Abstract
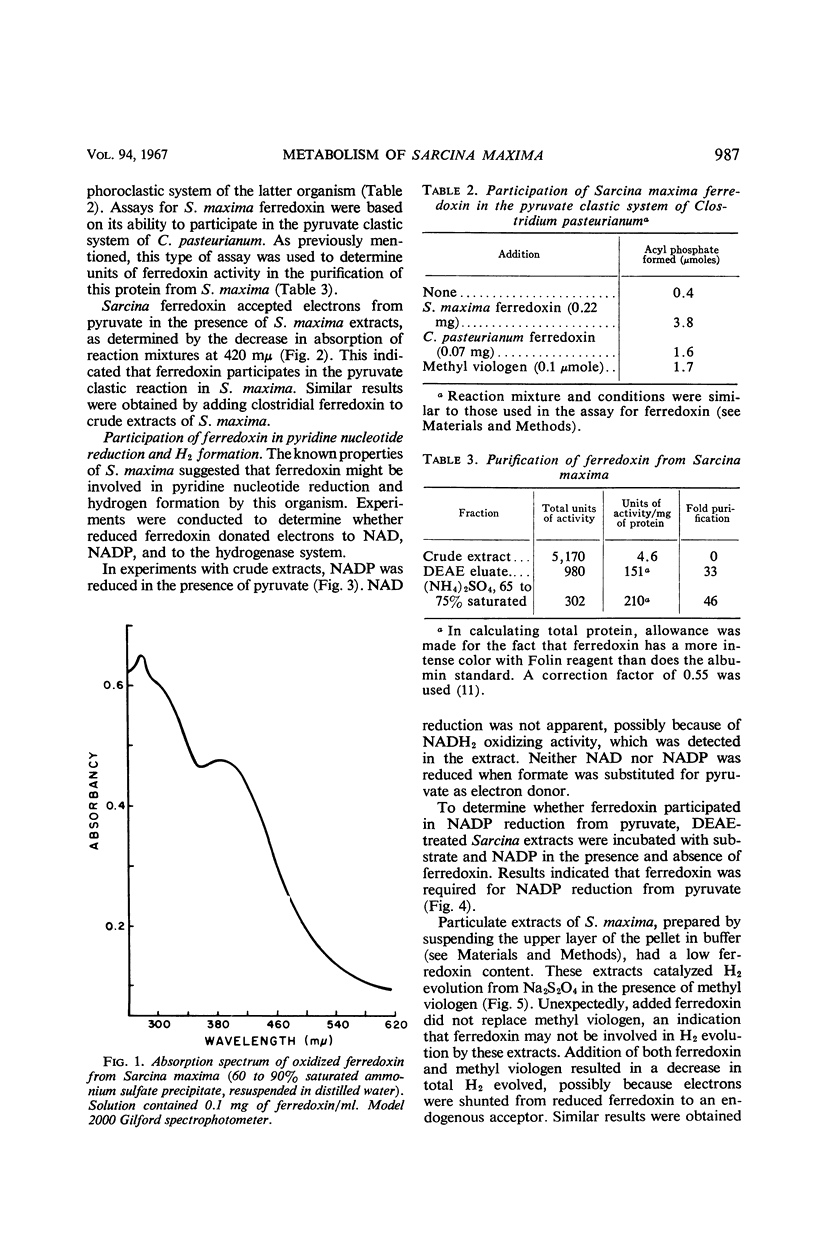
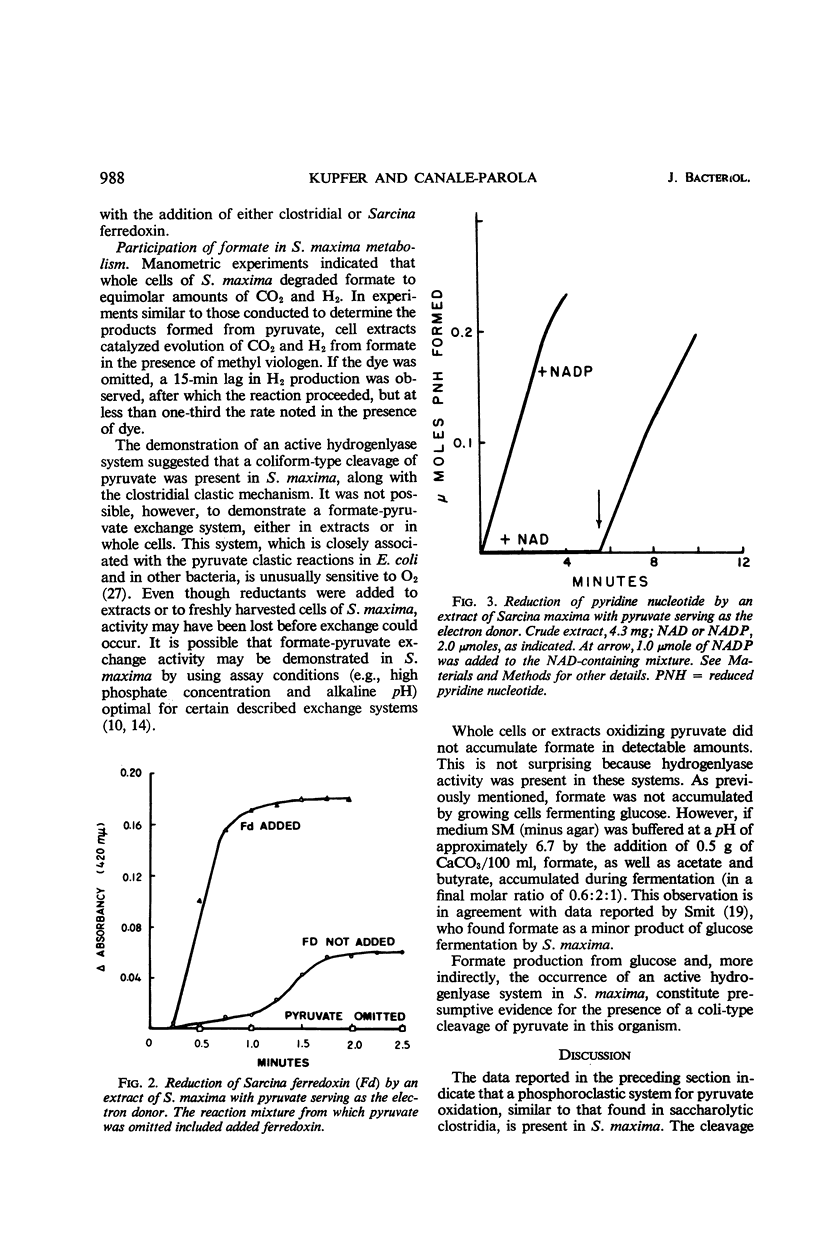
The mechanisms of pyruvate cleavage and hydrogen production by Sarcina maxima were studied. It was found that a phosphoroclastic system for pyruvate oxidation, similar to that occurring in saccharolytic clostridia, is present in S. maxima. Cleavage of pyruvate by extracts of the latter organism resulted in the formation of acetyl phosphate, CO2, and electrons which were transferred to ferredoxin. Formate was not an intermediate in this system. Pyruvate oxidation was coupled with ferredoxin-dependent nicotinamide adenine dinucleotide phosphate (NADP) reduction. A hydrogenase, active in particulate extracts of S. maxima, did not accept electrons from reduced ferredoxin. Formate was detected as a fermentation product when S. maxima was grown in media buffered with CaCO3. Whole cells and extracts degraded formate to H2 and CO2. The evidence suggests that electrons generated by ferredoxin-linked pyruvate oxidation by S. maxima are not used for H2 production, but that they serve for the reduction of NADP. Reduced NADP may be utilized by the organisms for synthesis of cell material. Production of H2 by S. maxima may occur through a pyruvate clastic system similar to that present in coliform bacteria.