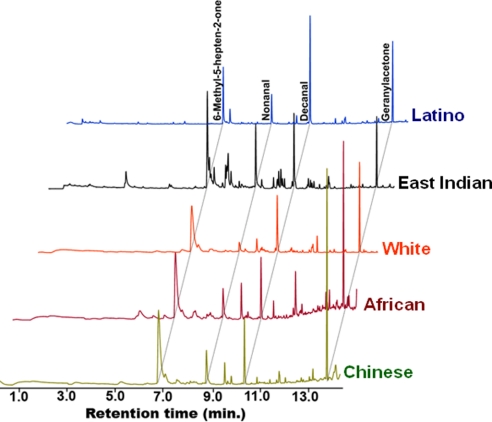
Odorant profiles of humans from various ethnic backgrounds, collected by solid phase microextraction (SPME) (Fig. S1), were dominated by four compounds, namely, 6-methyl-5-hepten-2-one, nonanal, decanal, and geranylacetone (Fig. 1). The ratio of these compounds differed across the sampling groups that comprised of Latino (three subjects; one male and two females), East Indian (one male), White (seven males and one female), Black (two males), and Chinese (two males), ranging from 20 to 55 years of age.
Fig. 1.
Representative odorant profiles from humans of various ethnicities. Total ion chromatogram obtained from human volunteers after SPME direct collection and GC-MS analysis. Regardless of ethnic background, all samples comprised of four major constituents whose peaks are labeled in the uppermost trace. Identification was confirmed with synthetic standards.
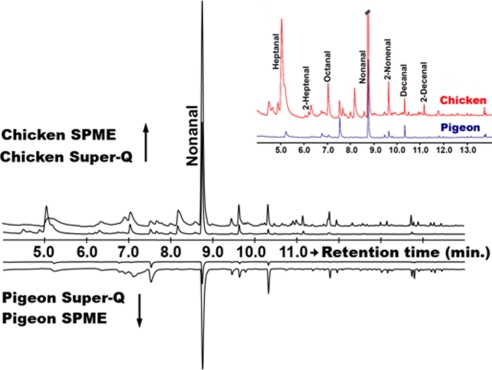
We then compared the human profiles with those obtained from birds, the principal hosts of Cx. quinquefasciatus. Attaching a SPME fiber proved difficult in birds due to perching behavior. Initially, we attached a SPME fiber under the wing and wrapped it around with a stretchable tape. The most dominant peak detected in these trials was nonanal. Next, we enclosed a live bird (chicken or pigeon) in a glass desiccator and trapped the headspace odor on a SPME fiber and a polymer adsorbent SuperQ in tandem. Nonanal was by far the major peak in the profiles of both chicken and pigeon, regardless of the collection method (Fig. 2). SPME and SuperQ profiles were remarkably similar both quantitatively and qualitatively (Fig. 2 Inset).
Fig. 2.
Odorant profiles from pigeon and chicken obtained by SuperQ and SPME extraction and GC-MS analysis. Nonanal was the major peak detected in chicken and pigeon samples collected by SPME and SuperQ polymer adsorbent. A series of straight chain, saturated and unsaturated aldehydes were detected in minor amounts (Inset).
By employing mosquito antenna as the sensing element (Fig. 3A), we next addressed the question on what constituents of bird and human headspace would be relevant to Cx. quinquefasciatus sensory ecology. Consistently, nonanal elicited response from Cx. quinquefasciatus antenna (Fig. 3B; Fig. S2). Injections of synthetic compounds identified in human and bird volatile collections elicited antennal responses, except for 6-methyl-5-hepten-2-one (Fig. 3C). Due to the prevalence of aldehydes as minor constituents of bird odor spectra (Fig. 2 Inset), we also tested a synthetic mixture of aliphatic straight-chain saturated C3-C10 aldehydes. Antennal responses to heptanal, octanal, nonanal, and decanal were reproducibly recorded (Fig. S3).
Fig. 3.
Analysis of bird- and human-derived odorants by GC-EAD. (A) A live nonbloodfed Cx. quinquefasciatus female was fixed in a truncated pipette tip and secured by modeling clay letting only the eyes and antenna exposed. (Inset) Ground electrode impaled in the eye and two antennae inserted into the recording electrode. Antennal responses to (B) chicken odorants collected on SuperQ polymer and fractionated by gas chromatography and (C) a synthetic mixture containing 50 ng of each human-derived odorants (from left to right: 6-methyl-5-heptene-2-one, nonanal, (E)-2-nonenal, decanal, indole, and geranylacetone). (E)-2-nonenal and indole were added as internal standards. Upper traces (blue) are EAD responses. Upward deflections are due to mechanical disturbances during the GC-EAD runs. Nonanal was the only EAD-active peak in chicken extracts (n = 3). Despite several trials (n = 5), we did not observe EAD response to 6-methyl-5-heptene-2-one. (Scale bar in both the GC-EAD runs, 0.2 mV.)
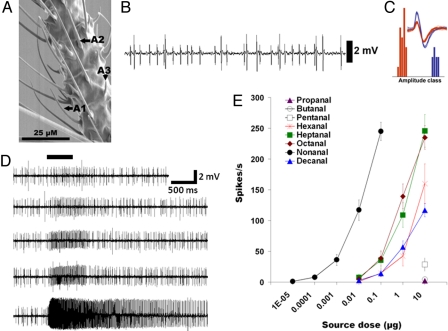
We surmised that Cx. quinquefasciatus antenna should be equipped with very sensitive ORNs to detect nonanal, the key headspace component of bird odors and also a major constituent of human headspace volatiles. We then performed single unit recordings from >700 olfactory sensilla on antennae to characterize ORNs that detect the identified volatiles, in particular, nonanal. We extended this analysis by adding a large array of volatiles described in literature as potentially involved in host- and sugar-seeking-behavior. Nonanal was detected by ORNs located in a trichoid sensillum (A2) (Fig. 4 A–C) with extreme sensitivity (Fig. 4 D and E). This sensillum houses two ORNs as inferred by the distinct spontaneous firing of cells with two spike amplitudes (Fig. 4 B and C). Other related aldehydes showed at least three log dose higher response threshold (Fig. 4E). The other colocated ORN in this sensillum responded to indole and o-cresol in a dose-dependent manner (Fig. S4). Of the 56 complete recordings from this type of sensilla, all but one responded to nonanal with very high sensitivity.
Fig. 4.
Cx. quinquefasciatus antenna, sensilla, and ORNs detecting nonanal, the key constituent of host odors. (A) A scanning electron micrograph of the 12th antennal segment showing three distinct classes of sensilla (named after ref. 5): thin sharp tipped (A1), thick blunt tipped sensilla (A2), and grooved pegs (A3), the shortest sensilla. (B) A2 type houses two ORNs with distinct spontaneous firing rates and amplitudes revealed by extracellular single-unit recordings. (C) Amplitude-frequency histograms with an inset showing two distinct neuronal spike clusters (blue and red). (D) Neurons of large amplitude responded to nonanal with extreme sensitivity. Excitatory neuronal response elicited by increasing dose of nonanal: 0.001 to 100 ng in decadic steps (top to bottom). (E) Dose-response curves for a homologous series of aldehydes, C3-C10 (n = 9–13 ± SEM).
Another sensillum type, A1 (Fig. 4A), houses two ORNs (Fig. S5A). We observed two morphologically distinct populations, namely, long (≈60 μm) and short (≈25 μm) trichoid A1 sensilla. Despite being challenged with >100 odorants, we were unable to stimulate long A1 sensilla, in marked contrast to short A1 trichoids. Therefore, all results reported here pertain to the short sensilla. We recorded from 112 sensilla that were sensitive to plant compounds (Table 1). Of notice, cell A responded to racemic linalool, and cell B was stimulated by skatole, whereas racemic 1-octen-3-ol elicited excitatory responses from both cells (Fig. S5A). In addition to plant compounds (Table 1; Fig. S6), this sensillum type responded to two of the constituents of human volatiles, geranylacetone (Fig. S6) and 6-methyl-5-hepten-2-one (Table 1) with strong and mild activity, respectively. Racemic linalool and skatole generated the strongest, dose-dependent responses (Figs. S5 B and C and S6), but their thresholds were at least three-log dose higher than nonanal threshold response by A2s (see above). In addition to these sensilla responding to plant and human compounds, we recorded also from two functionally distinct classes of A1 sensilla. One houses ORNs sensitive to the insect repellent DEET, whereas the other type responded to a mosquito oviposition pheromone (MOP) (10, 18).