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUCHANAN B. B., LOVENBERG W., RABINOWITZ J. C. A comparison of clostridial ferredoxins. Proc Natl Acad Sci U S A. 1963 Mar 15;49:345–353. doi: 10.1073/pnas.49.3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CANALE-PAROLA E., WOLFE R. S. Studies on Sarcina ventriculi. I. Stock culture method. J Bacteriol. 1960 Jun;79:857–859. doi: 10.1128/jb.79.6.857-859.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISHERWOOD F. A., HANES C. S. Separation and estimation of organic acids on paper chromatograms. Biochem J. 1953 Dec;55(5):824–830. doi: 10.1042/bj0550824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MORTENSON L. E., VALENTINE R. C., CARNAHAN J. E. An electron transport factor from Clostridium pasteurianum. Biochem Biophys Res Commun. 1962 Jun 4;7:448–452. doi: 10.1016/0006-291x(62)90333-9. [DOI] [PubMed] [Google Scholar]
- McCormick N. G., Ordal E. J., Whiteley H. R. DEGRADATION OF PYRUVATE BY MICROCOCCUS LACTILYTICUS I. : General Properties of the Formate-Exchange Reaction. J Bacteriol. 1962 Apr;83(4):887–898. doi: 10.1128/jb.83.4.887-898.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVELLI G. D. The exchange of H14COOH with the carboxyl group of pyruvate by Clostridium butylicum and Micrococcus lactilyticus. Biochim Biophys Acta. 1955 Dec;18(4):594–596. doi: 10.1016/0006-3002(55)90170-0. [DOI] [PubMed] [Google Scholar]
- PECK H. D., Jr, GEST H. A new procedure for assay of bacterial hydrogenases. J Bacteriol. 1956 Jan;71(1):70–80. doi: 10.1128/jb.71.1.70-80.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSE I. A., GRUNBERG-MANAGO M., KOREY S. R., OCHOA S. Enzymatic phosphorylation of acetate. J Biol Chem. 1954 Dec;211(2):737–756. [PubMed] [Google Scholar]