Table 1.
Response spectra of the Cx. quinquefasciatus ORNs elicited by various chemostimuli
| Sensillum type | Response category | Chemostimuli |
|---|---|---|
| Sharp trichoid (A1) | Strong | Rac. Linalool, Eucalyptol, αβ-Thujone, Skatole, Geranylacetone, Rac. 1-Octen-3-ol, Ethylhexanoate |
| Mild | Indole, o-Cresol, 6-Methyl-5-hepten-2-one, 5-Methyl-2-hexanone, 2-Heptanone, Citral, Nerol, Geraniol, 4-Methyl-cyclohexanol, Heptalactone | |
| Blunt trichoid (A2) | Strong | Nonanal, Indole, o-Cresol, Heptanal, Octanal |
| Mild | Phenol, (E)-2-hexenal, 3 & 6-Methyl indole, 7-Octenoic acid | |
| Grooved peg (A3) | Strong | Ammonia, Trimethylamine, Propylamine, Butylamine |
| Mild | Pentanoic acid, Hexanoic acid, Lactic acid, Cadaverine, Putrescine |
Responses were categorized based on the counts of action potentials on stimulation with a chemostimuli at 10 μg source dose. Mild, 25–50% increase; strong, 50–100% increase. Percentage increase is determined after the maximum percentage increase of the excitation of a nonanal sensitive ORN (250 spikes/s) in response to 100 ng source dose. All compounds generating strong responses were subsequently tested for dose-response function. Chemicals in boldface are those that were consistently present in human and/or birds. Chemostimuli in each row are arranged according to the response strength (decreasing).
It is worth mentioning that sometimes we observed reversal in the spike amplitudes of two ORNs colocalized within the same trichoid sensilla. For example, in A2, frequently ORN with the larger spike amplitude responded to nonanal, whereas the smaller spiking ORN was sensitive to indole. However, we also observed in a small, although significant population of these sensilla, small spike amplitude responses to nonanal (while indole eliciting large spikes) and even firing of both ORNs with similar amplitude. We did not observe any clear pattern to allow correlation of change in amplitude of the two colocated ORNs with the point of contact on or near the recording sensillum. Due to this unique phenomenon in trichoids, we did not categorize responses as excitation from “ORN A” or “ORN B,” but instead, we broadly grouped responses from sensilla to chemostimuli (Table 1). It is worth mentioning that response kinetics (rise and fall) and magnitude were consistent regardless of responses with small or large spike amplitude.
Grooved pegs were difficult to record due to their smaller size and changing amplitude with time. A total 50 sensilla were tested for all odorant panel and they responded to polar compounds. All of the grooved pegs responded by increasing their firing rate to homologous series of aliphatic carboxylic acids of C2-C8 chain length and amines of C3-C6 chain length. Three distinct ORNs were observed (Fig. S7) with cell A, B, and C responding to ammonia, propylamine, and the mosquito oviposition attractant trimethylamine (TMA) (10), respectively. ORNs with larger amplitude always responded to ammonia and alkyl amines, whereas carboxylic acids and trimethylamine elicited excitatory responses from ORN with smallest amplitude. Ammonia and TMA elicited dose-dependent excitatory responses (Figs. S7 and S8). Within alkylamines, the shorter the chain length the more active they were (Fig. S8).
The so called human specific acids, 7-octenoic acid and 3-methyl-2-hexenoic acid (19), elicited excitatory responses from the nonanal-detecting ORNs. The 7-octenoic acid responses were dose-dependent, but showed threshold three order of magnitude higher than nonanal. This compound elicited 20 ± 5 spikes/s (n = 4; mean ± SEM) at 100 ng, 22 ± 7 (1 μg), 59 ± 12 (10 μg), and 107 ± 22 (100 μg). Responses to racemic 3-methy-2-hexenoic acid were even lower, with a dose of 100 μg eliciting only 37 ± 10 spikes (n = 5; mean ± SEM).
In marked contrast to ORNs in maxillary palps that showed 100- to 1,000-fold selectivity to enantiomers of linalool and 1-octen-3-ol (20), antennal ORNs did not discriminate stereoisomers of these compounds. Racemic 1-octen-3-ol at source dose of 10 μg elicited strong responses in A1 type sensilla (165 ± 9 spikes/s), whereas (R)-(−)-1-octen-3-ol and (S)-(+)-1-octen-3-ol elicited comparable responses (145 ± 13 and 119 ± 13 spikes/s, respectively). A colocated ORN in these sensilla were also excited, but showed much lower responses: 48 ± 22, 50 ± 6, and 31 ± 10 spikes/s, respectively. Racemic linalool elicited 243 ± 25 spikes at a source dose of 10 μg, whereas l-(−)- and d-(+)-linalool generated 251 ± 13 and 230 ± 10 spikes/s, respectively.
Last, we tested whether the major human- and bird-derived odorant, nonanal, could be used as attractant for field populations of Culex mosquitoes. Encephalitis Vector Survey (EVS) traps baited with low dosage of nonanal (0.1 mg) captured significantly more Culex mosquitoes than control traps (Fig. 5A). Observations of mosquito behavior approaching nonanal-baited traps with low-light video recordings indicate that only a very small percentage of attracted mosquitoes were captured. This is not surprising, given that these traps were designed for CO2 bait. We then compared catches in traps baited with CO2 alone and those loaded with CO2 and nonanal. Nonanal and CO2 synergized, thus, leading to significantly higher captures in traps baited with the binary lure than with CO2 alone (Fig. 5B). Also, we compared in a separate set of trials captures of Culex mosquitoes in trap baited with single and binary lures. Again, the synergistic effect of a nonanal-CO2 lure was demonstrated by significantly higher catches in traps baited with binary bait as compared with single lures (Fig. 5C). When tested at 10× higher doses, the synergistic effect of nonanal was reduced (mean ± SEM: CO2, 1,626 ± 123 Culex mosquitoes per trap per night; CO2 plus nonanal, 1,881 ± 136; nonanal, 11 ± 1.9; blank, 0.7 ± 0.3; n = 6). Although catches in traps baited with binary lures were significantly higher than in traps loaded with CO2 alone (P = 0.23; Tukey HSD), the synergistic effect at this dosage led only to 16% increase in capture, as opposed to 50% (Fig. 5B) and 66% (Fig. 5C) with lower dosage of nonanal.
Fig. 5.
Captures of Culex mosquitoes in traps baited with nonanal, CO2, or a combination of these two attractants. (A) Nonanal-baited traps caught significantly more mosquitoes than control traps. (B) Synergistic effect of nonanal indicated by significantly higher captures (50% increase) in traps baited with a combination of CO2 and nonanal than those loaded with CO2 alone. (C) Similar results (66% increase) were obtained when the combined lure was compared with individual attractants in a separate location. Treatments followed by the same letters are not significantly different at the 5% level according to Tukey HSD.
Discussion
In our prospecting for mosquito attractants, we analyzed semiochemicals derived from humans and birds, which may be involved in long distance orientation of Culex mosquitoes to potential hosts. Surprisingly, we found chemical commonalities that may explain the well-documented host shifts in Culex (17), which lead to the transmission of WNV. We developed a simple system for collecting human odorants at the skin surface, employing SPME technology (18), and identified odorant profiles from various ethnic groups (Fig. 1). Of these compounds, nonanal, decanal, and geranylacetone showed strong electroantennographic detection (EAD) responses (Fig. 3). Our prospecting for bird odorants revealed a clearly dominant nonanal peak in the odorant profiles from pigeon and chicken (Fig. 2), which have been used as sentinels in surveillance programs because of their ability to attract Culex mosquitoes (13, 21–24). We also observed that the chromatograms, particularly from chicken, are dominated by a series of aldehydes (Fig. 2 Inset). With an improved GC-EAD system, in which a live mosquito was restrained to record high signal-to-noise antennal responses, nonanal was the only odorant from pigeon and chicken that elicited EAD response by the antennae of the Southern house mosquito. To identify that ORNs tuned to the reception of host-derived semiochemicals, possibly involved in host shifts, we carried out an extensive single sensillum recordings (SSR) from >700 antennal sensilla by challenging them with >100 odorants, including those identified from humans and birds. Cx. quinquefasciatus antennae are enodowed with ORNs that respond with remarkably high sensitivity to nonanal (Fig. 4) even higher than the gold standard pheromone-detecting neurons in moths. The nonanal-sensitive ORNs were located in blunt tipped trichoid sensilla (A2 type). This sensillum type is more abundant in ornithophilic species among Culex complex (5). Therefore, we suggest that the increased number and extreme sensitivity of this sensillum type enables Cx. quinquefasciatus long range host detection by smelling nonanal. Also, occurrence of nonanal as a key constituent of headspace odor in both humans and birds seems to facilitate the host shifts (17) that lead to WNV transmission from birds to humans. Of the total ≈1,300 olfactory sensilla on Cx. quinquefasciatus antenna, ≈500 are A2 type (5, 25), underlying the importance of this sensillum type in the sensory ecology of Culex mosquitoes. Another broad category of olfactory sensilla, A1, did not respond with extreme sensitivity to any host odor components identified in this study. The last category of antennal olfactory sensilla, A3 type, responded to “ubiquitous host odors.” We identified ORNs detecting amines and acids to be colocated in the grooved peg sensilla. ORNs detecting ammonia are found on almost all blood feeding insects studied so far (26–31). Lactic acid was previously shown to excite ORNs in Cx. pipiens (32). In our recordings, no strong responses were detected to lactic acid.