- STADTMAN E. R., BARKER H. A. Fatty acid synthesis by enzyme preparations of Clostridium kluyveri. VI. Reactions of acyl phosphates. J Biol Chem. 1950 Jun;184(2):769–793. [PubMed] [Google Scholar]
- TWAROG R., WOLFE R. S. Enzymatic phosphorylation of butyrate. J Biol Chem. 1962 Aug;237:2474–2477. [PubMed] [Google Scholar]
- VALENTINE R. C. BACTERIAL FERREDOXIN. Bacteriol Rev. 1964 Dec;28:497–517. doi: 10.1128/br.28.4.497-517.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VALENTINE R. C., BRILL W. J., SAGERS R. D. FERREDOXIN LINKED DPN REDUCTION BY PYRUVATE IN EXTRACTS OF CLOSTRIDIUM ACIDI-URICI. Biochem Biophys Res Commun. 1963 Aug 1;12:315–319. doi: 10.1016/0006-291x(63)90303-6. [DOI] [PubMed] [Google Scholar]
- VALENTINE R. C., WOLFE R. S. ROLE OF FERREDOXIN IN THE METABOLISM OF MOLECULAR HYDROGEN. J Bacteriol. 1963 May;85:1114–1120. doi: 10.1128/jb.85.5.1114-1120.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLFE R. S., O'KANE D. J. Cofactors of the carbon dioxide exchange reaction of Clostridium butyricum. J Biol Chem. 1955 Aug;215(2):637–643. [PubMed] [Google Scholar]
- Wilson J., Krampitz L. O., Werkman C. H. Reversibility of a phosphoroclastic reaction. Biochem J. 1948;42(4):598–600. doi: 10.1042/bj0420598. [DOI] [PMC free article] [PubMed] [Google Scholar]