Nonanal has been implicated in the chemical ecology of various blood feeding insects. In triatomine bugs, it elicited excitatory responses in one of the grooved peg sensillum and induced behavioral responses in combination with isobutyric acid (33). And in Aedes aegypti, it elicited strong responses from one of the blunt tipped (A2) sensilla (34). Nonanal also elicited EAG and behavioral response in host seeking adult Ae. aegypti females (35). Although nonanal was found in fresh and incubated sweat samples, no EAG activity was observed in Anopheles gambiae (36). Here, we demonstrated that nonanal per se is a mosquito attractant, as inferred by significantly higher captures of Culex mosquitoes in nonanal-baited than in control traps. Also, nonanal synergizes with the well-known mosquito bait, CO2; thus, significantly enhancing performance of traps baited with nonanal/CO2 binary lure.
WNV is now the dominant vector born disease in North America (37). While birds serve as reservoir, the virus is transmitted to dead end hosts like humans and horses by mosquitoes that had previously fed on birds. A clear preference for birds in Cx. quinquefasciatus was reported (15, 38) and comprehensively revised recently (39). However, Culex species are uniquely adapted to switch hosts based on host availability and/or abundance as clearly demonstrated by 7-fold shift in feeding preferences from birds to humans during late summer when bird hosts dispersed (17). Here, we identified that the odorant bouquet of birds and humans have a common semiochemical, nonanal, which is detected with remarkable sensitivity by the olfactory system of the Southern house mosquito. Given the acute sensitivity of the nonanal-detecting ORNs and the abundance of the sensilla (≈40% of all olfactory detectors in the antennae) housing these neurons, it is tempting to speculate that nonanal may be involved in the well documented host shifts between humans and birds that lead to WNV transmission.
Materials and Methods
Collection of Human Odorants.
Human subjects washed their forearm exhaustively with warm tap water for at least 10 min before the experiments. A gray SPME fiber where a polymeric stationary phase is coated onto fused silica fiber (StableFlex, divinylbenzene/Carboxen on polydimethylsiloxane; 50/30 μm coating; Supelco) was attached to the forearm by a Johnson & Johnson cloth tape. The forearm was wrapped loosely in a cylinder made of aluminum foil. The cylinder was carefully slipped over the arm without disturbing the SPME fiber and both ends were loosely attached to the skin with cloth tape (Fig. S1). Exposing SPME needle close to the skin surface for 1 h collected appreciable amounts of volatiles, as indicated by collection of ≈20 ng of decanal (Fig. 1) in the uppermost trace. In a limited sampling, we did not observe any significant differences between 30-min and 1-h exposure. We sampled Latinos (one male and two females), East Indian (one male), white (seven males and one female), black (two males from Ghana), and Chinese (two males).
Collection of Bird Odorants.
A live pigeon, Columba livia, of unknown age and sex (wild caught from Elk grove region, Sacramento-Yolo district, CA) or chicken, Gallus gallus (white leghorn chicken female 6-months-old) was placed in an airtight desiccator fitted with an inlet and outlet tubes. Charcoal-filtered room air entered through an inlet, and the outlet was connected to a glass cartridge containing 50 mg of SuperQ 80/100 60–80 mesh (Alltech). Laboratory vacuum service sucked air over the birds on to the adsorbent. Volatiles were desorbed with dichloromethane (HPLC grade; Acros). Also, a SPME fiber (above) was exposed in the air stream before the SuperQ trap for 1 h, retracted and immediately injected into GC-MS for the analysis.
Field Experiments.
EVS traps (Bioquip) baited with nonanal, CO2, nonanal plus CO2, or unbaited (control) were deployed in the Yolo Bypass Wildlife Area, Davis, CA, from July 17 to August 11, 2009. Dry ice (1 kg) was used as CO2 source, whereas nonanal was released from plastic pellets (4–5 mm in diameter) made of polyethylene-vinyl acetate (Fuji Flavor) incorporated with 0.1 or 1 mg of the attractant. Traps were hung on trees before sunset, with the height adjusted to have the collection bags 10 cm above the ground, and with an intertrap distance of at least 100 m. Nonanal was released by one pellet per trap, held in a Teflon perforated tube (diameter, 0.5 cm; height, 3 cm), and attached to the top of the trap's battery cover. Traps were inspected soon after sunrise, brought back to the lab, and redeployed in a rotated position in the evening. Capture data were transformed to log (× + 1) and analyzed by ANOVA and Tukey's honestly significant difference (HSD).
A separate set of traps were dedicated to record behavioral responses of approaching mosquitoes. Videos were recorded for 3 h during the peak activity, with a Sony Camcorder (DCR-DVD810) equipped with infrared light source, set on a tripod, and placed 50 cm away from the traps.
Additional materials and methods are described in the SI Text.
Supplementary Material
Acknowledgments.
We thank Bedoukian Research for providing enantiomeric samples of linalool, 1-octen-3-ol, and 7-octenoic acid; Dr. John A. Pickett (Rothamsted Research, Harpenden, United Kingdom) for a sample of 3-methyl-2-hexenoic acid; Dr. Anthony J. Cornel (Department of Entomology, University of California, Davis) for supplying mosquitoes; Sara Wheeler (Center for Vector Borne Disease, University of California, Davis) for providing birds; Dr. Gabrielle Nevitt (Department of Neurobiology, Physiology, and Behavior, University of California, Davis) for guidance in bird volatile collections and enlightening discussion; Tara Thiemann and Dr. William K. Reisen (Center for Vector Borne Disease, University of California, Davis); and Mary Schiedt (California Department of Fish and Game, Sacramento, CA) for logistic support of field experiments. Animal experiments followed approved protocols (IACUC 12877 and 12878). This work was funded in part by the National Institutes of Health, a cooperative research agreement with Bedoukian Research, and the National Science Foundation (0918177).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0906932106/DCSupplemental.
References
- 1.Dethier VG. The sensory physiology of blood-sucking arthropods. Exp Parasitol. 1957;6:68–122. doi: 10.1016/0014-4894(57)90009-7. [DOI] [PubMed] [Google Scholar]
- 2.Cork A. Olfactory basis of host location by mosquitoes and other haematophagous Diptera. Ciba Found Symp. 1996;200:71–84. doi: 10.1002/9780470514948.ch7. and discussion (1996) 200:84–88. [DOI] [PubMed] [Google Scholar]
- 3.Lehane JM. Biology of Blood Sucking Insects. London: Harper Collins Academia; 1991. [Google Scholar]
- 4.Gibson G, Torr SJ. Visual and olfactory responses of haematophagous Diptera to host stimuli. Med Vet Entomol. 1999;13:2–23. doi: 10.1046/j.1365-2915.1999.00163.x. [DOI] [PubMed] [Google Scholar]
- 5.McIver SB. Comparative study of antennal sense organs of female Culicine mosquitoes. Can Entomol. 1970;102:1258–1267. [Google Scholar]
- 6.Leal WS. Pheromone reception. Top Curr Chem. 2005;240:1–36. [Google Scholar]
- 7.Ishida Y, Cornel AJ, Leal WS. Identification and cloning of a female antenna-specific odorant-binding protein in the mosquito Culex quinquefasciatus. J Chem Ecol. 2002;28:867–871. doi: 10.1023/a:1015253214273. [DOI] [PubMed] [Google Scholar]
- 8.Ishida Y, Cornel AJ, Leal WS. Odorant-binding protein from Culex tarsalis, the most competent vector of West Nile virus in California. J Asia Pacific Entomol. 2003;6:1–4. [Google Scholar]
- 9.Pelletier J, Leal WS. Genome analysis and expression petterns of odorant-binding proteins from the Southern house mosquito Culex pipiens quinquefasciatus. PLoS ONE. 2009;4:e6237. doi: 10.1371/journal.pone.0006237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leal WS, et al. Reverse and conventional chemical ecology approaches for the development of oviposition attractants for Culex mosquitoes. PLoS ONE. 2008;3:e3045. doi: 10.1371/journal.pone.0003045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kramer LD, Styer LM, Ebel GD. A global perspective on the epidemiology of West Nile virus. Ann Rev Entomol. 2008;53:61–81. doi: 10.1146/annurev.ento.53.103106.093258. [DOI] [PubMed] [Google Scholar]
- 12.Granwehr BP, et al. West Nile virus: Where are we now? Lancet Infec Dis. 2004;4:547–556. doi: 10.1016/S1473-3099(04)01128-4. [DOI] [PubMed] [Google Scholar]
- 13.Komar N. In: West Nile Virus: Detection, Surveillance, and Control. White DJ, Morse DL, editors. Vol 951. New York: New York Academy of Sciences; 2001. pp. 58–73. [DOI] [PubMed] [Google Scholar]
- 14.McIver SB. Host preferences and discrimination by mosquitoes Aedes aegypti and Culex tarsalis (Diptera Culicidae) J Med Entomol. 1968;5:422–428. doi: 10.1093/jmedent/5.4.422. [DOI] [PubMed] [Google Scholar]
- 15.Allan SA, Bernier UR, Kline DL. Laboratory evaluation of avian odors for mosquito (Diptera : culicidae) attraction. J Med Entomol. 2006;43:225–231. doi: 10.1603/0022-2585(2006)043[0225:leoaof]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 16.Mboera LEG, Takken W. Odour-mediated host preference of Culex quinquefasciatus in Tanzania. Entomol Exp Appl. 1999;92:83–88. [Google Scholar]
- 17.Kilpatrick AM, Kramer LD, Jones MJ, Marra PP, Daszak P. West Nile virus epidemics in North America are driven by shifts in mosquito feeding behavior. PLoS Biol. 2006;4:606–610. doi: 10.1371/journal.pbio.0040082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Syed Z, Leal WS. Mosquitoes smell and avoid the insect repellent DEET. Proc Natl Acad Sci USA. 2008;105:13598–13603. doi: 10.1073/pnas.0805312105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Costantini C, et al. Electroantennogram and behavioural responses of the malaria vector Anopheles gambiae to human-specific sweat components. Med Vet Entomol. 2001;15:259–266. doi: 10.1046/j.0269-283x.2001.00297.x. [DOI] [PubMed] [Google Scholar]
- 20.Syed Z, Leal WS. Maxillary palps are broad spectrum odorant detectors in Culex quinquefasciatus. Chem Senses. 2007;32:727–738. doi: 10.1093/chemse/bjm040. [DOI] [PubMed] [Google Scholar]
- 21.Cherry B, et al. In: West Nile Virus: Detection, Surveillance, and Control. White DJ, Morse DL, editors. Vol 951. New York: New York Academy of Sciences; 2001. pp. 343–346. [Google Scholar]
- 22.Deegan CS, et al. Sentinel pigeon surveillance for West Nile virus by using lard-can traps at differing elevations and canopy cover classes. J Med Entomol. 2005;42:1039–1044. doi: 10.1093/jmedent/42.6.1039. [DOI] [PubMed] [Google Scholar]
- 23.Darbro JM, Harrington LC. Bird-baited traps for surveillance of West Nile mosquito vectors: Effect of bird species, trap height, and mosquito escape rates. J Med Entomol. 2006;43:83–92. doi: 10.1093/jmedent/43.1.83. [DOI] [PubMed] [Google Scholar]
- 24.Reisen WK, Hardy JL, Presser SB. Evaluation of domestic pigeons as sentinels for detecting arbovirus activity in southern California. Am J Trop Med Hyg. 1992;46:69–79. doi: 10.4269/ajtmh.1992.46.69. [DOI] [PubMed] [Google Scholar]
- 25.Hill SR, Hansson BS, Ignell R. Characterization of antennal trichoid sensilla from female Southern house mosquito, Culex quinquefasciatus Say. Chem Senses. 2009;34:231–252. doi: 10.1093/chemse/bjn080. [DOI] [PubMed] [Google Scholar]
- 26.Haggart DA, Davis EE. Ammonia sensitive neurons on the 1st tarsi of the tick, Rhipicephalus-Sanguineus. J Insect Physiol. 1980;26:517–523. [Google Scholar]
- 27.Meijerink J, Braks MAH, Van Loon JJA. Olfactory receptors on the antennae of the malaria mosquito Anopheles gambiae are sensitive to ammonia and other sweat-borne components. J Insect Physiol. 2001;47:455–464. doi: 10.1016/s0022-1910(00)00136-0. [DOI] [PubMed] [Google Scholar]
- 28.Pappenberger B, Geier M, Boeckh J. In: Olfaction in Mosquito-Host Interactions, Ciba Foundation Symposia. Bock GR, Cardew G, editors. Vol 200. New York: John Wiley and Sons; 1996. pp. 254–266. [Google Scholar]
- 29.Steullet P, Guerin PM. Identification of vertebrate volatiles stimulating olfactory receptors on tarsus-I of the tick Amblyomma variegatum Fabricius (Ixodidae). 2. Receptors outside the Hallers organ capsule. J Comp Physiol A. 1994;174:39–47. doi: 10.1007/BF00192003. [DOI] [PubMed] [Google Scholar]
- 30.Taneja J, Guerin PM. Ammonia attracts the haematophagous bug Triatoma infestans: Behavioural and neurophysiological data on nymphs. J Comp Physiol A. 1997;181:21–34. [Google Scholar]
- 31.van den Broek IVF, den Otter CJ. Odour sensitivity of antennal olfactory cells underlying grooved pegs of Anopheles gambiae s. s. and An. quadriannulatus. Entomol Exp Appl. 2000;96:167–175. [Google Scholar]
- 32.Bowen MF. Sensilla basiconica (Grooved pegs) on the antennae of female mosquitos - electrophysiology and morphology. Entomol Exp Appl. 1995;77:233–238. [Google Scholar]
- 33.Guerenstein PG, Guerin PM. Olfactory and behavioural responses of the blood-sucking bug Triatoma infestans to odours of vertebrate hosts. J Exp Biol. 2001;204:585–597. doi: 10.1242/jeb.204.3.585. [DOI] [PubMed] [Google Scholar]
- 34.Ghaninia M, Larsson M, Hansson BS, Ignell R. Natural odor ligands for olfactory receptor neurons of the female mosquito Aedes aegypti: Use of gas chromatography-linked single sensillum recordings. J Exp Biol. 2008;211:3020–3027. doi: 10.1242/jeb.016360. [DOI] [PubMed] [Google Scholar]
- 35.Logan JG, et al. Identification of human-derived volatile chemicals that interfere with attraction of Aedes aegypti mosquitoes. J Chem Ecol. 2008;34:308–322. doi: 10.1007/s10886-008-9436-0. [DOI] [PubMed] [Google Scholar]
- 36.Meijerink J, et al. Identification of olfactory stimulants for Anopheles gambiae from human sweat samples. J Chem Ecol. 2000;26:1367–1382. [Google Scholar]
- 37.LaDeau SL, Marra PP, Kilpatrick AM, Calder CA. West Nile Virus revisited: Consequences for North American ecology. Bioscience. 2008;58:937–946. [Google Scholar]
- 38.Hardy LH, Reeves CW. Experimental Studies on Infection in Vectors. Sacramento, CA: California Mosquito and Vector Control Association; 1990. [Google Scholar]
- 39.Cooperband MF, McElfresh JS, Millar JG, Carde RT. Attraction of female Culex quinquefasciatus Say (Diptera : Culicidae) to odors from chicken feces. J Insect Physiol. 2008;54:1184–1192. doi: 10.1016/j.jinsphys.2008.05.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.